Abstract
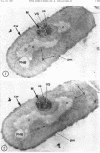
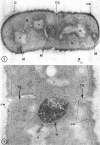
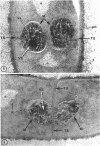
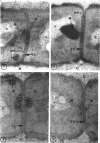
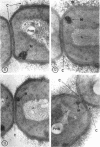
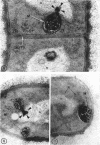
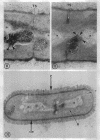
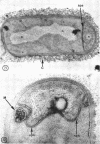
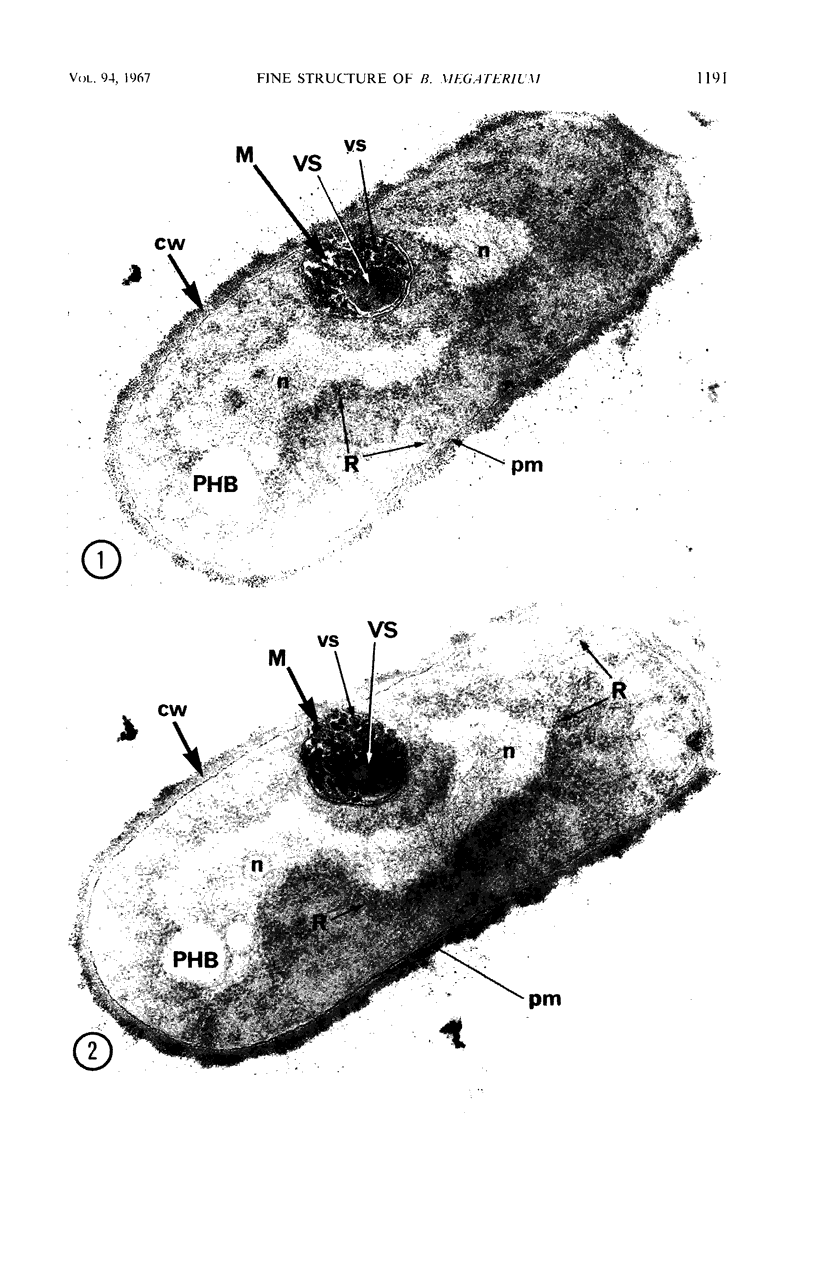
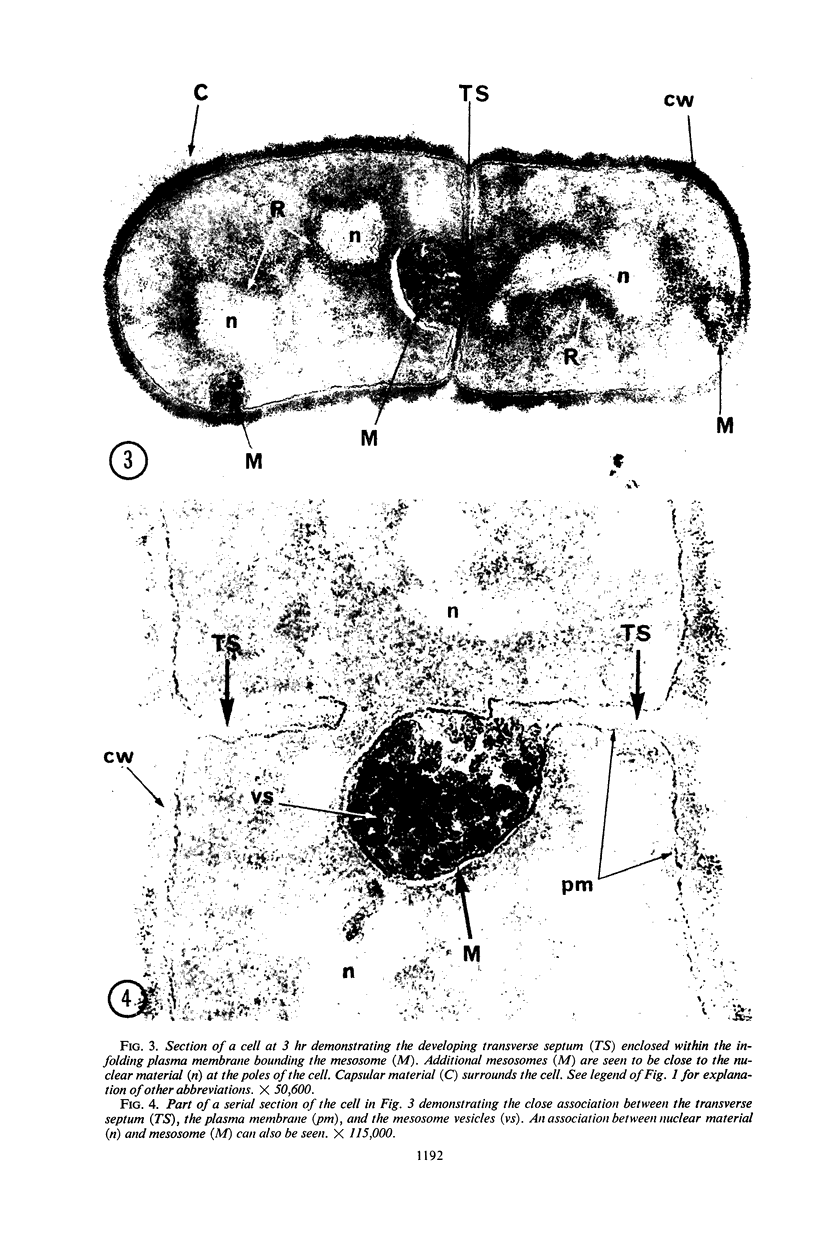
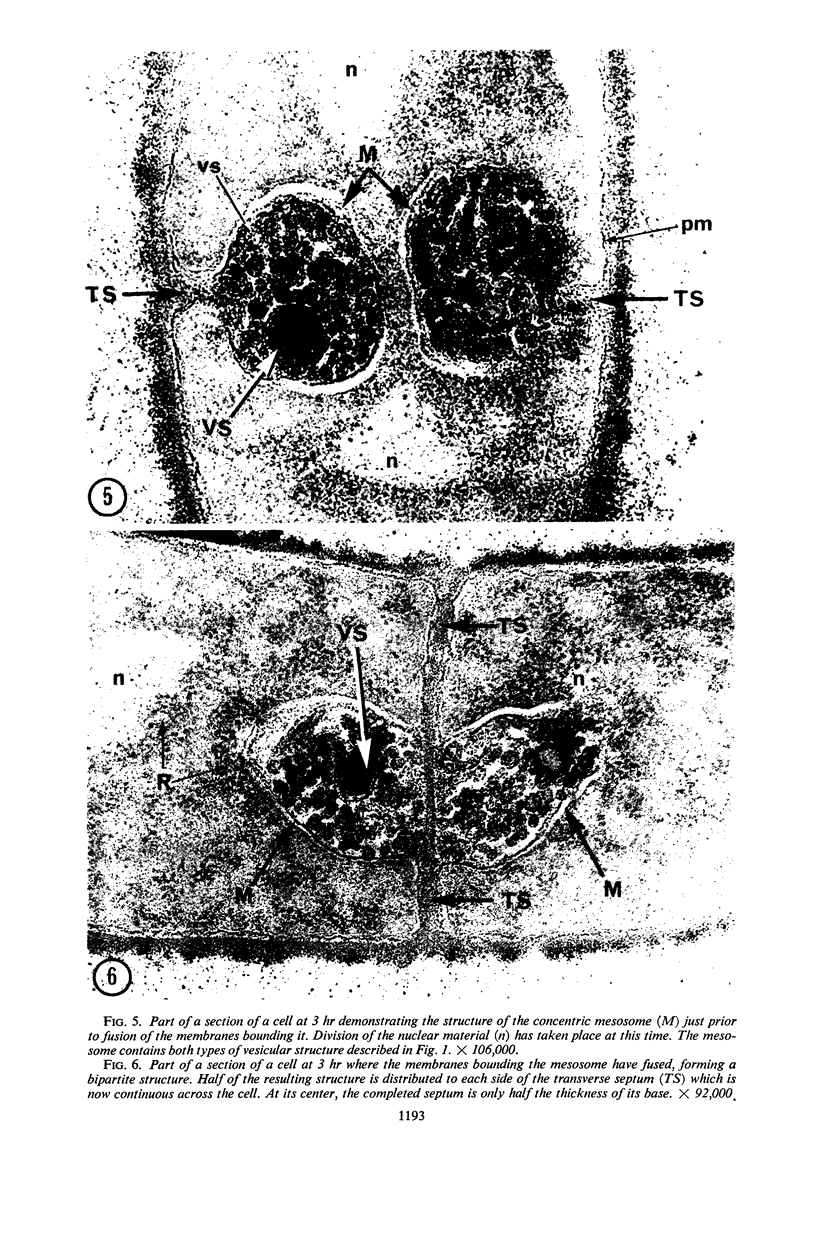
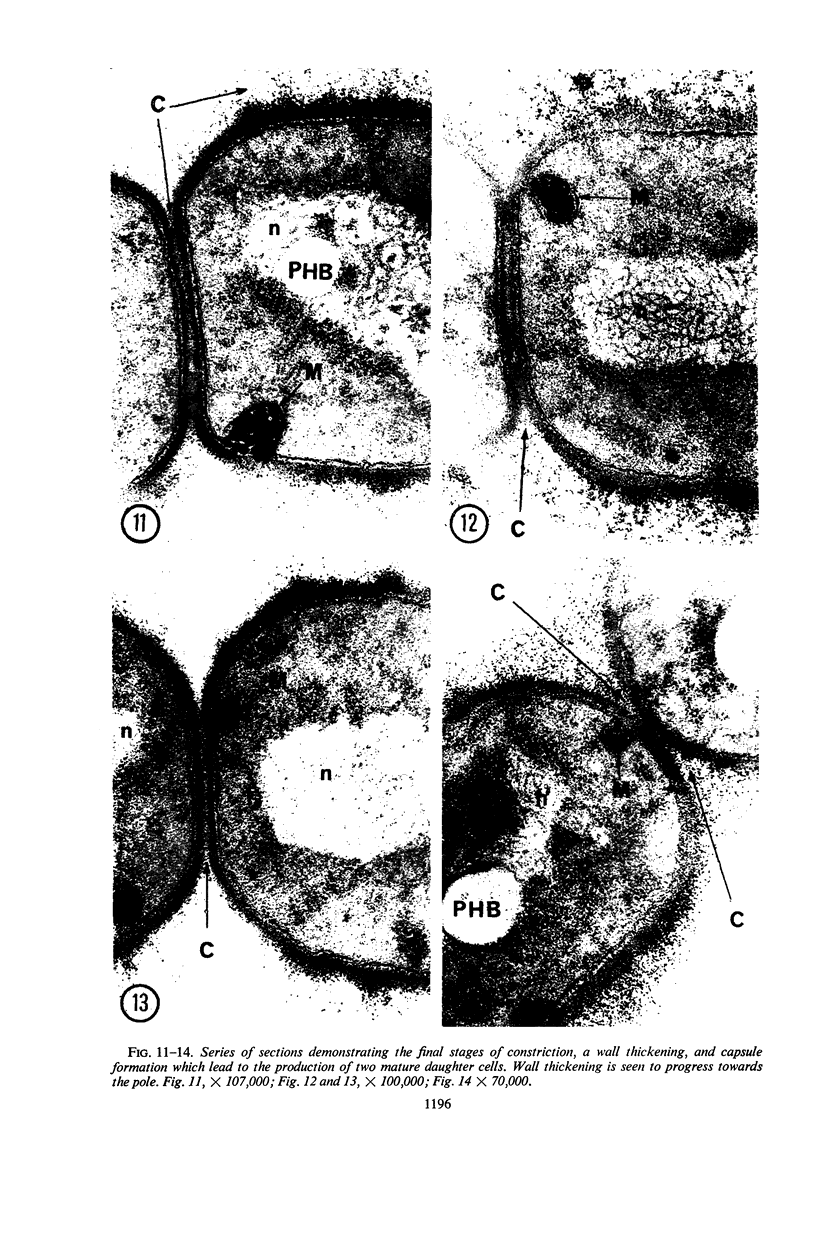
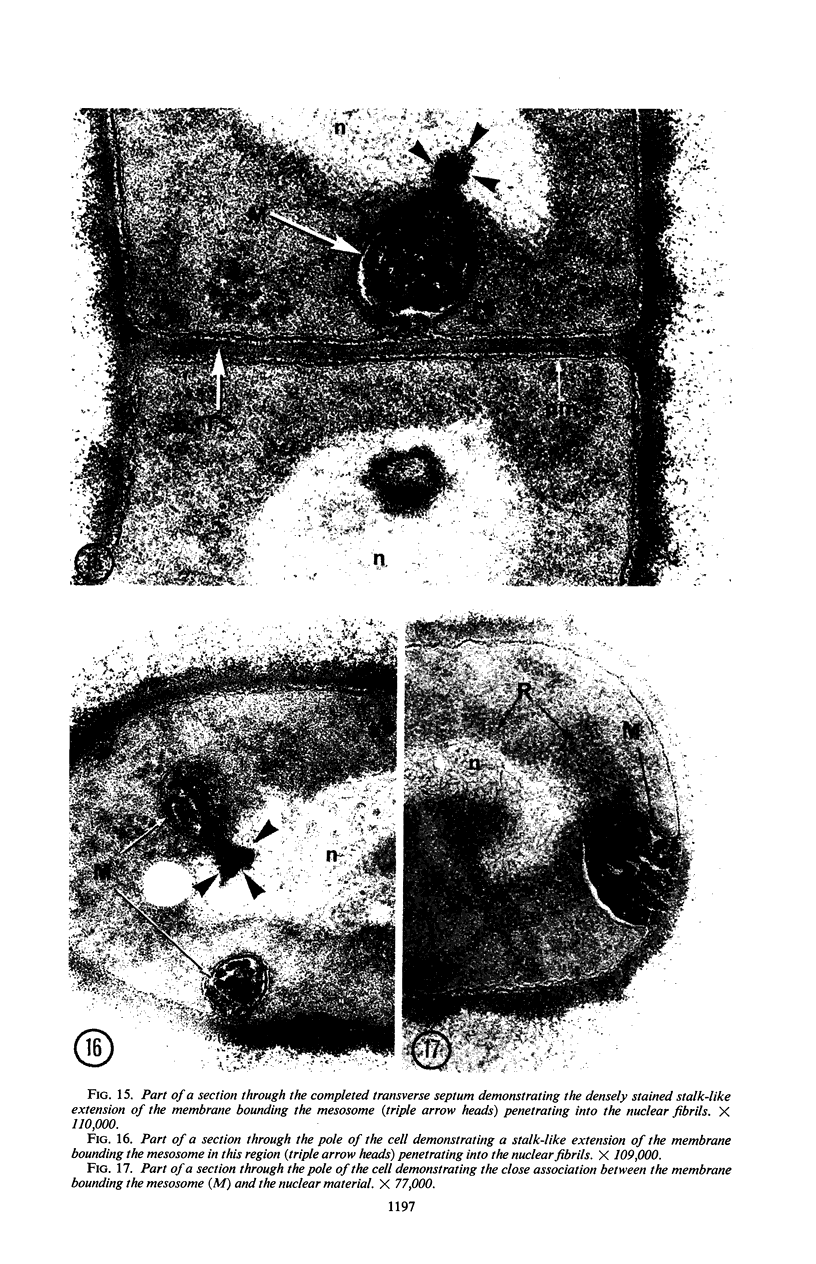
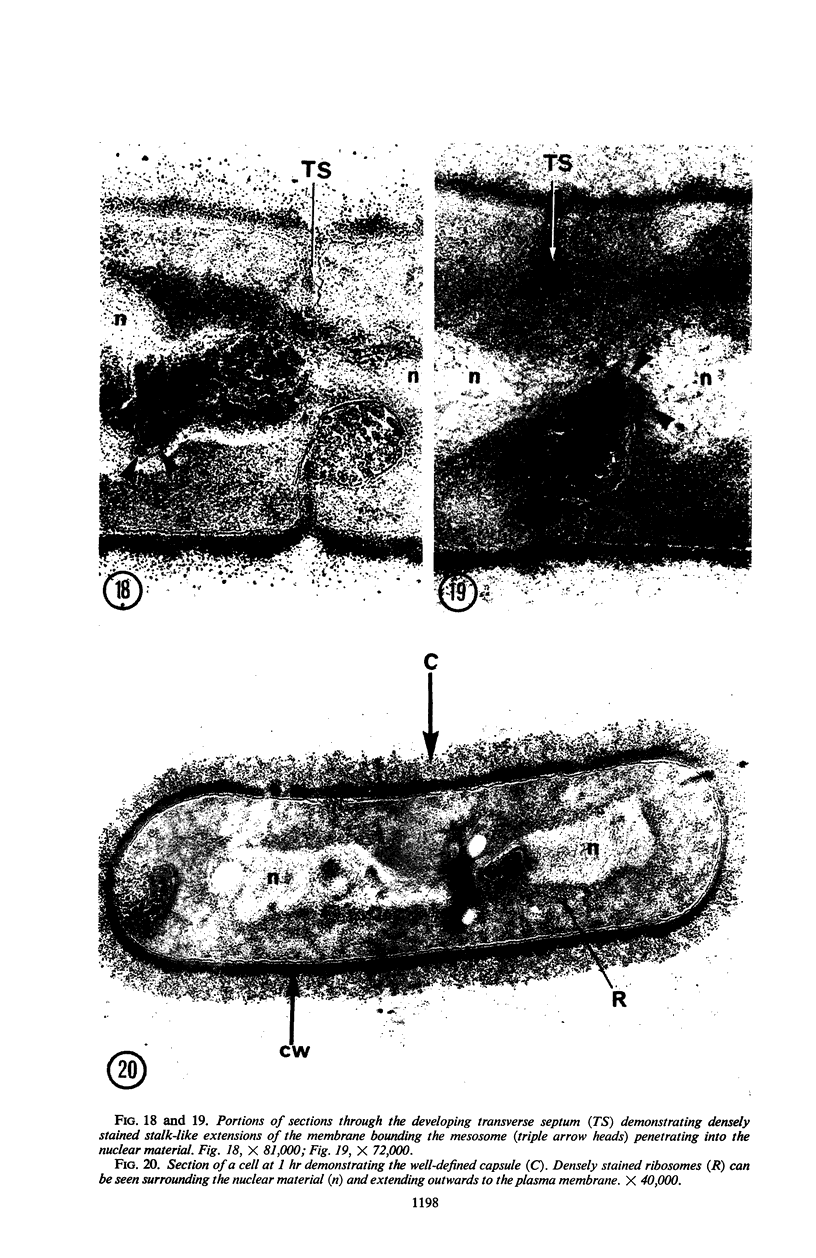
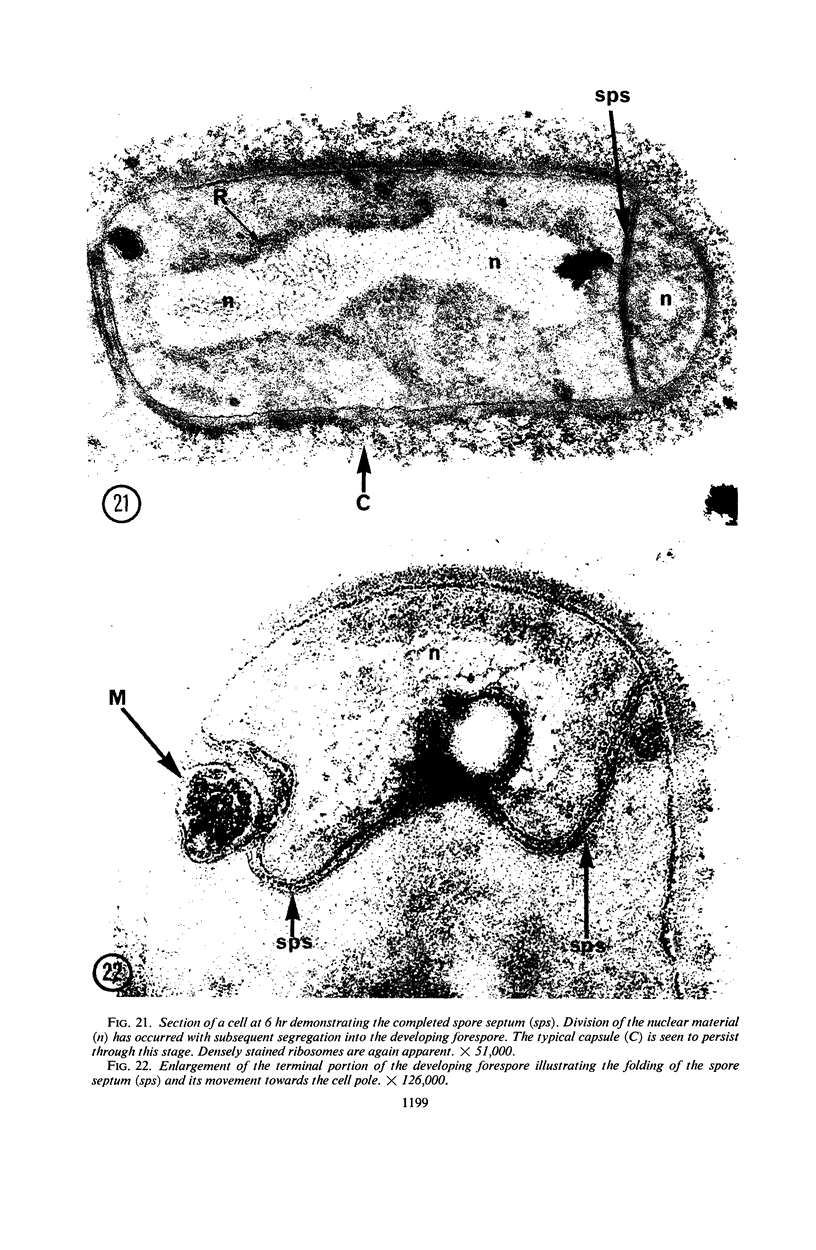
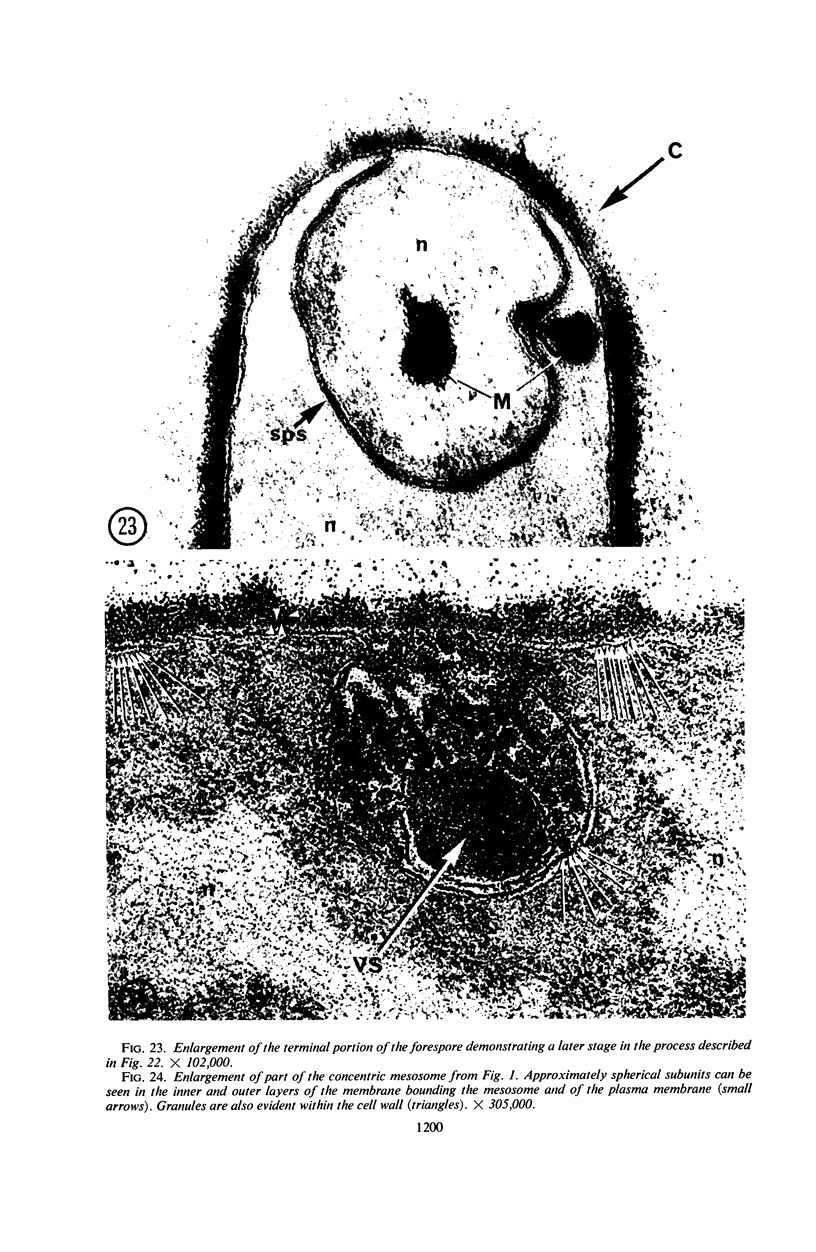
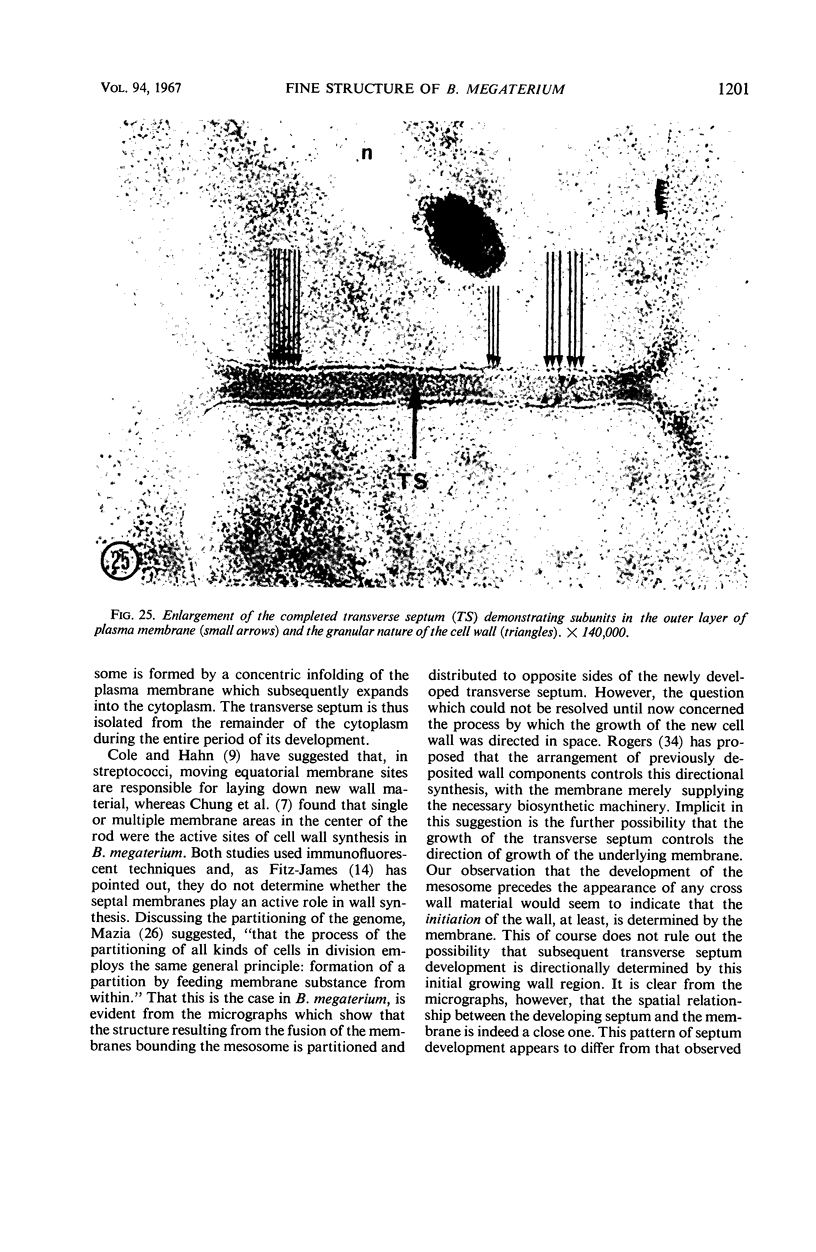
A fine-structure study of synchronously dividing Bacillus megaterium revealed the sequence of events involved in the division of the cell. First, a mesosome develops as a concentric fold of the plasma membrane at the site of septum formation. The mesosome contains membrane-bound vesicular structures, 300 to 500 A in diameter, plus a large membrane-bound structure, 2,000 A in diameter. These larger vesicles are peculiar to mesosomes in this stage of division and are not observed in the mesosomes involved in spore septum formation. The transverse septum originates within the mesosome and remains enclosed during its subsequent growth across the cell. An intimate association is observed between mesosome vesicles, mesosome membrane, and the growing edge of the transverse septum. Prior to completion of the septum, the membranes bounding the mesosome fuse, and further wall thickening occurs within the structure formed by this fusion. At this time, the septum only equals the parent cell wall in thickness. The doubling in thickness of the septum, which is required for the production of two normal daughter cell walls, occurs during a second phase of wall thickening, which is characterized by the appearance of a constriction at the base of the septum. As the constriction widens, the wall in this region thickens, forming the typical rounded poles of the daughter cells. Capsular synthesis at the poles occurs during this second phase of wall thickening. Throughout the division process, the nuclear material appears to be associated at one end with a mesosome at or near the pole of the cell and at the other end to the mesosome involved in septum formation. This association frequently takes the form of a stalklike extension of the mesosome penetrating into the chromatin fibrils.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D. ELECTRON MICROSCOPE OBSERVATIONS ON INTACT CELLS, PROTOPLASTS, AND THE CYTOPLASMIC MEMBRANE OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Mar;89:855–873. doi: 10.1128/jb.89.3.855-873.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BADDILEY J. TEICHOIC ACIDS AND THE BACTERIAL CELL WALL. Endeavour. 1964 Jan;23:33–37. [PubMed] [Google Scholar]
- Baker R. F., Loosli C. G. The ultrastructure of encapsulated Diplococcus pneumoniae type 1 before and after exposure to type specific antibody. Lab Invest. 1966 Apr;15(4):716–730. [PubMed] [Google Scholar]
- Blasie J. K., Dewey M. M., Blaurock A. E., Worthington C. R. Electron microscope and low-angle x-ray diffraction studies on outer segment membranes from the retina of the frog. J Mol Biol. 1965 Nov;14(1):143–152. doi: 10.1016/s0022-2836(65)80236-4. [DOI] [PubMed] [Google Scholar]
- CHAPMAN G. B. Electron microscope observations on the behavior of the bacterial cytoplasmic membrane during cellular division. J Biophys Biochem Cytol. 1959 Oct;6:221–224. doi: 10.1083/jcb.6.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B., HILLIER J. Electron microscopy of ultra-thin sections of bacteria I. Cellular division in Bacillus cereus. J Bacteriol. 1953 Sep;66(3):362–373. doi: 10.1128/jb.66.3.362-373.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG K. L., HAWIRKO R. Z., ISAAC P. K. CELL WALL REPLICATION. I. CELL WALL GROWTH OF BACILLUS CEREUS AND BACILLUS MEGATERIUM. Can J Microbiol. 1964 Feb;10:43–48. doi: 10.1139/m64-007. [DOI] [PubMed] [Google Scholar]
- COLE R. M., HAHN J. J. Cell wall replication in Streptococcus pyogenes. Science. 1962 Mar 2;135(3505):722–724. doi: 10.1126/science.135.3505.722. [DOI] [PubMed] [Google Scholar]
- Cole R. M. Symposium on the fine structure and replication of bacteria and their parts. 3. Bacterial cell-wall replication followed by immunofluorescence. Bacteriol Rev. 1965 Sep;29(3):326–344. doi: 10.1128/br.29.3.326-344.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISERLING F. A., ROMIG W. R. Studies of Bacillus subtilis bacteriophages. Structural characterization by electron microscopy. J Ultrastruct Res. 1962 Jun;6:540–546. doi: 10.1016/s0022-5320(62)80008-2. [DOI] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G. Fine structure of sporulation in Bacillus cereus grown in a chemically defined medium. J Bacteriol. 1966 Dec;92(6):1748–1764. doi: 10.1128/jb.92.6.1748-1764.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P., Hancock R. The initial structural lesion of penicillin action in Bacillus megaterium. J Cell Biol. 1965 Aug;26(2):657–667. doi: 10.1083/jcb.26.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhs G. W. Spherical subunits in photosynthetic membranes of two cyanophyceae and the bacterium Rhodospirillum rubrum. Arch Mikrobiol. 1966 Sep 8;54(3):253–265. doi: 10.1007/BF00408998. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., BRIEGER E. M., ALLEN J. M. The fine structure of vegetative cells of Bacillus subtilis. Exp Cell Res. 1961 Jan;22:73–85. doi: 10.1016/0014-4827(61)90087-8. [DOI] [PubMed] [Google Scholar]
- Imanaka H., Gillis J. R., Slepecky R. A. Synchronous growth and sporulation of Bacillus megaterium. J Bacteriol. 1967 May;93(5):1624–1630. doi: 10.1128/jb.93.5.1624-1630.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F., Ryter A., Cuzin F. On the association between DNA and membrane in bacteria. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):267–278. doi: 10.1098/rspb.1966.0029. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda T., Holden J. T., Utech N. M. Ultrastructure of the membrane system in Lactobacillus plantarum. J Bacteriol. 1967 Jan;93(1):472–482. doi: 10.1128/jb.93.1.472-482.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUYAMA Y., YANAGITA T. Physical methods for obtaining synchronous culture of Escherichia coli. J Bacteriol. 1956 May;71(5):542–546. doi: 10.1128/jb.71.5.542-546.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell G. G., Lawn A. M. Inheritance of capsule and the manner of cell-wall formation in Bacillus anthracis. J Gen Microbiol. 1965 Jun;39(3):423–427. doi: 10.1099/00221287-39-3-423. [DOI] [PubMed] [Google Scholar]
- Meynell G. G., Meynell E. The biosynthesis of poly d-glutamic acid, the capsular material of Bacillus anthracis. J Gen Microbiol. 1966 Apr;43(1):119–138. doi: 10.1099/00221287-43-1-119. [DOI] [PubMed] [Google Scholar]
- OHYE D. F., MURRELL W. G. Formation and structure of the spore of Bacillus coagulans. J Cell Biol. 1962 Jul;14:111–123. doi: 10.1083/jcb.14.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFISTER R. M., LUNDGREN D. G. ELECTRON MICROSCOPY OF POLYRIBOSOMES WITHIN BACILLUS CEREUS. J Bacteriol. 1964 Oct;88:1119–1129. doi: 10.1128/jb.88.4.1119-1129.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park R. B. Substructure of chloroplast lamellae. J Cell Biol. 1965 Oct;27(1):151–161. doi: 10.1083/jcb.27.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS H. J. THE BACTERIAL CELL WALL. THE RESULT OF ADSORPTION, STRUCTURE OR SELECTIVE PERMEABILITY? J Gen Microbiol. 1963 Jul;32:19–24. doi: 10.1099/00221287-32-1-19. [DOI] [PubMed] [Google Scholar]
- RYTER A. ETUDE MORPHOLOGIQUE DE LA SPORULATION DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1965 Jan;108:40–60. [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DE LA LIAISON ENTRE NOYAU ET M'ESOSOME CHEZ BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Sep;107:384–400. [PubMed] [Google Scholar]
- SLEPECKY R., FOSTER J. W. Alterations in metal content of spores of Bacillus megaterium and the effect on some spore properties. J Bacteriol. 1959 Jul;78(1):117–123. doi: 10.1128/jb.78.1.117-123.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASZ A., JAMIESON J. D., OTTOLENGHI E. THE FINE STRUCTURE OF DIPLOCOCCUS PNEUMONIAE. J Cell Biol. 1964 Aug;22:453–467. doi: 10.1083/jcb.22.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A., Nakamura K., Ueda M. Electron microscope studies of the intracytoplasmic membrane system in Clostridium tetani and Clostridium botulinum. Jpn J Microbiol. 1965 Sep;9(3):131–143. doi: 10.1111/j.1348-0421.1965.tb00282.x. [DOI] [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. Location and composition of spore mucopeptide in Bacillus species. J Cell Biol. 1963 Mar;16:593–609. doi: 10.1083/jcb.16.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weier T. E., Bisalputra T., Harrison A. Subunits in chloroplast membranes of Scenedesmus quadricauda. J Ultrastruct Res. 1966 Apr;15(1):38–56. doi: 10.1016/s0022-5320(66)80092-8. [DOI] [PubMed] [Google Scholar]
- van Iterson W. Symposium on the fine structure and replication of bacteria and their parts. II. Bacterial cytoplasm. Bacteriol Rev. 1965 Sep;29(3):299–325. doi: 10.1128/br.29.3.299-325.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]