Abstract
Latent membrane protein 2A (LMP2A) of Epstein-Barr virus (EBV) plays a key role in regulating viral latency and EBV pathogenesis by functionally mimicking signals induced by the B-cell receptor (BCR) altering normal B cell development. As c-Cbl ubiquitin ligase (E3) is a critical negative regulator in the BCR signal pathway, the role of c-Cbl in the function and formation of the LMP2A signalsome was examined. c-Cbl promoted LMP2A degradation through ubiquitination, specifically degraded the Syk protein tyrosine kinase in the presence of LMP2A, and inhibited LMP2A induction of the EBV lytic cycle. Our earlier studies indicated that LMP2A-dependent Lyn degradation was mediated by Nedd4-family E3s in LMP2A expressing cells. Combine with these new findings, we propose a model in which c-Cbl and Nedd4-family E3s cooperate to degrade target proteins at discrete steps in the function of the LMP2A signalosome.
Keywords: Epstein-Barr virus (EBV), Latent membrane protein 2A (LMP2A), c-Cbl, Lyn, Syk, ubiquitin, lytic replication
Introduction
Latent membrane protein 2A (LMP2A) is an Epstein-Barr virus (EBV) encoded protein that has been implicated in regulating viral latency and pathogenesis in EBV infections (Ikeda et al., 2005; Longnecker, 2000). LMP2A functions as a signalosome by constitutively associating and activating proteins normally associated with the B cell receptor (BCR) (Longnecker, 2000). The understanding of the molecular basis of LMP2A-mediated signaling is essential to clarify the involvement of LMP2A in EBV latent infections and EBV-related malignancies. The elucidation of differences between the BCR and LMP2A signaling may aide the development of novel therapeutic agents to treat EBV latent infections and EBV-associated cancers.
The LMP2A amino-terminal domain interacts and activates with the Src family protein tyrosine kinase (PTK) Lyn and the Syk PTK (Fruehling and Longnecker, 1997; Fruehling et al., 1998; Rovedo and Longnecker, 2008) in a constitutive manner mimicking a BCR providing development and survival signals in the absence of corresponding antigens (Caldwell et al., 1998). In contrast to the BCR, LMP2A contains two PY motifs (PPXY) that specifically associate with Nedd4-family ubiquitin-protein ligases (E3s) resulting in the downmodulation of LMP2A activity by ubiquitinating both LMP2A and LMP2A-associated PTKs (Ikeda et al., 2000; Ikeda et al., 2001; Winberg et al., 2000). In addition, LMP2A ubiquitination negatively regulates LMP2A signal transduction in B cell development (Ikeda et al., 2003; Ikeda et al., 2004). LMP2A ubiquitin-dependent processes are likely important for LMP2A function in EBV latent infection such as the modulation of LMP2A-induced signals which alter normal B cell development (Casola et al., 2004; Ikeda et al., 2004).
BCR stimulation triggers the activation of PTKs, which leads to the phosphorylation of numerous signal molecules such as adapter, docking and effecter proteins (Kurosaki, 2002). The phosphorylation of B cell signal molecules is critical for their recruitment to the plasma membrane and the formation of BCR signalosome. The proto-oncogenic protein c-Cbl and other Cbl-family proteins have been recognized as key players in the negative regulation of antigen receptor and other signaling pathways (Swaminathan and Tsygankov, 2006). c-Cbl is a RING-finger E3 that negatively regulates the BCR and other signal pathways by targeting multiple signal molecules for degradation. These targets include Src-family and Syk PTKs (Swaminathan and Tsygankov, 2006). Cbl proteins are multivalent adapter proteins capable of interacting with multiple signal components (Swaminathan and Tsygankov, 2006). The phosphorylation of Cbl proteins following signal stimulation is essential for the pivotal role of Cbl proteins for their adaptor function (Swaminathan and Tsygankov, 2006).
Several previous studies have shown that c-Cbl interacts with known LMP2A-associated proteins. Two Nedd4-family E3s, Nedd4 and AIP4/Itch, bind to three Cbl-family proteins and target them for degradation, which inhibits Cbl-mediated desensitization of activated EGFR and non-receptor c-Src PTKs (Courbard et al., 2002; Magnifico et al., 2003). Syk is a target of Cbl-mediated ubiquitination and degradation upon BCR stimulation (Rao et al., 2001). Cbl proteins preferentially interact with and target Src-family PTKs including Lyn, Fyn and Lck for degradation (Andoniou et al., 2000; Kaabeche et al., 2004; Rao et al., 2002; Sanjay et al., 2001). These interactions suggest that c-Cbl may interact with LMP2A-associated proteins with functional consequences. In addition, c-Cbl is constitutively phosphorylated in LMP2A-expressing LCLs (Engels et al., 2001). Taken together, these previous studies suggest c-Cbl adaptor functions in LMP2A signaling and the downmodulation of LMP2A signaling by c-Cbl E3 activity. In this paper, we demonstrate that c-Cbl promotes the degradation of LMP2A and LMP2A associated proteins. Furthermore, our results indicate that c-Cbl may be an important regulator of EBV latency by blocking LMP2A-mediated induction of the EBV lytic cycle as has been previously observed (Schaadt et al., 2005).
Results
c-Cbl inhibits the expression of LMP2A and LMP2A-associated proteins
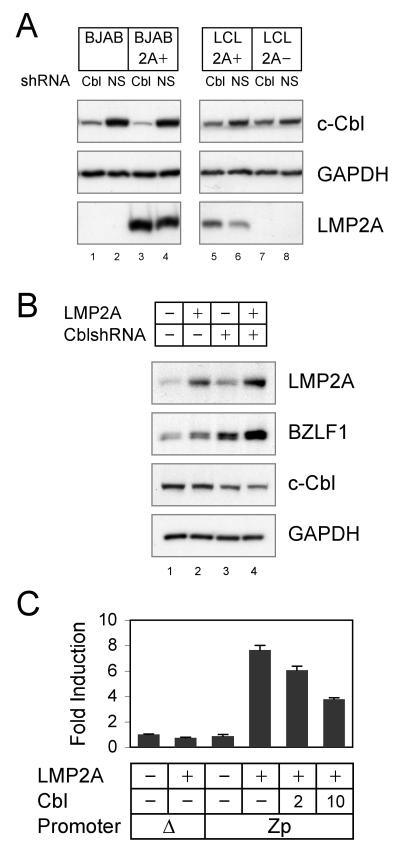
To examine whether c-Cbl downmodulates LMP2A-mediated signaling, c-Cbl specific shRNA was used to reduce expression of the c-Cbl protein. LMP2A+ and LMP2A− BJAB cells and EBV infected LCLs were infected with a lentivirus containing c-Cbl shRNAmir and transduced cells were selected by puromycin selection. In BJAB cells, the reduction of c-Cbl protein was at approximately 90% compared to nonspecific shRNAmir infected control cells (Fig. 1A, c-Cbl, lanes 1-4) as determined by densitometry. In LMP2A+ and LMP2A− LCLs, the c-Cbl reduction was approximately 50% (Fig. 1A, c-Cbl, lanes 5-8) also as determined by densitometry. In both BJAB cells and LCLs expressing LMP2A there was very modest decrease in LMP2A expression (Fig. 1A, LMP2A, compare lane 3 with lane 4 and lane 5 with lane 6). The levels of GAPDH were similar in all lysates (Fig. 1A, GAPDH).
Fig. 1. LMP2A degradation and LMP2A inhibition of EBV lytic replication by c-Cbl.
(A) BJAB, BJAB-LMP2A+, LMP2A-expressing LCLs and LMP2A-deleted LCLs were infected with c-Cbl shRNAmir or nonesilencing (NS) shRNAmir lentiviruses. Cell lysates were immunoblotted for c-Cbl and GAPDH. Immunoprecipates of LMP2A, Lyn and Syk were immunoblotted for each protein and for phosphotyrosine. (B) EREB2.5 cells were infected with c-Cbl shRNAmir or nonesilencing (NS) shRNAmir lentiviruses. Selected cells were depleted with estrogen and transfected with LMP2A-expressing plasmid or control vector. Cell lysates were immunoblotted for LMP2A, BZLF1, c-Cbl and GAPDH. (C) BJAB cells were transfected with 5 μg of reporter plasmid expressing firefly luciferase under the control of BZLF1 promoter (Zp) with 20 μg of LMP2A and indicated μg of c-Cbl. Average fold induction of luciferase activity to promoterless plasmid with no activator is indicated. Data averaged from three independent experiments are shown ± standard deviation. The data is the representative of three comparable experiments.
c-Cbl negatively regulates LMP2A-mediated EBV lytic induction
To further examine the c-Cbl role in LMP2A signaling, LMP2A-mediated EBV lytic induction was examined. It has been reported that transient expression of LMP2A can induce expression of the EBV immediate early protein BZLF1 using EREB2.5 cells (Schaadt et al., 2005). EREB2.5 cells were established by the infection of human B cells with a recombinant EBV expressing an EBNA2-estrogen receptor fusion protein (Kempkes et al., 1996). Upon estrogen depletion, EBNA2 is inactivated and EBNA2-dependent genes including LMP2A are downregulated. In the absence of EBNA2, these cells are highly permissive for virus reactivation. An LMP2A expressing plasmid was transiently transfected into freshly estrogen depleted EREB2.5 cells. In the cells without LMP2A transfection, the basal level of LMP2A expressed in the EREB2.5 cells was observed (Fig. 1B, LMP2A, lane 1). As previously reported, overexpression of LMP2A induced EBV lytic replication over the baseline levels of lytic replication as measured by BZLF1 (Fig. 1B, BZLF1, lane 2). In c-Cbl shRNAmir lentivirus infected EREB2.5 cells, c-Cbl silencing induced BZLF1 regardless of LMP2A transfection (Fig. 1B, BZLF1, lanes 1 and 3). Interestingly, LMP2A transfection into c-Cbl shRNA cells increased BZLF1 induction to a greater extent than when compared to LMP2A or c-Cbl shRNA only cells (Fig. 1B, BZLF1, compare lane 4 with lanes 2 and 3). Finally, we sought to determine whether LMP2A activates the BZLF1 promoter. The requited activating region (−221 to +12) of BZLF1 promoter (Zp) was cloned into the pGL3 Basic reporter vector, rendering expression of firefly luciferase dependent upon the Zp. BJAB cells were transiently transfected with the Zp-reporter and the LMP2A expression plasmid. Expression of LMP2A alone was sufficient to activate the Zp (Fig. 1C). LMP2A induced an eight-fold increase in the relative luciferase activity but did not induce luciferase activity from the promoterless pGL3 Basic reporter vector (Fig. 1C). The expression of c-Cbl significantly decreased the fold induction of LMP2A-mediated luciferase activity in a dose-dependent manner indicating that c-Cbl inhibits LMP2A-mediated BZLF1 induction.
Expression and degradation of LMP2A, Lyn, and Syk in the presence of c-Cbl
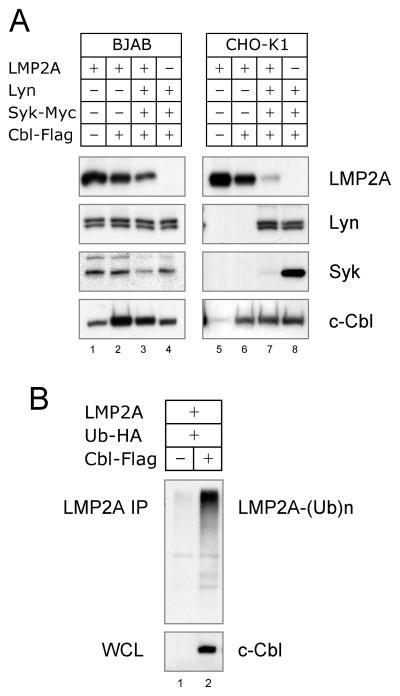
We next examined the molecular mechanism of Cbl-catalyzed degradation of the LMP2A complex. Expression plasmids for LMP2A, Lyn, Syk and c-Cbl were co-transfected in B cells and Chinese hamster ovary (CHO-K1) cells. The level of each protein in cell lysates was determined by immunoblotting with specific antibodies. The transient expression of LMP2A and c-Cbl was readily observed in BJAB and CHO-K1 cells, whereas significant increases of Lyn and Syk was only observed in the CHO-K1 cells (Fig 2A). Compatible with the c-Cbl silencing results, c-Cbl caused a decrease in LMP2A expression in the both cell lines (Fig. 2A, LMP2A, compare lane 1 with lane 2 and lane 5 with lane 6). c-Cbl also caused LMP2A ubiquitination (Fig. 2B), indicating that c-Cbl targeted LMP2A to be degraded by the proteasome. When Lyn and Syk were coexpressed with LMP2A and c-Cbl, LMP2A was degraded to a greater extent (Fig. 2A, LMP2A, lanes 3 and 7), indicating that LMP2A-associated signal molecules are critical for promoting LMP2A degradation. Interestingly, LMP2A caused a dramatic decrease in Syk in CHO-K1 and even in BJAB cells (Fig. 2A, Syk, compare lane 3 with lane 4 and lane 7 with lane 8). However, LMP2A did not alter the level of Lyn (Fig. 2A, Lyn, compare lane 3 with lane 4 and lane 7 with lane 8). This difference between the ability of LMP2A to target effectively target Lyn in transient transfections has previously been observed (Rovedo and Longnecker, 2008). This indicates that LMP2A specifically regulates the degradation of Syk which is a key player in LMP2A signaling (Merchant et al., 2000).
Fig. 2. Expression and ubiquitination of LMP2A signalosome.
(A) BJAB and CHO-K1 cells were transfected with 10 or 1 μg of LMP2A, Lyn, Syk-Myc and c-Cbl-Flag, respectively. Cell lysates were immunoblotted with anti-LMP2A, Lyn, Syk or c-Cbl antibody to detect both endogenous and transfected expression. (B) BJAB cells were transfected with 10 μg of LMP2A, Ub-HA and c-Cbl-Flag, respectively. LMP2A immunoprecipitates were immunoblotted with anti-HA antibody to detect ubiquitin-conjugated LMP2A. Cell lysates were immunoblotted with anti-c-Cbl antibody. The data is the representative of four comparable experiments.
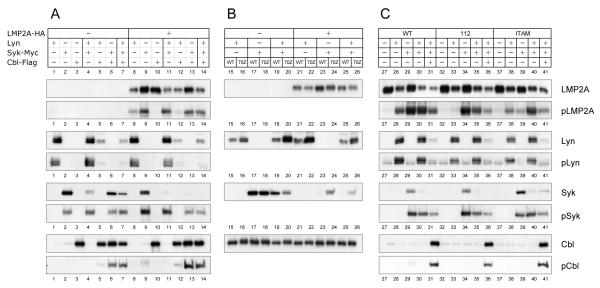
Since the results obtained with the B cell line were consistent with the CHO-K1 results, we chose to use this approach to examine the details of c-Cbl function in LMP2A signaling for their ease of transfection, the resulting high levels of protein expression, and reduced levels or lack of expression of Lyn, Syk and Cbl. Similar approaches using COS, 293T, NIH-3T3 and insect cells have been used to dissect the molecular mechanisms for a variety of receptors including the BCR, T cell receptor (TCR), and high affinity IgE receptor (FcεRI) (Saouaf et al., 1995; Scharenberg et al., 1995; Wossning and Reth, 2004; Yamamoto et al., 2001). Initially, LMP2A expression was examined in transfected CHO-K1 cells. Interestingly, a reduction of LMP2A was always observed when Lyn was coexpressed (Fig. 3A, LMP2A, compare lanes 9, 10, and 13 with lanes 8, 11, 12, and 14). However, Syk expression did not change levels of LMP2A (Fig. 3A, LMP2A). This indicates that Syk is not required for Lyn-dependent LMP2A degradation.
Fig. 3. Expression and phosphorylation of LMP2A, Lyn, Syk and c-Cbl.
CHO-K1 cells were transfected with 1 μg of wild type (WT) or mutant LMP2A-HA, Lyn, Syk-Myc, and wild type (WT) or mutant c-Cbl-Flag (70Z) as indicated. Cell lysates were immunoblotted with anti-LMP2A, Lyn, Syk or c-Cbl antibody to detect both endogenous and transfected expression. Immunoprecipates of LMP2A (HA), Lyn, Syk (Myc) and c-Cbl (Flag) were immunoblotted for phosphotyrosine. The data is the representative of three comparable experiments.
In the next series of experiments, Lyn expression was analyzed similarly. The reduction of Lyn was observed when c-Cbl was coexpressed (Fig. 3A, Lyn, compare lanes 1, 4, 8, and 11 with lanes 5, 7, 12, and 14). The Lyn reduction was not observed when the RING-finger mutant 70Z was coexpressed instead of wild type c-Cbl (Fig. 3B, Lyn, compare lane 15 with lane 16, lane 19 with lane 20, lane 21 with lane 22, and lane 25 with lane 26). Thus, Lyn levels are, at least in part, regulated by the RING-finger ubiquitin ligase activity of c-Cbl. However, neither LMP2A nor Syk changed the c-Cbl-mediated Lyn degradation (Fig. 3A, Lyn, compare lane 5 with lanes 7 and 12). Moreover, neither the Y112F nor the Y74/85F LMP2A mutants altered Lyn degradation (Fig. 3C, Lyn, lanes 31, 36 and 41). Previous studies have shown that both the Y112F mutant and the Y74/85F mutant are non-functional in B cells (Fruehling et al., 1998). The Y112F mutant is unable to bind Lyn whereas the Y74/85F which has a mutated ITAM does not bind Syk (Fruehling and Longnecker, 1997; Fruehling et al., 1998). This indicates that the Lyn degradation by c-Cbl was independent of LMP2A or LMP2A signalosome formation.
Finally, Syk levels were analyzed in the transfected cells. Although the c-Cbl-mediated reduction was similarly observed for Syk, Syk reduction was dependent on LMP2A in contrast to Lyn (Fig. 3A). The Syk reduction was relatively small when only c-Cbl was coexpressed, but in the presence of LMP2A coexpression with c-Cbl dramatically reduced Syk levels (Fig. 3A, Syk, lanes 2, 6 and 13). In addition to LMP2A coexpression, the additional coexpression of Lyn with LMP2A furthermore diminished this Syk reduction (Fig. 3A, Syk, compare lane 6 with 7 and lane 13 with lane 14). Moreover, the 70Z mutant restored the Syk levels in the presence of LMP2A although it did not in the absence of LMP2A (Fig. 3B, Syk, lanes 17-18 and 23-24). Thus, c-Cbl degrades Syk efficiently in the presence of LMP2A. In the absence of LMP2A, Lyn can modestly reduce Syk (Fig. 3A, Syk, lanes 2 and 4). This Lyn alteration on Syk expression was independent of the c-Cbl RING since the 70Z-Cbl did not restore the Syk expression (Fig. 3B, Syk, lanes 19 and 20). The mechanism of the latter observation remains to be elucidated.
Phosphorylation of LMP2A, Lyn, Syk, and c-Cbl
Since phosphorylation is critical for the formation of most receptor-mediated signalosomes, we examined the phosphorylation of LMP2A, Lyn, Syk and Cbl. In the absence of Lyn and Syk, LMP2A phosphorylation was negligible, indicating the key roles of these two proteins in LMP2A phosphorylation. Interestingly, Lyn or Syk was sufficient for LMP2A phosphorylation (Fig. 3A, pLMP2A, lanes 8 and 9) indicating that high levels of Syk expression can bypass the previously observed requirement for Lyn for the initial steps in LMP2A phosphorylation (Fruehling et al., 1998). As might be expected, the combination of both Lyn and Syk produced the highest levels of LMP2A phosphorylation (Fig. 3A, pLMP2A, lane 11). LMP2A binds to Lyn through phosphorylated Y112 while it binds to Syk through phosphorylated Y74 and Y85 of the LMP2A ITAM (Fruehling and Longnecker, 1997; Fruehling et al., 1998). As shown in Fig. 3C, the Y112F mutation in LMP2A caused the loss of LMP2A phosphorylation by Lyn (pLMP2A, compare lane 28 with lane 33), however it did not affect LMP2A phosphorylation mediated by Syk (pLMP2A, compare lane 29 with lane 34). Thus, Y112 is not only required for the interaction with Lyn but also essential for LMP2A phosphorylation mediated by Lyn. Although not as dramatic as observed for the Y112F mutation and Lyn, the Y74/85F mutation greatly reduced LMP2A phosphorylation by Syk (Fig. 3C, pLMP2A, compare lane 29 with lane 39), while the phosphorylation mediated by Lyn transfection was fairly similar to wild type LMP2A (Fig. 3C, pLMP2A compare lane 28 with lane 38). This emphasizes the necessity of the LMP2A ITAM for high level LMP2A phosphorylation.
In addition to phosphorylating LMP2A, Lyn transfection resulted in the phosphorylation of Syk, c-Cbl and Lyn (Fig. 3A, pSyk lane 4, pCbl lane 5, and pLyn lane 1). Syk expression resulted in the phosphorylation of c-Cbl as well as Syk phosphorylation (Fig. 3A, pCbl lane 6, pSyk lane 2). These results indicate, as might be expected that Lyn and Syk have differences in substrate specificity. As shown in Fig. 3A, in the presence of Lyn, Syk phosphorylation was increased (pSyk lanes 2 and 4), however in the presence of Syk, Lyn phosphorylation was not increased (pLyn lanes 1 and 4). In regard to the requirement of LMP2A for the observed phosphorylation, Lyn phosphorylation was independent of LMP2A (Fig. 3A, pLyn, lanes 1 and 8), whereas Lyn- and Syk-mediated Syk phosphorylation was considerably increased when LMP2A was present (Fig. 3A, pSyk, lanes 2 and 9). Moreover, c-Cbl phosphorylation in the presence of Syk was significantly enhanced by LMP2A (Fig. 3A, pCbl, lanes 6 and 13). These observations may indicate that the action of Lyn is an early requirement for the LMP2A signalosome formation as our earlier studies have suggested (Fruehling et al., 1998).
c-Cbl RING mediates Lyn degradation
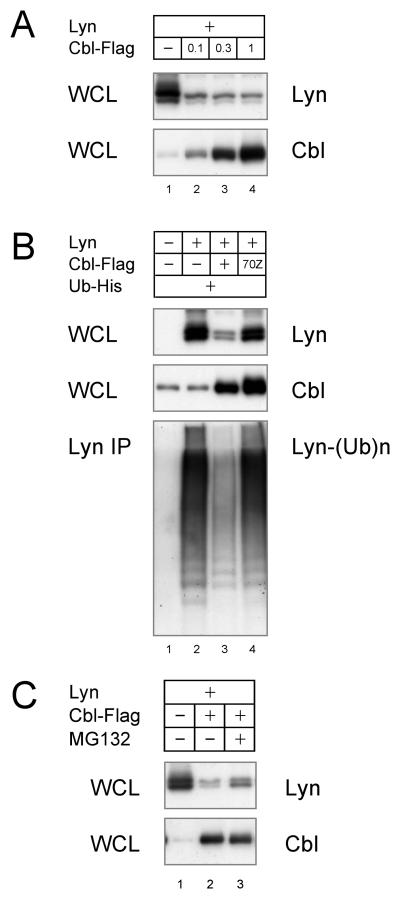
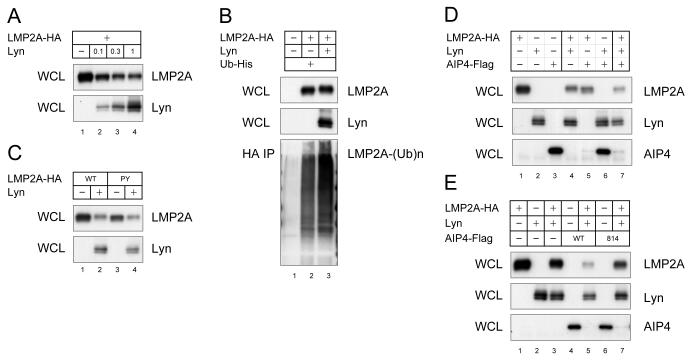
The molecular mechanism of Lyn degradation by c-Cbl was further examined. As shown in Fig. 3A, transfection of only c-Cbl was sufficient to degrade Lyn. This was verified by demonstrating a dose-dependent decrease in Lyn expression by reducing the amount of cCbl cotransfected with Lyn (Fig. 4A). We next tested whether Lyn was ubiquitinated by the c-Cbl E3 activity. As shown in Fig. 4B, Lyn was highly ubiquitinated without c-Cbl overexpression, but the levels of Lyn were not as greatly reduced as when c-Cbl was transfected. The Lyn ubiquitination observed in the absence of c-Cbl is likely mediated by ubiquitin ligases constitutively expressed in the CHO-K1 cells. To further investigate these observations, the c-Cbl 70Z mutant was expressed instead of wild type c-Cbl. Expression of this c-Cbl mutant increased the amount of Lyn and the ubiquitination as observed in the cells transfected with just Lyn (Fig. 4B, lane 4). This results are quite interestingly and suggest that overexpression of the c-Cbl mutant mediates more efficient Lyn degradation. This may be explained by the c-Cbl adapter ability, in which both Lyn and Nedd4-family E3s are recruited by c-Cbl, causing the degradation of Lyn by the Nedd4-family. Finally, we addressed the question whether the Lyn degradation is mediated by proteasomes. As shown in Fig. 4C, the proteasome inhibitor MG132 resulted in a modest increase in Lyn levels, indicating that a portion of Lyn is processed in proteasome. However, since there was not a complete restoration other mechanisms may be involved in the Lyn degradation and/or the inhibition of the proteoseome by MG132 may not be complete.
Fig. 4. Lyn degradation by c-Cbl RING.
(A) CHO-K1 cells were transfected with 1 μg of Lyn and indicated amount of c-Cbl-Flag. Cell lysates were immunoblotted for Lyn and c-Cbl. (B) CHO-K1 cells were transfected with Lyn, wild type or mutant c-Cbl-Flag, and Ub-His. Lyn immunoprecipitates were immunoblotted with anti-His antibody to detect ubiquitin-conjugated Lyn. (C) Transfected CHO-K1 cells were treated with 10 μM MG132 and immunoblotted for Lyn and c-Cbl. The data is the representative of three comparable experiments.
c-Cbl mediates LMP2A-dependent Syk degradation
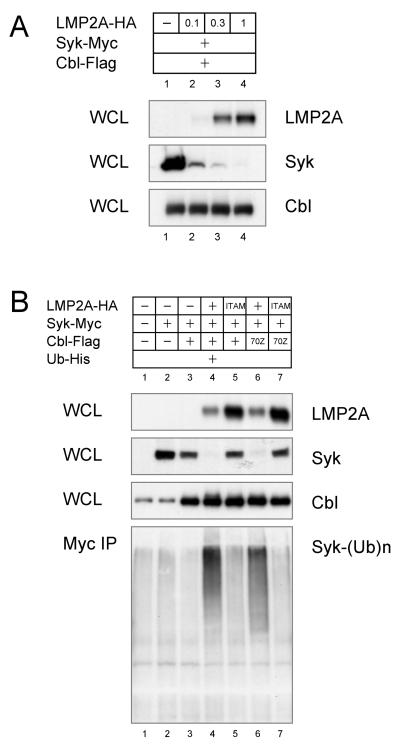
The role of LMP2A in Syk degradation was further analyzed in transfection by increasing the amount of LMP2A included in the transfection with c-Cbl and Syk. A dose-dependent increase in Syk degradation was observed when more LMP2A was included in the transfection (Fig. 5A). The amount of LMP2A-catalyzed Syk degradation correlated with increased Syk ubiquitination (Fig. 5B, lanes 3 and 4). In contrast to Lyn, Syk was not constitutively ubiquitinated even when c-Cbl was overexpressed (compare Fig. 5B, lanes 2 and 3 with Fig. 4B, lanes 2 and 3). Syk ubiquitination was only observed in the presence of LMP2A (Fig. 5B, lanes 2 and 4). Interestingly, Syk degradation did not appear to be dependent on c-Cbl since transfection of the c-Cbl mutant 70Z had no effect on Syk levels or Syk ubiquitination (Fig. 5B, lanes 4 and 6). Finally, by using the LMP2A Y74/85F ITAM mutant that does not bind Syk, it was apparent that the interaction of Syk with LMP2A is required for the LMP2A-mediated degradation of Syk (Fig. 5B, lanes 5 and 7).
Fig. 5. LMP2A-dependent Syk degradation by c-Cbl.
(A) CHO-K1 cells were transfected with indicated amount of LMP2A-HA, 1 μg of Syk-Myc, and 1 μg of c-Cbl-Flag. Cell lysates were immunoblotted for LMP2A, Syk and c-Cbl. (B) CHO-K1 cells were transfected with 1 μg of wild type or ITAM-mutant LMP2A-HA, Syk-Myc, c-Cbl-Flag and Ub-His. Anti-Syk (Myc) immunoprecipitates were immunoblotted with anti-His antibody to detect ubiquitin-conjugated Syk. The data is the representative of three comparable experiments.
Nedd4-family ubiquitin ligases are not required for Lyn-mediated LMP2A degradation
In our final series of experiments, we investigated the mechanism of Lyn-mediated degradation of LMP2A. The interaction between c-Cbl and Lyn (Kaabeche et al., 2004) raises the possibility that LMP2A recruits c-Cbl to degrade LMP2A signalosome by using Lyn as an adaptor protein. Indeed, Lyn increased LMP2A degradation as well as ubiquitination (Fig. 6A and 6B). To verify this model, the effect of the c-Cbl 70Z mutation on LMP2A degradation was tested. As shown in Fig. 3B, the c-Cbl 70Z mutation inhibited Lyn degradation (Lyn, compare lane 15 with lane 16, lane 19 with lane 20, lane 21 with lane 22, and lane 25 with lane 26), however the c-Cbl mutant did not increase LMP2A degradation (LMP2A, compare lane 21 with lane 22, lane 23 with lane 24, and lane 25 with lane 26). Instead, it appeared that increased Lyn expression resulted in a decrease of LMP2A (Fig. 3B, LMP2A, lanes 22 and 26). Thus in this experiment, c-Cbl seemed to inhibit the negative regulator role of Lyn in LMP2A expression. In addition, our data suggests that the Lyn interaction with LMP2A is unnecessary for Lyn-dependent LMP2A degradation. As shown in Fig. 3C, the Lyn-binding deficient LMP2A mutant Y112F in which the LMP2A tyrosine that binds Lyn is mutated or the Syk-binding deficient Y74/85F mutant in which the LMP2A ITAM that binds Syk has been mutated, still demonstrate Lyn-dependent LMP2A degradation (LMP2A, lanes 28, 33 and 38). Thus, the exact molecular mechanism that Lyn utilizes to promote LMP2A degradation is not clear. Despite these observations, the significant increase in LMP2A degradation and ubiquitination by Lyn indicate the involvement of ubiquitin ligases in Lyn-mediated LMP2A degradation. Previous studies have shown that LMP2A is ubiquitinated by Nedd4-family E3s and that Src-family PTKs interact with Nedd4-family E3s, suggesting that Lyn increases the Nedd4 E3 activity by stabilizing the Nedd4 interaction with LMP2A. However, the Nedd4-interaction deficient PY1PY2 mutation within LMP2A does not reduce Lyn-mediated LMP2A degradation (Fig. 6C, lane 4). This indicates that Nedd4 E3s have little role in Lyn-mediated LMP2A degradation. Instead, Nedd4 E3s likely degrade LMP2A in addition to the enhancement of LMP2A degradation observed when Lyn is transfected. As shown in Fig. 6D, the Nedd4-family member AIP4/Itch increased induced LMP2A degradation when coexpressed with Lyn (LMP2A, lanes 4 and 7). Interestingly, the degradation of the AIP4/Itch like Syk is also greatly enhanced in the presence of LMP2A compared to transfections in which LMP2A is omitted (Fig. 6D, AIP4, compare lane 3 with lane 5, and lane 6 with lane 7). The HECT-domain mutation C814A within AIP4 was not sufficient to restore LMP2A expression to the level without Lyn coexpression (Fig. 6E, LMP2A, lanes 1, 3, 5 and 7). Thus, dominant-negative AIP4 did not suppress Lyn-mediate LMP2A degradation suggesting that both Lyn and Nedd4 regulate LMP2A levels in parallel.
Fig. 6. Lyn-mediated LMP2A ubiquitination.
(A) CHO-K1 cells were transfected with 1 μg of LMP2A-HA and indicated amount of Lyn. Cell lysates were immunoblotted for LMP2A and Lyn. (B) CHO-K1 cells were transfected with LMP2A-HA, Lyn, and Ub-His. HA immunoprecipitate was immunoblotted with anti-His antibody to detect ubiquitin-conjugated LMP2A. (C) CHO-K1 cells were transfected with wild type or PY1PY2 mutant LMP2A-HA, and Lyn. Cell lysates were immunoblotted for LMP2A and Lyn. (D & E) CHO-K1 cells were transfected with LMP2A-HA, Lyn, and wild type (WT) or mutant AIP4 (C814A). Cell lysates were immunoblotted for LMP2A, Lyn and AIP4/Itch. The data is the representative of three comparable experiments.
Discussion
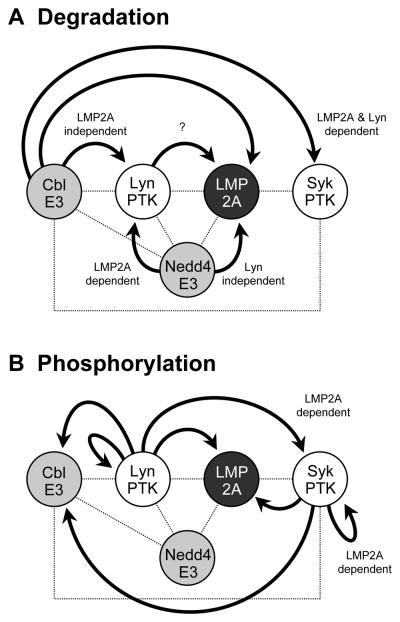
LMP2A development and survival signals are critical for the establishment of EBV latent infections and EBV-related malignant transformation. However, the molecular basis of LMP2A signaling and its regulation are not well understood. Here, we show that c-Cbl E3 downregulates the LMP2A signalsome and LMP2A induction of the viral lytic cycle. The Cbl-family proteins play a critical role in negatively regulating signals from BCR. Therefore, the involvement of c-Cbl in LMP2A signaling is not unexpected since LMP2A in many ways mimics a functional BCR. We demonstrated in this study that c-Cbl reduced Lyn expression in the LMP2A-independent manner while c-Cbl induced Syk degradation only in the presence of LMP2A. Thus, c-Cbl specifically downregulates Syk levels in the presence of LMP2A. This may be particular important for signals that emanate from LMP2A signalosome since this signal does not fully resemble an activated BCR (Portis and Longnecker, 2004a). In contrast to the dependence of c-Cbl to downregulate Syk levels, our earlier studies demonstrated Lyn specific degradation in LMP2A expressing cells is mediated by the Nedd4-family E3s. Taken together, these results allow us to propose a model in which c-Cbl and Nedd4-family ubiqutin ligases cooperate to degrade LMP2A target proteins at discrete steps in the function of the LMP2A signalosome (Fig. 7A). Nedd4-family E3s specifically degrade Lyn in the initiation of LMP2A signaling while c-Cbl strictly targets Syk as the LMP2A signalosome signal expands to target additional proteins which we have identified such as Btk, PI3Kinase, Akt, NF-kB, and Ras (Merchant and Longnecker, 2001; Portis and Longnecker, 2004b; Scholle et al., 2000; Swanson-Mungerson et al., 2005; Wang et al., 2006). This downmodulation of the LMP2A signalosome by c-Cbl and Nedd4 family E3s may be in particularly important in regulating the strength of the LMP2A signal. Future studies will focus on the importance of the ubiquitin ligases for the regulation of downstream targets of the LMP2A signalosome.
Fig. 7. Schematic model for the specific degradation and phosphorylation of LMP2A signalosome.
The LMP2A signalsome consists of a large complex mediated by a variety of protein-protein and phosphotyrosine-SH2 interactions (indicated by dotted lines in both Panels A and B). By forming a signalosome, LMP2A manipulates normal BCR signaling by targeting proteins in the LMP2A signalosome for degradation (indicated by arrows in panel A) and/or phosphorylation (indicated by arrows in panel B). (A) c-Cbl and Nedd4-family E3s respectively target specific proteins recruited to the LMP2A signalosome. Syk is specifically degraded as a result of constitutive LMP2A tyrosine phosphorylation mediated by the Lyn and Syk PTKs resulting in the phosphorylation and recruitment of c-Cbl to the LMP2A signalsome. This recruitment results in the degradation of Syk by c-Cbl. Thus, LMP2A and LMP2A phosphorylation are critical for the Syk degradation by c-Cbl. c-Cbl is also capable of degrading Lyn, however, LMP2A is unnecessary for this degradation. In contrast, Lyn is specifically downregulated by Nedd4-family ubiquitin ligases in the presence of LMP2A. Thus, the two key tyrosine kinases Lyn and Syk in LMP2A signaling are downregulated by two different ubiquitin ligases. In addition, although LMP2A is degraded by Nedd4-family ubiquitin ligases, we show in this study that Lyn enhances LMP2A degradation. Although the exact mechanism is unknown, our previous results indicate that lipid raft-mediated endocytosis may be key (Ikeda and Longnecker, 2007). (B) Lyn is able to induce the activation and phosphorylation of a number of substrate proteins independent of LMP2A including Lyn, c-Cbl, and LMP2A. In contrast, the activation of Syk by Lyn is dependent on LMP2A. Interestingly, the activation and phosphorylation of c-Cbl by Syk is independent of LMP2A whereas the activation and phosphorylation of Syk is dependent on LMP2A. The observations are of interest since they indicate a remarkable degree of specificity of the tyrosine kinases that are recruited to the LMP2A signalsome for targeting proteins contained within the LMP2A signalosome. Circled P indicates tyrosine phosphorylation.
In addition to the importance of c-Cbl E3 activity, many cellular events mediated by c-Cbl are dependent on c-Cbl adapter function. c-Cbl has been shown to form complexes with numerous proteins via its various binding domains. Our results indicate a potential importance of c-Cbl adapter function in LMP2A signalosome function. For example, the LMP2A Y74/85F mutant is deficient in Syk association and only Lyn and not Syk is able to bind this LMP2A mutant. However, when Syk is expressed along with Lyn, there is an increase in the phosphorylation of Y74/85F mutant. This indicates that Syk is able to recognize this LMP2A mutant through an interaction independent of binding of the tandem Syk SH2 domains with the LMP2A ITAM comprised of LMP2A Y74 and Y85. Since it is has been previously shown that c-Cbl interacts with Src-family protein tyrosine kinases and Nedd4-family ubiquitin ligases (Andoniou et al., 2000; Courbard et al., 2002; Kaabeche et al., 2004; Magnifico et al., 2003; Rao et al., 2002; Sanjay et al., 2001), it is reasonable to speculate that the interaction of c-Cbl with either Lyn or Nedd4 ubiquitin liagase enables Syk to interact with LMP2A and mediate the observed phosphorylation (Fig. 7B). Our attempts to detect a direct or indirect c-Cbl interaction with LMP2A by immunoprecipitation failed (data not shown). This suggests that the LMP2A interaction with c-Cbl is weaker than the interaction of LMP2A with Lyn, Syk, or Nedd4-family ubiquitin ligases. Additionally, detergent-sensitive cellular structures such as lipid rafts may provide a platform to recruit LMP2A and c-Cbl for their subsequent and continued interaction.
In addition to the factors such as c-Cbl that regulate the LMP2A signalosome, we made the interesting observation that expression of Lyn can also induce LMP2A degradation. Although our results suggest that this degradation was directed by c-Cbl, our recent studies on cholesterol-dependent LMP2A degradation may be informative (Ikeda and Longnecker, 2007). Previously, we demonstrated that cholesterol depletion from plasma membrane dramatically increases LMP2A abundance (Ikeda and Longnecker, 2007). In these studies, we hypothesized that this was a result of blocking endocytosis of the LMP2A signalosome. The cholesterol depletion also blocked LMP2A phosphorylation and ubiquitination. As Lyn and LMP2A are constitutively present in lipid rafts (Dykstra et al., 2001), the block of LMP2A phosphorylation by cholesterol depletion can be explained by the disruption of lipid rafts and the resulting dissociation of LMP2A and Lyn. The increase of LMP2A ubiquitination by Lyn observed in this study correlates nicely with our previous study. Therefore, it may be reasonable to conclude that the interaction of Lyn with LMP2A promotes endocytosis of the LMP2A signalosome. Overall, the results presented in this study provide new information into formation of the LMP2A signalosome and subsequent regulation of signals that are transduced by LMP2A.
Materials and Methods
Cell culture
All cell lines were maintained in medium supplemented with 10% serum, 100 U/ml penicillin and 100 μg/ml streptomycin. RPMI1640 medium was used for the culture of B cell lines. LCL1 is a B95-8 EBV-transformed lymphoblastoid cell line (LCL) expressing wild-type LMP2A (Longnecker and Kieff, 1990). ES1 is a LCL harboring LMP2A-deleted EBV genome (Longnecker et al., 1993). BJAB is an EBV negative B lymphoma cell line obtained from ATCC (Rockville, MD). The BJAB cell line expressing LMP2A (BJAB-LMP2A+) was previously described (Longnecker and Kieff, 1990). EREB2.5 is an estrogen-dependent LCL and grown in the medium with 1 μM estrogen and was kindly provided by Bettina Kempkes. Chinese hamster ovary (CHO)-K1 cells were grown in Ham's F12 medium. Human Embryonic Kidney (HEK) 293T cells were grown in DMEM.
Antibodies
The anti-LMP2A rat monoclonal antibody (14B7) was previously described (Fruehling et al., 1996). The anti-HA mouse monoclonal antibody (12CA5) was from Covance. The anti-Myc mouse monoclonal antibody (9E11) was purified at the Northwestern University Monoclonal Antibody Facility. The anti-Flag mouse monoclonal antibody (M2) was from Sigma. The anti-Lyn, Syk, and Cbl rabbit polyclonal antibodies were from Santa Cruz. The anti-GAPDH mouse monoclonal antibody was from Abcam. The anti-phosphotyrosine mouse monoclonal antibody (PY20) was from Santa Cruz. The anti-BZLF1 mouse monoclonal antibody was described previously (Young et al., 1991). All horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Amersham.
Plasmids
The wild type or HA-tagged LMP2A cDNA was subcloned in the mammal expression vector pSG5 (Fruehling et al., 1998). The LMP2A mutant constructs of Y112F, Y74/85F, and PY1PY2 were described previously (Fruehling and Longnecker, 1997; Fruehling et al., 1998; Ikeda et al., 2001). The Lyn cDNA was amplified by PCR from pSV7c-Lyn and subcloned into pcDNA3.1 Neo. Myc-tagged Syk expression plasmid, cloned in pcDNA3.1 Neo, was obtained from Dr. S. Latour (Latour et al., 1996). The wild type c-Cbl and oncogenic mutant 70Z cDNAs, obtained from Dr. Y. Yarden, were Flag-tagged by PCR amplification and subcloned into pcDNA3.1 Neo. The His-tagged ubiquitin expression plasmid was obtained from Dr. D. Bohmann. The Flag-tagged AIP4 and C814A mutant plasmids were described previously (Ikeda et al., 2000). The Gag-Pol expression plasmid psPAX2 was obtained from Addgene. The VSV-G expression plasmid pVSV-G was purchased from Clontech. The Cbl shRNAmir (V2LHS 48407) and none-silencing shRNAmir (RHS4346) cloned in pGIPZ lentiviral vector were purchased from Open Biosystems (Huntsville, AL). The region from −221 to +12 of BZLF1 promoter (Zp) was PCR amplified and cloned into the pGL3 Basic plasmid (Promega) as a SacI/BglII fragment.
Transfection
CHO-K1 cells were transfected by using Lipofectamine 2000 (GIBCO/BRL). 70% confluent CHO-K1 cells in 6-well plate were incubated with DNA in lipid micelles for 6 hours and were analyzed after 18 hours. BJAB cell lines and LCLs were electroporated with DNA by using Amaxa nucleofector II (Amaxa). A total of 5 × 106 cells were electroporated with the solution C and the program X-001 and were analyzed after 24 hours.
Packaging and Infection of shRNA lentiviruses
For packaging lentiviruses, 293T cells were transfected with 12 μg of pGIPZ shRNAmir, 6 μg of psPAX2, and 0.375 μg of pVSV-G plasmids by using Lipofectamine 2000. Following four-hour incubation, the media of transfected cells were replaced with fresh media and cultured for additional 24 hours. Produced lentiviruses were prepared by filtrating the culture and stored with 4 μg/ml polybrene as stocks. For lentiviral infection, cells were incubated with the viral stock for 24 hours and then cultured in the complete medium. Infected cells were selected with 400 μg/ml puromycin and used for further analysis.
Luciferase assays
BJAB cells were transfected with Gene Pulser (Bio-Rad) at 210 V and 960 μF. Twenty-four hours post-transfection, cells were washed with sterile PBS and lysed with passive lysis buffer (Promega) for 30-min with rocking. Relative luciferase activity was measured with the Promega Luciferase Reporter Assay System in a Visibottom 96-well plate using a Victor plate reader according to the kit instructions.
Immunoprecipitation
A total of 1×107 cells were harvested and lysed in 1 ml of Triton X-100 lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 10% glycerol, 10 mM NaF, 1 mM Na3VO4, 2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin, 10 μg/ml leupeptin). Cleared lysates were incubated with the appropriate antibody for 1 hour at 4°C. Immune complexes were captured with 20 μl of Protein A or G-Sepharose (Pharmacia) for 1 hour at 4°C. Following three washes with lysis buffer, immunoprecipitated proteins were re-suspended in 2X SDS-PAGE sample buffer.
Immunoblotting
Protein samples were resolved by SDS-PAGE, transferred to Immobilon, and blocked with 4% skim milk or 4% bovine serum albumin (BSA) in TBST (10 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.05% Tween 20) at room temperature for 1 hour. Membranes were then incubated in 4% skim milk or 4% BSA in TBST with primary antibody for 1 hour, and then with appropriate secondary antibody for 1 hour. Following incubation, the membranes were washed with TBST, and the blot was visualized using ECL (Amersham).
Acknowledgements
R.L. is John Edward Porter Professor in Biomedical Research and supported by the Public Health Service grants CA62234 and CA73507 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andoniou CE, Lill NL, Thien CB, Lupher ML, Jr., Ota S, Bowtell DD, Scaife RM, Langdon WY, Band H. The Cbl proto-oncogene product negatively regulates the Src-family tyrosine kinase Fyn by enhancing its degradation. Molecular & Cellular Biology. 2000;20(3):851–67. doi: 10.1128/mcb.20.3.851-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9(3):405–11. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5(3):317–27. doi: 10.1038/ni1036. Epub 2004 Feb 1. [DOI] [PubMed] [Google Scholar]
- Courbard JR, Fiore F, Adelaide J, Borg JP, Birnbaum D, Ollendorff V. Interaction between two ubiquitin-protein isopeptide ligases of different classes, CBLC and AIP4/ITCH. Journal of Biological Chemistry. 2002;277(47):45267–75. doi: 10.1074/jbc.M206460200. [DOI] [PubMed] [Google Scholar]
- Dykstra ML, Longnecker R, Pierce SK. Epstein-Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity. 2001;14(1):57–67. doi: 10.1016/s1074-7613(01)00089-9. [DOI] [PubMed] [Google Scholar]
- Engels N, Merchant M, Pappu R, Chan AC, Longnecker R, Wienands J. Epstein-Barr virus latent membrane protein 2A (LMP2A) employs the SLP-65 signaling module. J Exp Med. 2001;194(3):255–64. doi: 10.1084/jem.194.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruehling S, Lee SK, Herrold R, Frech B, Laux G, Kremmer E, Grasser FA, Longnecker R. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on Blymphocyte surface immunoglobulin signal transduction. J Virol. 1996;70(9):6216–26. doi: 10.1128/jvi.70.9.6216-6226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology. 1997;235(2):241–51. doi: 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- Fruehling S, Swart R, Dolwick KM, Kremmer E, Longnecker R. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J Virol. 1998;72(10):7796–806. doi: 10.1128/jvi.72.10.7796-7806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Caldwell RG, Longnecker R, Ikeda M. Itchy, a Nedd4 ubiquitin ligase, downregulates latent membrane protein 2A activity in B-cell signaling. J Virol. 2003;77(9):5529–34. doi: 10.1128/JVI.77.9.5529-5534.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Merchant M, Lev L, Longnecker R, Ikeda M. Latent membrane protein 2A, a viral B cell receptor homologue, induces CD5+ B-1 cell development. J Immunol. 2004;172(9):5329–37. doi: 10.4049/jimmunol.172.9.5329. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Fukuda M, Longnecker R. Function of Latent Membrane Protein 2 A. In: Robertson E, editor. Epstein-Barr Virus. Caister Academic Press; 2005. pp. 531–50. [Google Scholar]
- Ikeda M, Ikeda A, Longan LC, Longnecker R. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology. 2000;268(1):178–91. doi: 10.1006/viro.1999.0166. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Ikeda A, Longnecker R. PY motifs of Epstein-Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins. J Virol. 2001;75(12):5711–8. doi: 10.1128/JVI.75.12.5711-5718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Longnecker R. Cholesterol is critical for Epstein-Barr virus latent membrane protein 2A trafficking and protein stability. Virology. 2007;360(2):461–8. doi: 10.1016/j.virol.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaabeche K, Lemonnier J, Le Mee S, Caverzasio J, Marie PJ. Cbl-mediated degradation of Lyn and Fyn induced by constitutive fibroblast growth factor receptor-2 activation supports osteoblast differentiation. Journal of Biological Chemistry. 2004;279(35):36259–67. doi: 10.1074/jbc.M402469200. [DOI] [PubMed] [Google Scholar]
- Kempkes B, Zimber-Strobl U, Eissner G, Pawlita M, Falk M, Hammerschmidt W, Bornkamm GW. Epstein-Barr virus nuclear antigen 2 (EBNA2)-estrogen receptor fusion proteins complement the EBNA2-deficient Epstein-Barr virus strain P3HR1 in transformation of primary B cells but suppress growth of human B cell lymphoma lines. Journal of General Virology. 1996;77(Pt 2):227–37. doi: 10.1099/0022-1317-77-2-227. [DOI] [PubMed] [Google Scholar]
- Kurosaki T. Regulation of B-cell signal transduction by adaptor proteins. Nat Rev Immunol. 2002;2(5):354–63. doi: 10.1038/nri801. [DOI] [PubMed] [Google Scholar]
- Latour S, Chow LM, Veillette A. Differential intrinsic enzymatic activity of Syk and Zap-70 protein-tyrosine kinases. Journal of Biological Chemistry. 1996;271(37):22782–90. doi: 10.1074/jbc.271.37.22782. [DOI] [PubMed] [Google Scholar]
- Longnecker R. Epstein-Barr virus latency: LMP2, a regulator or means for Epstein-Barr virus persistence? Adv Cancer Res. 2000;79:175–200. doi: 10.1016/s0065-230x(00)79006-3. [DOI] [PubMed] [Google Scholar]
- Longnecker R, Kieff E. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J Virol. 1990;64(5):2319–26. doi: 10.1128/jvi.64.5.2319-2326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R, Miller CL, Tomkinson B, Miao XQ, Kieff E. Deletion of DNA encoding the first five transmembrane domains of Epstein-Barr virus latent membrane proteins 2A and 2B. J Virol. 1993;67(8):5068–74. doi: 10.1128/jvi.67.8.5068-5074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnifico A, Ettenberg S, Yang C, Mariano J, Tiwari S, Fang S, Lipkowitz S, Weissman AM. WW domain HECT E3s target Cbl RING finger E3s for proteasomal degradation. Journal of Biological Chemistry. 2003;278(44):43169–77. doi: 10.1074/jbc.M308009200. [DOI] [PubMed] [Google Scholar]
- Merchant M, Caldwell RG, Longnecker R. The LMP2A ITAM is essential for providing B cells with development and survival signals in vivo. J Virol. 2000;74(19):9115–24. doi: 10.1128/jvi.74.19.9115-9124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant M, Longnecker R. LMP2A survival and developmental signals are transmitted through Btk-dependent and Btk-independent pathways. Virology. 2001;291(1):46–54. doi: 10.1006/viro.2001.1187. [DOI] [PubMed] [Google Scholar]
- Portis T, Longnecker R. Epstein-Barr virus (EBV) LMP2A alters normal transcriptional regulation following B-cell receptor activation. Virology. 2004a;318(2):524–33. doi: 10.1016/j.virol.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Portis T, Longnecker R. Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene. 2004b;23(53):8619–28. doi: 10.1038/sj.onc.1207905. [DOI] [PubMed] [Google Scholar]
- Rao N, Ghosh AK, Ota S, Zhou P, Reddi AL, Hakezi K, Druker BK, Wu J, Band H. The non-receptor tyrosine kinase Syk is a target of Cbl-mediated ubiquitylation upon B-cell receptor stimulation. EMBO Journal. 2001;20(24):7085–95. doi: 10.1093/emboj/20.24.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N, Miyake S, Reddi AL, Douillard P, Ghosh AK, Dodge IL, Zhou P, Fernandes ND, Band H. Negative regulation of Lck by Cbl ubiquitin ligase. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):3794–9. doi: 10.1073/pnas.062055999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovedo M, Longnecker R. Epstein-Barr virus latent membrane protein 2A preferentially signals through the Src family kinase Lyn. J Virol. 2008;82(17):8520–8. doi: 10.1128/JVI.00843-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjay A, Houghton A, Neff L, DiDomenico E, Bardelay C, Antoine E, Levy J, Gailit J, Bowtell D, Horne WC, Baron R. Cbl associates with Pyk2 and Src to regulate Src kinase activity, alpha(v)beta(3) integrin-mediated signaling, cell adhesion, and osteoclast motility. Journal of Cell Biology. 2001;152(1):181–95. doi: 10.1083/jcb.152.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saouaf SJ, Kut SA, Fargnoli J, Rowley RB, Bolen JB, Mahajan S. Reconstitution of the B cell antigen receptor signaling components in COS cells. J Biol Chem. 1995;270(45):27072–8. doi: 10.1074/jbc.270.45.27072. [DOI] [PubMed] [Google Scholar]
- Schaadt E, Baier B, Mautner J, Bornkamm GW, Adler B. Epstein-Barr virus latent membrane protein 2A mimics B-cell receptor-dependent virus reactivation. Journal of General Virology. 2005;86(Pt 3):551–9. doi: 10.1099/vir.0.80440-0. [DOI] [PubMed] [Google Scholar]
- Scharenberg AM, Lin S, Cuenod B, Yamamura H, Kinet JP. Reconstitution of interactions between tyrosine kinases and the high affinity IgE receptor which are controlled by receptor clustering. Embo J. 1995;14(14):3385–94. doi: 10.1002/j.1460-2075.1995.tb07344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholle F, Bendt KM, Raab-Traub N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol. 2000;74(22):10681–9. doi: 10.1128/jvi.74.22.10681-10689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan G, Tsygankov AY. The Cbl family proteins: ring leaders in regulation of cell signaling. Journal of Cellular Physiology. 2006;209(1):21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- Swanson-Mungerson MA, Caldwell RG, Bultema R, Longnecker R. Epstein-Barr virus LMP2A alters in vivo and in vitro models of B-cell anergy, but not deletion, in response to autoantigen. J Virol. 2005;79(12):7355–62. doi: 10.1128/JVI.79.12.7355-7362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Nicholas MW, Conway KL, Sen P, Diz R, Tisch RM, Clarke SH. EBV latent membrane protein 2A induces autoreactive B cell activation and TLR hypersensitivity. J Immunol. 2006;177(5):2793–802. doi: 10.4049/jimmunol.177.5.2793. [DOI] [PubMed] [Google Scholar]
- Winberg G, Matskova L, Chen F, Plant P, Rotin D, Gish G, Ingham R, Ernberg I, Pawson T. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol Cell Biol. 2000;20(22):8526–35. doi: 10.1128/mcb.20.22.8526-8535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wossning T, Reth M. B cell antigen receptor assembly and Syk activation in the S2 cell reconstitution system. Immunol Lett. 2004;92(12):67–73. doi: 10.1016/j.imlet.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuda T, Junicho A, Kishi H, Yoshimura A, Muraguchi A. Hematopoietic cell-specific adapter proteins, SLP-76 and BLNK, effectively activate NF-AT as well as NF-kappaB by Syk and Tec PTKs in nonlymphoid cell lines. FEBS Lett. 2001;491(3):272–8. doi: 10.1016/s0014-5793(01)02208-6. [DOI] [PubMed] [Google Scholar]
- Young LS, Lau R, Rowe M, Niedobitek G, Packham G, Shanahan F, Rowe DT, Greenspan D, Greenspan JS, Rickinson AB. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. Journal of Virology. 1991;65(6):2868–74. doi: 10.1128/jvi.65.6.2868-2874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]