Abstract
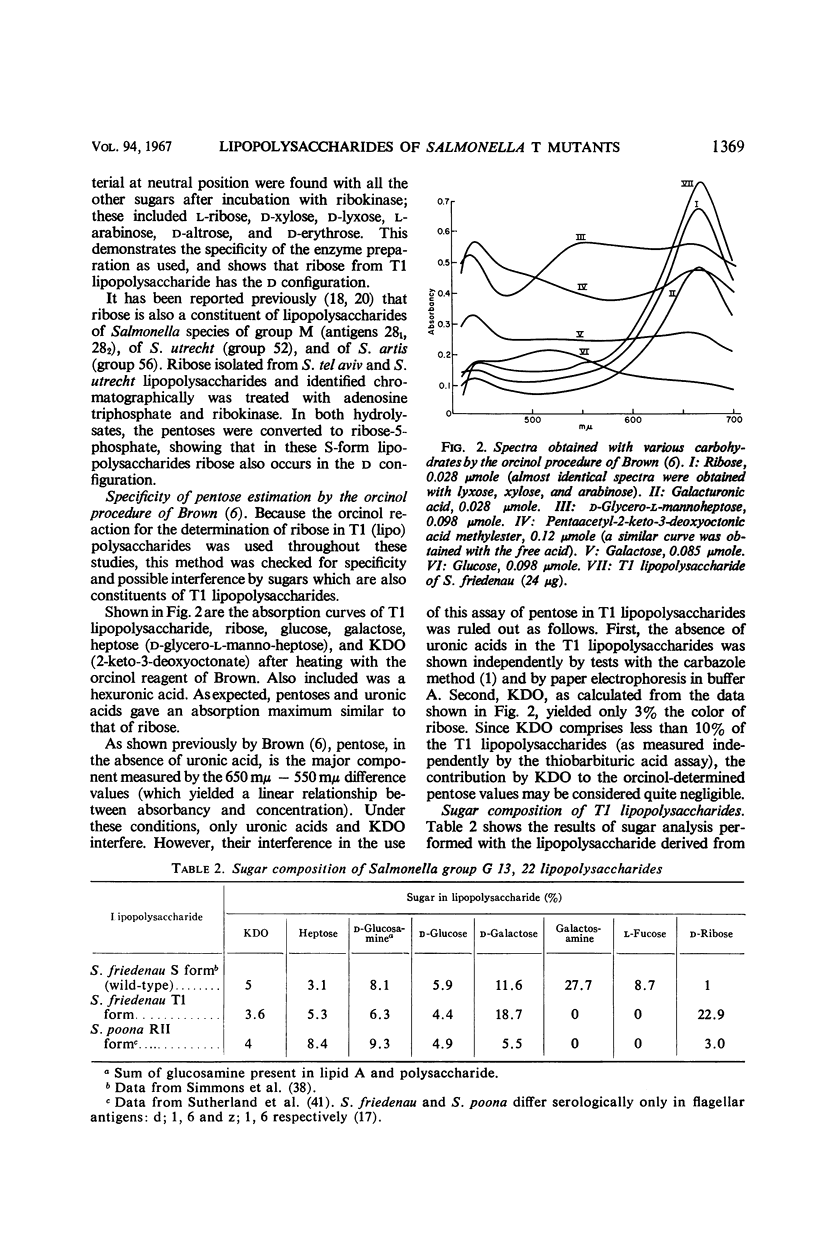
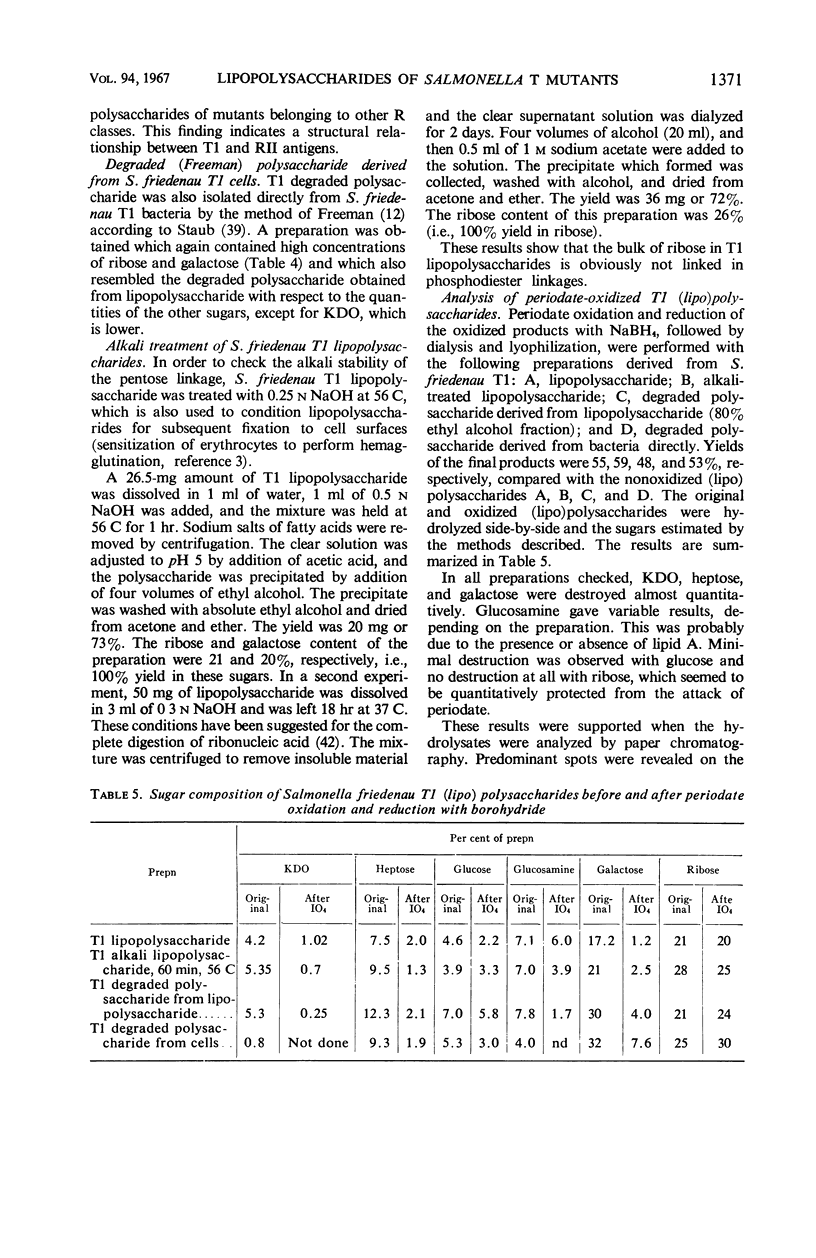
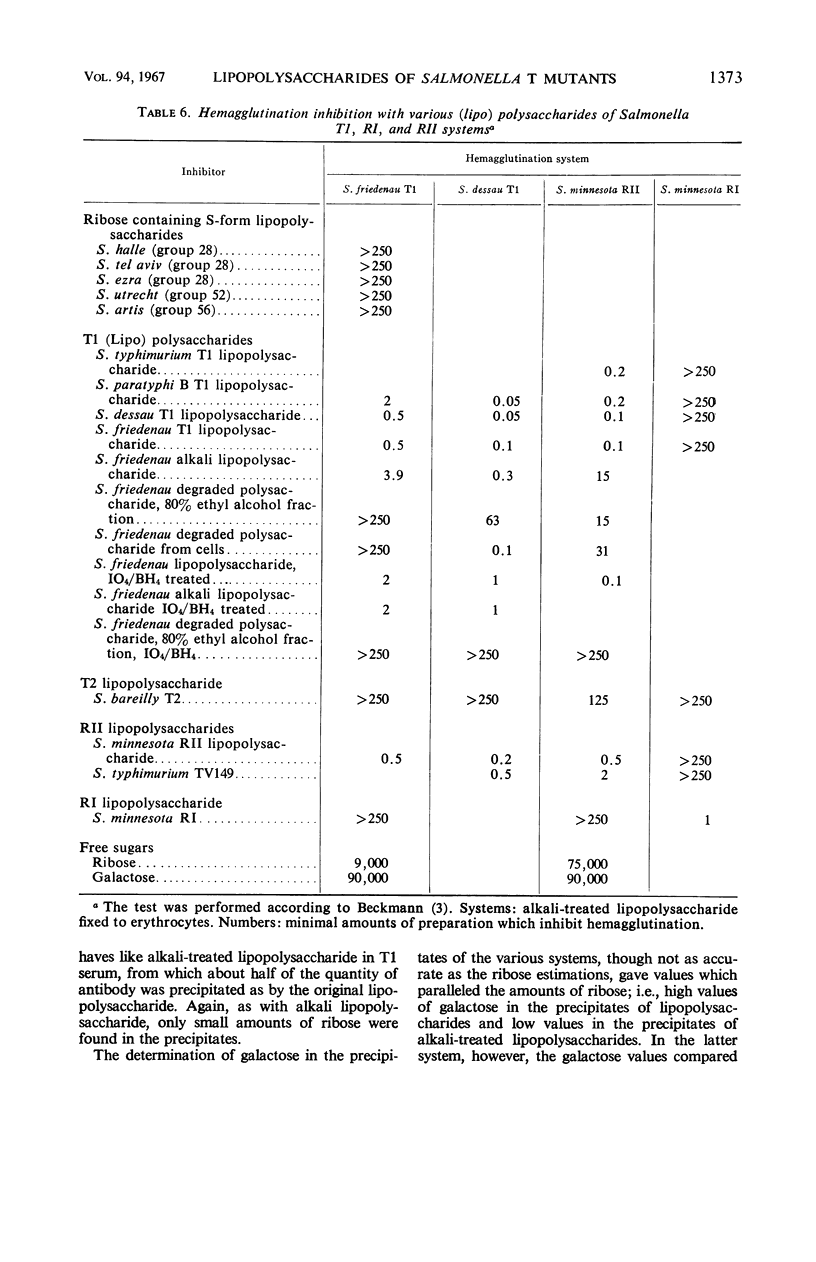
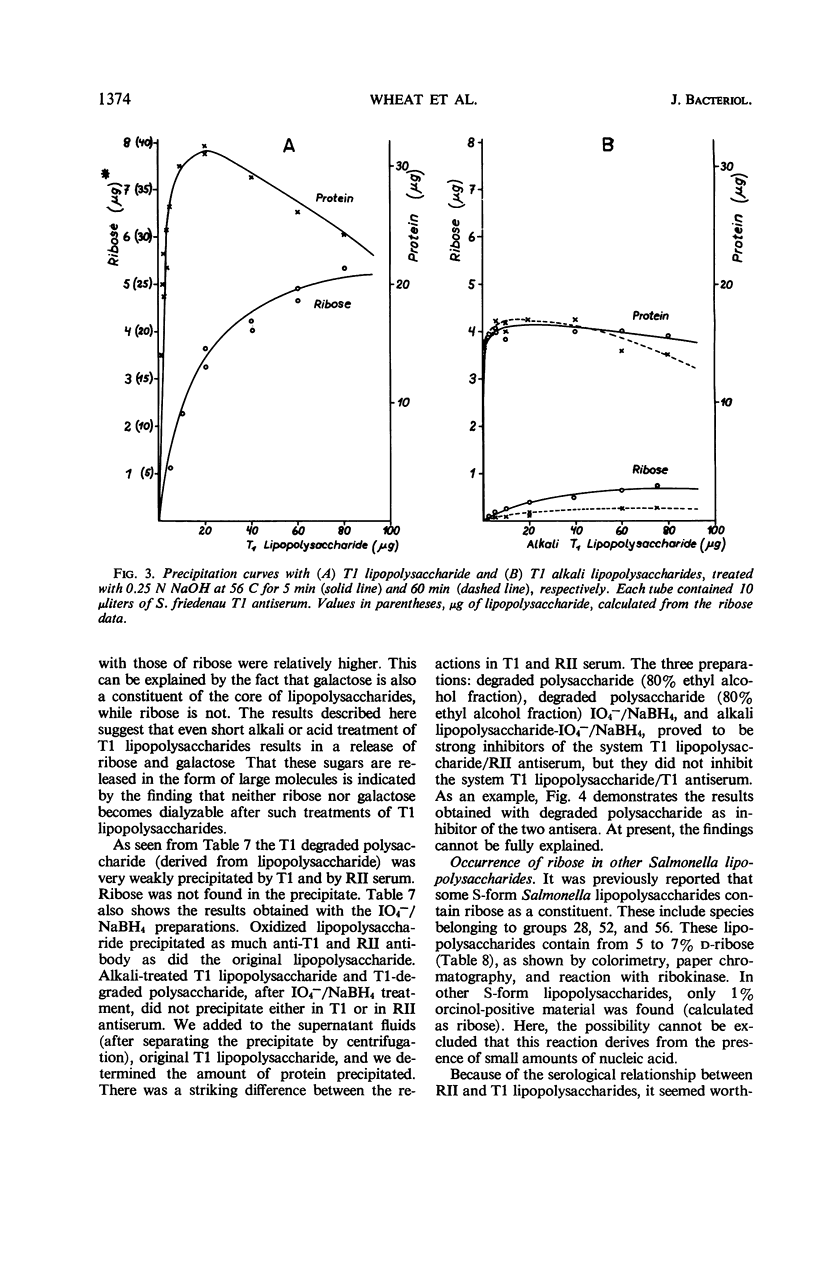
The composition of lipopolysaccharides derived from various Salmonella T forms was studied. All T1-form lipopolysaccharides examined contained 14 to 22% each of both d-galactose and pentose in addition to 4 to 9% each of ketodeoxyoctonic acid, heptose, d-glucosamine, and d-glucose. The pentose was identified as d-ribose. The T2-form lipopolysaccharide examined did not contain a significant amount of pentose, nor more than the usual amounts of d-galactose. Periodate oxidation of T1 (lipo) polysaccharides followed by NaBH4 reduction revealed that ribose was almost quantitatively protected, galactose was destroyed, and threitol and mannose were newly formed. The latter two products probably originated from 4-linked galactose and heptose, respectively. Ribose and galactose were found in specific precipitates of T1 lipopolysaccharide with anti-T1 antiserum but were not found in specific precipitates of alkali-treated T1 lipopolysaccharide and of Freeman degraded polysaccharide with anti-T1 serum Ribose and galactose are present in these degraded preparations in the form of nondialyzable polymers. The T1-form mutant lipopolysaccharides lacked the O-specific sugars constituting the side-chains in the wild-type antigens. They did not produce the soluble O-specific haptenic polysaccharide known to be accumulated in RI strains. With these properties, T1 lipopolysaccharides resemble RII lipopolysaccharides. Like RII degraded polysaccharides, T1-degraded polysaccharides also contained glucosamine. Furthermore, strong cross-reactions were found to exist between T1 and RII lipopolysaccharides in both hemagglutination inhibition assays and in precipitation tests. It is proposed that T1 lipopolysaccharides represent RII lipopolysaccharides to which polymers consisting of ribose and galactose are attached.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKMANN I., LUEDERITZ O., WESTPHAL O. ZUR IMMUNCHEMIE DER SOMATISCHEN ANTIGENE VON ENTEROBACTERIACEAE. IX. SEROLOGISCHE TYPISIERUNG VON SALMONELLA-R-ANTIGENEN. Biochem Z. 1964 May 22;339:401–415. [PubMed] [Google Scholar]
- BECKMANN I., SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. II. SEROLOGICAL AND CHEMICAL INVESTIGATIONS. Nature. 1964 Mar 28;201:1299–1301. doi: 10.1038/2011299a0. [DOI] [PubMed] [Google Scholar]
- Bagdian G., Dröge W., Kotelko K., Lüderitz O., Westphal O. Vorkommen zweier Heptosen in Lipopolysacchariden enterobakterieller Zellwände: L-Glycero-und D-Glycero-D-mannoheptose. Biochem Z. 1966 Mar 28;344(2):197–211. [PubMed] [Google Scholar]
- Bourne E. J., Hutson D. H., Weigel H. Oligosaccharides in dextran-producing cultures of Streptococcus bovis. Biochem J. 1961 Jun;79(3):549–553. doi: 10.1042/bj0790549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., SHETTLES L. B. A new spectrophotometric test for the detection of methylpentose. J Biol Chem. 1951 Oct;192(2):579–582. [PubMed] [Google Scholar]
- FISCHER F. G., DORFEL H. Die papierchromatographische Trennung und Bestimmung der Uronsäuren. Hoppe Seylers Z Physiol Chem. 1955 Sep 2;301(4-6):224–234. [PubMed] [Google Scholar]
- FISCHER W., ZAPF J. QUANTITATIVE BESTIMMUNG DER GALAKTOSE MITTELS GALATOSEOXYDASE AUS DACTYLIUM DENDROIDES. I. Hoppe Seylers Z Physiol Chem. 1964;337:186–195. doi: 10.1515/bchm2.1964.337.1.186. [DOI] [PubMed] [Google Scholar]
- FOSTER A. B., DAVIES D. A., CRUMPTON M. J. Action of periodate on some polysaccharides containing aldoheptose sugars. Nature. 1958 Feb 8;181(4606):412–413. doi: 10.1038/181412a0. [DOI] [PubMed] [Google Scholar]
- Freeman G. G. The preparation and properties of a specific polysaccharide from Bact. typhosum Ty(2): With an addendum by J. St L. Philpot, From the Department of Biochemistry, Oxford. Biochem J. 1942 Apr;36(3-4):340–356. doi: 10.1042/bj0360340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalambor M. A., Levine E. M., Heath E. C. The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. 3. The isolation and characterization of 3-deoxyoctulosonic acid. J Biol Chem. 1966 Jul 10;241(13):3207–3215. [PubMed] [Google Scholar]
- KAUFFMANN F. A new antigen of Salmonella paratyphi B and Salmonella typhi murium. Acta Pathol Microbiol Scand. 1956;39(4):299–304. doi: 10.1111/j.1699-0463.1956.tb03405.x. [DOI] [PubMed] [Google Scholar]
- KAUFFMANN F., KRUEGER L., LUEDERITZ O., WESTPHAL O. [On the immunochemistry of the O-antigen of Enterobacteriaceae. VI. Comparison of the sugar components of polysaccharides from S and R forms of Salmonella]. Zentralbl Bakteriol. 1961 May;182:57–66. [PubMed] [Google Scholar]
- KAUFFMANN F., LUEDERITZ O., STIERLIN H., WESTPHAL O. [On the immunochemistry of O antigens of Enterobacteriaceae. I. Analysis of the sugar component of Salmonella O antigens]. Zentralbl Bakteriol. 1960 May;178:442–458. [PubMed] [Google Scholar]
- KAUFFMANN F. On the T antigen of Salmonella bareilly. Acta Pathol Microbiol Scand. 1957;40(4):343–344. [PubMed] [Google Scholar]
- KRUEGER L., LUEDERITZ O., STROMINGER J. L., WESTPHAL O. [On the immunochemistry of O antigens of Enterobacteriaceae. VII. The relation of hexoses and 6-desoxyhexoses in Salmonella lipopolysaccharides to the D and L group]. Biochem Z. 1962;335:548–558. [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUEDERITZ O., KAUFFMANN F., STIERLIN H., WESTPHAL O. [On the immunochemistry of O antigens of enterobacteriaceae. II. Comparison of the sugar componet of the S. R and T forms of Salmonella]. Zentralbl Bakteriol. 1960 Jun;179:180–186. [PubMed] [Google Scholar]
- LUEDERITZ O., RISSE H. J., SCHULTE-HOLTHAUSEN H., STROMINGER J. L., SUTHERLAND I. W., WESTPHAL O. BIOCHEMICAL STUDIES OF THE SMOOTH-ROUGH MUTATION IN SALMONELLA MINNESOTA. J Bacteriol. 1965 Feb;89:343–354. doi: 10.1128/jb.89.2.343-354.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUEDERITZ O., SIMMONS D. A., WESTPHAL O., STROMINGER J. L. A SPECIFIC MICRODETERMINATION OF GLUCOSAMINE AND THE ANALYSIS OF OTHER HEXOSAMINES IN THE PRESENCE OF GLUCOSAMINE. Anal Biochem. 1964 Nov;9:263–271. doi: 10.1016/0003-2697(64)90184-8. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Risse H. J., Ruschmann E., Schlecht S., Schmidt G., Schulte-Holthausen H., Wheat R., Westphal O., Schlosshardt J. Structural relationship of Salmonella O and R antigens. Ann N Y Acad Sci. 1966 Jun 30;133(2):349–374. doi: 10.1111/j.1749-6632.1966.tb52376.x. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAIDE Y., NIKAIDO H., MAEKELAE P. H., WILKINSON R. G., STOCKER B. A. SEMIROUGH STRAINS OF SALMONELLA. Proc Natl Acad Sci U S A. 1965 Jan;53:147–153. doi: 10.1073/pnas.53.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., ZELEZNICK L. D., HORECKER B. L. LIPOPOLYSACCHARIDE OF THE GRAM-NEGATIVE CELL WALL. Science. 1964 Aug 21;145(3634):783–789. doi: 10.1126/science.145.3634.783. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMMLER D. H., RABINOWITZ J. C. A procedure for the microdetermination of formic acid in periodate oxidation mixtures. Anal Biochem. 1962 Aug;4:116–123. doi: 10.1016/0003-2697(62)90027-1. [DOI] [PubMed] [Google Scholar]
- Sarvas M., Mäkelä P. H. The production, by recombination, of Salmonella forms with both T-1 and O specifities. Acta Pathol Microbiol Scand. 1965;65(4):654–656. doi: 10.1111/apm.1965.65.4.654. [DOI] [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Wachstum und Lipopolysaccharid (O-Antigen)-Gehalt von Salmonellen bei Züchtung auf Agarnährböden. Zentralbl Bakteriol Orig. 1966 Jun;200(2):241–259. [PubMed] [Google Scholar]
- VOLKIN E., COHN W. E. Estimation of nucleic acids. Methods Biochem Anal. 1954;1:287–305. doi: 10.1002/9780470110171.ch11. [DOI] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]


