Abstract
Glutamate transporters are responsible for clearing synaptically released glutamate from the extracellular space. By this action, they maintain low levels of ambient glutamate, thus preventing excitotoxic damage, and contribute to shaping synaptic currents. We show that up-regulation of the glutamate transporter GLT-1 by ceftriaxone severely impaired mGluR-dependent long-term depression (LTD), induced at rat mossy fibre (MF)–CA3 synapses by repetitive stimulation of afferent fibres. This effect involved GLT-1, since LTD was rescued by the selective GLT-1 antagonist dihydrokainate (DHK). DHK per se produced a modest decrease in fEPSP amplitude that rapidly regained control levels after DHK wash out. Moreover, the degree of fEPSP inhibition induced by the low-affinity glutamate receptor antagonist γ-DGG was similar during basal synaptic transmission but not during LTD, indicating that in ceftriaxone-treated rats LTD induction did not alter synaptic glutamate transient concentration. Furthermore, ceftriaxone-induced GLT-1 up-regulation significantly reduced the magnitude of LTP at MF–CA3 synapses but not at Schaffer collateral–CA1 synapses. Postembedding immunogold studies in rats showed an increased density of gold particles coding for GLT-1a in astrocytic processes and in mossy fibre terminals; in the latter, gold particles were located near and within the active zones. In both CEF-treated and untreated GLT-1 KO mice used for verifying the specificity of immunostaining, the density of gold particles in MF terminals was comparable to background levels. The enhanced expression of GLT-1 at release sites may prevent activation of presynaptic receptors, thus revealing a novel mechanism by which GLT-1 regulates synaptic plasticity in the hippocampus.
Glutamate transporters (GluTs) are responsible for clearing synaptically released glutamate at excitatory synapses. GluTs reduce spillover, thus ensuring a spatially restricted action of glutamate and preventing its accumulation in the extracellular space (Conti & Weinberg, 1999; Danbolt, 2001; Amara & Fontana, 2002; Scanziani, 2002). By these actions, GluTs contribute to sculpting excitatory postsynaptic currents and to modulating synaptic plasticity (Tzingounis & Wadiche, 2007). Five GluTs have been characterized: GLAST, GLT-1, EAAC1, EAAT4 and EAAT5. GLT-1, particularly its GLT-1a isoform, exhibits the highest level of expression and is responsible for the largest proportion of glutamate transport in hippocampus (Danbolt, 2001). GLT-1 down-regulation increases extracellular glutamate levels which in turn cause excitotoxicity (Danbolt, 2001); conversely, an elevated expression of GLT-1 may be neuroprotective. Along these lines, it has been demonstrated that chronic treatment of rats with the β-lactam antibiotic ceftriaxone induces GLT-1 up-regulation (but not of the other GluTs) by enhancing transcription of the GLT-1 gene (Rothstein et al. 2005; see also Chu et al. 2007; Ouyang et al. 2007; Bellesi et al. 2009).
Here, ceftriaxone-treated rats were used to investigate the functional role of GLT-1 in synaptic plasticity in the hippocampus. We focused mainly on long-term depression (LTD) at mossy fibre (MF)–CA3 synapses, known to depend on the activation of presynaptic metabotropic glutamate receptors (mGluRs; Kobayashi et al. 1996; Yokoi et al. 1996). We hypothesized that increasing GLT-1 would reduce extracellular glutamate levels, thus preventing mGluR activation and LTD induction. We found that ceftriaxone-treated animals did not express LTD and that this effect was associated with an increased density of gold particles coding for GLT-1 near and within active zones of MF terminals and in astrocytic processes. In addition, ceftriaxone treatment significantly reduced the magnitude of LTP at MF–CA3 but not at Schaffer collateral–CA1 synapses.
Methods
Animals and hippocampal slice preparation
Experiments were carried out in accordance with EC Council Directives (Nov 24, 1986) and approved by the local authority's veterinary service; they also comply with the policies and regulations of The Journal of Physiology (Drummond, 2009). Animals were kept under a 12 h dark–light cycle and permitted food and water ad libitum.
Male Wistar rats (8–9 weeks old) and wild-type (WT) and GLT-1 KO mice (P15) received a daily intraperitoneal (i.p.) injection of saline or ceftriaxone (Rocefin, Roche; 200 mg kg−1 day−1 dissolved in saline) for 8 days. Twenty-four hours after the final injection, animals used for electrophysiological studies were anaesthetized with an i.p. injection of urethane (2 g kg−1) and killed.
Hippocampal slices from saline- or ceftriaxone-treated rats were prepared as described (Rosato-Siri et al. 2006). Briefly, brains were quickly removed from the skull and transverse hippocampal slices (400 μm thick) were obtained by cutting each hemisphere in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mm): NaCl 126, KCl 3.5, NaH2PO4 1.2, NaHCO3 25, MgCl2 1.3, CaCl2 2.5, glucose 25, saturated with 95% O2–5% CO2 (pH 7.3–7.4). After a recovery period of at least 1 h, single slices were placed in the recording chamber and continuously superfused with oxygenated ACSF (2–3 ml min−1 at 33–34°C). Slices were firstly used for electrophysiological studies and then for immunocytochemical investigations.
Animals used for electron microscope studies (4 rats: 2 controls and 2 CEF-treated; and 8 mice: 2 untreated WT, 2 ceftriaxone-treated wild-type, 2 GLT-1 KO (Tanaka et al. 1997) and 2 GLT-1 KO treated with CEF) were anaesthetized with chloral hydrate (300 mg kg−1) 24 h after the final injection and perfused through the ascending aorta with a flush (∼1 min) of saline followed by 4% paraformaldehyde (PFA). Brains were removed and postfixed for 7 days before cutting; these sections were used for immunogold studies.
Western blotting studies
Eight rats were used; four received ceftriaxone and four saline. Rats were anaesthetized and decapitated and the hippocampus was dissected free. Tissue was homogenized and both cell extracts and crude synaptic membranes were prepared (Danbolt et al. 1990; Varoqui et al. 2000). The total amount of protein in each homogenate was determined by the method of Bradford (Bradford, 1976) using the Bio-Rad Protein Assay (Bio-Rad Laboratories) and a Beckman DU 530 spectrophotometer (Beckman Coulter; 3–4 measurements/homogenate).
Since housekeeping proteins are sensitive to experimental treatments and have limitations for use as internal standards (Ferguson et al. 2005), three to six measurements per animal were made to yield the mean value per animal; to minimize procedural variables, homogenates from treated and control rats were loaded onto the same gel. Curves of increasing concentration were drawn to define a linear range for densitometric analysis (Bragina et al. 2006). Homogenate aliquots (1.5 μg of total protein) were subjected to SDS-PAGE; separated proteins were electroblotted onto nitrocellulose filters. Nitrocellulose filters were initially washed in phosphate buffered saline with 0.1% Tween 20 (PBS-T; pH 7.4); subsequently, they were exposed first to a blocking buffer solution (5% Bio-Rad non-fat dry milk in PBS-T; 1 h) and next to a solution of 0.1% BSA in PBS-T containing primary antibodies (2 h at room temperature and then overnight at 4°C). Primary antibodies were polyclonal antibodies directed against a synthetic peptide corresponding to amino acids 559–573 (SADCSVEEEPWKREK) of rat GLT-1a C-terminus (Rothstein et al. 1994; see also Chen et al. 2004); they were generously provided by Dr J. D. Rothstein, Johns Hopkins University (batch no. 080706; 0.02 μg ml−1). The following day, filters were washed with PBS-T and then exposed to secondary antibodies (Vector Laboratories, Inc., Burlingame, CA, USA) dissolved in PBS-T. Bands were visualized by Bio-Rad Chemidoc and Quantity One software using the SuperSignal West Pico chemiluminescent substrate (Bragina et al. 2006).
Immunocytochemical studies
Immunofluorescence studies
Nine slices were used; five were from ceftriaxone-treated animals and four from controls. After completion of electrophysiological experiments, slices were postfixed in 4% PFA for 7 days and processed in parallel to minimize procedural variables. They were incubated first in a solution containing GLT-1a antibodies (0.3 μg ml−1) and then in one containing affinity-purified tetramethylrhodamine isothiocyanate-conjugated antibodies (Molecular Probes). Slices were examined with a Bio-Rad confocal microscope.
Microscopic fields from stratum lucidum of slices were randomly selected and z-axis image stacks were acquired with high-resolution parameters (Melone et al. 2009) using the confocal microscope. Analysis of puncta was performed in randomly selected 20 × 20 μm fields from each image (24 fields from controls and 24 from ceftriaxone-treated animals). Images collected on the surface of stained sections were used for analysis. Threshold values (Melone et al. 2005) did not differ between groups. For each field the mean intensity level was determined using Adobe Photoshop CS2; number and mean size of GLT-1a+ puncta were estimated by transforming threshold processed images to binary images and calculated using ImageJ v.1.38 (Bozdagi et al. 2000).
Immunogold studies
Sections from control and ceftriaxone-treated animals were processed for the osmium-free method (Phend et al. 1995). Chips including the stratum lucidum of CA3 were cut and sectioned, and ultrathin sections (60–80 nm) were mounted on nickel grids. All rinse and diluent solutions were filtered through a 0.45 μm membrane filter before use. After treatment with 4% para-phenyenediamine in Tris-buffered saline (0.1 m Tris, pH 7.6, with 0.005% Tergitol N P-10 (TBST)), grids were washed in distilled water, incubated for 15 min in blocking solution (1% bovine serum albumine (BSA) in TBST, pH 7.6) and then transferred in TBST (pH 7.6) solution containing GLT-1a antibodies (6 μg ml−1). The next day, grids were washed in TBST pH 7.6, incubated for 15 min in blocking solution (1% BSA in TBST pH 8.2), transferred in TBST (pH 8.2; 2 h) containing secondary antibodies conjugated to 12 nm gold particles (1: 20; 111-205-144, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA), washed, stained with uranyl acetate and Sato's lead (Sato, 1968) and examined with a Zeiss EM 900 electron microscope. Gold particles were not detected when the primary antiserum was omitted; when normal serum was substituted for the immune serum, sparse and scattered particles were observed. Optimal concentration of anti-GLT-1a antibodies was sought by testing several dilutions; the concentration yielding the lowest background labelling and immunopositive elements was used for final studies.
Electron micrographs (original magnification: 50 000–85 000×; 3 grids/animal) were taken from fields including at least one immunolabelled astrocytic profile and/or MF terminal associated with an asymmetric synapse with a clear active zone–postsynaptic density (PSD) complex (Peters et al. 1991; Henze et al. 2000; Tyler & Pozzo-Miller, 2001), except for GLT-1 KO mice. To determine the relative density of GLT-1a, gold particles within astrocytic profiles, MF terminals and cell nuclei were counted and areas calculated using ImageJ. Background was calculated by estimating labelling density over nuclei (Kharazia & Weinberg, 1997). Particle density was then compared with background. Gold particles were considered membrane-associated if they were within 15 nm of its extracellular side, and cytoplasmic if they were >25 nm from the membrane extracellular side. Localization of a membrane-associated gold particle with respect to the active zone margin was defined as described (Kharazia & Weinberg, 1997). The lateral position of a gold particle was defined as the distance along the membrane from the active zone edge to the centre of the particle (Valtschanoff & Weinberg, 2001); distance was measured using ImageJ.
Electrophysiological studies
Field excitatory postsynaptic potentials (fEPSPs) were obtained from stratum lucidum in the hippocampal CA3 region and from stratum radiatum in the CA1 region in response to stimulation of dentate gyrus granule cells or Schaffer collateral, respectively. Electrical stimuli (100 μs square pulses at 0.1 Hz) were delivered through bipolar twisted NiCr-insulated electrodes (stimulus strength was adjusted to evoke a response equal to 40% of the maximal fEPSP). The recording micropipettes (resistance 2–5 MΩ) were pulled from thick-wall borosilicate glass capillaries (Hilgenberg, Malsfeld, Germany) and filled with NaCl (2 m). fEPSPs were recorded using a DAM-800 differential amplifier (World Precision Instruments, Sarasota, FL, USA), low-pass filtered at 1 kHz, digitized at 10 kHz (DigiData 1200, Axon Instruments/Molecular Devices, Sunnyvale, CA, USA). Signals were acquired with the WinLTP software package (courtesy of W. W. Anderson, Bristol University, UK) and analysed off-line using Clampfit 10.1 software (Axon Instruments/Molecular Devices). After establishing a stable baseline for at least 10 min, LTD was induced by low frequency stimulation (LFS; 1 Hz for 15 min) of afferent inputs. After LTD, fEPSP amplitudes were normalized to mean baseline values (obtained before LTD) and plotted against time using the LTP software package. MF fEPSPs were identified on the basis of their sensitivity to the group II mGluR agonist 2-(2,3-dicarboxycyclopropyl)glycine (DCG-IV) and on their short-term frequency-dependent facilitation (Salin et al. 1996). Paired stimuli (50 ms interval) were delivered to afferent pathways and the paired pulse ratio (PPR) was calculated as the ratio of the amplitude of the second fEPSP to the first one. In some experiments, LTP was induced at MF–CA3 synapses or Schaffer collateral–CA1 synapses by two high-frequency stimulation trains (100 pulses at 100 Hz each, 10 s apart) delivered either to the granule cells in the dentate gyrus or to the Schaffer collateral. Unless otherwise stated, all electrophysiological experiments were performed at 34–35°C.
Drugs
Drugs were dissolved in ACSF and applied through a three-way tap system by changing the superfusion solution to one that differed only in drug content. The ratio of flow rate to bath volume ensured complete exchange within 1 min. We used: (RS)-α-methyl-4-carboxyphenylglycine (MCPG), d-(–)-2-amino-5-phosphonopentanoic acid (d-APV), dihydrokainate (DHK), (RS)-α-methyl-4-carboxyphenylglycine (MCPG), 2-(2,3-dicarboxycyclopropyl) glycine (DCG-IV) and γ-d-glutamylglycine (γ-DGG) purchased from Tocris Cookson (Bristol, UK).
Statistical analysis
All values are means ±s.e.m. Statistical significance for western blotting, immunocytochemical and immunogold studies was assessed by a two-sided Student's t-test; for electrophysiological studies by a two-sided Wilcoxon's signed rank test and Wilcoxon–Mann–Whitney test for paired and unpaired samples, respectively. The significance level was P < 0.05.
Results
Chronic treatment with ceftriaxone up-regulates GLT-1a
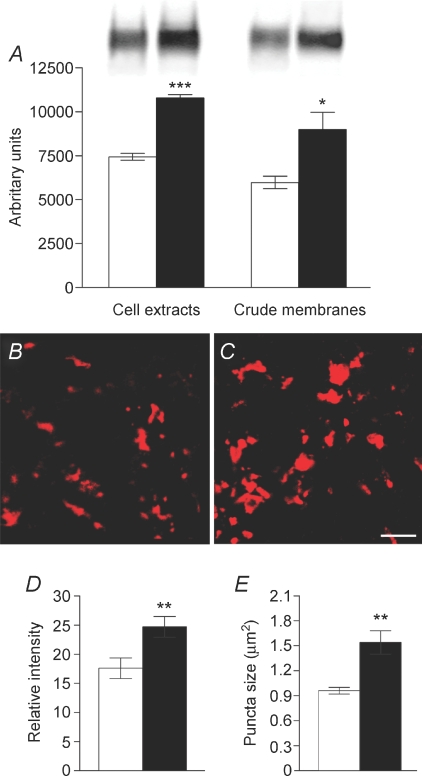
In cell extracts and crude synaptic membranes from the hippocampus of ceftriaxone-treated and control rats, GLT-1a antibodies recognized a single band of 70 kDa; densitometric analysis showed that ceftriaxone significantly raised GLT-1a expression by 45 ± 1.8% in cell extracts (P < 0.0001; n= 8) and 50.3 ± 11% in synaptic membranes (P= 0.02; n= 8). The difference between the amount of GLT-1 detected in cell extracts and synaptic membranes was comparable in controls and ceftriaxone-treated animals (P= 0.8), suggesting that ceftriaxone increases both membrane-bound and cytoplasmic GLT-1 (Fig. 1A). Immunofluorence analysis of slices used for electrophysiological recordings revealed a significant increase in intensity (40.2 ± 10.1%; P= 0.007) and size (61 ± 14.1%; P= 0.02), but not density, of GLT-1a+ puncta in stratum lucidum of ceftriaxone-treated animals compared to controls (Fig. 1B–E), indicating that ceftriaxone increases GLT-1 expression in the very layer in which electrophysiological recordings were performed.
Figure 1. Ceftriaxone-induced GLT-1a up-regulation.
A, GLT-1a expression in cell extracts and crude synaptic membranes of the hippocampus of control (open columns) and ceftriaxone-treated (filled columns) rats. B and C, examples of GLT-1a+ puncta in stratum lucidum of slices from control (B) and ceftriaxone-treated (C) rats. Scale bar: 5 μm. D and E, intensity and size of GLT-1a+ puncta are increased in ceftriaxone rats (filled bars). *P < 0.05; **P < 0.01; ***P < 0.0001.
Ceftriaxone treatment alters the distribution of GLT-1a coding particles at MF–CA3 synapses
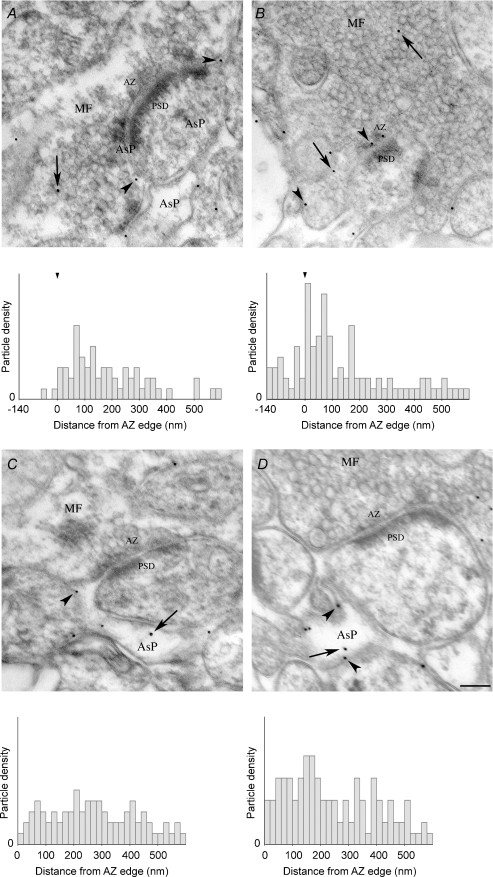
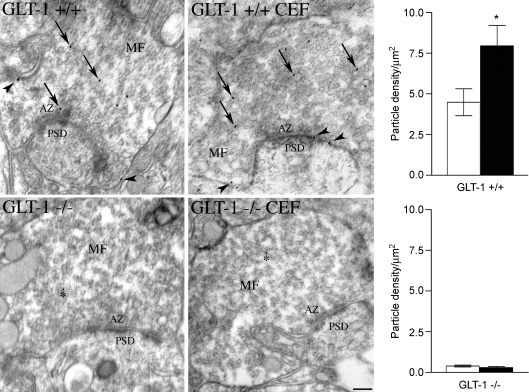
Next we studied the density and distribution of gold particles coding for GLT-1a at MF–CA3 asymmetric synapses using postembedding immunogold electron microscopy. In MF terminals and astrocytic processes from stratum lucidum of control and ceftriaxone-treated rats, gold particles density was significantly higher than background (online Supplemental Material, Table S1). Interestingly, the density of gold particles was significantly higher on the plasma membrane than on the cytoplasm (Table S1). In addition, in MF terminals and astrocytic processes of ceftriaxone-treated rats, the density of gold particles in both membrane and cytoplasmic compartments was significantly higher than in controls (Table S1). Analysis of distribution of membrane-associated gold particles in relation to active zones showed that the mean distance of gold particles from the active zone edges on the plasma membrane was significantly reduced in MF terminals of treated rats (controls: 184.9 ± 20 nm (n= 58); ceftriaxone: 116.5 ± 17.9 nm (n= 102); P= 0.01), and that in ceftriaxone-treated rats gold particles were more numerous at active zones (Fig. 2A and B), whereas no significant changes were seen in astrocytic processes (controls: 281.6 ± 18.9 nm (n= 68); ceftriaxone: 243.7 ± 13.9 nm (n= 113) (Fig. 2C and D). To verify the specificity of MF terminal immunostaining, we studied GLT-1a immunoreactivity in untreated wild-type and GLT-1 KO mice, as well as in ceftriaxone-treated wild-type and GLT-1 KO mice (Tanaka et al. 1997). This analysis showed that the density of gold particles in MF terminals from ceftriaxone-treated wild-type mice was significantly higher than that measured in untreated wild-type mice (online supplemental material, Table S2; and Fig. 3, upper row), whereas in both ceftriaxone-treated KO mice and untreated KO mice it fell to background levels (Table S2 and Fig. 3, lower row).
Figure 2. Ceftriaxone treatment alters the distribution of GLT-1a coding particles at MF–CA3 synapses.
Examples and distribution of gold particles coding for GLT-1a in MF terminals (A) and AsPs (C) of controls and of ceftriaxone-treated rats (B and D, respectively) in relation to active zone. Note the presence of gold particles within the active zone in B. Density/bin (20 nm width) was higher in MF terminals (P= 0.026) and AsPs (P= 0.002) of ceftriaxone-treated rats than in controls. MF, mossy fibre terminal; AsP, astrocytic process; AZ, active zone; PSD, postsynaptic density. Arrows indicate cytoplasmic gold particles, arrowheads membrane-associated particles. Scale bar: 150 nm (for A–D).
Figure 3. Immunogold studies of GLT-1a positive MF terminals of untreated (GLT-1 +/+) and ceftriaxone-treated (GLT-1 +/+ CEF) WT mice, and of untreated (GLT-1 −/−) and ceftriaxone-treated GLT-1 KO (GLT-1 −/− CEF) mice.
Values used for the bar charts (open column, untreated; filled column, treated) are reported in online supplemental material Table S2. MF, mossy fibre terminal; AZ, active zone; PSD, postsynaptic density. Arrows indicate cytoplasmic gold particles; arrowheads point to membrane-associated particles; asterisks indicate background labelling. *P < 0.05. Scale bar: 150 nm.
Ceftriaxone treatment alters frequency-dependent short-term plasticity at MF–CA3 synapses
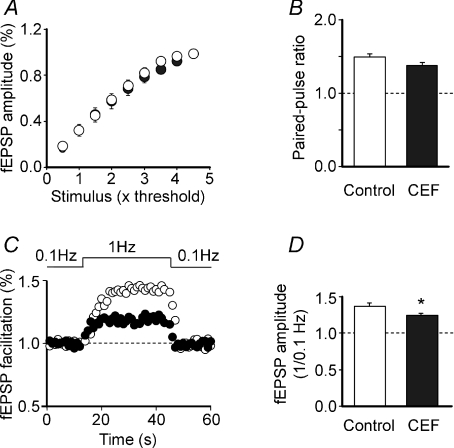
In a first set of experiments the strength of synaptic transmission was analysed by delivering single test stimuli (at 0.1 Hz) of graded intensity to granule cells in the dentate gyrus in both controls and ceftriaxone-treated rats. Overall, the shape and amplitude of fEPSPs were similar in treated and untreated animals. The input–output curves obtained in the two groups of animals by plotting the amplitude of the fEPSPs versus stimulation strength completely overlapped (the slopes of the curves obtained by fitting data points with a sigmoid function were 0.69 ± 0.03 and 0.68 ± 0.07 in control and CEF rats, respectively; P= 0.89; n= 10 for both groups; Fig. 4A). CEF treatment did not alter the sensitivity of MF responses to DCG-IV. The relative amplitude of fEPSP in DCG-IV (1 μm) was 0.3 ± 0.02 and 0.3 ± 0.04 in controls and ceftriaxone-treated rats, respectively (n= 10 for both groups). In addition, similar values of PPR, largely used to evaluate changes in probability of transmitter release (Zucker & Regehr, 2002), were found in both controls and CEF-treated animals (1.50 ± 0.05 and 1.39 ± 0.03, respectively; n= 14 for both groups; P= 0.063; Fig. 4B). Furthermore, changing stimulation frequency from 0.1 Hz to 1 Hz caused a transient increase in amplitude of fEPSPs that was significantly larger in controls than in CEF-treated rats (1.35 ± 0.04; n= 11 and 1.23 ± 0.03; n= 18, respectively; P= 0.03; Fig. 4C and D). These experiments suggest that CEF treatment does not modify cell excitability or the probability of glutamate release but affects frequency-dependent short-term facilitation (Salin et al. 1996).
Figure 4. Effect of CEF treatment on cell excitability and short-term plasticity at MF–CA3 synapses.
A, fEPSP amplitude as a function of stimulation intensity in control (open symbols; n= 10) and CEF rats (closed symbols; n= 10). Data points obtained in different experimental conditions completely overlapped. B, CEF treatment did not modify the paired-pulse ratio, indicating that the probability of Glu release was not affected. C and D, frequency facilitation at MF–CA3 synapses. Changing stimulation frequency from 0.1 Hz to 1 Hz for 30 s caused an increase in fEPSP amplitude that was significantly larger in controls (n= 11) compared to CEF-treated rats.
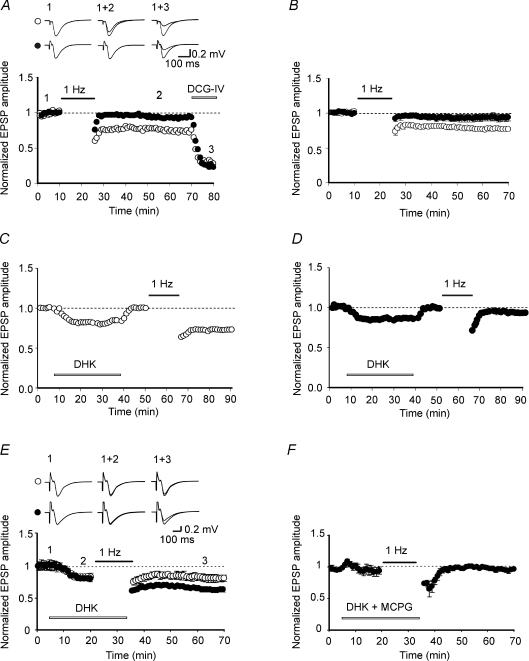
Ceftriaxone treatment severely impairs LTD at MF–CA3 synapses
To test whether at MF–CA3 synapses up-regulation of GLT-1 in ceftriaxone-treated rats interfered with LTD, which at these synapses is mGluR dependent (Kobayashi et al. 1996; Yokoi et al. 1996), we recorded fEPSPs in stratum lucidum before and after LFS of granule cells in the dentate gyrus for 15 min. LFS induced LTD in controls, but failed to modify synaptic efficacy in ceftriaxone-treated rats. The relative amplitude of fEPSP 40 min after LFS was 0.77 ± 0.02 (P= 0.031; n= 7) in controls and 0.94 ± 0.02 (P= 0.33; n= 9) in ceftriaxone-treated animals (Fig. 5A and B). The impairment of LTD observed in CEF-treated rats may be related to the reduced clearance of Glu following CEF-induced up-regulation of GLT-1. Since Glu uptake is greatly affected by temperature (Asztely et al. 1997), in some experiments (n= 6) LFS was delivered to hippocampal slices (from CEF-treated rats) kept at room temperature (22–24°C). Even in these cases, LFS failed to induce LTD (the relative amplitude of fEPSPs measured 40 min after LFS was 0.99 ± 0.04; data not shown). To test whether a longer induction protocol (30 min, 1 Hz LFS) could overwhelm the enhanced Glu uptake allowing LTD induction, additional experiments were performed from hippocampal slices from CEF treated rats. In these cases, the strong stimulation protocol induced a transient depression of the fEPSPs of 0.78 ± 0.003 that slowly stabilized to a steady state level (30 min after LFS the relative amplitude of fEPSP was 0.91 ± 0.003; P= 0.005; n= 6; online supplemental material, Fig. S1).
Figure 5. Impairment of LTD at MF–CA3 synapses in ceftriaxone-treated rats.
A and B, MF stimulation (filled bar) induced LTD in control (open symbols) but not in ceftriaxone-treated rats (closed symbols). A, representative data from control and ceftriaxone-treated rats for LTD induction and effect of DCG-IV. B, summary data for 7 controls and 9 ceftriaxone-treated rats. C and D, application of DHK (15 μm; open bar) produced a modest but consistent decrease in fEPSP amplitude which rapidly recovered upon washout of the drug in both controls (n= 10; C) and ceftriaxone-treated rats (n= 10; D). E, LFS of MF (1 Hz, filled bar) delivered in the presence of DHK (open bar) rescued LTD in ceftriaxone-treated rats (closed symbols, n= 6). In controls, the magnitude of LTD was not significantly different from that induced in the absence of DHK (○, n= 6). F, the effect of DHK on ceftriaxone-treated rats was blocked by MCPG (500 μm; n= 5, open bar). Insets in A and E are fEPSPs in control and in ceftriaxone-treated rats taken at the points shown in the graph.
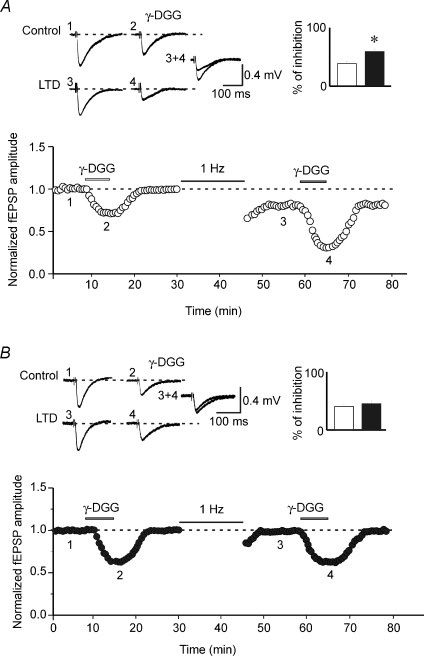
To further assess whether the lack of LTD in ceftriaxone-treated rats depended on GLT-1 up-regulation, GLT-1 was blocked with the selective antagonist dihydrokainate (DHK; Arriza et al. 1994). DHK (15 μm) per se produced a modest but consistent decrease in fEPSP amplitude of 18 ± 2% and 14 ± 1% in controls (n= 6) and ceftriaxone-treated rats (n= 6), respectively (P= 0.26; Fig. 5C,D). The amplitude of fEPSPs rapidly regained control levels after washing out the drug. Interestingly, in ceftriaxone-treated rats LFS delivered in the presence of DHK was able to restore LTD. Forty minutes after LFS, the relative fEPSP magnitude was 0.70 ± 0.03 (n= 6; P= 0.002; Fig. 5E). fEPSP amplitude relative to DHK was 0.79 ± 0.03 (P= 0.01). In controls, the relative fEPSP amplitude obtained by delivering LFS to MF in the presence of DHK was 0.84 ± 0.05 (P= 0.05; n= 6; Fig. 5E). Although this value was lower in respect to CEF, it did not significantly differ from that obtained in the absence of the drug (P= 0.1). In addition, the effect of DHK on LTD was prevented by MCPG (500 μm), a broad-spectrum mGluR antagonist, in both controls (not shown) and ceftriaxone-treated rats (relative fEPSP magnitude: 0.96 ± 0.004, n= 5; P= 0.68; Fig. 5F), indicating that mGluR activation is essential for LTD induction at MF–CA3 synapses. To probe changes in synaptic glutamate transient concentrations, slices from both groups were exposed before and after LTD induction to γ-DGG, a low affinity glutamate receptor antagonist (Liu et al. 1999). Because of the low affinity of γ-DGG, during synaptic glutamate transient a certain fraction of synaptic receptors will replace bound γ-DGG for glutamate, thus allowing assessment of changes in glutamate transient concentration in the synaptic cleft during basal synaptic transmission and LTD. Compared to controls, inhibition of fEPSP amplitude induced by γ-DGG (1 mm) was 0.38 ± 0.03 and 0.41 ± 0.03 in saline-treated (n= 5) and ceftriaxone-treated rats (n= 5), respectively. These values were not significantly different (P= 0.36). However, 15 min after LFS, the degree of γ-DGG-induced inhibition relative to controls was 0.59 ± 0.04 in untreated and 0.45 ± 0.03% in ceftriaxone-treated rats, respectively (Fig. 6). Mean values of fEPSPs inhibition obtained before and after LTD were significantly different in controls (P= 0.045) but not in ceftriaxone-treated rats (P= 0.74; see insets in Fig. 5). These results indicate that in controls (but not in ceftriaxone-treated rats), LTD was associated with a reduction in glutamate concentration in the cleft, further supporting its presynaptic locus of expression.
Figure 6. LTD involves a reduction in glutamate release in controls but not in ceftriaxone-treated rats.
Graphs in A and B show the degree of γ-DGG inhibition before and 15 min after LFS stimulation (1 Hz) in two hippocampal slices obtained from control (open symbols, A; n= 5) and in ceftriaxone-treated animals (closed symbols, B; n= 5). Note that in A LTD was associated with an increase in γ-DGG inhibition (see superimposed traces obtained before and after LTD). Insets in A and B are averaged fEPSPs obtained in the same slices in the absence or in the presence of γ-DGG, before and after LFS (taken at the points shown in the graph). The columns on the right represent the mean inhibition of fEPSPs induced by γ-DGG before (open) and after LTD induction (filled). *P= 0.045.
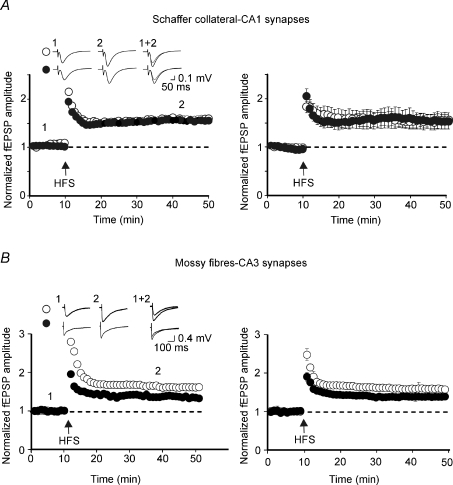
Ceftriaxone treatment affects LTP at MF–CA3 synapses
Although this study was mainly focused on LTD, additional experiments were performed to examine whether the up-regulation of GLT-1 by ceftriaxone affects also LTP whose expression at MF–CA3 synapses is presynaptic (Nicoll & Malenka, 1995). In control animals, two high frequency stimulation (HFS) trains delivered to granule cells in the dentate gyrus (10 s apart) in the presence of the selective NMDA receptor antagonist d-APV induced LTP. The relative amplitude of fEPSPs measured 40 min after the induction was 1.58 ± 0.01 (n= 5; Fig. 7A). In CEF-treated rats, LTP was significantly lower than controls (40 min after LTP induction the relative amplitude of fEPSPs was 1.38 ± 0.001 n= 5; Fig. 7A). Differences in LTP amplitude (controls versus CEF) were statistically significant (P= 0.0002). The effects of CEF on LTP were selective for MF–CA3 synapses, since similar experiments performed at Schaffer collateral–CA1 synapses, which express a postsynaptic form of LTP (Nicoll & Malenka, 1995), failed to cause any modification in LTP. On average, 40 min after the induction the relative amplitude of fEPSPs was 1.53 ± 0.14 and 1.54 ± 0.16, in controls (n= 5) and CEF rats (n= 6), respectively (Fig. 7B). These data indicate that the up-regulation of GLT-1 following chronic CEF treatment affects only LTP occurring at MF–CA3 synapses, where both induction and expression are mainly presynaptic.
Figure 7. Ceftriaxone treatment reduces the magnitude of LTP at MF–CA3 but not at Schaffer collateral–CA1 synapses.
A, high frequency stimulation of granule cells in the dentate gyrus (arrow) induced LTP whose magnitude was larger in control than in ceftriaxone-treated rats. On the left, single examples from a control (open symbols) and a ceftriaxone-treated rat (closed symbols); on the right, summary data for 5 controls and 5 ceftriaxone-treated rats. B, as in A but for Schaffer collateral–CA1 synapses. Note in this case no differences between treated and untreated rats. Insets in A and B are fEPSPs in control and in ceftriaxone-treated rats taken at the points shown in the graph.
Discussion
We showed that enhancing transcription of the GLT-1 gene by ceftriaxone changed GLT-1a expression and distribution and significantly impaired LTD and LTP at MF–CA3 synapses. These results reveal a novel mechanism by which GLT-1 regulates synaptic plasticity.
Post-embedding immunogold studies showed that at MF–CA3 synapses, gold particles coding for GLT-1a were significantly increased in MF terminals and astrocytic processes, and that the mean distance of gold particles from active zone edges on the plasma membrane was significantly reduced in MF terminals of treated rats. Assuming that gold particle density is linearly correlated to the number of functional molecules (Bergersen et al. 2008), the increased density of membrane-associated GLT-1a and its presence closer or within glutamate release sites indicates that at MF–CA3 synapses of ceftriaxone-treated rats glutamate clearance is more efficient. This would prevent activation of mGlu and kainate receptors, leading to LTD and LTP impairment.
Electrophysiological experiments clearly demonstrated that chronic treatment with ceftriaxone did not affect AMPA-mediated fEPSPs, which were similar in amplitude and shape to those observed in untreated animals. In addition, this treatment did not modify cell excitability (the input–output curves overlapped in both groups of animals) or the probability of glutamate release as suggested by the observation that the paired pulse ratio, considered an index of presynaptic release probability (Zucker & Regehr, 2002), had similar values in both treated and untreated animals. However, ceftriaxone significantly reduced frequency-dependent short-term facilitation, a hallmark of MF–CA3 synapses (Salin et al. 1996). This short-term form of synaptic plasticity relies on specialized intrinsic properties of ‘giant’ boutons present on MF terminals and on the tonic activation of presynaptic receptors by endogenous ligands present in the extracellular space (Bischofberger et al. 2006; Nicoll & Schmitz, 2005). Among these, kainate autoreceptors have been shown to play a crucial role in facilitating glutamate release from MF terminals (Lauri et al. 2001; Schmitz et al. 2001; but see Kwon & Castillo, 2008). Therefore, it is likely that the up-regulation of GLT-1 in CEF animals reduces the extracellular levels of Glu, thus preventing activation of presynaptic kainate receptors leading to a reduced synaptic facilitation.
The up-regulation of GLT-1 and the enhanced clearance of Glu were able to prevent also the activation of mGluRs present on MF terminals (responsible for LTD induction, Kobayashi et al. 1996; Yokoi et al. 1996) leading to a severe impairment of LTD. The possibility of rescuing LTD with the selective GLT-1 antagonist DHK suggests that in ceftriaxone-treated rats a dysregulation of GLT-1 was indeed responsible for the lack of LTD. This effect was unaltered by lowering the temperature, known to reduce Glu uptake and to facilitate extrasynaptic diffusion of this neurotransmitter (Asztely et al. 1997). The experiments with the low affinity antagonist γ-DGG, aimed at assessing changes in glutamate transient concentrations in the synaptic cleft, clearly demonstrated that while during basal synaptic transmission the degree of γ-DGG inhibition was similar in both controls and ceftriaxone-treated rats, during LTD it was clearly increased only in controls further confirming that in ceftriaxone-treated animals LTD induction did not alter synaptic glutamate.
The geometry of synaptic cleft and the position of GluTs relative to release sites are crucial for preventing glutamate spillover and activation of perisynaptic mGluRs. Hippocampal MFs give rise to large en passant swellings and terminal expansions on CA3 principal neurons seen as giant boutons with the electron microscope (Amaral & Dent, 1981). These fibres adapt to specialized postsynaptic elements on proximal dendrites of CA3 principal cells, called thorny excrescences. Thorny excrescences form a tortuous cleft interface that slows down glutamate clearance (Savtchenko & Rusakov, 2004), thus facilitating mGluR activation. Interestingly, immunocytochemical and EM experiments showed that group II mGluRs, including mGluR2, are primarily localized at preterminal regions of MF terminals (Shigemoto et al. 1997). It is conceivable that owing to the remote location of mGluRs, glutamate must diffuse for some distance to reach mGluRs; indeed, mGluRs are not activated during low frequency stimulation (0.05 Hz), but they do become activated at higher frequencies (1 Hz), when release is facilitated and glutamate spreads from release sites (Nicoll & Schmitz, 2005). Our observation that in ceftriaxone-treated animals it was possible to rescue at least partially LTD by a stronger stimulation protocol is in line with this view. Thus, in the present experiments, both changes in GLT-1a distribution (closer to the active zone; AZ) and density at MF terminals may prevent glutamate diffusion and activation of group II mGluRs. Glutamate clearance would be further facilitated by the increased expression of GLT-1 in astrocytic processes ensheathing synapses. Interestingly, Wadiche & Jahr (2005) reported that in cerebellar Purkinje cells the efficacy of glutamate clearance mediated by the neuronal glutamate transporter EAAT4 varies in different cerebellar regions, and found that in regions expressing high EAAT4 levels the more efficient clearance of glutamate prevents activation of perisynaptic mGluRs and LTD induction.
Chronic treatment with ceftriaxone did not alter only LTD but also LTP, which at MF–CA3 synapses involves presynaptic mechanisms and is associated with an increase of glutamate release (Nicoll & Malenka, 1995; Kawamura et al. 2004). This may lead to long-lasting activation of presynaptic kainate receptors, a mechanism which imposes associative properties to MF synapses, because the activity in neighbouring synapses influences the threshold for inducing LTP (Schmitz et al. 2003; see also Bortolotto et al. 1999). Therefore, it is not surprising that an increased glutamate clearance following ceftriaxone-induced up-regulation of GLT-1 led to a reduced LTP at MF–CA3 but not at Schaffer collateral–CA1 synapses whose site of induction is postsynaptic (Nicoll & Malenka, 1995).
In conclusion, GLT-1 up-regulation, besides preventing excitotoxic damage by increasing glutamate clearance, may impair activity-dependent synaptic plasticity by preventing the activation of presynaptic autoreceptors which control transmitter release. This may affect the information coding and may lead to pathological states.
Acknowledgments
This work was supported by grants from Ministero Istruzione Universita’ e Ricerca (PRIN) to E.C. and F.C. A.Z. was supported by the Program for Training and Research of the International Center for Theoretical Physics (Trieste, Italy). We thank L. Ballerini for useful suggestions and R.J. Weinberg (UNC at Chapel Hill) for critically reading a previous version of the manuscript.
Glossary
Abbreviations
- AZ
active zone
- CEF
ceftriaxone
- DCG-IV
2-(2,3-dicarboxycyclopropyl)glycine
- γ-DGG,
γ-d-glutamylglycine
- d-APV
d-(–)-2-amino-5-phosphonopentanoic acid;
- DHK
dihydrokainate
- fEPSP
field excitatory postsynaptic potential
- GLT-1
glutamate transporter-1
- HFS
high frequency stimulation
- LFS
low frequency stimulation
- LTD
long-term depression
- LTP
long-term potentiation
- MCPG
(RS)-α-methyl-4-carboxyphenylglycine
- MF
mossy fibre
- mGluR
metabotropic glutamate receptor
- PPR
paired pulse ratio
- PSD
post-synaptic density
Author contributions
A.O. and V.S. performed electrophysiological experiments, M.B. performed western and immunofluorescence studies, M.M. carried out immunogold studies, T.A. and K.T. performed KO studies. F.C. and E.C. designed experiments and wrote the paper. All authors discussed the results and commented on the manuscript.
Supplemental material
References
- Amara SG, Fontana AC. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int. 2002;41:313–318. doi: 10.1016/s0197-0186(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Dent JA. Development of the mossy fibres of the dentate gyrus: I. A light and electron microscopic study of the mossy fibres and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- Bellesi M, Melone M, Gubbini A, Battistacci S, Conti F. GLT-1 upregulation impairs prepulse inhibition of the startle reflex in adult rats. Glia. 2009;57:703–713. doi: 10.1002/glia.20798. [DOI] [PubMed] [Google Scholar]
- Bergersen LH, Storm-Mathisen J, Gundersen V. Immunogold quantification of amino acids and proteins in complex subcellular compartments. Nat Protoc. 2008;3:144–152. doi: 10.1038/nprot.2007.525. [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Frotscher M, Jonas P. Timing and efficacy of transmitter release at mossy fibre synapses in the hippocampal network. Pflugers Arch. 2006;453:361–372. doi: 10.1007/s00424-006-0093-2. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bragina L, Melone M, Fattorini G, Torres-Ramos M, Vallejo-Illarramendi A, Matute C, Conti F. GLT-1 down-regulation induced by clozapine in rat frontal cortex is associated with synaptophysin up-regulation. J Neurochem. 2006;99:134–141. doi: 10.1111/j.1471-4159.2006.04030.x. [DOI] [PubMed] [Google Scholar]
- Chen W, Mahadomrongkul V, Berger UV, Bassan M, DeSilva T, Tanaka K, Irwin N, Aoki C, Rosenberg PA. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. J Neurosci. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, Kim SJ, Park DK, Jung KH, Song EC, Lee SK, Kim M, Roh JK. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke. 2007;38:177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- Conti F, Weinberg RJ. Shaping excitation at glutamatergic synapses. Trends Neurosci. 1999;22:451–458. doi: 10.1016/s0166-2236(99)01445-9. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Pines G, Kanner BI. Purification and reconstitution of the sodium- and potassium-coupled glutamate transport glycoprotein from rat brain. Biochemistry. 1990;29:6734–6740. doi: 10.1021/bi00480a025. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RE, Carroll HP, Harris A, Maher ER, Selby PJ, Banks RE. Housekeeping proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics. 2005;5:566–571. doi: 10.1002/pmic.200400941. [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fibre pathway: a review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Manita S, Nakamura T, Inoue M, Kudo Y, Miyakawa H. Glutamate release increases during mossy-CA3 LTP but not during Schaffer-CA1 LTP. Eur J Neurosci. 2004;19:1591–1600. doi: 10.1111/j.1460-9568.2004.03258.x. [DOI] [PubMed] [Google Scholar]
- Kharazia VN, Weinberg RJ. Tangential synaptic distribution of NMDA and AMPA receptors in rat neocortex. Neurosci Lett. 1997;238:41–44. doi: 10.1016/s0304-3940(97)00846-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Manabe T, Takahashi T. Presynaptic long-term depression at the hippocampal mossy fibre-CA3 synapse. Science. 1996;273:648–650. doi: 10.1126/science.273.5275.648. [DOI] [PubMed] [Google Scholar]
- Kwon HB, Castillo PE. Role of glutamate autoreceptors at hippocampal mossy fibre synapses. Neuron. 2008;60:1082–1094. doi: 10.1016/j.neuron.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Delany C, J Clarke VR, Bortolotto ZA, Ornstein PL, Isaac JTR, Collingridge GL. Synaptic activation of a presynaptic kainate receptor facilitates AMPA receptor-mediated synaptic transmission at hippocampal mossy fibre synapses. Neuropharmacology. 2001;41:907–915. doi: 10.1016/s0028-3908(01)00152-6. [DOI] [PubMed] [Google Scholar]
- Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron. 1999;22:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Melone M, Burette A, Weinberg RJ. Light microscopic identification and immunocytochemical characterization of glutamatergic synapses in brain sections. J Comp Neurol. 2005;492:495–509. doi: 10.1002/cne.20743. [DOI] [PubMed] [Google Scholar]
- Melone M, Bellesi M, Conti F. Synaptic localization of GLT-1a in the rat somatic sensory cortex. Glia. 2009;57:108–117. doi: 10.1002/glia.20744. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H. The Fine Structure of Nervous System: Neurons and their Supporting Cells. New York: Oxford University Press; 1991. [Google Scholar]
- Phend KD, Rustioni A, Weinberg RJ. An osmium-free method of epon embedment that preserves both ultrastructure and antigenicity for post-embedding immunocytochemistry. J Histochem Cytochem. 1995;43:283–292. doi: 10.1177/43.3.7532656. [DOI] [PubMed] [Google Scholar]
- Rosato-Siri M, Cattaneo A, Cherubini E. Nicotine-induced enhancement of synaptic plasticity at CA3–CA1 synapses requires GABAergic interneurons in adult anti-NGF mice. J Physiol. 2006;576:361–377. doi: 10.1113/jphysiol.2006.114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. A modified method for lead staining of thin sections. J Electron Microsc (Tokyo) 1968;17:158–159. [PubMed] [Google Scholar]
- Savtchenko LP, Rusakov DA. Glutamate escape from a tortuous synaptic cleft of the hippocampal mossy fibre synapse. Neurochem Int. 2004;45:479–484. doi: 10.1016/j.neuint.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Scanziani M. Competing on the edge. Trends Neurosci. 2002;25:282–283. doi: 10.1016/s0166-2236(02)02155-0. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fibre synapses. Science. 2001;291:1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Breustedt J, Nicoll RA. Presynaptic kainate receptors impart an associative property to hippocampal mossy fibre long-term potentiation. Nat Neurosci. 2003;6:1058–1063. doi: 10.1038/nn1116. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ. Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci. 2001;21:1211–1217. doi: 10.1523/JNEUROSCI.21-04-01211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqui H, Zhu H, Yao D, Ming H, Erickson JD. Cloning and functional identification of a neuronal glutamine transporter. J Biol Chem. 2000;275:4049–4054. doi: 10.1074/jbc.275.6.4049. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8:1329–1334. doi: 10.1038/nn1539. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Impairment of hippocampal mossy fibre LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.