Abstract
Pesticide exposure has been suggested as an increased risk factor in developing Parkinson’s disease (PD). While the molecular mechanism underlying this association is not clear, several studies have demonstrated a role for mitochondrial dysfunction and oxidative damage in PD. Although data on specific pesticides associated with PD are often lacking, several lines of evidence point to the potential involvement of the organochlorine class of pesticides. Previously, we have found that the organochlorine pesticide methoxychlor (mxc) causes mitochondrial dysfunction and oxidative stress in isolated mitochondria. Here, we sought to determine whether mxc-induced mitochondrial dysfunction results in oxidative damage and dysfunction of the dopamine system. Adult female CD1 mice were dosed with either vehicle (sesame oil) or mxc (16, 32, or 64 mg/kg/day) for 20 consecutive days. Following treatment, we observed a dose-related increase in protein carbonyl levels in non-synaptic mitochondria, indicating oxidative modification of mitochondrial proteins which may lead to mitochondrial dysfunction. Mxc exposure also caused a dose-related decrease in striatal levels of dopamine (16–31%), which were accompanied by decreased levels of the dopamine transporter (DAT; 35–48%) and the vesicular monoamine transporter 2 (VMAT2; 21–44%). Because mitochondrial dysfunction, oxidative damage, and decreased levels of DAT and VMAT2 are found in PD patients, our data suggests that mxc should be investigated as a possible candidate involved in the association of pesticides with increased risk for PD, particularly in highly-exposed populations.
Keywords: Methoxychlor, Parkinson’s disease, dopamine, complex I, mitochondria, neurodegeneration, dopamine transporter, vesicular monoamine transporter 2, oxidative stress
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurological disorder characterized by abnormal posture, bradykinesia, rigidity, akinesia and resting tremor. Although the vast majority of affected patients have no clear causative factors, both genetic and environmental influences have been implicated (Warner and Schapira, 2003; Kamel and Hoppin, 2004; BenMoyal-Segal and Soreq, 2006; Brown et al., 2006). Several epidemiological studies (Priyardarshi et al., 2000; Ascherio et al., 2006; Frigerio et al., 2006; Kamel et al., 2007) have shown an association between pesticide exposure and increased risk of developing PD. Although the mechanism behind this association is unclear, oxidative stress and brain mitochondrial dysfunction, which has been implicated in neurodegenerative diseases, particularly PD (for reviews see Fiskum et al., 2003; Lin and Beal, 2006), has been proposed as a possible mechanism for the association of pesticides with PD.
In support of a role for mitochondrial dysfunction in PD, inhibition of complex I of the electron transport chain (ETC) has been determined to be the mechanism of action of 1-Methyl-4-phenylpyridinium (MPP+) (Nicklas and Heikkila, 1985; Richardson et al., 2007), the active metabolite of 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP) which has been demonstrated to induce parkinsonism (Langston et al., 1983). Evidence linking pesticides and complex I in PD was provided following the observation that rats chronically exposed to the complex I inhibitor rotenone reproduces the nigrostriatal neurodegeneration associated with PD (Betarbet et al., 2000). In the rotenone model, oxidative damage resulting from complex I inhibition has been demonstrated to be the key mechanism responsible for dopaminergic degeneration by rotenone both in vivo and in vitro (Sherer et al., 2003). Taken in concert, these data suggest that other pesticides that disrupt proper mitochondrial function may damage the dopamine system and increase vulnerability to PD.
In an earlier study, we demonstrated that the organochlorine pesticide 1,1,1-trichloro-2,2-bis(p-methoxyphenyl)ethane (methoxychlor, mxc) inhibited complex I of the ETC of isolated non-synaptosomal rat brain mitochondria in vitro and following repeated in vivo mxc exposure (Schuh et al., 2005). Based on our previous findings, and the association of complex I dysfunction in PD, we sought to test the hypothesis that mxc-induced mitochondrial dysfunction leads to oxidative damage and dysfunction of the dopamine system. Increased protein carbonylation, loss of dopamine (DA) and its metabolites 3,4-dihydroyxphenylacetic acid (DOPAC) and homovanillic acid (HVA) and changes in dopamine transporter levels were observed suggesting that mxc may act as an environmental neurotoxicant capable of promoting neurodegeneration.
MATERIALS AND METHODS
Chemicals and reagents
1,1,1-trichloro-2,2-bis(p-methoxyphenyl)ethane (methoxychlor, mxc) was purchased from ChemService, (Westchester, PA) in a powdered form and was 99% pure. All other reagents were purchased from Sigma (St Louis, MO) unless otherwise stated.
Animals
Female CD-1 (Charles River Laboratories, Charles River, CA) mice (25 g, 39 days old) were housed five animals per cage at the University of Maryland School of Medicine Central Animal Facility and provided food and water ad libitum. Animals were subjected to 12-h light-dark cycles. Mice were weighed daily to assess the amount of mxc to give them, then dosed via intraperitoneal injection with 16, 32, or 64 mg/kg/day mxc, or sesame oil (vehicle) for 20 continuous days. The mice were sacrificed when in estrus to minimize variability due to hormonal fluctuations within 24 h after the final mxc treatment. The gender, dosing regimen and route of administration was selected based on our previous studies (Schuh et al., 2005; Gupta et al., 2006) and had been determined to cause no overt toxicity as demonstrated by a lack of tremor or lethargy and no significant weight loss in the mxc-treated animals. The University of Maryland School of Medicine Institutional Animal Use and Care Committee approved all procedures involving animal care, euthanasia and tissue collection.
Striatal isolation
Following decapitation, striata were dissected from the mouse brains on ice, and then the left striata were weighed and immediately frozen in liquid nitrogen until high performance liquid chromatography could be performed. The right striata from the same mouse brains were homogenized in ice-cold mannitol-sucrose (MS) buffer pH 7.4 (225 mM mannitol, 75 mM sucrose, 5 mM Hepes, 1 mg/ml fatty acid free BSA) plus protease inhibitor cocktail (Calbiochem, San Diego, CA). Right striatal homogenates were centrifuged at 2,000 ×g for 5 min, then, the supernatants were further centrifuged at 30,000 × g for 30 min. The final pellet was resuspended in homogenization buffer then frozen and stored at −80°C until Western blots could be performed. Protein concentrations were determined by the method described by Lowry et al., (1951) and ranged from 8 – 17 mg/ml.
Mitochondrial isolation
The fresh mouse forebrain tissues were processed to isolate non-synaptosomal mitochondria using the percoll isolation method as previously described (Sims, 1990). Briefly, the tissue was placed in ice-cold MS buffer pH 7.4 (225 mM mannitol, 75 mM sucrose, 5 mM Hepes, 1 mg/ml fatty acid free BSA and 1 mM EGTA). The brain was homogenized and then centrifuged twice at 1,317 g for 3 min. Following a further 10 min centrifugation at 21,074 g, the pellet was resuspended in 15% percoll (Amersham Biosciences, Piscataway, NJ) and then layered on a discontinuous percoll gradient and centrifuged at 29,718 g for 8 min. The mitochondrial fraction was centrifuged at 16,599 g for 10 min, the pellet resuspended and then spun at 6,668 g for 10 min. The mitochondrial pellet was resuspended in the above buffer but without BSA or EGTA. Protein concentrations were determined by the method described by Lowry et al., (1951) and ranged from 8 –17 mg/ml.
Protein carbonyl determination
Isolated mitochondria were lysed in buffer containing 50 mM potassium phosphate (pH 7.8), 1 mM EDTA, 0.1% Triton X-100 (2:1 volume ratio of mitochondria: lysis buffer), then subjected to two freeze/thaw cycles. Protein concentrations were remeasured as described above. Mitochondrial lysates (15 μg) were assessed for oxidative modification using an OxyBlot kit (Chemicon, Temecula, CA) that recognizes carbonylated proteins. Carbonylation was determined according to the manufacturer’s protocol.
High performance liquid chromatography (HPLC)
Supernatants from mouse left striata were analyzed for DA and its metabolites DOPAC and HVA, and serotonin (5HT) and its metabolite 5-hydroxyindoleacetic acid (5HIAA) as performed previously (Richardson and Miller, 2004).
Western blot procedure
Proteins (15 μg) as determined by the Lowry method (1951) from mouse right striatal lysates were resolved using sodium dodedcyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 10% precast NuPage gels (Invitrogen, Carlsbad, CA) and transferred to a polyvinylidene difluoride membrane. Immunoblotting for Dopamine transporter (DAT), Vesicular monoamine transporter 2 (VMAT2), Tyrosine hydroxylase (TH) (Chemicon), and Glial fibrillary acidic protein (GFAP) (Sigma) was performed as previously described (Richardson et al., 2008). Briefly, nonspecific sites were blocked in 7.5% nonfat dry milk in Tris-buffered saline (135 mM NaCl, 2.5 mM KCl, 50 mM Tris and 0.1% Tween 20, pH 7.4). Membranes were then incubated overnight in DAT monoclonal antibody and detected using a goat anti-rat horseradish peroxidase secondary antibody and enhanced chemiluminescence. The luminescence signal was captured on an Alpha Innotech Fluorochem Imager and stored as a digital image. Membranes were stripped for 15 min at room temperature with Pierce stripping buffer (Rockford, IL) and sequentially reprobed with polyclonal antibodies against VMAT2, TH and GFAP. β-actin (Sigma) blots were used to ensure equal protein loading.
Statistical analysis
Data are expressed as means ± SE and the comparisons between experimental groups were made using SPSS statistical software (SPSS, Inc., Chicago, IL) using one-way analysis of variance (ANOVA). Holm-Sidak test was used for post-hoc analysis. Statistical significance was assumed at p < 0.05.
RESULTS
Previous studies have shown that exposure of mice to low levels of the organochlorine insecticide dieldrin induces oxidative stress resulting in altered dopamine transporter levels within the nigrostriatal dopamine system (Richardson et al., 2006; Hatcher et al., 2007) and decreased dopamine metabolites (Hatcher et al., 2007). Methoxychlor (mxc), another organochlorine insecticide has also been shown to induce oxidative stress in both mouse reproductive tissue (Gupta et al., 2006) and brain (Schuh et al., 2005). Inhibition of mitochondrial respiration and stimulation of hydrogen peroxide (H2O2) production were observed when non-synaptosomal mitochondria isolated from normal rat brain were exposed to mxc (Schuh et al., 2005). In addition, we found that repeated exposure of mice to mxc resulted in significant inhibition of mitochondrial respiration (Schuh et al., 2005) which can affect measures of mitochondrial oxidative stress. In order to verify that this treatment regimen induces oxidative stress, we measured the extent of protein carbonylation in these mitochondria. As shown in Fig 1, a dose-dependent increase in mitochondrial protein carbonyl groups was observed following chronic exposure of mice to mxc.
Fig 1.
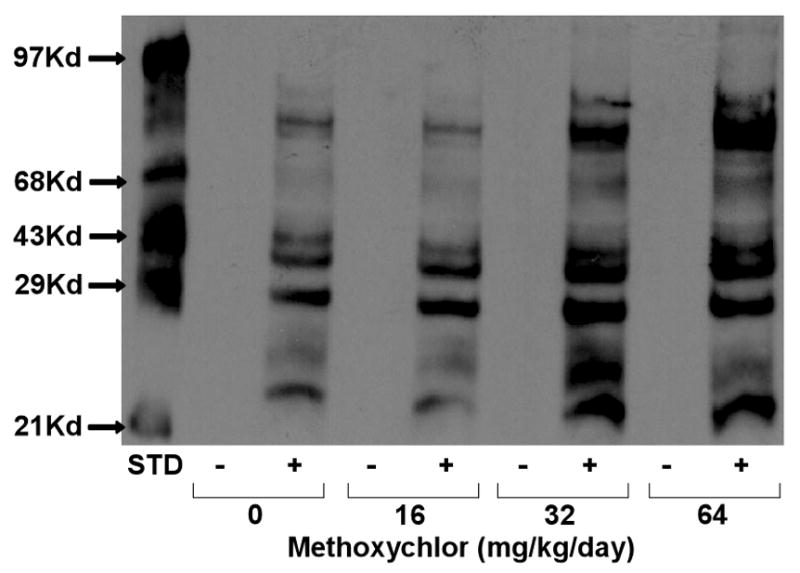
Methoxychlor Increases Protein Carbonyl Formation in Mitochondria. Representative western blot of carbonylated proteins in non-synaptosomal mitochondria isolated from mouse brain following in vivo methoxychlor (0, 16, 32, 64 mg/kg/day) exposure. All samples were either derivatized (+) or not (−) prior to loading on gels. STD = standard.
To determine whether mxc-induced oxidative stress perturbed the nigrostriatal dopamine system, adult female CD1 mice were dosed daily for 20 days via intraperitoneal injection with mxc (0, 16, 32 or 64 mg/kg). Within 24 h of the final mxc exposure, striata from the mice were dissected out for HPLC analysis of dopamine and its metabolites DOPAC and HVA.
Striatal dopamine (DA) and metabolite levels are summarized in Fig 2. There was a dose-dependent decrease in DA levels, 16% at the lower doses and 31% at the highest mxc dose, which was significant (p < 0.05 as compared to control) (Fig 2A). The dopamine metabolite DOPAC also significantly decreased ~ 44% at all mxc doses (p < 0.05 as compared to control) (Fig 2B). HVA levels were not significantly altered with mxc treatment (p = 0.38) as compared to control animals but showed a trend toward decreased levels (Fig 2C). Serotonin (5HT) (Fig 3A) and its metabolite 5-hydroxyindoleacetic acid (5HIAA) (Fig 3B) were not affected at any mxc dose suggesting that this effect was specific to dopamine and its metabolites. Additionally, the DOPAC/DA ratio was significantly decreased (p < 0.05) as compared to control animals with the 16 and 32 mg/kg exposure (Table 1). Interestingly, the HVA/DA ratio was not affected at the lower doses but was significantly increased (p < 0.01) with the highest dose (Table 1).
Fig 2.
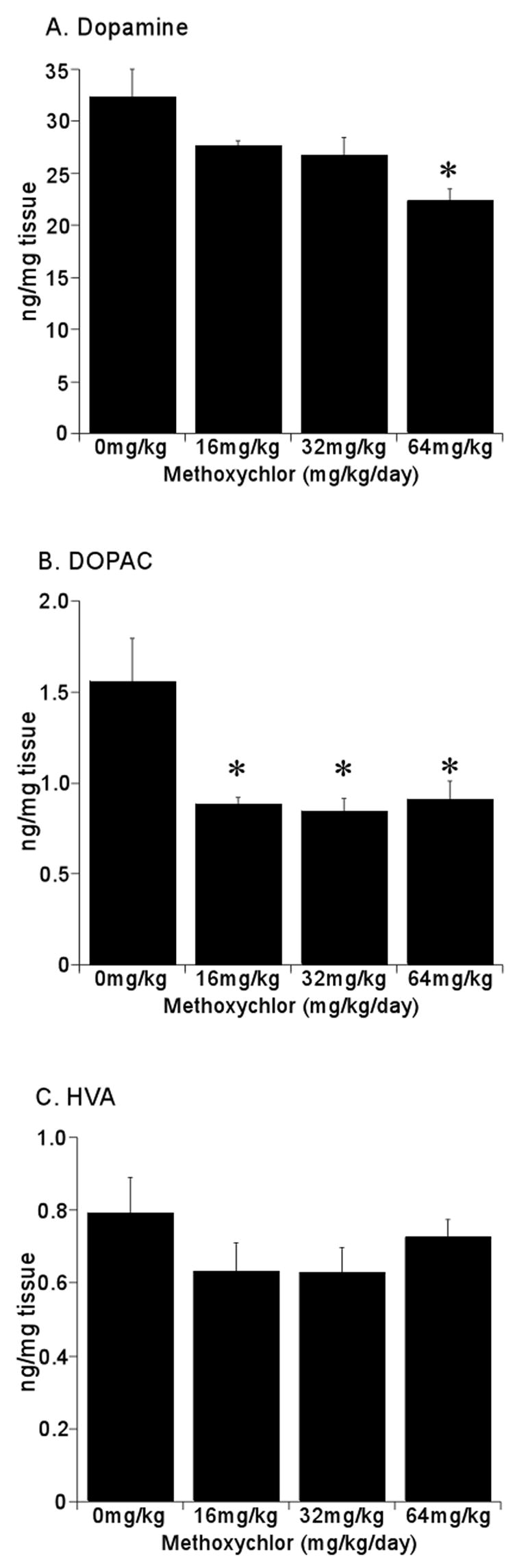
Methoxychlor Decreases Striatal Dopamine and DOPAC, but not HVA Levels. Striata were removed from mice administered 0, 16, 32 or 64mg/kg/day for 20 days. Analysis of dopamine (A), DOPAC (B) and HVA (C) levels was determined by HPLC. Data are expressed as mean ng/mg tissue ± SEM (n = 3–4 separate animals per treatment group). *Significantly different (p < 0.05) from sesame vehicle control as determined by one-way ANOVA followed by the Holm-Sidak test for post-hoc analysis.
Fig 3.
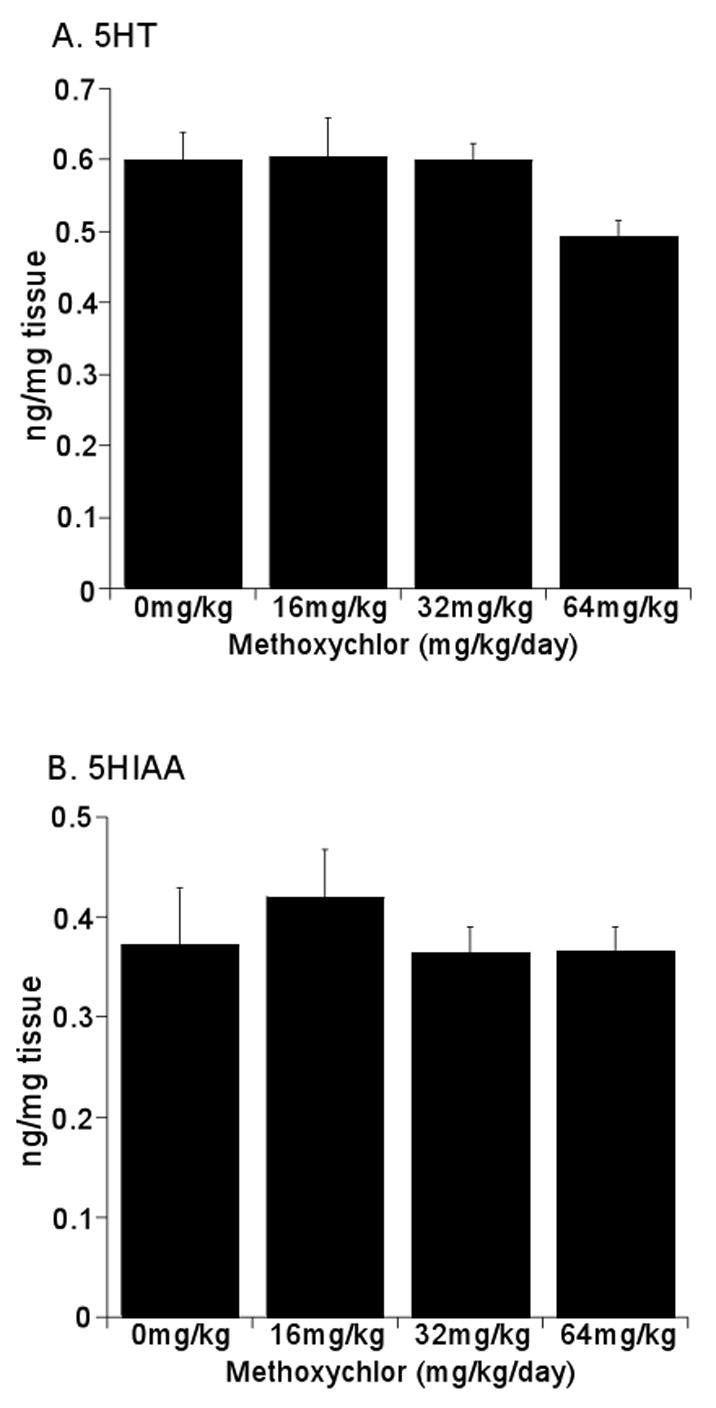
Methoxychlor Does Not Alter 5HT and 5HIAA Levels in the Striatum. Striata were removed from mice administered 0, 16, 32 or 64mg/kg/day for 20 days. Analysis of 5HT (A) and 5HIAA (B) levels was determined by HPLC. Data are expressed as mean ng/mg tissue ± SEM (n = 3–4 separate animals per treatment group). There was no statistically significant difference between treatment and sesame vehicle control groups as determined by one-way ANOVA followed by the Holm-Sidak test for post-hoc analysis.
Table 1.
Ratio of DOPAC and HVA to striatal dopamine
| DOPAC/DA | HVA/DA | |
|---|---|---|
| 0mg/kg | 4.81 ± 0.61 | 2.43 ± 0.17 |
| 16mg/kg | 3.21 ± 0.10* | 2.28 ± 0.25 |
| 32mg/kg | 3.18 ± 0.14* | 2.34 ± 0.15 |
| 64mg/kg | 4.03 ± 0.24 | 3.24 ± 0.09* |
Data expressed as ratios of raw data ± SEM (n = 3–4).
Significantly different (p < 0.05) from sesame vehicle control as determined by one-way ANOVA followed by the Holm-Sidak test for post-hoc analysis.
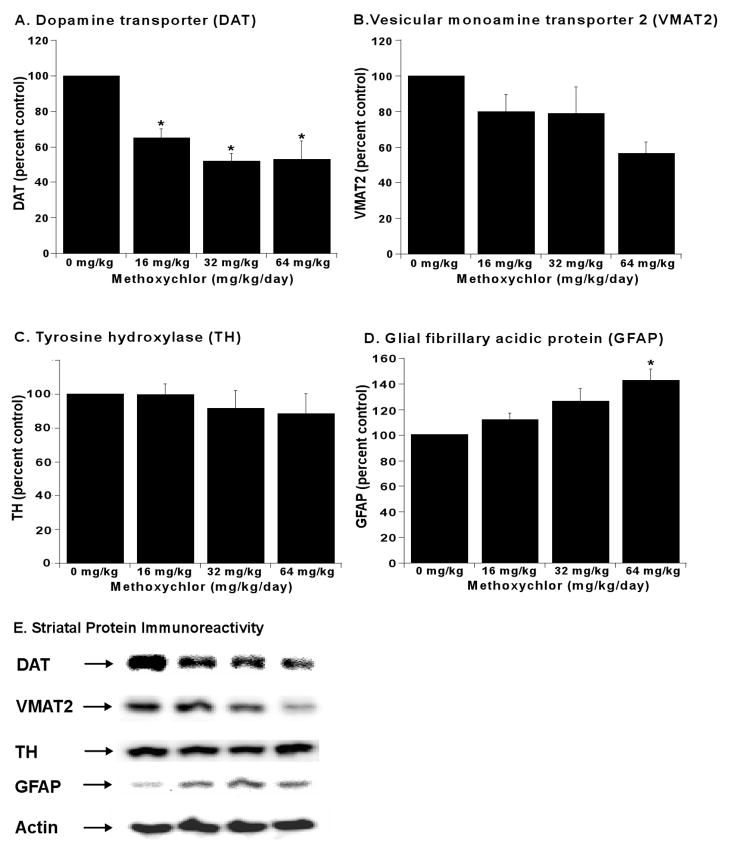
In addition to altered levels of DA and its metabolites, dopamine transport and repackaging into vesicles for later release have also been determined to be perturbed following pesticide exposure (Hatcher et al., 2007). Therefore, we examined the changes in dopamine transporter levels in mxc-treated animals. The left striata were dissected for HPLC analysis described above, and the right striata from the same mouse brains were processed for Western blot analysis of the dopamine transporter (DAT) and vesicular monoamine transporter (VMAT2). DAT immunoreactivity was decreased 48% at 32 and 64 mg/kg doses (p < 0.05) and 35% at the lowest dose (p < 0.05) as compared to control (Fig 4A,E). Additionally, VMAT2 immunoreactivity was decreased 21% at 16 and 32 mg/kg doses and 44% at 64 mg/kg as compared to control (p = 0.06, Fig 4B,E). Interestingly, Glial fibrillary acidic protein (GFAP) immunoreactivity dose-dependently increased, becoming significant at 64 mg/kg (p < 0.05, Fig 4D,E). Tyrosine hydroxylase (TH) immunoreactivity was not significantly affected at any mxc dose (p = 0.74) as compared to control (Fig 4C,E).
Fig 4.
Methoxychlor Decreases Striatal Dopamine Transporter and Increases Glial Activation. Dopamine transporter (DAT), vesicular monoamine transporter 2 (VMAT2), and tyrosine hydroxylase (TH) levels were determined in the striatum by western blot. Glial activation was determined by measuring glial fibrillary acidic protein (GFAP) levels. Quantitation of DAT (A), VMAT2 (B), TH (C), and GFAP immunoreactivity (D). Data are expressed as percent of control from 3–4 separate animals per treatment group ± SEM. *Significantly different (p < 0.05) from sesame vehicle control as determined by one-way ANOVA followed by the Holm-Sidak test for post-hoc analysis. (E) Representative western blots for DAT, VMAT2, TH, GFAP, and β-actin to ensure equal loading of gels.
DISCUSSION
Numerous epidemiological studies have identified pesticide exposure as a risk factor for PD (Priyardarshi et al., 2000; Ascherio et al., 2006; Frigerio et al., 2006; Kamel et al., 2007). Elevated levels of 1,1-dichloro-2,2-bis(p-chloro-phenyl)ethylene (p,p′-DDE), the major metabolite of the banned pesticide dichlorodiphenoxytrichloroethane (DDT) have been found in postmortem PD brains, suggesting that organochlorine pesticides may play a role in the dopaminergic neurodegeneration observed in PD (Corrigan et al., 2000; Pennell et al., 2006). Here, we found that sub-chronic exposure of mice to the currently used organochlorine pesticide methoxychlor (mxc) results in oxidative damage to mitochondria, decreased levels of striatal dopamine, and decreased levels of the dopamine transporter (DAT) and vesicular monoamine transporter 2 (VMAT2). Given the integral roles of mitochondrial dysfunction, oxidative damage, and dopaminergic dysfunction in PD, our data suggest that mxc may be an additional candidate for pesticides contributing to PD pathogenesis by causing damage to the dopamine system, particularly to those that may be occupationally exposed to high levels.
Although DDT has long been banned, mxc, the p,p′-dimethoxy analog of DDT, is a current use pesticide that is thought to have a similar mechanism of action as DDT (Smith, 2001). Mxc is generally thought of as a relatively safe pesticide (EPA Class IV) owing to its high LD50 (~2,000 mg/kg for mice) and its lack of persistence in the environment compared to DDT (ATSDR, 2002). Mxc is currently registered for use on a variety of food crops, forage crops, and forests. However, the major use of mxc is in the control of ectoparasites on cattle and livestock. Although there has been much attention paid to the potential endocrine disrupting effects of mxc, relatively little is known about its neurotoxic potential and whether it may damage the dopamine system.
Previously, we demonstrated that mxc preferentially inhibits brain mitochondrial respiration at the level of complex I of the ETC and generates reactive oxygen species in vitro (Schuh et al., 2005). This is particularly relevant to dopaminergic neurodegeneration as inhibition of complex I has been demonstrated to cause nigrostriatal degeneration in the rotenone model of PD (Sherer et al., 2003). Partial inhibition of complex I, as produced by rotenone and as observed in PD, has been found to enhance production of reactive oxygen species (Hasegawa et al., 1990; Hensley et al., 1998), similar to that we previously observed in isolated mitochondria exposed to mxc (Schuh et al., 2005). Here, we found increased protein carbonylation, a marker of oxidative stress, in brain mitochondria isolated from mice repeatedly exposed to mxc. Elevated levels of protein carbonyls have also been found in the mitochondria of animals exposed to the complex I inhibitors rotenone and MPTP (Lee et al., 2000; Testa et al., 2005). Using the same dosing paradigm as employed in the current study, Gupta and co-workers (2006) found that oxidative stress was responsible for growth inhibition and atresia of antral follicles following mxc treatment. In that study, mxc treatment decreased Cu/Zn superoxide dismutase, glutathione peroxidase and catalase levels whereas the antioxidant N-acetyl cysteine ameliorated the effects on the antral follicles. Thus, it appears that mxc not only targets the reproductive system through oxidative stress, but the brain as well.
Because mitochondrial dysfunction and oxidative damage by complex I inhibition has been linked to dopaminergic neurodegeneration (Sherer et al., 2003; Richardson et al., 2007), we next sought to determine the effect of mxc treatment on the dopamine system. Mxc dose-dependently decreased striatal dopamine (16–31%) and its metabolite DOPAC (~ 44%). However, levels of the dopamine metabolite, homovanillic acid (HVA), were unaffected. These results are similar to that observed in mice exposed to another organochlorine pesticide, dieldrin (Hatcher et al., 2007), although dieldrin acts through a different mechanism to cause toxicity. Levels of tyrosine hydroxylase, the rate limiting step in dopamine synthesis, were not affected following mxc treatment, suggesting that reduced dopamine levels are likely not the result of loss of dopamine synthesis. Further, the significant decrease in DOPAC levels suggests that increased dopamine degradation is likely not responsible for the reduction in dopamine levels. However, the effects of mxc on dopamine synthesis and metabolism will have to be determined in future studies to conclusively rule out these possibilities.
In addition to synthesis and degradation, dopamine levels are under the tight and concerted control of the dopamine transporter (DAT), which terminates dopamine action by removing it from the synaptic cleft, and the vesicular monoamine transporter 2 (VMAT2), which packages dopamine for subsequent release. Indeed, mice lacking or possessing reduced levels of DAT and VMAT2 have significantly decreased striatal dopamine levels, while DOPAC and HVA are less affected (Giros et al., 1996; Wang et al., 1997). Decreased levels of DAT and VMAT2 have also been shown to be sensitive and early indicators of dopaminergic damage (Brooks et al., 2003). Here, we found that DAT (35–48%) and VMAT2 (21–44%) levels were decreased in mice repeatedly exposed to mxc. A similar reduction in DAT and VMAT2 levels has been demonstrated in mice exposed to MPTP (Richardson et al., 2007) and environmental toxicants that damage the dopamine system such as dieldrin and polychlorinated biphenyls (PCBs; Richardson and Miller, 2004; Caudle et al., 2006; Hatcher et al., 2007). Because PCBs (Lee and Opanashuk, 2004; Lyng and Seegal, 2008), dieldrin (Kanthasamy et al., 2005; Hatcher et al., 2007) and mxc (this study) all generate oxidative stress, this may provide insight into the decreased levels of DAT and VMAT2 observed following mxc treatment. Indeed, oxidative and nitrative stress has been demonstrated to result in decreased levels of both DAT and VMAT2 by directly oxidizing or nitrating amino acid residues in both proteins (Park et al., 2002; Eyerman and Yamamoto, 2007). Interestingly, rotenone, a classic complex I inhibitor, has also been demonstrated to reduce both DAT and VMAT2 levels as a result of oxidative damage (Maragos et al., 2002; Watabe and Nakaki, 2008). Additionally, we observed increased neurotoxicity following mxc treatment as defined by increased GFAP levels, as has been observed in animals treated with the complex I inhibitor MPTP (Richardson et al., 2006). Thus, it appears that a likely mechanism for the effects of mxc on the dopamine system is through inhibition of complex I which leads to oxidative damage and decreased levels of DAT and VMAT2, and ultimately loss of dopamine.
There is ever increasing evidence that pesticide exposure can cause damage to the dopamine system and is associated with increased risk of PD. The data reported here build upon an increasing body of evidence that organochlorine pesticides may contribute significantly to these effects. However, our data with mxc do differ because of the lack of environmental persistence of mxc compared to DDT and dieldrin. Although elevated levels of DDT have been found in postmortem PD brains, recent data from animal studies suggest that neither DDT nor its metabolite DDE cause damage to the dopamine system when administered to mice (Hatcher et al., 2008). Although commonly assumed that mxc and DDT share a similar mechanism of action based on structure, our data demonstrate the ability of mxc to inhibit complex I and cause oxidative damage, whereas DDT has no effect on complex I (Ferreira et al., 1997). This finding could explain the differences between the two studies and suggest that careful consideration should be given before assuming a common mechanism of action of DDT and mxc. Interestingly, we have recently found that serum levels of mxc in a small cohort of PD patients was slightly elevated compared to age-matched controls (Richardson, unpublished data). However, how the mxc levels used in this study relate to those that exist in humans is difficult to assess, as virtually no data on mxc levels in human tissue are available. Taken in concert, our data support the idea that pesticides that disrupt mitochondrial function can lead to dopaminergic dysfunction and may ultimately contribute to the pathogenic process in PD patients exposed to such pesticides.
Acknowledgments
VA Research Service, REAP Fellowship (R.A.S.). This work was supported in part by National Institute of Environmental Health Sciences (NIEHS) grant P30ES005022; National Institutes of Health (NIH) grants R01ES015991, and R21ES013828 to J.R.R., R01ES12893 to J.A.F., and R01NS34152 and P01HD016595 to G.F.
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- DOPAC
3,4-dihydroyxphenylacetic acid
- mxc
methoxychlor, 1,1,1-trichloro-2,2-bis(p-methoxyphenyl)ethane
- HVA
homovanillic acid
- VMAT2
vesicular monoamine transporter
- 5HT
serotonin
- 5HIAA
5-hydroxyindoleacetic acid
- GFAP
glial fibrillary acidic protein
- TH
tyrosine hydroxylase
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ascherio A, Chen H, Weisskopf MG, O’Reilly E, McCullough ML, Calle EE, Schwarzschild MA, Thun MA. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60 (2):197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) U.S. Dept. of Health and Human Services-Public Health Service; 2002. Toxicological profile for DDT, DDE, and DDD. http://www.atsdr.cdc.gov/toxprofiles/phs35.html. [PubMed] [Google Scholar]
- BenMoyal-Segal L, Soreq H. Gene-environment interactions in sporadic Parkinson’s disease. J Neurochem. 2006;97:1740–1755. doi: 10.1111/j.1471-4159.2006.03937.x. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3 (12):1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Frey KA, Marek KL, Oakes D, Paty D, Prentice R, Shults CW, Stoessl AJ. Assessment of neuroimaging techniques as biomarkers of the progression of Parkinson’s disease. Exp Neurol. 2003;184 (Suppl 1):S68–79. doi: 10.1016/j.expneurol.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson’s disease-is there a link? Environ Health Perspect. 2006;114 (2):156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Delea KC, Guillot TS, Wang M, Pennell KD, et al. Toxicol Sci. 2006;92:490–499. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J Toxicol Environ Health A. 2000;59 (4):229–234. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem. 2007;103(3):1219–1227. doi: 10.1111/j.1471-4159.2007.04837.x. [DOI] [PubMed] [Google Scholar]
- Ferreira FM, Madeira VM, Moreno AJ. Interactions of 2,2-bis(p-chlorophenyl)-1,1-dichloroethylene with mitochondrial oxidative phosphorylation. Biochem Pharmacol. 1997;53(3):299–308. doi: 10.1016/s0006-2952(96)00689-2. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Starkov A, Polster B, Chinopoulos C. Mitochondrial mechanisms of neural cell death and neuroprotective interventions in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:111–119. doi: 10.1111/j.1749-6632.2003.tb07469.x. [DOI] [PubMed] [Google Scholar]
- Frigerio R, Sanft KR, Grossardt BR, Peterson BJ, Elbaz A, Bower JH, Ahlskog JE, de Andrade M, Maraganore DM, Rocca WA. Chemical exposures and Parkinson’s disease: a population-based case-control study. Mov Disord. 2006;21 (10):1688–92. doi: 10.1002/mds.21009. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Schuh RA, Fiskum G, Flaws JA. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol Appl Pharmacol. 2006;216 (3):436–45. doi: 10.1016/j.taap.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Hatcher JM, Delea KC, Richardson JR, Pennell KD, Miller GW. Disruption of dopamine transport by DDT and its metabolites. Neurotoxicology. 2008:682–690. doi: 10.1016/j.neuro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher JM, Richardson JR, Guillot TS, McCormack AL, Di Monte DA, Jones DP, Pennell KD, Miller GW. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol. 2007;204 (2):619–30. doi: 10.1016/j.expneurol.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Pye QN, Maidt ML, Stewart CA, Robinson KA, Jeffrey F, Floyd RA. Interaction of alpha-phenyl-N-tert-butyl nitrone and alternative electron acceptors with complex I indicates a substrate reduction site upstream from the rotenone binding site. J Neurochem. 1998;71(6):2549–2557. doi: 10.1046/j.1471-4159.1998.71062549.x. [DOI] [PubMed] [Google Scholar]
- Kamel F, Hoppin JA. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect. 2004;112 (9):950–958. doi: 10.1289/ehp.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel F, Tanner CM, Umbach DM, Hoppin JA, Alavanja MC, Blair A, Comyns K, Goldman SM, Korell M, Langston JW, Ross GW, Sandler DP. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol. 2007;165(4):364–74. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Dieldrin-induced neurotoxicity: relevance to Parkinson’s disease pathogenesis. Neurotoxicology. 2005;26:701–719. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219 (4587):979–80. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lee CS, Han ES, Jang YY, Han JH, Ha HW, Kim DE. Protective effect of harmalol and harmaline on MPTP neurotoxicity in the mouse and dopamine-induced damage of brain mitochondria and PC12 cells. J Neurochem. 2000;75(2):521–531. doi: 10.1046/j.1471-4159.2000.0750521.x. [DOI] [PubMed] [Google Scholar]
- Lee DW, Opanashuk LA. Polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress plays a role in dopaminergic cell injury. Neurotoxicology. 2004;25(6):925–939. doi: 10.1016/j.neuro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443 (7113):787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193 (1):265–75. [PubMed] [Google Scholar]
- Lyng GD, Seegal RF. Polychlorinated biphenyl-induced oxidative stress in organotypic co-cultures: experimental dopamine depletion prevents reduction in GABA. Neurotoxicology. 2008;29(2):301–308. doi: 10.1016/j.neuro.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos WF, Zhu J, Chesnut MD, Dwoskin LP. Mitochondrial toxin inhibition of [(3)H]dopamine uptake into rat striatal synaptosomes. Biochem Pharmacol. 2002;63(8):1499–1505. doi: 10.1016/s0006-2952(02)00910-3. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36 (26):2503–8. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Park SU, Ferrer JV, Javitch JA, Kuhn DM. Peroxynitrite inactivates the human dopamine transporter by modification of cysteine 342: potential mechanism of neurotoxicity in dopamine neurons. J Neurosci. 2002;22(11):4399–4405. doi: 10.1523/JNEUROSCI.22-11-04399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell KD, Hatcher JM, Caudle WM, Richardson JR, Gearing M, Levey AI, et al. Elevated levels of dieldrin are associated with Parkinson’s disease. Preprints for Extended Abstracts; American Chemical Society 232nd National Meeting; San Francisco, CA. 2006. special Symposium on Persistent, Bioaccumulative and Toxic Chemicals, Division of Environmental Chemistry. [Google Scholar]
- Priyardarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson’s disease and exposure to pesticides. Neurotoxicology. 2000;(4):435–40. [PubMed] [Google Scholar]
- Richardson JR, Miller GW. Acute exposure to aroclor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol Lett. 2004;148 (1–2):29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. FASEB J. 2006;20 (10):1695–7. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Guillot TS, Watson JL, Nakamaru-Ogiso E, Seo BB, Sherer TB, Greenamyre JT, Yagi T, Matsuno-Yagi A, Miller GW. Obligatory role for complex I inhibition in the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicol Sci. 2007;95(1):196–204. doi: 10.1093/toxsci/kfl133. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental heptachlor exposure increases susceptibility of dopamine neurons to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in a gender-specific manner. Neurotoxicology. 2008;29:855–863. doi: 10.1016/j.neuro.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh RA, Kristián T, Gupta RK, Flaws JA, Fiskum G. Methoxychlor inhibits brain mitochondrial respiration and increases hydrogen peroxide production and CREB phosphorylation. Toxicol Sci. 2005;(2):495–504. doi: 10.1093/toxsci/kfi334. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22 (16):7006–15. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CR, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23 (34):10756–64. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179(1):9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using percoll density gradient centrifugation. J Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Smith AG. DDT and its analogs. In: Krieger RI, editor. Handbook of Pesticide Toxicology. San Diego: Academic Press; 2001. pp. 1305–1355. [Google Scholar]
- Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134 (1):109–18. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Wang YM, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, Bock CB, Miller GW, Wightman RM, Caron MG. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19(6):1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- Warner TT, Schapira AH. Genetic and environmental factors in the cause of Parkinson’s disease. Ann Neurol. 2003;53 (3):S16–S25. doi: 10.1002/ana.10487. [DOI] [PubMed] [Google Scholar]
- Watabe M, Nakaki T. Mitochondrial complex I inhibitor rotenone inhibits and redistributes vesicular monoamine transporter 2 via nitration in human dopaminergic SH-SY5Y cells. Mol Pharmacol. 2008 Jul 3; doi: 10.1124/mol.108.048546. Epub ahead of print. [DOI] [PubMed] [Google Scholar]