Abstract
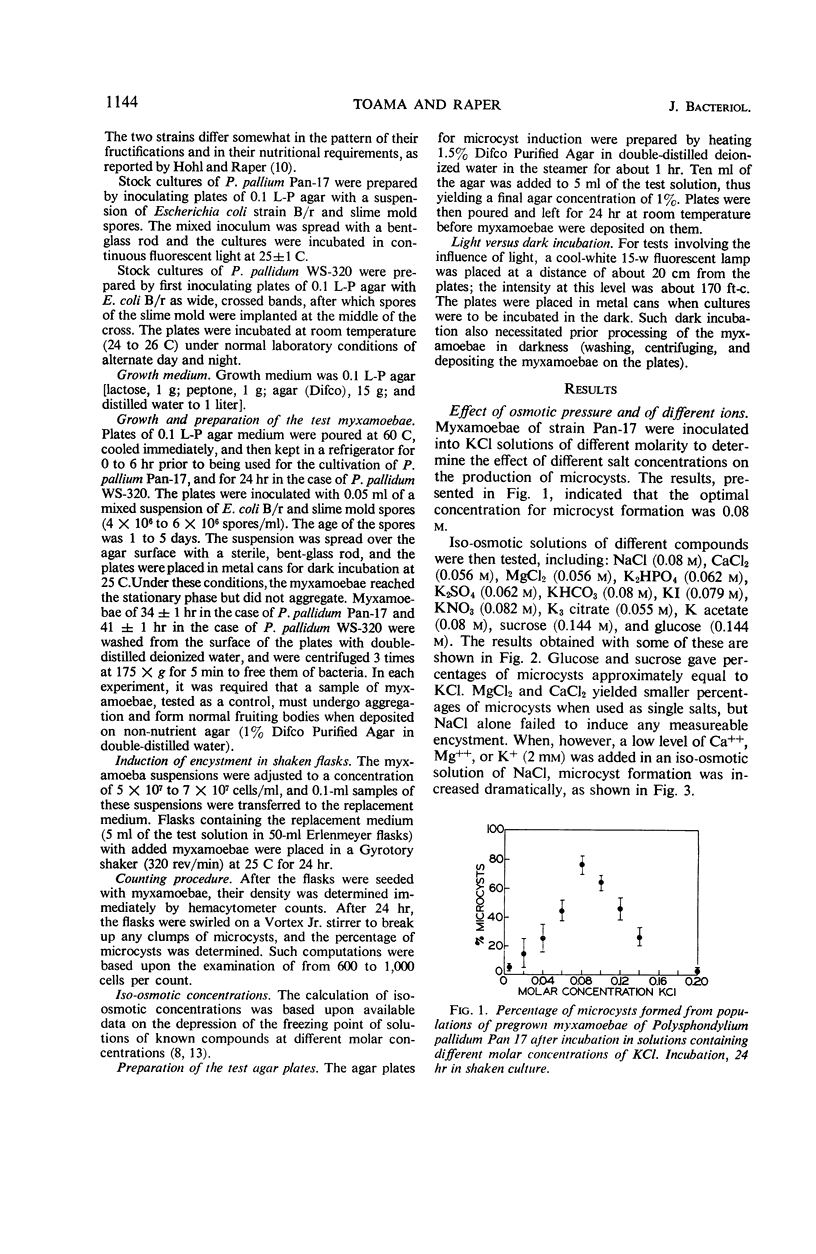
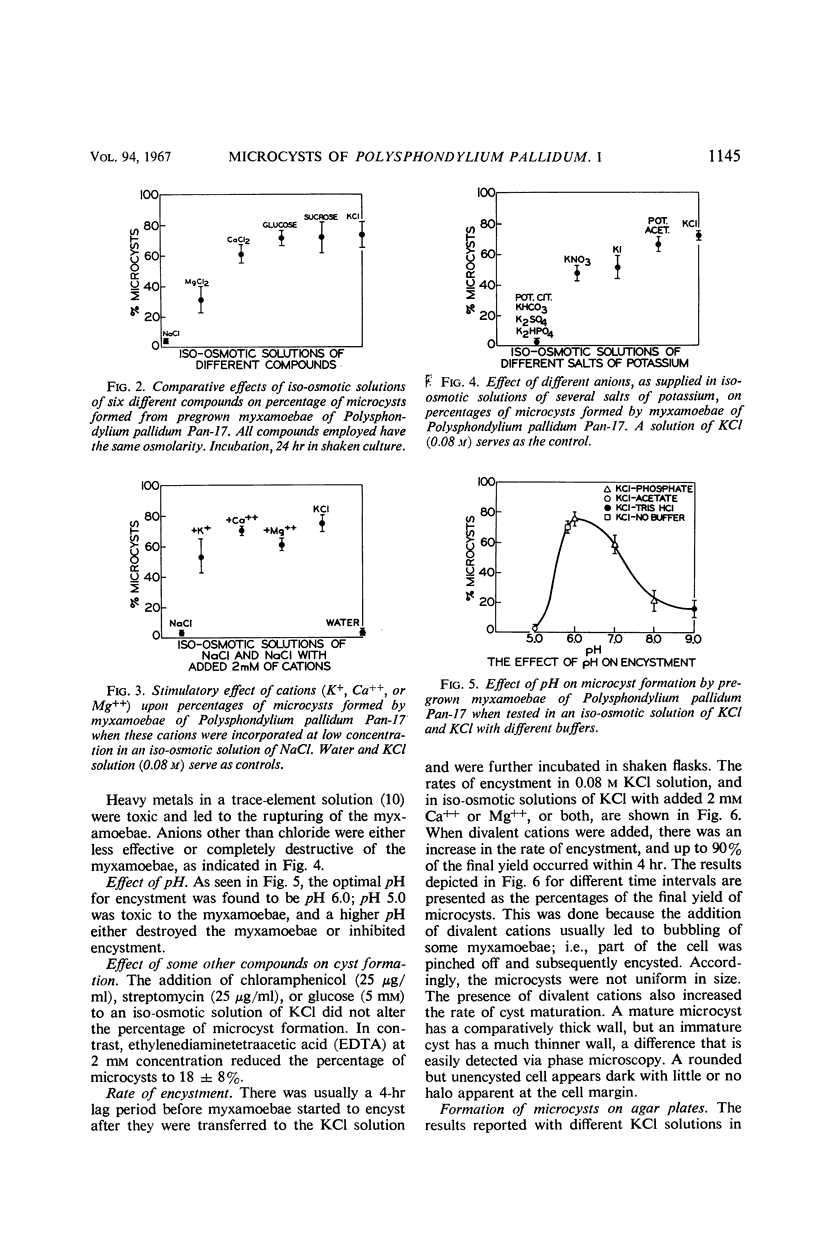
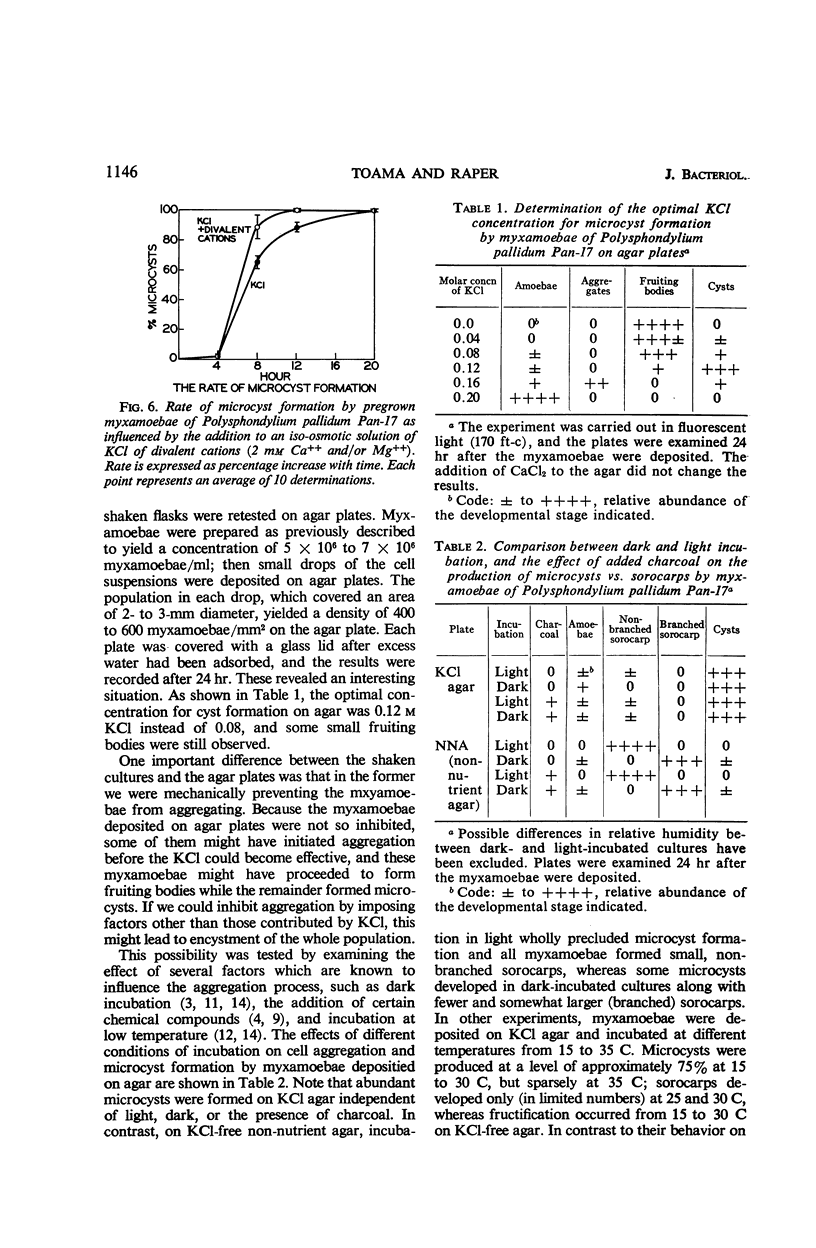
Microcyst formation can be induced by increasing the osmotic pressure of the surrounding medium. Certain ions such as K+, Ca++, or Mg++ may be needed in the encystment process, and the presence of divalent cations increases the rate of encystment and cyst maturation. Chloride of potassium is optimal for encystment, but other anions of potassium are either less effective or toxic. The optimal pH for encystment was found to be pH 6.0. The use of agar plates containing KCl revealed the importance to the encystment process of inhibiting cell aggregation. When myxamoebae of Polysphondylium pallidum strain Pan-17 are deposited on KCl-agar plates, approximately 20% of the population proceeds through aggregation to sorocarp formation at the concentration of KCl optimal for microcyst formation. However, the same proportion of myxamoebae remains unaligned, or forms defective aggregation centers, if synergistic inhibitors (such as incubation in darkness or at low temperature) are employed in addition to KCl. The possibility that this is due to heterocytosis has been excluded. Accordingly, it is suggested that during the stationary phase approximately 20% of the population becomes committed to forming component cells of fruiting bodies, and that these myxamoebae cannot be induced to form microcysts by exposure to KCl. In P. pallidum strains WS-320 on the other hand, the imposition of synergistic inhibitors leads to the total encystment of the cell population. This suggests that, in contrast to Pan-17, the myxamoebae of the latter strain remain potentially equal and exhibit minimal presumptive specialization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONNER J. T., HOFFMAN M. E. EVIDENCE FOR A SUBSTANCE RESPONSIBLE FOR THE SPACING PATTERN OF AGGREGATION AND FRUITING IN THE CELLULAR SLIME MOLDS. J Embryol Exp Morphol. 1963 Sep;11:571–589. [PubMed] [Google Scholar]
- FAUST R. G., FILOSA M. F. Permeability studies on the amoebae of the slime mold, Dictyostelium mucoroides. J Cell Comp Physiol. 1959 Dec;54:297–298. doi: 10.1002/jcp.1030540313. [DOI] [PubMed] [Google Scholar]
- Francis D. Acrasin and the development of Polysphondylium pallidum. Dev Biol. 1965 Dec;12(3):329–346. doi: 10.1016/0012-1606(65)90001-1. [DOI] [PubMed] [Google Scholar]
- HIRSCHBERG E., RUSCH H. P. Effects of compounds of varied biochemical action on the aggregation of a slime mold, Dictyostelium discoideum. J Cell Physiol. 1950 Aug;36(1):105–113. doi: 10.1002/jcp.1030360108. [DOI] [PubMed] [Google Scholar]
- HOHL H., RAPER K. B. NUTRITION OF CELLULAR SLIME MOLDS. III. SPECIFIC GROWTH REQUIREMENTS OF POLYSPHONDYLIUM PALLIDUM. J Bacteriol. 1963 Dec;86:1314–1320. doi: 10.1128/jb.86.6.1314-1320.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konijn T. M. Chemotaxis in the cellular slime molds. I. The effect of temperature. Dev Biol. 1965 Dec;12(3):487–497. doi: 10.1016/0012-1606(65)90011-4. [DOI] [PubMed] [Google Scholar]


