Abstract
OBJECTIVE
Macrophages play an important role in the pathogenesis of insulin resistance via the production of proinflammatory cytokines. Our goal is to decipher the molecular linkage between proinflammatory cytokine production and insulin resistance in macrophages.
RESEARCH DESIGN AND METHODS
We determined cytokine profiles in cultured macrophages and identified interleukin (IL)-1β gene as a potential target of FoxO1, a key transcription factor that mediates insulin action on gene expression. We studied the mechanism by which FoxO1 mediates insulin-dependent regulation of IL-1β expression in cultured macrophages and correlated FoxO1 activity in peritoneal macrophages with IL-1β production profiles in mice with low-grade inflammation or insulin resistance.
RESULTS
FoxO1 selectively promoted IL-1β production in cultured macrophages. This effect correlated with the ability of FoxO1 to bind and enhance IL-1β promoter activity. Mutations of the FoxO1 binding site within the IL-1β promoter abolished FoxO1 induction of IL-1β expression. Macrophages from insulin-resistant obese db/db mice or lipopolysaccharide-inflicted mice were associated with increased FoxO1 production, correlating with elevated levels of IL-1β mRNA in macrophages and IL-1β protein in plasma. In nonstimulated macrophages, FoxO1 remained inert with benign effects on IL-1β expression. In response to inflammatory stimuli, FoxO1 activity was augmented because of an impaired ability of insulin to phosphorylate FoxO1 and promote its nuclear exclusion. This effect along with nuclear factor-κB acted to stimulate IL-1β production in activated macrophages.
CONCLUSIONS
FoxO1 signaling through nuclear factor-κB plays an important role in coupling proinflammatory cytokine production to insulin resistance in obesity and diabetes.
Low-grade inflammation and insulin resistance are two key components that are interwoven in control subjects with obesity and type 2 diabetes. The former is accompanied by markedly elevated production of proinflammatory cytokines in the body, whereas the latter is characterized by significantly diminished responsiveness of peripheral tissues to normal plasma insulin levels. Although there is a lack of consensus regarding the cause-and-effect relationship between chronic exposure to low-grade inflammation and onset of insulin resistance, the intangible association between these two pathological traits forebodes a cross-talking mechanism that integrates abnormal production of inflammatory cytokines to the development of insulin resistance in obesity and type 2 diabetes (1–5). Indeed, amelioration of insulin resistance with insulin sensitizers is associated with reduction of inflammation in obesity and type 2 diabetes (6–9). Likewise, anti-inflammatory therapies result in improved insulin sensitivity in subjects with chronic inflammation (10–13). Insulin exerts an independent inhibitory effect on proinflammatory cytokine production in human aortic endothelial cells and mononuclear cells of obese control subjects (14–16).
Crucial for proinflammatory cytokine production are macrophages, a cell type that is differentiated from monocytes in response to inflammatory stimuli. Rampant macrophage infiltration into peripheral tissues such as liver and adipose tissues are seen in human subjects with metabolic syndrome as well as in animal models of obesity and type 2 diabetes (17–22). These tissue-resident macrophages play important roles in instigating insulin resistance by promoting the production of proinflammatory cytokines (17,23,24). Nonetheless, the molecular basis linking insulin resistance to increased proinflammatory cytokine production remains incompletely understood.
Among proinflammatory cytokines that are detrimental to insulin signaling are interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). These proinflammatory cytokines act in an autocrine or paracrine manner to interfere with insulin signaling in peripheral tissues and macrophages (1–3). In an attempt to decipher the molecular link between abnormal proinflammatory production and insulin resistance, we determined the effect of FoxO1 on cytokine production profiles in cultured macrophages. FoxO1 is a forkhead transcriptional factor that acts to mediate insulin action on target gene expression in peripheral cells (25–27). Inappropriately increased FoxO1 activity, resulting from impaired insulin signaling, is associated with altered metabolism in obesity and type 2 diabetes (28–30). We show that FoxO1 selectively stimulated macrophage production of IL-1β, a prominent proinflammatory cytokine whose overproduction exerts a deleterious effect on peripheral insulin signaling and β-cell function (31–33). Increased macrophage IL-1β production is closely associated with insulin resistance in obesity and type 2 diabetes (31,34). There is clinical evidence that blockage of IL-1β signaling via its receptor antagonist contributes significantly to improved insulin sensitivity in subjects with type 2 diabetes (31). Likewise, administration of neutralizing IL-1β antibodies results in improved glycemic control in diet-induced obese mice (34). These data together with our observations spur the hypothesis that FoxO1 plays a significant role in linking proinflammatory cytokine IL-1β production to insulin resistance.
In this study, we addressed this hypothesis in cultured macrophages as well as in normal mice with lipopolysaccharide (LPS)-elicited inflammation and obese db/db mice with insulin resistance. We show that FoxO1 was capable of binding to the IL-1β promoter, augmenting IL-1β promoter activity. This effect contributed to increased IL-1β expression and was counteracted by insulin. Mutations of the FoxO1 target site within the IL-1β promoter abolished FoxO1-mediated induction of IL-1β expression. Under inflammatory conditions, FoxO1 expression was upregulated, correlating with increased production of IL-1β protein in cultured macrophages. Furthermore, increased FoxO1 activity along with marked upregulation of IL-1β production was detectable in peritoneal macrophages of normal mice with prior exposure to LPS and insulin-resistant mice with genetic predisposition to obesity. Intriguingly, FoxO1 remained inert with benign effects on IL-1β expression in the absence of inflammatory cues. In response to inflammation, FoxO1 activity was augmented along with the activation of nuclear factor (NF)-κB, which in turn acted with FoxO1 in a synergistic manner to promote IL-1β overproduction in macrophages. While unrestrained IL-1β production is associated with impaired insulin action in the face of chronic low-grade inflammation (1,31,34), the underlying mechanism remains elusive. Our data underscore the importance of FoxO1 in regulating macrophage production of IL-1β, suggesting that FoxO1 signaling through NF-κB plays a significant role in coupling proinflammatory cytokine production to insulin resistance in obesity and type 2 diabetes.
RESEARCH DESIGN AND METHODS
Male homozygous db/db and age- or sex-matched heterozygous littermates db/+ were obtained from the Jackson Laboratory (Bar Harbor, MI). Male Balb/c mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were housed in standard shoebox cages supplied with standard rat diet and water ad libitum in a pathogen-free facility with a 12-h light/dark cycle. For LPS treatment, male Balb/c mice (∼25 g) were injected intraperitoneally with LPS at the dose of 4 mg/kg body weight for 2 consecutive days, as described (35). To isolate peritoneal macrophages, mice were killed and peritoneal lavage fluid was collected by washing the peritoneum with 5-ml ice-cold Hank's balanced salt solution (HBSS; Gibco, Carlsbad, CA). Subsequent preparation of peritoneal macrophages was according to the protocol as described (35). All procedures were approved by the Institutional Animal Care and Usage Committee of Children's Hospital of Pittsburgh. Other methods including the statistics are described in the supplemental materials available in an online appendix at http://diabetes.diabetesjournals.org/cgi/full/db09-0232/DC1.
RESULTS
Effect of FoxO1 on cytokine production in cultured macrophages.
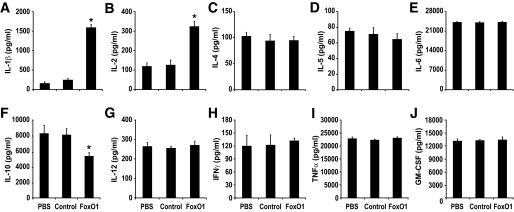
Aberrant proinflammatory cytokine production is intertwined with insulin resistance in obesity and type 2 diabetes. To decipher the molecular basis that links proinflammatory cytokine production to insulin resistance, we determined the effect of FoxO1 on cytokine production in macrophages, a cell type that plays a pivotal role in mediating whole-body insulin resistance in the face of low-grade inflammation. Macrophage RAW264.7 cells were transduced with Adv-FoxO1 or Adv-null vector at a predefined MOI (200 pfu/cell), followed by the determination of cytokine concentrations in conditioned medium. As shown in Fig. 1, transduction of RAW264.7 cells by FoxO1 vector resulted in a significant induction of IL-1β and IL-2 production without alterations in the production of other cytokines including IL-4, IL-5, IL-6, IL-12, IFN-γ, TNF-α, and granulocyte monocyte colony-stimulating factor. In contrast, IL-10 expression was significantly downregulated in response to FoxO1 production in RAW264.7 cells. In the absence of LPS, the expression of proinflammatory cytokines remained at low basal levels independently of FoxO1 action (supplemental Table S1). These data underscore the importance of FoxO1 in regulating cytokine production in LPS-activated macrophages. Because of the close association between abnormal production of IL-1β in macrophages and the pathogenesis of insulin resistance, we focused on the delineation of the molecular basis underlying FoxO1-mediated induction of IL-1β in this study.
FIG. 1.
Effect of FoxO1 on cytokine production profiles in cultured macrophages. RAW264.7 cells were mock-treated with PBS, or transduced with Adv-FoxO1 or control Adv-null vector at a fixed dose (MOI, 200 pfu/cell). After 24-h incubation in the presence of 100 ng/ml LPS, conditioned medium was harvested for profiling the production of cytokine IL-1β (A), IL-2 (B), IL-4 (C), IL-5 (D), IL-6 (E), IL-10 (F), IL-12 (G), IFN-γ (H), TNF-α (I), and granulocyte monocyte colony-stimulating factor (GM-CSF) (J). Data were obtained from three independent experiments. *P < 0.001 vs. controls.
Impact of FoxO1 on IL-1β production in peritoneal macrophages in LPS-treated mice.
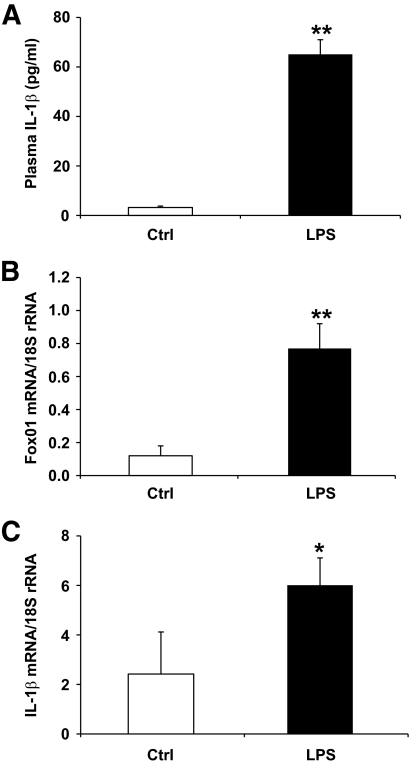
To investigate the potential role of FoxO1 in macrophage production of IL-1β in vivo, we treated Balb/C mice with once-daily intraperitoneal administration of LPS (4 mg/kg) for 2 days. This treatment elicited acute inflammation in mice. Compared to PBS-treated control mice (n = 8), LPS-treated mice (n = 8) exhibited significantly elevated plasma IL-1β levels (Fig. 2A). Two days after LPS treatment, mice were killed and peritoneal macrophages were retrieved for real-time qRT-PCR analysis. LPS treatment resulted in a significant induction of FoxO1 expression (Fig. 2B), correlating with significantly elevated IL-1β mRNA levels in peritoneal macrophages (Fig. 2C).
FIG. 2.
FoxO1 expression is upregulated in peritoneal macrophages of LPS-treated mice. Male Balb/C mice (8 weeks old, n = 8 per group) were treated with once-daily intraperitoneal injection of LPS (4 mg/kg body weight) or mock-treated with PBS for 2 days. Aliquots of blood (50 μl) were sampled from the tail vein for the determination of plasma IL-1β levels (A). Two days after LPS treatment, peritoneal macrophages were isolated from individual mice for the preparation of total RNA, which was subjected to real-time qRT-PCR assay for the determination of FoxO1 (B) and IL-1β (C) mRNA levels using 18S rRNA as control. *P < 0.05 and **P < 0.001 vs. control.
FoxO1 and IL-1β expression in peritoneal macrophages in obese db/db mice.
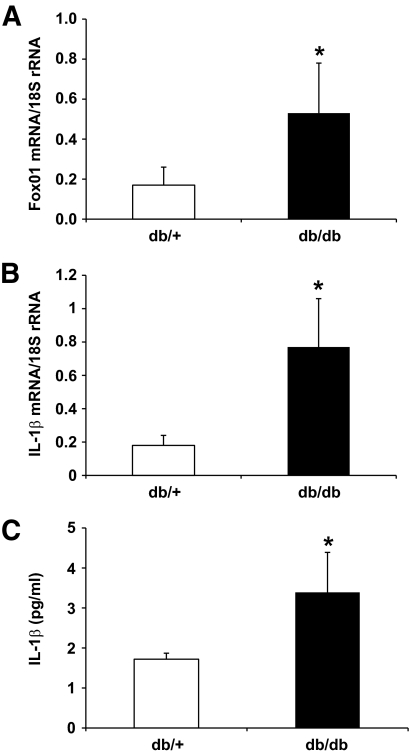
To determine whether FoxO1 plays a role in linking insulin resistance to aberrant IL-1β expression, we studied FoxO1 expression in peritoneal macrophages in obese db/db mice. Because of the lack of leptin receptor, db/db mice are genetically obese, developing insulin resistance and hyperinsulinemia at weaning. Two groups of male db/db mice (n = 7, male, 10 weeks old) and age- or sex-matched heterozygous db/+ lean littermates (n = 7) were fasted for 16 h, as described (30). Peritoneal macrophages were isolated from individual mice and subjected to real-time qRT-PCR analysis. As shown in Fig. 3A, FoxO1 expression was significantly upregulated in peritoneal macrophages of insulin-resistant obese db/db mice. Furthermore, db/db mice were associated with significantly higher levels of IL-1β mRNA in peritoneal macrophages (Fig. 3B) and IL-1β protein in plasma (Fig. 3C), which was indicative of chronic inflammation in insulin-resistant obese db/db mice.
FIG. 3.
Covariation of FoxO1 and IL-1β expression in peritoneal macrophages of insulin-resistant db/db mice. Peritoneal macrophages were isolated from insulin-resistant obese db/db mice (n = 7) and control db/+ littermates (n = 7). Total macrophage RNA was prepared and subjected to real-time qRT-PCR assay for the determination of FoxO1 (A) and IL-1β (B) mRNA levels. Plasma IL-1β levels were determined by anti–IL-1β ELISA (C). *P < 0.05 vs. control.
Effect of FoxO1 on IL-1β expression in macrophages.
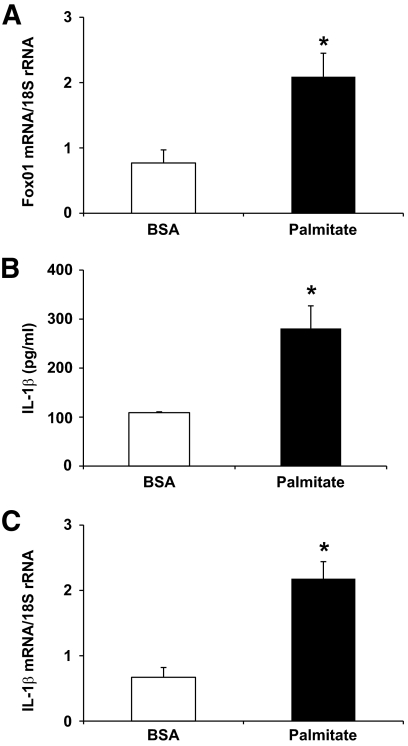
We hypothesized that the IL-1β gene is a FoxO1 target, and FoxO1 promotes IL-1β expression in macrophages in response to inflammation. To test this hypothesis, we treated RAW264.7 cells with palmitate, the predominant saturated form of free fatty acid that is known to elicit low-grade inflammation. As shown in Fig. 4, this treatment resulted in significant induction of IL-1β production, as reflected in markedly elevated levels of IL-1β protein in conditioned medium and IL-1β mRNA in cells. This effect was accompanied by significantly increased FoxO1 expression in palmitate-treated macrophages.
FIG. 4.
FoxO1 expression in palmitate-treated macrophages. RAW264.7 cells were incubated in culture medium in the presence of 0.2 mmol/l palmitate or BSA as control for 24 h. Cells were harvested and subjected to real-time qRT-PCR assay for the determination of FoxO1 (A) and IL-1β (B) mRNA levels. Conditioned medium was used for the determination of IL-1β concentrations by anti–IL-1β ELISA (C). *P < 0.05 vs. control.
Mechanism of FoxO1-mediated induction of IL-1β expression.
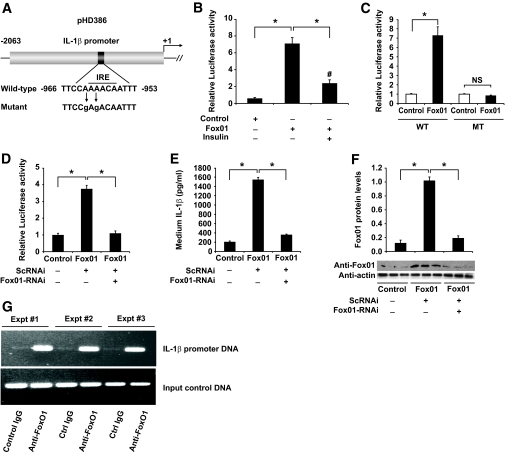
To characterize the underlying mechanism, we addressed whether FoxO1 targets the IL-1β promoter for trans-activation. A 2-kb DNA fragment harboring the mouse IL-1β promoter was cloned into the pGL3-Basic luciferase reporter system (Fig. 5A). The resulting plasmid pHD386 encoding the IL-1β promoter–directed luciferase was transfected via electroporation into RAW264.7 cells. IL-1β promoter activity was determined in response to adenovirus-mediated FoxO1 production in the presence and absence of insulin. As shown in Fig. 5B, we detected a more than sixfold induction of the IL-1β promoter activity in RAW264.7 cells that were pretransduced with FoxO1 vector. This effect was counteracted by insulin, correlating with the ability of FoxO1 to undergo insulin-dependent translocation from the nucleus to cytoplasm (25,27).
FIG. 5.
Effect of FoxO1 on IL-1β promoter activity in RAW264.7 cells. A: A schematic depiction of the IL-1β promoter–directed luciferase reporter system in pHD386. A consensus IRE DNA motif and its mutant form are indicated. B: Effect of FoxO1 on wild-type IL-1β promoter activity. RAW264.7 cells in six-well microplates were transduced with Adv-FoxO1 or control Adv-null vectors at a fixed dose (MOI, 200 pfu/cell), followed by transfection with 1-μg pHD386 plus 1-μg pCA35-LacZ in the presence of 100 ng/ml LPS in culture medium. After 24-h incubation in the absence or presence of insulin (300 nmol/l), cells were harvested for the determination of luciferase and β-galactosidase activities. The relative luciferase activity was calculated by setting the ratio of luciferase/β-galactosidase activity in control cells to 1. C: Effect of FoxO1 on mutant IL-1β promoter activity. RAW264.7 cells were transfected with wild-type (WT) or mutant (MT) IL-1β promoter–directed luciferase system in the absence or presence of adenovirus-mediated FoxO1 production. After 24-h incubation in the presence of 100 ng/ml LPS, cells were harvested for the determination of relative luciferase activities using β-galactosidase activity as internal controls. D: Effect of FoxO1 knockdown on IL-1β promoter activity. RAW264.7 cells were cotransfected with pHD386 and pCA35-LacZ in the absence or presence of adenovirus-mediated FoxO1 production. One subset of cells was transduced with 200 MOI of Adv-FoxO1-RNAi encoding FoxO1-specific RNAi or control Adv-ScRNAi encoding scrambled RNAi. After 24-h incubation in the presence of 100 ng/ml LPS, cells were harvested for the determination of relative luciferase activities using β-galactosidase activity as internal controls. E: Effect of FoxO1 knockdown on IL-1β production. The conditioned medium from the experiment in D was used for the determination of IL-1β levels. F: FoxO1 protein levels. Cells obtained from the experiment in D were subjected to semiquantitative immunoblot analysis for the determination of total FoxO1 protein levels, using intracellular actin protein as control. G: Interaction of FoxO1 with IL-1β promoter DNA. RAW264.7 cells incubated in six-well dishes in the presence of LPS (100 ng/ml) for 24 h. Cells were fixed with 1% formaldehyde, followed by ChIP assay using rabbit anti-FoxO1 or control IgG (rabbit anti–β-galactosidase). Immunoprecipitates were analyzed by PCR assay for the detection of DNA, using primers flanking the consensus FoxO1 binding site within the IL-1β promoter. Data in B–F were obtained from three to five independent experiments. *P < 0.001 and #P < 0.05 vs. control. NS, not significant.
To corroborate these findings, we altered the insulin-responsive element (IRE) motif within the IL-1β promoter via site-directed mutagenesis (supplemental Fig. S1). The resulting mutant IL-1β promoter was analyzed for its ability to mediate the stimulatory effect of FoxO1 on IL-1β expression. As shown in Fig. 5C, alterations within the IRE DNA motif rendered the mutant IL-1β promoter unresponsive to FoxO1 production in RAW264.7 cells. As control, adenovirus-mediated FoxO1 production resulted in a significant induction of wild-type IL-1β promoter activity (Fig. 5C).
Effect of FoxO1 knockdown on IL-1β expression.
To further prove that FoxO1 contributed to IL-1β regulation, we employed an RNA interference (RNAi)-mediated gene silencing approach to knockdown FoxO1 expression in cultured macrophages. RAW264.7 cells were transfected with pHD386 expressing the IL-1β promoter-directed luciferase reporter gene, followed by transduction of adenoviral vectors expressing FoxO1-specific RNAi or control scrambled RNAi, as described (30). After 24-h incubation, cells were subjected to luciferase activity assay. As shown in Fig. 5D, RNAi-mediated FoxO1 knockdown resulted in abolition of FoxO1-mediated induction of IL-β promoter activity in LPS-stimulated RAW264.7 cells. These results were corroborated when the conditioned medium was used for the determination of IL-1β protein. As shown in Fig. 5E, medium IL-1β concentration was markedly induced by FoxO1. This effect was abolished by RNAi-mediated FoxO1 knockdown, as reflected in ∼80% of reduction in FoxO1 protein levels in RAW264.7 cells that were pretransduced with FoxO1-RNAi vector (Fig. 5F).
Molecular interaction of FoxO1 with IL-1β promoter DNA.
The above results spurred the idea that FoxO1 stimulates IL-1β expression via direct binding to the IL-1β promoter. Consistent with this idea, the IL-1β promoter contains an IRE (Fig. 5A), a consensus DNA motif that is responsible for binding and mediating FoxO1 induction of target gene expression (25,27). Interestingly, similar FoxO1 consensus sites were detected in the regulatory region of both human and rat IL-1β promoters, suggesting an evolutionally conserved mechanism (supplemental Fig. S2). To determine the ability of the conserved IRE motif to mediate the stimulatory effect of FoxO1 on IL-1β promoter activity, we performed chromatin immunoprecipitation (ChIP) assay to analyze the potential interaction of FoxO1 with IL-1β promoter DNA in RAW264.7 cells. Because of low FoxO1 expression in unstimulated RAW264.7 cells, we transduced RAW264.7 cells with FoxO1 vector in the presence of LPS (100 ng/ml) in culture medium. After 24-h incubation, cells were subjected to ChIP assay using rabbit anti-FoxO1 antibody or control rabbit IgG. The resulting immunoprecipitates were subjected to PCR analysis, using primers flanking the IRE motif within the IL-1β promoter. A 457-bp DNA corresponding to the IRE region (−1,359/−902 nt) of the IL-1β promoter was produced from anti-FoxO1 immunoprecipitates (Fig. 5F). In contrast, the immunoprecipitates derived from control IgG were negative in the same PCR assay.
Effect of LPS or palmitate treatment on insulin signaling in macrophages.
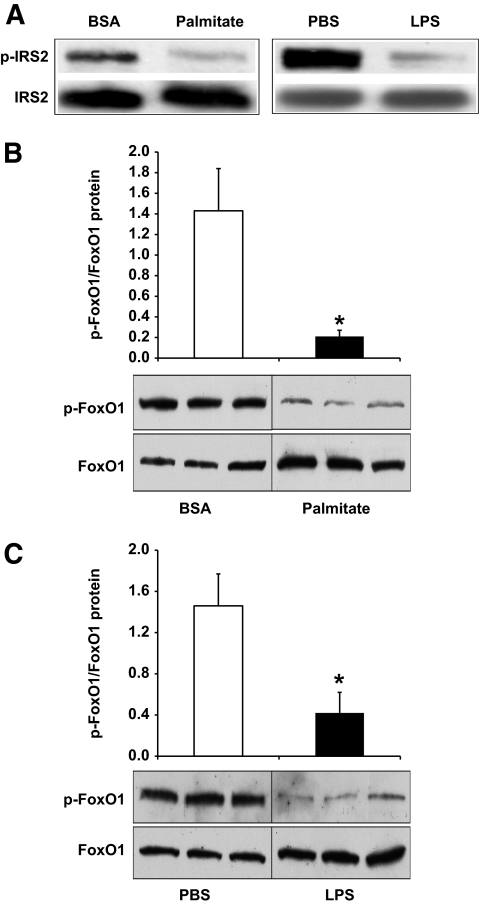
To further illustrate the role of FoxO1 in linking aberrant IL-1β production to insulin resistance, we determined the effect of LPS or palmitate treatment on insulin signaling in cultured macrophages. RAW264.7 cells were incubated in the presence of LPS (100 ng/ml) or palmitate (0.2 mmol/l) for 36 h, followed by the addition of insulin (300 nmol/l) into culture medium. The dose of insulin used in culture medium was based on our preliminary studies and data in the literature (36). Cells were harvested 45 min after insulin action for the determination of insulin-stimulated phosphorylation of insulin receptor substrates (IRSs) and FoxO1, both of which are key signaling molecules downstream of insulin action. Compared to mock-treated controls, RAW264.7 cells pretreated with LPS or palmitate treatment were associated with a significant reduction in insulin-stimulated phosphorylation of IRS2 (Fig. 6A). Because of extremely low levels of IRS1 expression in RAW264.7 cells, we were unable to detect insulin-stimulated phosphorylation of IRS1. Similar findings are reported by Welham et al. (37), who show that IRS1 is undetectable and IRS2 is the sole substrate downstream of insulin action in primary murine macrophages. LPS or palmitate treatment also resulted in marked elevations of FoxO1 production in RAW264.7 cells (Fig. 6B and C). However, the ratio of phosphorylated versus total FoxO1 levels was significantly reduced, reflecting an impaired ability of insulin to promote FoxO1 phosphorylation in LPS- or palmitate-treated RAW264.7 cells (Fig. 6B and C).
FIG. 6.
Effect of LPS and palmitate on FoxO1 production in cultured macrophages. RAW264.7 in six-well dishes were treated with either 100 ng/ml LPS or 0.2 mmol/l palmitate. Control wells of cells were mock-treated with PBS or BSA. After 24 h of incubation, insulin (300 nmol/l) was added to both treatment and control wells of cells. After 45-min incubation, cells were harvested for the preparation of total cell lysates. Aliquots of cell lyates were immunoprecipitated by anti-IRS2 antibody, followed by immunoblot assay for the determination of phosphorylated IRS2 using antiphosphotyrosine antibody (A). In addition, aliquots of cell lysates were subjected to immunoblot analysis for the determination of phosphorylated and total FoxO1 in response to palmitate (B) or LPS treatment (C). Data were obtained from three independent measurements. *P < 0.05.
FoxO1 signaling through NF-κB stimulates IL-1β expression.
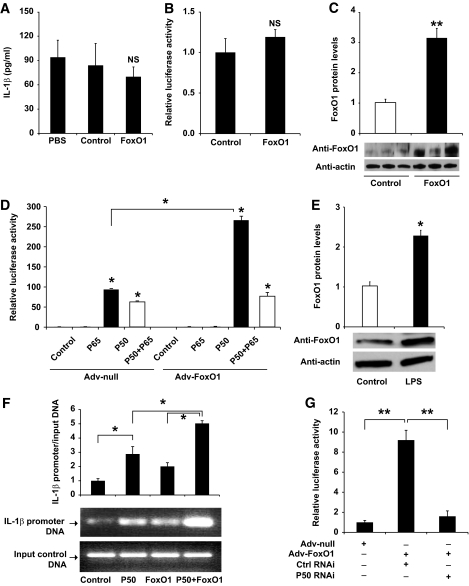
Our findings of FoxO1-stimulated IL-1β production in inflammation-inflicted macrophages pose two potential mechanisms: 1) FoxO1 exerts an independent effect on IL-1β production, and 2) alternatively, FoxO1 promotes IL-1β overproduction through NF-κB, a well-characterized pathway that is responsible for mediating proinflammatory cytokine production in macrophages under inflammatory or insulin-resistant conditions. To distinguish these two possibilities, we chose to study the impact of FoxO1 in nonstimulated macrophages. Because of a concomitant induction of endogenous FoxO1 and NF-κB production in LPS- or palmitate-treated macrophages, unstimulated RAW264.7 cells were transduced with FoxO1 or control vector to determine the net effect of FoxO1 on IL-1β expression in the absence of NF-κB activation. Unexpectedly, FoxO1 failed to stimulate IL-1β production, as the amount of immunoreactive IL-1β protein remained unchanged in FoxO1 versus control vector–treated RAW264.7 cells (Fig. 7A). Under the same condition, adenovirus-mediated FoxO1 production resulted in significant induction of IL-1β production in RAW264.7 cells that were pre-exposed to LPS (Fig. 1A). To ascertain this finding, we transfected pHD386 encoding the IL-1β promoter-directed luciferase reporter system into nonstimulated RAW264.7 cells. No significant induction of luciferase activities in RAW264.7 cells was detected in basal states (Fig. 7B), despite a threefold elevation in FoxO1 protein levels in naïve RAW264.7 cells that were pretransduced with FoxO1 vector (Fig. 7C).
FIG. 7.
FoxO1 signaling via NF-κB in regulating IL-1β expression in cultured macrophages. A: RAW264.7 cells were transduced with FoxO1 or control null vectors. After 24-h incubation in the absence of LPS, the conditioned medium was collected for the determination of IL-1β concentrations. B: RAW264.7 cells were transfected with pHD386 encoding the IL-1β promoter–directed luciferase expression system in the presence or absence of FoxO1 production. After 24-h incubation in the absence of LPS, the relative luciferase activity in cells was determined using β-galactosidase for the normalization of transfection efficiency. C: FoxO1 protein levels. Cells from the experiment in B were subjected to immunoblot analysis for the determination of nuclear FoxO1 protein levels. D: RAW264.7 cells were transfected with pHD386 encoding the IL-1β promoter–directed luciferase reporter in the presence of FoxO1 plus P50 or P65 production, using the dual luciferase system. After 24-h incubation in the absence of LPS, the relative promoter activity, defined as the ratio of Firefly and Renilla luciferase activities, was determined. E: Effect of LPS on FoxO1 production in RAW264.7 cells. RAW264.7 cells were incubated in the absence and presence of LPS (100 ng/ml) for 24 h, followed by immunoblot analysis using anti-FoxO1 and anti-actin antibodies. F: Impact of NF-κB P50 on interaction of FoxO1 with IL-1β promoter DNA. RAW264.7 cells were transduced with FoxO1 vector in the presence or absence of P50 production. Control cells were either transfected with P50-expressing plasmid alone or mock-treated. After 24-h incubation in the absence of LPS, cells were fixed with 1% formaldehyde, followed by ChIP assay using rabbit anti-FoxO1 antibody. The immunoprecipitates were subjected to semiquantitative PCR assay using primers flanking the conserved IRE DNA motif within the IL-1β promoter. After normalizing to input DNA controls, the amount of immunoprecipitated IL-1β promoter DNA fragments was compared among different conditions. G: Effect of NF-κB P50 knockdown on IL-1β promoter activity. RAW264.7 cells were transfected with pHD386 encoding the IL-1β promoter–directed luciferase expression system in the presence or absence of FoxO1 production, using the dual luciferase system. One subset of cells was cotransfected with siRNA that is targeted to the NF-κB P50 subunit. Control cells were transfected with control siRNA. After 24-h incubation, cells were harvested for the determination of luciferase activity. Data in A–G were from three independent experiments. *P < 0.05 and **P < 0.001 vs. control. NS, not significant.
To investigate whether FoxO1 contributes to IL-1β production via a mechanism that is dependent on NF-κB, we determined the effect of FoxO1 on IL-1β promoter activity in the presence and absence of vector-mediated production of P50 or P65, two subunits of NF-κB. In keeping with our earlier observations (Fig. 7A), adenovirus-mediated FoxO1 production exerted little effects on IL-1β promoter activity in nonstimulated RAW264.7 cells. However, cotransfection of FoxO1 along with P50, but not with P65, resulted in a significant induction of IL-1β promoter activity (Fig. 7D). These results suggest that FoxO1 associates with P50 in stimulating IL-1β expression in macrophages, or, alternatively, P50 is required for the recruitment of FoxO1 to its target site to stimulate IL-1β promoter activity.
To address the first hypothesis, we studied the potential association of FoxO1 with P50 using a coimmunoprecipitation assay. We show that LPS, known to stimulate NF-κB activation, also promoted FoxO1 production in RAW264.7 cells (Fig. 7E). Thus, to determine the net impact of FoxO1 and P50 interaction on IL-1β expression, we chose to perform the studies in unstimulated RAW264.7 cells with vector-mediated production of FoxO1 and P50 proteins. FoxO1-expressing RAW264.7 cells were transfected with plasmid p50 encoding the human NF-κB P50 subunit, followed by immunoprecipitation using anti-FoxO1 or anti-P50 antibodies. The resulting immunoprecipitates were analyzed by immunoblot assay for FoxO1 and P50, respectively. We detected the presence of FoxO1 or P50 in immunoprecipitates that were derived from its cognate antibody, validating the method of immunoprecipitation. However, neither FoxO1 nor P50 was reciprocally coimmunoprecipitated by anti-P50 or anti-FoxO1 antibodies (data not shown). These results argue against the notion that FoxO1 formed a complex with P50 in promoting IL-1β expression in macrophages.
We then addressed the second hypothesis that FoxO1 binding to its target site within the IL-1β promoter depends on P50 in cells. Implicit in this hypothesis is the presence of a highly conserved NF-κB P50-binding site (5′-GGGAGCATCC-3′) downstream of the IRE, the characteristic DNA motif that is responsible for FoxO1 binding (Fig. 5A). We performed ChIP assay on RAW264.7 cells in the presence of FoxO1 or P50 expression alone or in combination. The resulting immunoprecipitates were analyzed by semiquantitative PCR assay using specific primers flanking the FoxO1 binding site in the IL-1β promoter. As shown in Fig. 7F, positive interactions between FoxO1 and IL-1β promoter DNA were detected in response to FoxO1 or P50 production in RAW264.7 cells. However, coproduction of FoxO1 and P50 resulted in a synergistic effect on FoxO1 binding to its target site within the IL-1β promoter. This effect correlated with the costimulatory effect of FoxO1 and P50 in combination on IL-1β promoter activity (Fig. 7D).
Effect of siRNA-mediated P50 knockdown on macrophage IL-1β expression.
To consolidate our findings that FoxO1 signaling through P50 regulates IL-1β production in macrophages, we used the siRNA-mediated gene-silencing approach to knockdown P50 expression, followed by determining the ability of FoxO1 to stimulate IL-1β expression in LPS-stimulated RAW264.7 cells. As shown in Fig. 7G, adenovirus-mediated FoxO1 production resulted in a marked induction of luciferase activity, which was significantly reduced after siRNA-mediated P50 knockdown in LPS-stimulated RAW264.7 cells. These results suggest that depletion of NF-κB P50 attenuated the ability of FoxO1 to induce IL-1β promoter activity in macrophages.
DISCUSSION
Our goal is to understand the molecular basis that links insulin resistance to abnormal production of proinflammatory cytokine IL-1β in macrophages. We show that FoxO1 stimulated IL-1β production in activated macrophages. This effect was inhibited by insulin, which is correlated with the ability of FoxO1 to undergo insulin-dependent phosphorylation and nuclear exclusion. FoxO1 was shown to bind to the IL-1β promoter and enhance IL-1β promoter activity. In accordance with these observations, there is a highly conserved FoxO1 binding site within the IL-1β promoter of humans, rats, and mice. Mutations of the FoxO1 consensus site abrogated FoxO1-mediated induction of IL-1β promoter activity. In LPS- or palmitate-treated macrophages, FoxO1 expression along with its nuclear localization was increased, contributing to augmented FoxO1 activity and elevated IL-1β production. LPS or palmitate treatment also impaired the ability of insulin to promote IRS2 and FoxO1 phosphorylation in cultured macrophages. These data characterize FoxO1 at the interface between abnormal production of proinflammatory cytokine IL-1β and the pathogenesis of insulin resistance in macrophages.
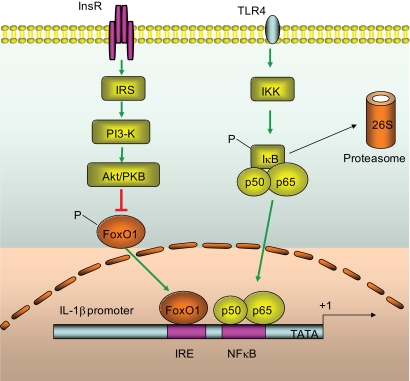
Macrophage production of IL-1β is governed by NF-κB, a master regulator that integrates inflammatory signals to cytokine gene expression (11,38). In unstimulated cells, NF-κB is bound by IκB and is sequestered in the cytoplasm. In the presence of inflammatory stimuli, IκB is phosphorylated by IκB kinase and is destined for proteasome-mediated degradation. As a result, NF-κB is dissociated from IκB and is translocated to the nucleus for promoting target gene expression (38). Consistent with this notion is the conservation of a consensus NF-κB target site within the IL-1β promoter among different species (supplemental Fig. S2). However, this mechanism falls short of explaining why insulin resistance provokes the induction of IL-1β production in activated macrophages. To address this fundamental issue, several independent studies show that activation of the PI3 kinase-Akt pathway is effective in limiting LPS-stimulated inflammatory cytokine production with uncharacterized mechanisms (39–41). Here we show that macrophage IL-1β expression is regulated by FoxO1, a key nuclear transcriptional factor that mediates the inhibitory effect of insulin on target gene expression. In the absence of insulin, FoxO1 acts in the nucleus as an enhancer for promoting target gene expression, whereas in the presence of insulin, FoxO1 is phosphorylated by Akt/PKB, resulting in its nuclear exclusion and contributing to the inhibition of target gene expression. This phosphorylation-dependent protein trafficking mechanism is critical for insulin to regulate FoxO1 transcriptional activity in cells (25–27,30). Our present studies shed light on the molecular basis that couples insulin resistance to the induction of proinflammatory cytokines. It follows that in insulin-resistant states, loss of insulin inhibition of FoxO1 activity consequently results in unrestrained IL-1β expression in activated macrophages (Fig. 8). Consistent with this interpretation, we show that FoxO1 activity was significantly increased, correlating with impaired insulin action and elevated IL-1β production in LPS- and palmitate-stimulated macrophages. Furthermore, addition of insulin resulted in only partial suppression of IL-1β promoter activity, which is indicative of insulin resistance in LPS-stimulated RAW264.7 cells (Fig. 5). Likewise, primary peritoneal macrophages retrieved from LPS-treated mice and insulin-resistant db/db mice were invariably associated with increased FoxO1 activity, accompanied by elevated IL-1β levels in plasma. Tripathy et al. (42) show that acute elevation of free fatty acid directly provokes inflammation, culminating in enhanced NF-κB activity and increased proinflammatory cytokine expression in mononuclear cells in healthy individuals. These findings together with our data support the notion that circulating mononuclear cells in insulinresistant obese subjects are in a proinflammatory state (43). Indeed, treatment with thiazolidenediones, a class of insulin sensitizers for improving peripheral insulin resistance, reduces mononuclear NF-κB activity and ameliorates inflammation in obese subjects (44–46).
FIG. 8.
Convergence of the FoxO1 and NF-κB pathways in IL-1β gene expression. FoxO1 targets at the IRE DNA motif of the IL-1β promoter for trans-activation. Under insulin-resistant or inflammatory conditions, FoxO1 activity is increased because of impaired abilities of insulin to phosphorylate and translocate FoxO1 from the nucleus to cytoplasm. This effect along with the activation of the NF-κB pathway synergistically promotes macrophage production of proinflammatory cytokine IL-1β. The evolutionally conserved IRE DNA motif and NF-κB binding site are indicated within the promoter-proximal region.
Although FoxO1 targeted IL-1β promoter for trans-activation, FoxO1 did not elicit a significant induction of IL-1β expression in cultured RAW264.7 cells in the absence of inflammatory stimuli. In response to inflammatory signals such as LPS or palmitate treatment, FoxO1 protein levels were markedly upregulated, contributing to enhanced FoxO1 activity. This effect was mirrored by the activation of NF-κB in LPS- or palmitate-activated macrophages. Interestingly, we show that FoxO1 in concert with activated NF-κB produced a synergistic effect on the induction of IL-1β overproduction in the presence of inflammation. Together these results suggest that augmented FoxO1 activity serves to amplify the action of NF-κB in stimulating macrophage IL-1β production under inflammatory conditions (Fig. 8). This interpretation is underpinned by three lines of evidence. First, the productive interaction between FoxO1 and IL-1β promoter DNA was significantly enhanced in the presence of P50 production, correlating with the costimulatory effect of FoxO1 and P50 on IL-1β promoter activity in cultured macrophages. Second, siRNA-mediated knockdown of P50 significantly attenuated the ability of FoxO1 to stimulate IL-1β expression in LPS-stimulated macrophages. Third, the IL-1β promoter contains both FoxO1 and NF-κB target sites that are juxtaposed within the promoter-proximal region of the Il1b gene in mice, rats, and humans (supplemental Fig. S2), implying an evolutionally conserved mechanism for FoxO1 signaling through the NF-κB pathway in regulating proinflammatory cytokine IL-1β production in macrophages in response to insulin resistance.
In conclusion, we characterized the Il1b gene as a FoxO1 target, demonstrating that macrophage production of IL-1β was stimulated by FoxO1 and inhibited by insulin. Under insulin-resistant or inflammatory conditions, FoxO1 activity was augmented, because of an impaired ability of insulin to phosphorylate FoxO1 and promote its nuclear exclusion. This effect along with active NF-κB contributed to macrophage overproduction of IL-1β. Although insulin resistance is associated with low-grade inflammation in obesity and type 2 diabetes, the underlying mechanism remains obscure. Our data suggest that FoxO1 signaling through NF-κB plays a significant role in coupling insulin resistance to proinflammatory cytokine IL-1β production in obesity and type 2 diabetes.
Supplementary Material
Acknowledgments
This study was supported in part by American Diabetes Association and National Health Institute Grant DK-066301.
No potential conflicts of interest relevant to this article were reported.
We thank Dr. Steve Ringquist for the critical reading of this manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Hotamisligil GS: Inflammation and metabolic disorders Nature 2006; 444: 860– 867 [DOI] [PubMed] [Google Scholar]
- 2.Schenk S, Saberi M, Olefsky JM: Insulin sensitivity: modulation by nutrients and inflammation J Clin Invest 2008; 118: 2992– 3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilg H, Moschen AR: Inflammatory mechanisms in the regulation of insulin resistance Mol Med 2008; 14: 222– 231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guest CB, Park MJ, Johnson DR, Freund GG: The implication of proinflammatory cytokines in type 2 diabetes Front Biosci 2008; 13: 5187– 5194 [DOI] [PubMed] [Google Scholar]
- 5.Dandona P, Aljada A, Bandyopadhyay A: Inflammation: the link between insulin resistance, obesity and diabetes Trends Immunol 2004; 25: 4– 7 [DOI] [PubMed] [Google Scholar]
- 6.Rosenson RS, Huskin AL, Wolff DA, Helenowski IB, Rademaker AW: Fenofibrate reduces fasting and postprandial inflammatory responses among hypertriglyceridemia patients with the metabolic syndrome Atherosclerosis 2008; 198: 381– 388 [DOI] [PubMed] [Google Scholar]
- 7.Morin-Papunen L, Rautio K, Ruokonen A, Hedberg P, Puukka M, Tapanainen JS: Metformin reduces serum C-reactive protein levels in women with polycystic ovary syndrome J Clin Endocrinol Metab 2003; 88: 4649– 4654 [DOI] [PubMed] [Google Scholar]
- 8.Rautio K, Tapanainen JS, Ruokonen A, Morin-Papunen LC: Rosiglitazone treatment alleviates inflammation and improves liver function in overweight women with polycystic ovary syndrome: a randomized placebo-controlled study Fertil Steril 2007; 87: 202– 206 [DOI] [PubMed] [Google Scholar]
- 9.Samaha FF, Szapary PO, Iqbal N, Williams MM, Bloedon LT, Kochar A, Wolfe ML, Rader DJ: Effects of rosiglitazone on lipids, adipokines, and inflammatory markers in nondiabetic patients with low high-density lipoprotein cholesterol and metabolic syndrome Arterioscler Thromb Vasc Biol 2006; 26: 624– 630 [DOI] [PubMed] [Google Scholar]
- 10.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI: Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes J Clin Invest 2002; 109: 1321– 1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoelson SE, Lee J, Goldfine AB: Inflammation and insulin resistance J Clin Invest 2006; 116: 1793– 1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleischman A, Shoelson SE, Bernier R, Goldfine AB: Salsalate improves glycemia and inflammatory parameters in obese young adults Diabetes Care 2008; 31: 289– 294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoelson SE, Herrero L, Naaz A: Obesity, inflammation, and insulin resistance Gastroenterology 2007; 132: 2169– 2180 [DOI] [PubMed] [Google Scholar]
- 14.Aljada A, Saadeh R, Assian E, Ghanim H, Dandona P: Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide J Clin Endocrinol Metab 2000; 85: 2572– 2575 [DOI] [PubMed] [Google Scholar]
- 15.Aljada A, Ghanim H, Saadeh R, Dandona P: Insulin inhibits NFκB and MCP-1 expression in human aortic endothelial cells J Clin Endocrinol Metab 2001; 86: 450– 453 [DOI] [PubMed] [Google Scholar]
- 16.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S: Insulin inhibits intranuclear nuclear factor κB and stimulates IκB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 2001; 86: 3257– 3265 [DOI] [PubMed] [Google Scholar]
- 17.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr: Obesity is associated with macrophage accumulation in adipose tissue J Clin Invest 2003; 112: 1796– 1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H: Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance J Clin Invest 2003; 112: 1821– 1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortega Martinez de Victoria E, Xu X, Koska J, Francisco AM, Scalise M, Ferrante AW, Jr, Krakoff J: Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians Diabetes 2009; 58: 385– 393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S: In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue J Clin Invest 2008; 118: 710– 721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N, Rudich A: Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity J Clin Endocrinol Metab 2007; 92: 2240– 2247 [DOI] [PubMed] [Google Scholar]
- 22.Coenen KR, Gruen ML, Chait A, Hasty AH: Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue Diabetes 2007; 56: 564– 573 [DOI] [PubMed] [Google Scholar]
- 23.Odegaard JI, Chawla A: Mechanisms of macrophage activation in obesity-induced insulin resistance Nat Clin Pract Endocrinol Metab 2008; 4: 619– 626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A: Alternative M2 activation of Kupffer cells by PPARΔ ameliorates obesity-induced insulin resistance Cell Metab 2008; 7: 496– 507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Accili D, Arden KC: FoxOs at the crossroads of cellular metabolism, differentiation, and transformation Cell 2004; 117: 421– 426 [DOI] [PubMed] [Google Scholar]
- 26.Barthel A, Schmoll D, Unterman TG: FoxO proteins in insulin action and metabolism Trends Endocrinol Metab 2005; 16: 183– 189 [DOI] [PubMed] [Google Scholar]
- 27.Kamagate A, Dong HH: FoxO1 integrates insulin signaling to VLDL production Cell Cycle 2008; 7: 3162– 3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH: Foxo1 mediates insulin action on ApoC-III and triglyceride metabolism J Clin Invest 2004; 114: 1493– 1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu S, Altomonte J, Perdomo G, He J, Fan Y, Kamagate A, Meseck M, Dong HH: Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism Endocrinology 2006; 147: 5641– 5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH: FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice J Clin Invest 2008; 118: 2347– 2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY: Interleukin-1-receptor antagonist in type 2 diabetes mellitus N Engl J Med 2007; 356: 1517– 1526 [DOI] [PubMed] [Google Scholar]
- 32.Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M: Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans Science 1986; 232: 1545– 1547 [DOI] [PubMed] [Google Scholar]
- 33.Mandrup-Poulsen T: The role of interleukin-1 in the pathogenesis of IDDM Diabetologia 1996; 39: 1005– 1029 [DOI] [PubMed] [Google Scholar]
- 34.Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T: Treatment with an interleukin-1β antibody improves glycemic control in diet-induced obesity Cytokine 2008; 44: 141– 148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK: Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages J Biol Chem 2006; 281: 33019– 33029 [DOI] [PubMed] [Google Scholar]
- 36.O'Rourke L, Gronning LM, Yeaman SJ, Shepherd PR: Glucose-dependent regulation of cholesterol ester metabolism in macrophages by insulin and leptin J Biol Chem 2002; 277: 42557– 42562 [DOI] [PubMed] [Google Scholar]
- 37.Welham MJ, Bone H, Levings M, Learmonth L, Wang LM, Leslie KB, Pierce JH, Schrader JW: Insulin receptor substrate-2 is the major 170-kDa protein phosphorylated on tyrosine in response to cytokines in murine lymphohemopoietic cells J Biol Chem 1997; 272: 1377– 1381 [DOI] [PubMed] [Google Scholar]
- 38.Li X, Stark GR: NFκB-dependent signaling pathways Exp Hematol 2002; 30: 285– 296 [DOI] [PubMed] [Google Scholar]
- 39.Guha M, Mackman N: The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells J Biol Chem 2002; 277: 32124– 32132 [DOI] [PubMed] [Google Scholar]
- 40.Molnarfi N, Gruaz L, Dayer JM, Burger D: Opposite regulation of IL-1β and secreted IL-1 receptor antagonist production by phosphatidylinositide-3 kinases in human monocytes activated by lipopolysaccharides or contact with T cells J Immunol 2007; 178: 446– 454 [DOI] [PubMed] [Google Scholar]
- 41.Tsukamoto K, Hazeki K, Hoshi M, Nigorikawa K, Inoue N, Sasaki T, Hazeki O: Critical roles of the p110 β subtype of phosphoinositide 3-kinase in lipopolysaccharide-induced Akt activation and negative regulation of nitrite production in RAW264.7 cells J Immunol 2008; 180: 2054– 2061 [DOI] [PubMed] [Google Scholar]
- 42.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P: Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects Diabetes 2003; 52: 2882– 2887 [DOI] [PubMed] [Google Scholar]
- 43.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P: Circulating mononuclear cells in the obese are in a proinflammatory state Circulation 2004; 110: 1564– 1571 [DOI] [PubMed] [Google Scholar]
- 44.Ghanim H, Garg R, Aljada A, Mohanty P, Kumbkarni Y, Assian E, Hamouda W, Dandona P: Suppression of nuclear factor-κB and stimulation of inhibitor κB by troglitazone: evidence for an anti-inflammatory effect and a potential antiatherosclerotic effect in the obese J Clin Endocrinol Metab 2001; 86: 1306– 1312 [DOI] [PubMed] [Google Scholar]
- 45.Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, Al-Haddad W, Dhindsa S, Dandona P: Evidence for a potent antiinflammatory effect of rosiglitazone J Clin Endocrinol Metab 2004; 89: 2728– 2735 [DOI] [PubMed] [Google Scholar]
- 46.Ghanim H, Dhindsa S, Aljada A, Chaudhuri A, Viswanathan P, Dandona P: Low-dose rosiglitazone exerts an antiinflammatory effect with an increase in adiponectin independently of free fatty acid fall and insulin sensitization in obese type 2 diabetics J Clin Endocrinol Metab 2006; 91: 3553– 3558 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.