Abstract
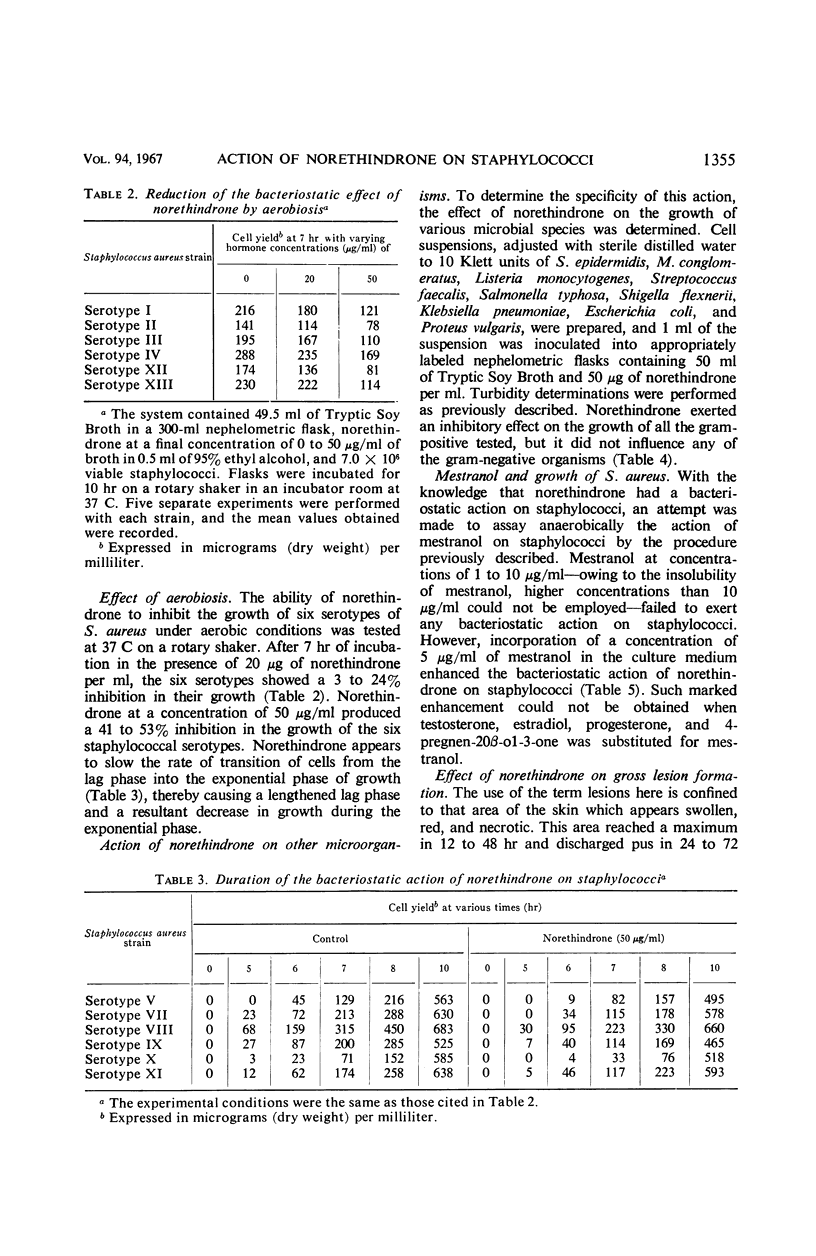
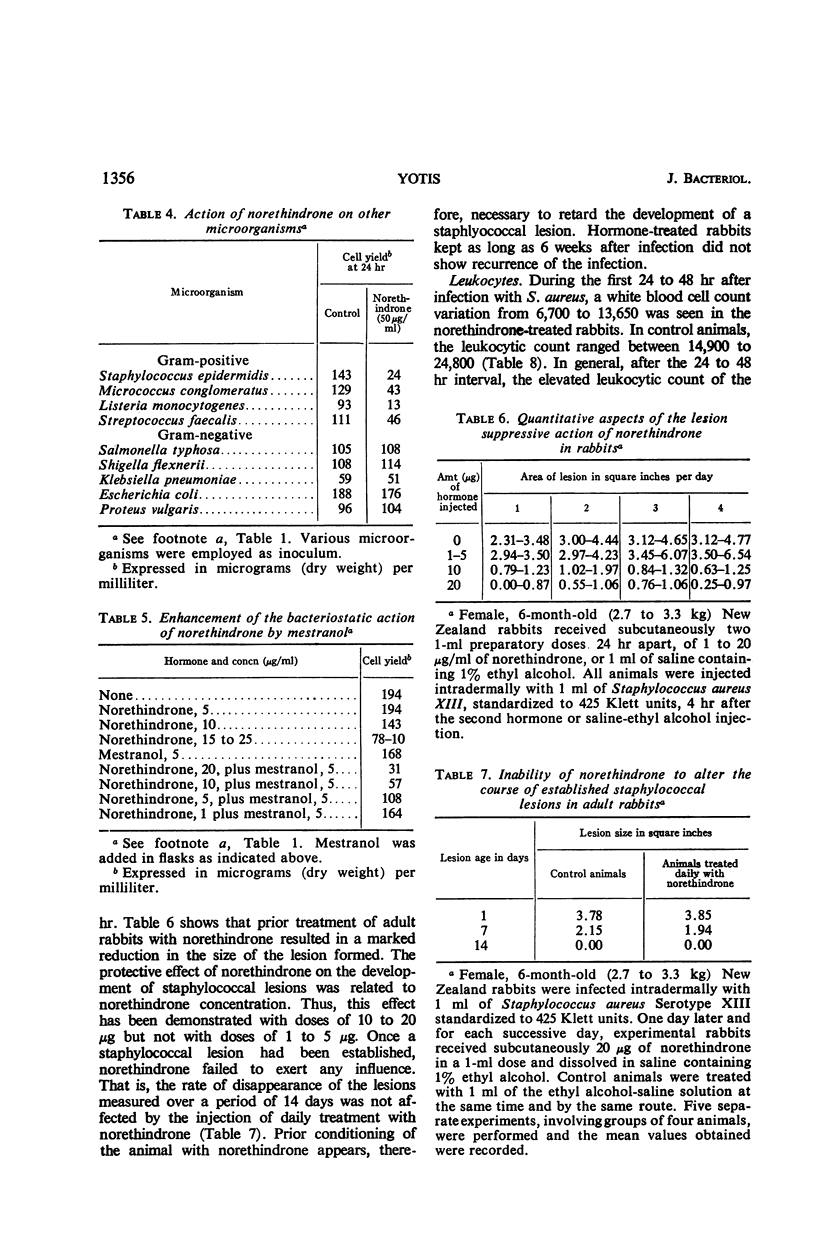
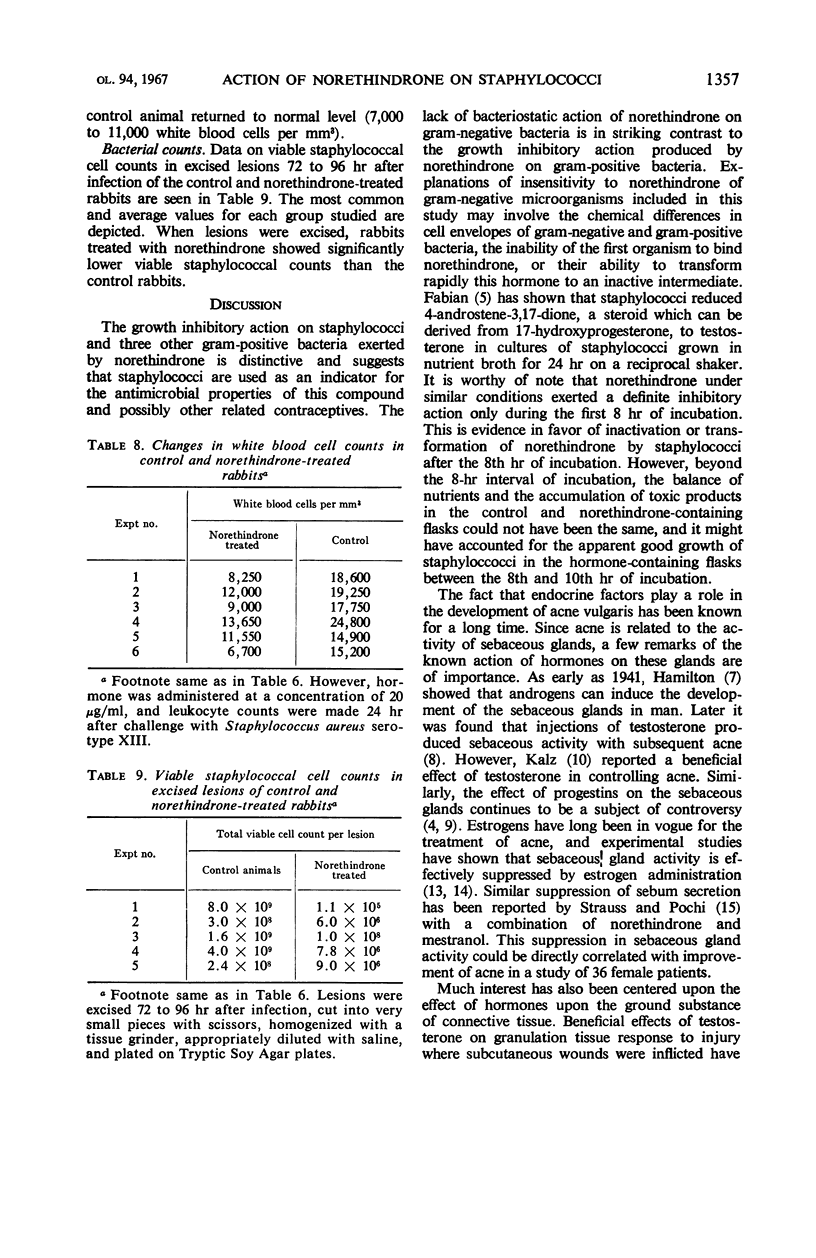
Norethindrone has been examined in vitro for antibacterial activity against 10 microorganisms. Turbidimetric techniques were used to assay the antibacterial activity of norethindrone. The organisms tested included Staphylococcus aureus, S. epidermidis, Micrococcus conglomeratus, Listeria monocytogenes, Streptococcus faecalis, Salmonella typhosa, Shigella flexnerii, Klebsiella pneumoniae, Escherichia coli, and Proteus vulgaris. Bacteriostatic action was shown only against the gram-positive microorganisms when they were grown anaerobically in Tryptic Soy Broth containing 10 to 50 μg of norethindrone per ml. The bacteriostatic action of norethindrone was exerted primarily during the first 8 hr of incubation and it was reduced by the presence of oxygen. Mestranol at a concentration of 1 to 10 μg/ml failed to exert any significant action on S. aureus. However, incorporation of 5 μg of mestranol per ml in the culture medium enhanced the bacteriostatic action of norethindrone on staphylococci. Enhancement of the bacteriostatic action of norethindrone could not be obtained by the addition of a concentration of 5 μg/ml of testosterone, 17α-estradiol, and 17β-estradiol. Progesterone and 4-pregnen-20β-ol-3-one under similar conditions showed an additive bacteriostatic effect when they were incorporated into the culture medium containing norethindrone. In vivo studies indicated that female, adult New Zealand rabbits, injected subcutaneously with two injections of 10 to 20 μg of norethindrone, 24 hr apart, and challenged intradermally with S. aureus 4 hr after the second injection, had fewer lesions with smaller areas of swelling and erythema as compared to control, nontreated rabbits. The protective effect of norethindrone on the development of staphylococcal lesion seemed related to hormone concentration. Thus, it was demonstrated with doses of 20, 15, and 10 μg, but not with doses of 1 and 5 μg. When the lesions were excised 48 to 92 hr after infection and when viable cell counts were made, rabbits treated with norethindrone showed significantly lower staphylococcal counts than the control rabbits. During the 1st day after infection with S. aureus, leukocytic counts of the norethindrone-treated rabbits remained normal, whereas control animals showed elevated leukocytic counts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DORFMAN A., SCHILLER S. Effects of hormones on the metabolism of acid mucopolysaccharides of connective tissue. Recent Prog Horm Res. 1958;14:427–456. [PubMed] [Google Scholar]
- EBLING F. J. Failure of progesterone to enlarge sebaceous glands in the female rat. Br J Dermatol. 1961 Feb;73:65–68. doi: 10.1111/j.1365-2133.1961.tb14409.x. [DOI] [PubMed] [Google Scholar]
- FABIAN J. CONTRIBUTION ON THE DIAGNOSIS OF STAPHYLOCOCCUS AUREUS ROSENBACH. Folia Microbiol (Praha) 1964 Sep;50:310–311. doi: 10.1007/BF02873311. [DOI] [PubMed] [Google Scholar]
- HAMILTON J. B. Effect of castration in adolescent and young adult males upon further changes in the proportions of bare and hairy scalp. J Clin Endocrinol Metab. 1960 Oct;20:1309–1318. doi: 10.1210/jcem-20-10-1309. [DOI] [PubMed] [Google Scholar]
- HASKIN D., LASHER N., ROTHMAN S. Some effects of ACTH, cortisone, progesterone and testosterone on sebaceous glands in the white rat. J Invest Dermatol. 1953 Mar;20(3):207–212. doi: 10.1038/jid.1953.24. [DOI] [PubMed] [Google Scholar]
- KALZ F. Hormonal influences on sebaceous gland activity and on acne vulgaris. Can Med Assoc J. 1958 Nov 15;79(10):852–855. [PMC free article] [PubMed] [Google Scholar]
- KASS E. H., FINLAND M. Adrenocortical hormones in infection and immunity. Annu Rev Microbiol. 1953;7:361–388. doi: 10.1146/annurev.mi.07.100153.002045. [DOI] [PubMed] [Google Scholar]
- LUDWIG A. W., BOAS N. F. The effects of testosterone on the connective tissue of the comb of the cockerel. Endocrinology. 1950 Mar;46(3):291–298. doi: 10.1210/endo-46-3-291. [DOI] [PubMed] [Google Scholar]
- STRAUSS J. S., KLIGMAN A. M., POCHI P. E. The effect of androgens and estrogens on human sebaceous glands. J Invest Dermatol. 1962 Aug;39:139–155. doi: 10.1038/jid.1962.94. [DOI] [PubMed] [Google Scholar]
- STRAUSS J. S., POCHI P. E. THE HUMAN SEBACEOUS GLAND: ITS REGULATION BY STEROIDAL HORMONES AND ITS USE AS AN END ORGAN FOR ASSAYING ANDROGENICITY IN VIVO. Recent Prog Horm Res. 1963;19:385–444. [PubMed] [Google Scholar]
- Strauss J. S., Pochi P. E. Effect of cyclic progestin-estrogen therapy on sebum and acne in women. JAMA. 1964 Nov 30;190(9):815–819. [PubMed] [Google Scholar]
- TOMKINS G. M., MAXWELL E. S. SOME ASPECTS OF STEROID HORMONE ACTION. Annu Rev Biochem. 1963;32:677–708. doi: 10.1146/annurev.bi.32.070163.003333. [DOI] [PubMed] [Google Scholar]


