Abstract
γδ T cell subsets, defined by their Vγ chain usage, have been shown in various disease models to have distinct functional roles. While a role for total γδ T cells in collagen-induced arthritis (CIA) has been studied, the γδ T cell subsets involved have not been well characterized. Therefore, we examined the two main peripheral γδ T cell subsets, the Vγ1+ and Vγ4+ cells, and found that both subsets increased in number during CIA, but only the Vγ4+ cells were activated. Mice depleted of Vγ4+ cells showed a significant reduction in total IgG and IgG2a anti-collagen antibodies, disease severity, and incidence of arthritis. Surprisingly, the Vγ4+ cells appeared to be antigen-selected, based on preferential Vγ4/Vδ4 pairing and very limited TCR junctions. During the height of their activation, in both the draining lymph node and the joints, the vast majority of the Vγ4/Vδ4+ cells produced IL-17, which may explain their pathogenicity.
Collagen-induced arthritis (CIA) is a murine model of chronic inflammation that shares many hallmarks with rheumatoid arthritis (RA) (reviewed in1). For example, there is a strong association with the MHC Class II allele HLA-DR4 (DRB1*0401) in humans and IAq in mice2,3 and both class II molecules bind the same immunodominant collagen type II (CII) peptide4. In addition, anti-collagen antibodies play a critical role in the development of CIA (reviewed in1) and complement-fixing IgG2a has been shown to dominate the anti-collagen response and be essential for pathogenesis5. Finally, αβ T cells have been shown to be essential in CIA6.
There is also evidence that γδ T cells play a role in CIA7,8. γδ T cells are resident in the synovium of mice and their proportion in the joints rises dramatically when mice develop CIA7,8. Additionally, γδ T cells are increased in the peripheral blood and synovium of patients with RA9-11. However, studies in mice genetically deficient for T cells have shown that γδ T cells are neither necessary nor sufficient for the development of CIA6. Yet, when mice were temporarily depleted of γδ T cells, an effect on disease was noted. Depleting mice of γδ T cells prior to immunization with CII significantly delayed the onset of arthritis and severity. In contrast, antibody administered 40 days after the immunization resulted in rapid and severe exacerbation of CIA7. This differential effect on the development of CIA could be explained if distinct γδ T cell subsets were involved.
Previous studies have demonstrated that the two main peripheral γδ T cell subsets12,13, Vγ1 and Vγ4, have different functional roles in various disease models (reviewed in14). In the CIA model, we found while both Vγ1+ and Vγ4+ cells increased, only the Vγ4+ cells were activated, as measured by surface marker expression. Depletion of Vγ4+ cells during CIA resulted in less severe disease indicating a pathogenic role for these cells. Because the proinflammatory cytokine, IL-17, has been shown to play an important pathogenic role in autoimmune diseases such as experimental allergic encephalomyelitis (EAE) and CIA (reviewed in15), we also examined whether γδ T cell subsets could produce IL-17. We found that the vast majority of the responding Vγ4+ cells produced IL-17 and co-expressed Vδ4. Sequence analysis revealed limited γ and δ junctional regions, indicating that these cells were antigen-selected.
Results
γδ T cell subsets respond differentially in CIA
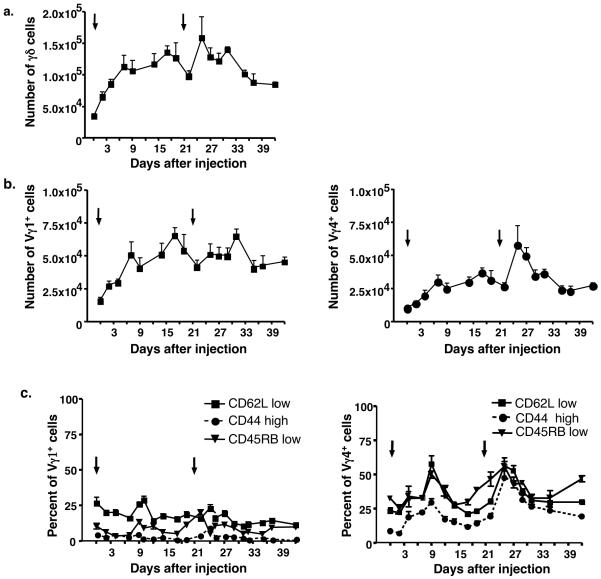
To further define the role of γδ T cells in CIA, we analyzed the two main lymphoid γδ T cell subsets in mice on various days after collagen/CFA injection. Nine days after the first injection, total γδ T cells were increased approximately three-fold when compared to untreated mice (day 0) (Fig. 1a). Within 3-4 days following the second immunization, total γδ T cells increased again (Fig. 1a). The responses of both the Vγ1+ and Vγ4+ γδ T cells mirrored that of total γδ T cells, and both increased in numbers to approximately the same degree after the first collagen/CFA injection. However, Vγ4+ cells increased rapidly after the second injection, while Vγ1+ cells increased more slowly and less vigorously (Fig. 1b).
Figure 1.
The total numbers of γδ T cells (a), Vγ1+ cells, and Vγ4+ cells (b) obtained from the lymph nodes of mice that had received collagen/CFA injections on days 0 and 21 (black arrows). On the indicated days following the initial injection, the draining lymph nodes (inguinal, brachial and popliteal) were removed and cells were stained for γδ T cell subsets. Using FACs analysis, the total number of γδ cells and individual subsets were calculated. Each time point represents the average + SEM for at least 8 different mice. (c) On designated days after collagen/CFA injections (black arrows), γδ T cells were isolated and stained for Vγ1 and Vγ4 expression and for levels of CD62L, CD44, or CD45RB. The mean percentage + SEM of cells having an “activated” phenotype (CD62L low, CD44 high, CD45RB low) is shown.
The loss of CD62L and CD45RB expression along with the gain of CD44 have been shown to correlate with αβ T cell activation/memory16. Therefore, we also stained the γδ T cell subsets for these markers at various time points after CII immunization. As shown in Figure 1c, the percentage of Vγ4+ cells that expressed high levels of CD44 increased (more than 10 fold) within the first 9 days of the disease course. A reciprocal loss of CD62L and CD45RB expression was also seen. These “activated” cells were transient and returned to near-baseline levels during the first three weeks of the disease process. Following the second immunization, the percent of “activated” Vγ4+ cells again increased. In contrast, Vγ1+ cells exhibited little change in expression of CD44, CD45RB and CD62L, even though Vγ1+ cell numbers increased during CIA (Fig. 1c). Therefore, the Vγ4+ subset appeared to be specifically responsive to the immunizations, whereas the Vγ1+ subset did not.
Vγ4+ γδ T cells are pathogenic
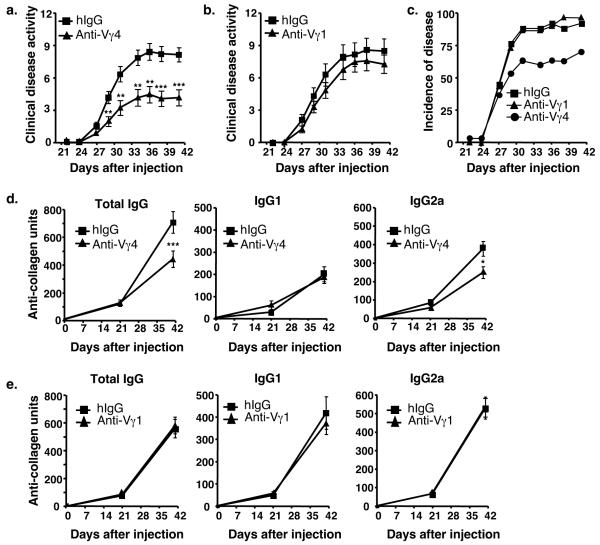
In order to determine the contribution of the Vγ1+ and Vγ4+ subsets to the development of CIA, mice were injected intravenously on day 17 with an anti-Vγ4 mAb or anti-Vγ1 mAb, to deplete the Vγ4+ or Vγ1+ subset, respectively, before the second injection of collagen/CFA. A control group of mice, injected with hamster IgG, was included in each experiment. Less than 1% of the relevant subset remained detectable in the blood after depletion (data not shown). As shown in Figure 2a, Vγ4-depleted mice showed significantly less clinical disease as compared to control mice. In contrast, clinical disease scores were not significantly changed in Vγ1-depleted mice (Fig. 2b). The overall incidence of disease was also lower in the Vγ4-depleted mice but not in the Vγ1-depleted animals (Fig. 2c). On day 41, the mice were sacrificed and the joints from the Vγ4-depleted, Vγ1-depleted, and hamster IgG treated mice were examined for changes in inflammation, pannus, cartilage damage and bone damage. The Vγ4-depleted mice showed a 42% decrease in total score for all histological parameters examined when compared with those obtained from hamster IgG treated mice (Table 1). In agreement with the overall disease scores, Vγ1-depleted mice showed no statistical difference in any of their histological scores when compared to control mice (Table 1).
Figure 2.
(a) Clinical disease activity in mice with collagen-induced arthritis. Mice were immunized with collagen/CFA on days 0 and 21. On day 17, mice were given either anti-Vγ4 antibody (black triangle, n=30) or hamster IgG (hIgG) (black square, n=26) intravenously. (b) In a separate experiment, mice were treated with anti-Vγ1 antibody (black triangle, n=30) or hamster IgG (black square, n=25). Clinical disease was assessed three times a week, starting on day 21. Values represent the mean ± SEM of 2 separate experiments (maximum score = 16). **p < 0.01, ***p<.001. Statistical significance was determined using the Mann-Whitney test. (c) Incidence of disease for each of the groups as described in (a & b). The incidence of disease for each hIgG treated group in 2 experiments was pooled and is shown as one group. (d) Anti-collagen antibody levels in mice with CIA that were depleted with an anti-Vγ4 antibody, as compared to hIgG-injected control animals. Mean ± SEM is shown for total IgG, IgG1, and IgG2a antibodies. Mice were treated on day 17 with hIgG (black square, n = 26) or a specific anti-Vγ subset antibody (black triangle, n = 30). *p < 0.05, ***p < 0.001. Data represent the mean ± SEM of 2 separate experiments. (e) Same as in (d) except mice were depleted with an anti-Vγ1 antibody and compared to hIgG. Again, mean ± SEM is shown for 2 separate experiments.
Table 1.
Histopathology scores in mice with collagen-induced arthritis treated with either an anti-Vγ4 antibody or an anti-Vγ1 antibody*
| Parameter | Hamster IgG (26 mice) | Anti-Vγ4 (30 mice) | P† |
|---|---|---|---|
| Inflammation | 2.59 ± 0.24 | 1.57 ± 0.27 | 0.007 |
| Pannus | 2.13 ± 0.25 | 1.20 ± 0.22 | 0.007 |
| Cartilage damage | 2.55 ± 0.24 | 1.47 ± 0.26 | 0.004 |
| Bone damage | 2.13 ± 0.25 | 1.20 ± 0.22 | 0.007 |
| Total score | 9.38 ± 0.97 | 5.43 ± 0.96 | 0.005 |
| Parameter | Hamster IgG (25 mice) | Anti-Vγ1 (30 mice) | P† |
|---|---|---|---|
| Inflammation | 2.43 ± 0.37 | 2.31 ± 0.26 | 0.783 |
| Pannus | 1.86 ± 0.29 | 1.65 ± 0.21 | 0.553 |
| Cartilage damage | 2.43 ± 0.38 | 2.22 ± 0.27 | 0.642 |
| Bone damage | 1.86 ± 0.29 | 1.65 ± 0.21 | 0.553 |
| Total score | 8.58 ± 1.33 | 7.83 ± 0.94 | 0.637 |
The inflammation, pannus, cartilage damage, and bone damage scores were determined for each joint examined (3-4 per animal). Changes were scored on a 0-5 scale. A mean score for each animal was determined for each parameter, and these were averaged to determine group means. Values shown are the group means ± SEM from 2 separate experiments. Statistical analysis of histopathologic parameters were done by comparing group means using the Student’s t test with significance set at 5%.
anti-Vγ4 or anti-Vγ1 versus hamster IgG treatment.
Next, total IgG, IgG1 and IgG2a anti-collagen antibody levels were measured in the sera from the treated and control mice to determine if γδ T cell subsets contributed to anti-collagen antibody production. No anti-collagen antibodies were detectable on day 0. On day 21, four days after the anti-Vγ4 or anti-Vγ1 treatment was given, the levels of total IgG, IgG1, and IgG2a anti-collagen antibodies were still equivalent to those seen in hamster IgG-treated control animals. However, by day 41 there was a significant decrease in the total IgG and pathogenic IgG2a levels of anti-collagen antibodies in the Vγ4-depleted mice (Fig. 2d). In contrast, mice depleted of Vγ1+ cells showed no change in antibody levels (Fig. 2e). The level of IgG1 anti-collagen antibodies did not differ from control groups in either Vγ4-depleted or Vγ1-depleted animals.
Vγ4+ γδ T cells produce IL-17 in the draining lymph nodes and joints
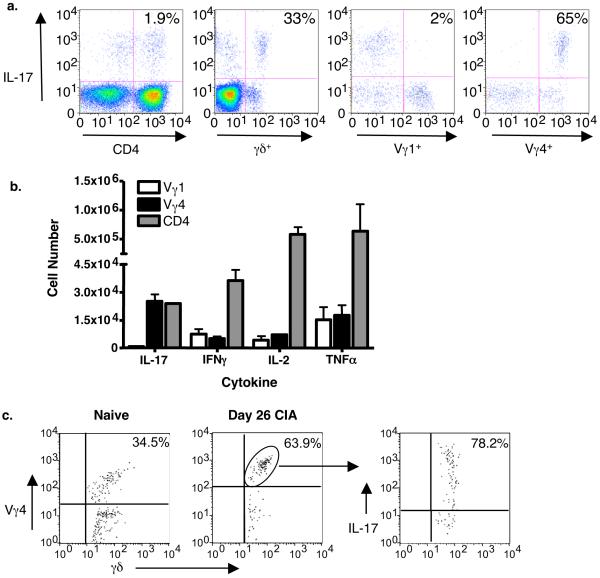
The results described above suggest that Vγ4+ cells are pathogenic in CIA. To determine how Vγ4+ γδ T cells mediate their effect, we assessed their cytokine potential. Draining lymph nodes were harvested on day 26, when the total number of Vγ4+ cells reaches its peak, and intracellular cytokine staining was used to detect IFNγ, IL-2, TNFα, and IL-17 production. In naïve mice, 6% of total γδ T cells, less than 1% of Vγ1+ cells, and 20% of Vγ4+ cells produced IL-17 (data not shown). However, in CIA mice, 33% of γδ T cells produced IL-17 (Fig. 3a). When the γδ T cell subsets were analyzed, only 2% of Vγ1+ cells as compared to 65% of Vγ4+ cells produced IL-17 (Fig. 3a). In fact, Vγ4+ cells represented over 90% of the total γδ T cells that produced IL-17 in CIA. The fraction (not shown) and number of Vγ1+ and Vγ4+ cells that produced TNFα, IL-2, and IFNγ were similar (Fig. 3b). Since IL-17 is an inflammatory cytokine produced by activated CD4+ αβ T cells (Th17 cells)17-20, we also compared the number of CD4+ cells and Vγ4+ cells that produced IL-17 in our model of CIA. Remarkably, despite its small size, the Vγ4+ population contained as many or more IL-17 producers than all CD4+ αβ T cells taken together, suggesting that Vγ4+ cells are a critical source of IL-17 (Fig. 3b). We also characterized the cytokine potential of γδ T cells from the joints of normal DBA/1 mice and CIA mice. We found a substantial percentage of TCR γδ+ cells among T cells in the joints of normal animals (15%) and even more, approximately 23%, in the joints of diseased paws. The percentage of Vγ4+ cells was also increased in the diseased joints, while the percentage of Vγ1+ cells was decreased (Supplementary Fig. 1 online). In addition, a large fraction of Vγ4+ cells taken from the joints produced IL-17 at day 26 of the disease process (Fig. 3c).
Figure 3.
Intracellular cytokine staining of T cells from the draining lymph nodes on day 26. (a) The percentages of CD4+, γδ+, Vγ1+, or Vγ4+ T cells that can produce IL-17 are shown, first gating on cells that stained with CD3. The percentage of Vγ1+ and Vγ4+ cells was then visualized by next gating on cells that stained with a pan-γδ reactive mAb. (b) The total number of Vγ1+, Vγ4+, or CD4+ cells stimulated to produce IL-17, IFNγ, IL-2, or TNFα as determined by intracellular cytokine staining. The total number was calculated based on the percentage that stained in (a). (c) The percentage of Vγ4+ cells in the joints of naïve mice versus CIA mice on day 26 is depicted on the left. The percentage of γδ+/Vγ4+cells that can produce IL-17 is shown on the right.
CIA-elicited Vγ4+ cells preferentially express Vδ4
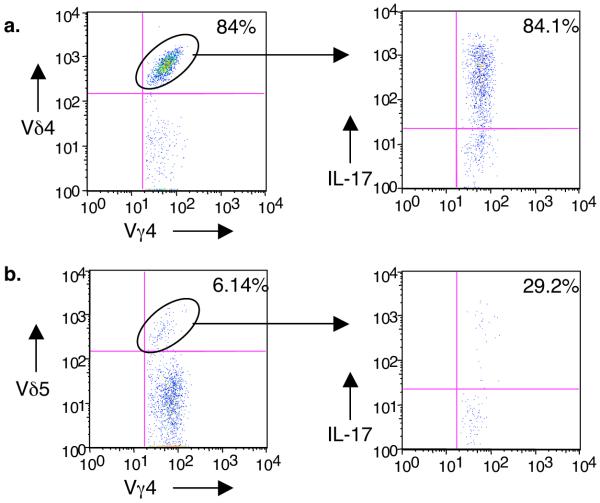
While the function of γδ T cells has been shown to primarily segregate with Vγ chain usage, a recent study by Shin et al. implied that some γδ T cells recognize their ligand primarily through the junctional region of the delta chain21. Therefore, we looked at the delta chains co-expressed by CIA-elicited Vγ4+ cells. Surprisingly, we found that 84% of the CIA-elicited Vγ4+ cells co-expressed Vδ4, and that these cells also represented the vast majority of the IL-17 producers (Fig. 4a). In naïve animals, the frequency of Vγ4+ cells co-expressing Vδ4+ was approximately 20% (data not shown). Of the few Vγ4/Vδ5+ cells in the lymph nodes of the CIA mice, a small percentage produced IL-17 (Fig. 4b). Very few Vγ4/Vδ6.3+ cells were detected (data not shown). Sequence analysis of day 26 lymph node cDNA revealed a strikingly limited junctional region in the Vγ4 chain, suggesting an antigen-driven clonal response (Fig. 5a). Specifically, 88% of the Vγ4+ sequences (37/42) encoded identical CDR3 regions which contained a leucine, encoded by N or P-nucleotides, in the V-J junctional region. Multiple codon triplets were found encoding this leucine, suggesting that this population did not result from a single clonal expansion. Instead, the oligoclonal response may have been driven by specific ligand recognition. The Vδ4 sequences were also limited in variability, with most showing both length conservation and exclusive use of a single Dδ2 reading frame (Fig. 5b). In addition, two conserved arginine codons were found in nearly all sequences, the first encoded by either the 3′ end of the Vδ4 gene or by N-additions, and the second encoded by the 3′ end of the Dδ2 gene. Small groups of identical Vδ4 clones were also evident. In contrast, Vγ4 and Vδ4 sequences from naive DBA/1 mice were highly variable (Supplementary Fig. 2 & 3 online). Importantly, no identical Vδ clones were found in the naïve animals.
Figure 4.
Vδ usage by Vγ4+ cells in CIA animals. Lymph nodes were analyzed by flow cytometry as for Fig. 3. Cells were triple stained for γδ TCR, Vγ4, and either Vδ4 (a), Vδ5 (b), or Vδ6.3 (data not shown) and the percentage of each V delta subset determined. Then, each Vδ-defined subset (circled population) was examined intracellularly for IL-17 production. The majority of the IL-17-producing Vγ4+ cells co-expressed Vδ4. Vγ4/Vδ6.3+ cells represented less than 0.5% of the Vγ4+ population, and did not produce IL-17 (not shown).
Figure 5.
Vγ4 and Vδ4 sequences from CIA-elicited γδ T cells. (a) In the CIA-elicited cells, 37/42 (88%) of the Vγ4+ clones encoded a leucine between the V and the J, and four of the six possible codons were used. In addition, when the codon “cta” was used to form leucine, we found different N/P nucleotides also flanking it. (b) The Vδ4 sequences from CIA-elicited cells revealed a striking length conservation (5-6 amino acids between V and J), a single Dδ2 reading frame, and the conservation of the two arginines, one at the end of the Vδ4 gene and one at the end of the Dδ2 gene. Both arginines were encoded by multiple codons as well.
Discussion
Our results imply that Vγ4+, but not Vγ1+ cells, are pathogenic in CIA. First, although Vγ1+ and Vγ4+ cells both increased during CIA after the second injection, the Vγ4+ cells increased more rapidly, and to a greater extent, than the Vγ1+ subset. As well, many of the Vγ4+ γδ T cells expressed markers of activation following the collagen/CFA injections, while the Vγ1+ cells appeared unresponsive. Second, depletion of the Vγ4+ cells before the second collagen/CFA injection led to a decrease in the severity and incidence of CIA, which was not seen following depletion of Vγ1+ cells. Finally, only the Vγ4+ subset produced IL-17, which is associated with inflammatory damage in CIA. Since surface markers of activation have not been studied extensively on γδ T cells, it is possible that characteristics of activation for Vγ1+ cells are different. Moreover, removing Vγ1+ cells before induction of CIA or at another time point of the disease process might uncover a role for this subset as well.
While the function of γδ T cells often segregates with their Vγ chain usage, recent studies have demonstrated an important role for the TCRδ chain in ligand recognition. Shin et al. found that a γδ T cell clone, G8, recognized its ligand, T22b, almost exclusively through the Dδ2 portion of the δ chain. Using a T22b-tetramer to identify T22b-specific γδ T cells, they found that a particular motif in the δ chain CDR3 was sufficient to confer T22b binding21. This motif was generated by the use of Dδ2 in only one of three possible reading frames [encoding (S)EGYE], flanked by two other conserved residues, a preceding tryptophan encoded by either the 3′ end of Vδ6.3 or by Dδ1, and a following leucine encoded by N or P-nucleotide additions. A variety of CDR3δ lengths were permitted among T22b-binding γδ TCRs, and the required motif could be generated almost entirely from germline-encoded components.
Our findings suggest that γδ T cells bearing particular TCRs are preferentially expanded by an antigen present during CIA. However, the requirements for binding this putative antigen appear to include elements of both the γ and δ chains, because activated cells expressing the Vγ4/Vδ4 combination predominated. We also found a recurrent motif in the CDR3 regions of the TCR-δ chain, including a single reading frame for Dδ2 among all CIA-elicited Vδ4s [(I)GGIR] (30/30 clones). Although the (I)GGIR reading frame is normally somewhat more common than the (S)EGYE reading frame, only 5/13 clones derived from naïve mice used the (I)GGIR reading frame (Supplementary Fig. 3). The Dδ2 was preceded by an arginine in 27/30 clones, encoded by either Vδ4 or N/P nucleotides, which may explain the preference for this V delta. Also, a second arginine, encoded by the 3′ end of Dδ2, was found in all 30 CIA-elicited Vδ4 clones, compared to only 4/13 naïve clones. Unlike the T22b-reactive δ-chains, the lengths of the CIA-elicited δ-chain CDR3s also seemed to be restricted, ranging between 5-6 amino acids between V and J in 23/30 clones.
The CDR3 of the CIA-elicited Vγ4s was also very limited. 37/42 clones contained only a single amino acid, leucine, between V and J, and four of the six possible leucine codons were found, consistent with the selective expansion of Vγ4/Vδ4+ cells bearing a particular motif in the γ-CDR3 as well. This contrasts markedly with the findings for T22b-binding γδ TCRs, in which the γ chain appeared to be uninvolved in ligand interaction21. Indeed, the γδ TCR restrictions associated with the CIA-selected γδ T cells are reminiscent of those common for αβ TCRs specific for a given ligand. Therefore, γδ T cells in the CIA model appear to be selected in a manner different from the T22b-binding cells, and more akin to the selection of αβ T cells. Thus, the question of whether γδ TCR ligand recognition differs fundamentally from that of αβ TCRs remains open.
Most of the molecules identified so far as ligands for γδ TCRs, including T22b, appear to be host-encoded molecules whose expression is induced by inflammation or stress (reviewed in23). However, the observed expansion of Vγ4/Vδ4+ cells in our model of CIA could be due to a response to the CFA, which is used in the immunizations. Therefore, we have examined mice immunized with PBS/CFA, which does not cause CIA in DBA/1 mice. Similar to CII-injected mice, the total number of γδ, Vγ1+ and Vγ4+ T cells increased after each PBS/CFA injection and moreover, the Vγ4+ subset showed the same “activated” phenotype (high CD44, low CD62L, and low CD45RB expression) as before (data not shown). However, the timing of the response was different. Despite similar initial responses, the maximal response measured by the percentage of “activated” Vγ4+ cells after the second PBS/CFA immunization was delayed, peaking at 6 days versus 4 days in CIA mice (data not shown), perhaps indicating that different stimuli are triggered in CIA than by PBS/CFA treatment alone. Hybridomas are now being produced to further investigate whether Vγ4/Vδ4+ cells respond to collagen, or another host-derived molecule that is induced by the immunization.
Opposing roles for Vγ1+ and Vγ4+ cells in various disease models have been previously noted. For example, Vγ4+ cells suppress allergic airway hyperresponsiveness (AHR)24 while Vγ1+ cells enhance AHR25. In addition, Vγ4+ cells promote myocarditis in a coxsackievirus B3 model, whereas Vγ1+ cells are protective26. This difference was attributed to skewing of the Th1/Th2 αβ T cell response by the γδ T cells. Interestingly, when using the BALB/c mouse in an effort to look at IL-4 producing CD4+ cells, infection with a strain of coxsackievirus B3 that promotes myocarditis resulted in an expansion of Vγ4/Vδ4+ cells27. Intracellular cytokine staining of these Vγ4/Vδ4+ cells revealed that a large proportion (50%) produced IFNγ (IL-17 was not measured). In our model of CIA, only 4% of the Vγ4/Vδ4+ cells produced IFNγ (data not shown). These results suggest Vγ4/Vδ4+ cells have the potential to produce Th1 and/or Th17 cytokines, and differences in the disease models may determine which type of cytokine is produced.
Two recent papers previously identified γδ T cells as a potent source of IL-1728,29. Stark et al. found that in B6 mice, both αβ and γδ T cells produced IL-17. More recently, Lockhart et al., studying Mycobacterium tuberculosis infection of the mouse lung, found that γδ T cells were in fact the dominant source of IL-17, rather than CD4+ αβ T cells29. Our results showed that in CIA, Vγ4/Vδ4+ cells predominated and the vast majority of these cells in both the draining lymph node and the joints of mice could be stimulated to produce IL-17. Moreover, there were as many IL-17+ Vγ4+ cells in the lymph node as CD4+ αβ TCR+IL-17+ cells. Based on studies in RA and EAE, IL-17 is now considered a major player in chronic autoimmune diseases. Studies in CIA have shown that disease is markedly suppressed in IL-17 “knocked-out” mice30, and neutralization of IL-17 after the onset of CIA reduces joint inflammation, cartilage destruction and bone erosion31. Depleting Vγ4+ cells in our model and thus, removing a large source of IL-17, may explain why these mice had less severe arthritis and a lower incidence of disease.
In this study, we demonstrate an antigen-driven oligoclonal response by the Vγ4/Vδ4+ γδ T cell subset. These cells are a potent source of IL-17 in the lymph nodes and joints of CIA mice and contribute to disease development. Therefore, it may be possible to reduce chronic inflammation in diseases such as CIA by preventing or eliminating the response of certain subsets of γδ T cells. Better understanding of the contribution of γδ T cells to the pathogenesis of autoimmune and allergic diseases may lead to therapies that target this small population of cells.
Methods
Animals
8-10 week old DBA/1 lac J male mice (Jackson Laboratories, Bar Harbor, ME) were used for this study.
Immunizations
Bovine type II collagen (CII) (Elastin Products, Owensville, MO) was diluted in 0.01M acetic acid to a final concentration of 4 mg/ml and stored at -70°C. Before injection, an equal volume of CII was emulsified with Freund’s incomplete adjuvant (Difco, Detroit, MI), to which 4 mg/ml inactivated Mycobacterium tuberculosis (H37Ra; Difco) had been added to generate Complete Freund’s Adjuvant (CFA). The emulsion was kept on ice during both preparation and use. The mice were injected intradermally at the base of the tail with 100μl of the emulsion, containing 200 μg of CII and 200 μg of M. tuberculosis, on day 0 and day 21. Mice were scored for severity of disease every other day starting on day 21 until they were sacrificed on day 41. The following scale was used: 0, no redness or swelling; 1, 1 digit swollen; 2, 2 digits swollen; 3, 3 digits swollen; and 4, entire paw swollen with ankylosis. The scores for each of 4 paws were added together to give a final score, such that the maximal severity score was 16.
Analysis of γδ T cells
Throughout this paper, we have used the simple numbering system of Heilig and Tonegawa for the murine γ and δ genes32. Official nomenclature equivalents are shown in parentheses22: Vγ1 (GV5S1), Vγ4 (GV3S1), Vδ4 (DV104S1), Vδ5 (DV105S1), and Vδ6.3 (ADV7S1). On various days after the first or second collagen/CFA immunization, mice were sacrificed, and the draining (inguinal, popliteal, and brachial) lymph nodes removed for flow cytometric analysis. A cell suspension from the lymph nodes was made using mesh screens, and T cells were enriched by passage over nylon wool33. Nylon wool non-adherent cells were stained for γδ T cells subsets using a FITC-labeled pan Cδ antibody (GL3,34), followed by biotinylated anti-Vγ1 (2.11,13) or anti-Vγ4 (UC3-10A6,35) antibodies plus strepavidin-APC, and PE-conjugated anti-CD62L, anti-CD45RB, or anti-CD44 antibodies (BD Biosciences, San Jose, CA). All samples were analyzed on a FACScalibur or FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ), and the data processed using FlowJo 6.4.1 software (Tree Star, Inc. Stanford, CA).
Treatment with depleting antibodies
Mice were injected with 200 μg of either an anti-Vγ4 antibody (UC3), an anti-Vγ1 antibody (clone 2.11), or with hamster IgG, as a control, on day 17, four days before the booster immunization with CII/CFA. On day 21, blood lymphocytes were tested to check for the depletion of the appropriate γδ T cell subset by flow cytometry. Briefly, heparinized blood samples were incubated in Gey’s solution for 10 minutes to lyse the red blood cells. The remaining cells were passed over nylon-wool columns in order to enrich for T cells. Nylon wool non-adherent cells were then stained with anti-CD3 (KT3,36), an anti-Cδ antibody (GL3) and either anti-Vγ1 (2.11) or anti-Vγ4 (UC3) antibodies to verify the depletion of the appropriate subset. All samples were analyzed on a FACScan or FACScalibur flow cytometer and the data processed using FlowJo 6.4.1 software.
Measurement of anti-collagen antibodies
Serum from each mouse was obtained by aspiration of retroorbital blood on days 0 and 21, at the time of the first and just before the second CII injection. On day 41, mice were tail bled before being sacrificed. Each serum sample was analyzed for the level of total IgG, IgG1, and IgG2a antibodies to type II collagen using modifications of published ELISA methods. Briefly, Immunlon II ELISA plates were coated with 5 μg/ml of CII (Chondrex, Seattle, WA) overnight at 4°C. Plates were washed three times with PBS containing 0.05% Tween and 1% bovine serum albumin (BSA), then blocked with PBS and 1% BSA at 4°C for 4 hours. The blocking solution was removed and 50 μl of a 1:9000 dilution of each serum sample was added in duplicate and the plates were incubated overnight at 4°C. The plates were washed with PBS containing 0.05% Tween, and 50 μl of horseradish peroxidase-conjugated goat-anti mouse IgG (diluted 1:3000 in PBS), IgG1 (diluted 1:2000), or IgG2a (diluted 1:2000) antibody (Invitrogen, Carlsbad, CA) was added to each well and incubated for 4 hours at 4°C. The plates were washed again with PBS containing 0.05% Tween before 50 μl of TMB substrate was added. The plates were developed for 5 minutes before the reaction was stopped with the addition of 25 μl of 2N H2SO4. Absorbance was measured at 450nm on a VERSAmax microplate reader and the data analyzed using Softmax Pro 4.7.1 software (Molecular Devices, Sunnyvale, CA). A standard pool of anti-collagen antibodies was obtained by combining sera from several mice with severe disease. The levels of IgG, IgG1, and IgG2a anti-collagen antibodies in this pool of sera were set as equivalent to 1000 units/ml.
Histology
On day 41, forepaws and hind paws (including the paw and ankle) were surgically removed and fixed immediately in 10% buffered formalin. Tissue was prepared and histological analyses were performed as previously described37. The treatment and clinical disease activity score of each sample was not disclosed to the trained observer who scored the slides. Sections were scored for mean inflammation, pannus formation, cartilage damage, and bone damage, and the overall score was based on a set of 3-4 joints per animal. All were scored on a 0-5 scale, as previously described37.
Isolation of cells from the joint
A modified version of a lung digestion protocol was used to obtain a cell suspension from the joints of mice38. Briefly, the skin was first removed from the mouse paws and then the paws were dissected into small pieces. The pieces were placed in an enzymatic digestion mixture containing 0.125% dispase II (Roche, Indianapolis, IN), 0.2% collagenase II (Sigma-Aldrich, St. Louis, MO), and 0.2% collagenase IV (Sigma-Aldrich) and shaken for 75 min at 37°C. After digestion, the supernatant was removed and the joint pieces were pushed through a cellector tissue sieve (Bellco Glass, Inc. Vineland, NJ) to disperse the cells. The cell suspension was then treated with Gey’s solution to remove red blood cells and passed over nylon wool columns to enrich for T cells.
Intracellular Cytokine staining
Nylon wool non-adherent cells were cultured at 1 × 106 cells/ml in culture medium39 containing 10 μg/ml Brefeldin A (Sigma-Aldrich), 50 ng/ml PMA (Sigma-Aldrich), and 1 μg/ml ionomycin (Sigma-Aldrich) at 37°C for 4 hours. After activation, cells were washed once in staining buffer and then stained with anti-CD3-APC-AF750 (EBioscience, San Diego, CA), FITC-labeled anti-TCRδ (GL3), biotinylated or FITC-labeled anti-Vγ1 (2.11) or anti-Vγ4 (UC3-10A6), and biotinylated anti-Vδ4 (GL2,34), anti-Vδ5 (F45.152,40), or anti-Vδ6.3 (17C,41) antibodies and detected with strepavidin-APC (BD Biosciences). The cells were then fixed in 1% paraformaldehyde for at least 20 minutes at 4°C. Fixed cells were permeabilized for 10 minutes at 4°C in 5% saponin/PBS buffer. Cells were then spun down and stained with PE-conjugated anti-cytokine antibodies (IL-2, IL-17, IFNγ, and TNFα; BD Biosciences) or an isotype control for 30 minutes at 4°C. Cells were washed once in saponin buffer and once in staining buffer before fixation in 1% paraformaldehyde. Samples were analyzed on a FACScan (Becton Dickinson) and the data processed using FlowJo v6.4.1 software.
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 4 (GraphPad Software, San Diego, CA). Statistical significance for the clinical disease activity was determined using the Mann-Whitney test. The histological data were analyzed by comparing group means using the Student’s t-test with significance set at 5%. For the anti-collagen antibodies, statistical significance was determined using an unpaired 2-tailed Student’s t-test to compare the two treatment groups.
Sequencing of TCRs
Total cell RNA was isolated from nylon wool non-adherent cells obtained from the lymph nodes of mice using the PicoPure RNA Isolation Kit (Arcturus Bioscience, Mountain View, CA). Reverse transcription-PCR was performed using primers specific for Vγ4/Cγ1, 2 and Vδ4/Cδ as previously described39. PCR-amplified transcripts were then cloned into a TA vector (Invitrogen, Carlsbad, CA) and individual clones sequenced to determine the frequency of specific TCR sequences.
Supplementary Material
Acknowledgements
We thank N. Banda (UCHSC) for technical assistance with establishing the disease model and R. Kedl (NJMRC) for taking the time to read this manuscript and for his constructive comments. This study was supported by an Investigator Award from the Arthritis Foundation (to C. L. R.), an award from the American Heart Association (to J. D. F.), and by NIH grants 2R01 A1044920 (to R. L. O.) and 2R01 HL65410 (to W. K. B.)
Footnotes
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org
Bibliography
- 1.Luross JA, Williams NA. The genetic and immunopathological processes underlying collagen-induced arthritis. Immunology. 2001;103:407–16. doi: 10.1046/j.1365-2567.2001.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–13. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 3.Wooley PH, Whalen JD, Chapdelaine JM. Collagen-induced arthritis in mice. VI. Synovial cells from collagen arthritic mice activate autologous lymphocytes in vitro. Cell Immunol. 1989;124:227–38. doi: 10.1016/0008-8749(89)90127-5. [DOI] [PubMed] [Google Scholar]
- 4.Andersson EC, et al. Definition of MHC and T cell receptor contacts in the HLA-DR4 restricted immunodominant epitope in type II collagen and characterization of collagen-induced arthritis in HLA-DR4 and human CD4 transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7574–9. doi: 10.1073/pnas.95.13.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson WC, Townes AS. Genetic susceptibility to murine collagen II autoimmune arthritis. Proposed relationship to the IgG2 autoantibody subclass response, complement C5, major histocompatibility complex (MHC) and non-MHC loci. J. Exp. Med. 1985;162:1878–91. doi: 10.1084/jem.162.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corthay A, Johansson A, Vestberg M, Holmdahl R. Collagen-induced arthritis development requires alpha beta T cells but not gamma delta T cells: studies with T cell-deficient (TCR mutant) mice. Int. Immunol. 1999;11:1065–73. doi: 10.1093/intimm/11.7.1065. [DOI] [PubMed] [Google Scholar]
- 7.Peterman GM, Spencer C, Sperling AI, Bluestone JA. Role of γδ T cells in murine collagen-induced arthritis. J. Immunol. 1993;151:6546–6558. [PubMed] [Google Scholar]
- 8.Arai K, et al. Extrathymic differentiation of resident T cells in the joints of mice with collagen-induced arthritis. J. Immunol. 1996;157:5170–7. [PubMed] [Google Scholar]
- 9.Holoshitz J, Koning F, Coligan JE, De Bruyn J, Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989;339:226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- 10.Kjeldsen-Kragh J, et al. T γδ cells in juvenile rheumatoid arthritis and rheumatoid arthritis. Scand. J. Immunol. 1990;32:651–660. doi: 10.1111/j.1365-3083.1990.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 11.Brennan FM, et al. T cells expressing γδ chain receptors in rheumatoid arthritis. J. Autoimmun. 1988;1:319–326. doi: 10.1016/0896-8411(88)90002-9. [DOI] [PubMed] [Google Scholar]
- 12.Sperling AI, Cron RQ, Decker DC, Stern DA, Bluestone JA. Peripheral T cell receptor γδ variable gene repertoire maps to the T cell receptor loci and is influenced by positive selection. J. Immunol. 1992;149:3200–3207. [PubMed] [Google Scholar]
- 13.Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of Vγ1-expressing γ/δ T lymphocytes in normal mice. J. Exp. Med. 1995;182:1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien RL, Lahn M, Born WK, Huber SA. T cell receptor and function cosegregate in gamma-delta T cell subsets. Chem. Immunol. 2001;79:1–28. doi: 10.1159/000058829. [DOI] [PubMed] [Google Scholar]
- 15.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 16.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao Z, et al. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 1995;155:5483–6. [PubMed] [Google Scholar]
- 18.Fossiez F, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Infante Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 2000;165:6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 20.Spriggs MK. Interleukin-17 and its receptor. J. Clin. Immunol. 1997;17:366–9. doi: 10.1023/a:1027360106635. [DOI] [PubMed] [Google Scholar]
- 21.Shin S, et al. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308:252–5. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 22.Arden B, Clark SP, Kabelitz D, Mak TW. Mouse T-cell receptor variable gene segment families. Immunogenetics. 1995;42:501–530. doi: 10.1007/BF00172177. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien R,L, et al. gammadelta T-cell receptors: functional correlations. Immunol. Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 24.Hahn YS, et al. V gamma 4+ gamma delta T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J. Immunol. 2003;171:3170–8. doi: 10.4049/jimmunol.171.6.3170. [DOI] [PubMed] [Google Scholar]
- 25.Hahn YS, et al. Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J. Immunol. 2004;172:2894–902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 26.Huber SA, Graveline D, Newell MK, Born WK, O’Brien RL. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J. Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 27.Huber SA, Graveline D, Born WK, O’Brien RL. Cytokine production by Vgamma(+)-T-cell subsets is an important factor determining CD4(+)-Th-cell phenotype and susceptibility of BALB/c mice to coxsackievirus B3-induced myocarditis. J. Virol. 2001;75:5860–9. doi: 10.1128/JVI.75.13.5860-5869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stark MA, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–94. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662–9. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 30.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003;171:6173–7. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 31.Lubberts E, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–9. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 32.Heilig JS, Tonegawa S. T-cell γ gene is allelically but not isotypically excluded and is not required in known functional T-cell subsets. Proc. Natl. Acad. Sci. U. S. A. 1987;84:8070–8074. doi: 10.1073/pnas.84.22.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur. J. Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 34.Goodman T, LeCorre R, Lefrancois L. A T-cell receptor γδ-specific monoclonal antibody detects a Vγ5 region polymorphism. Immunogenetics. 1992;35:65–68. doi: 10.1007/BF00216631. [DOI] [PubMed] [Google Scholar]
- 35.Dent AL, et al. Self-reactive γδ T cells are eliminated in the thymus. Nature. 1990;343:714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- 36.Tomonari K. A rat antibody against a structure functionally related to the mouse T cell receptor/T3 complex. Immunogenetics. 1988;28:455–458. doi: 10.1007/BF00355379. [DOI] [PubMed] [Google Scholar]
- 37.Bendele AM, et al. Combination benefit of treatment with the cytokine inhibitors interleukin-1 receptor antagonist and PEGylated soluble tumor necrosis factor receptor type I in animal models of rheumatoid arthritis. Arthritis Rheum. 2000;43:2648–59. doi: 10.1002/1529-0131(200012)43:12<2648::AID-ANR4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 38.Lahn M, et al. MHC class I-dependent Vgamma4+ pulmonary T cells regulate alpha beta T cell-independent airway responsiveness. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8850–5. doi: 10.1073/pnas.132519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien RL, et al. Heat shock protein Hsp-60 reactive γδ cells: A large, diversified T lymphocyte subset with highly focused specificity. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereira P, et al. Developmentally regulated and lineage-specific rearrangement of T cell receptor Valpha/delta gene segments. Eur. J. Immunol. 2000;30:1988–97. doi: 10.1002/1521-4141(200007)30:7<1988::AID-IMMU1988>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 41.Belles C, et al. Bias in the γδ T cell response to Listeria monocytogenes. Vδ6.3+ cells are a major component of the γδ T cell response to Listeria monocytogenes. J. Immunol. 1996;156:4280–4289. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.