Abstract
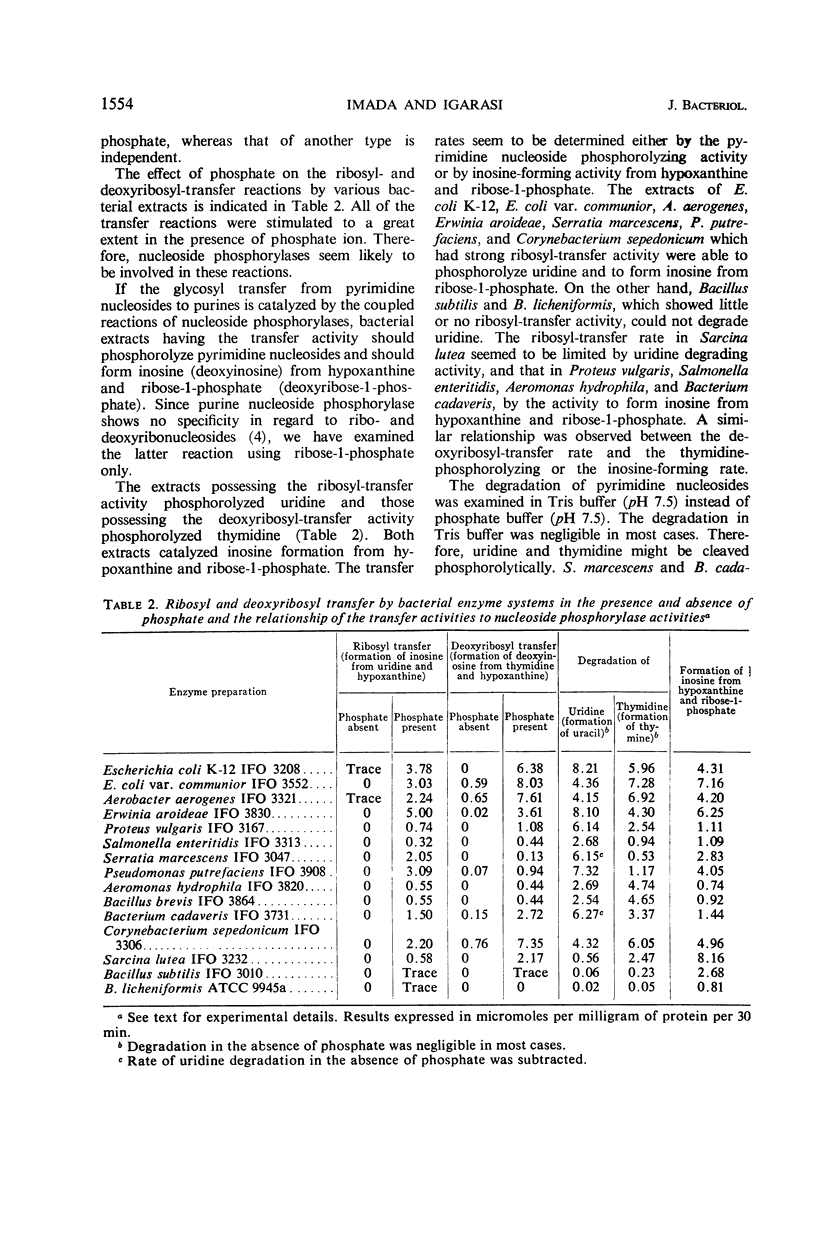
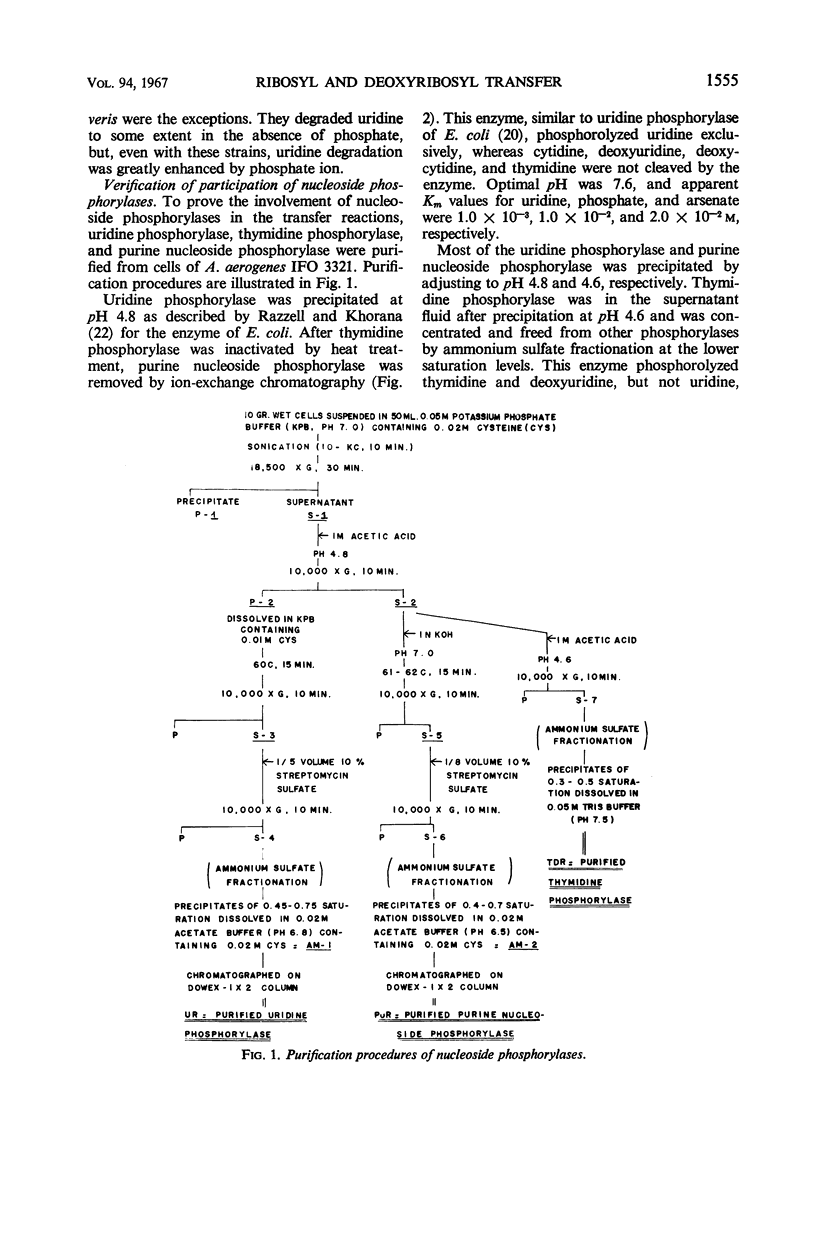
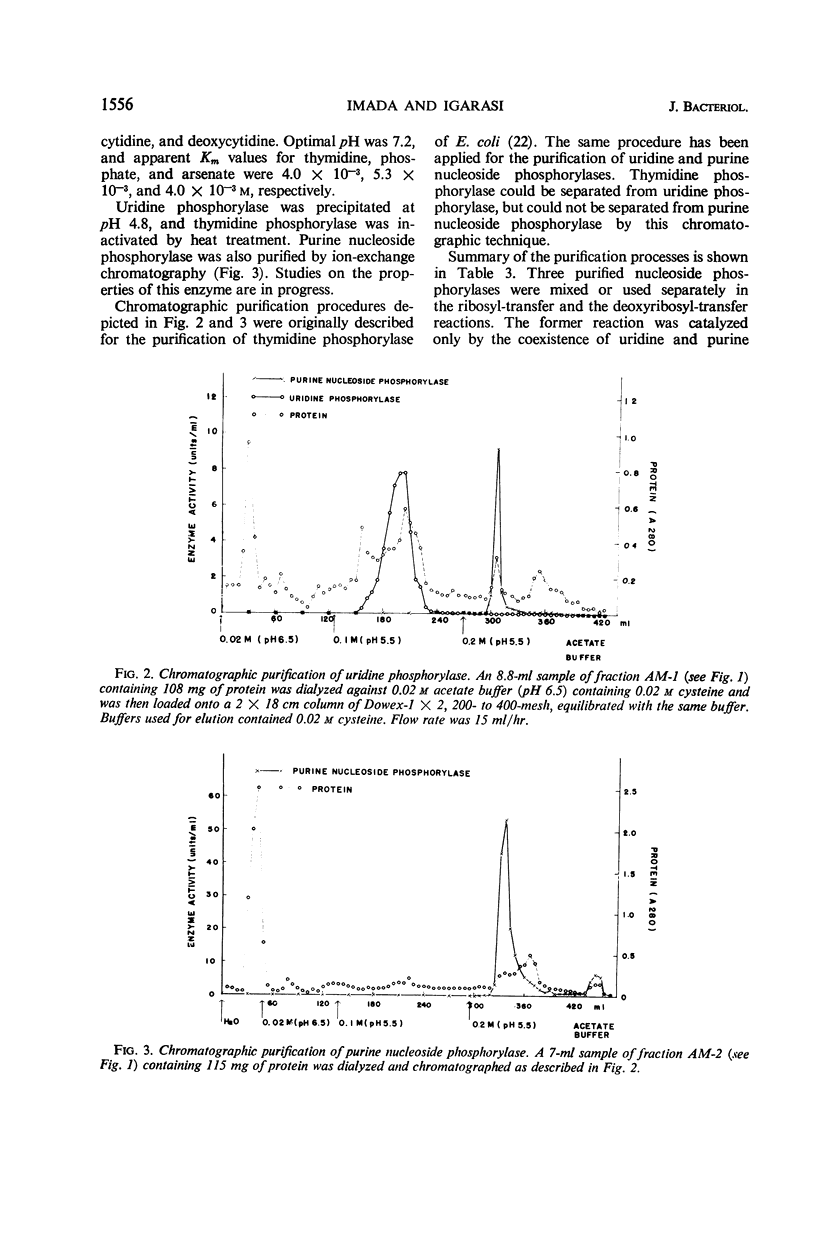
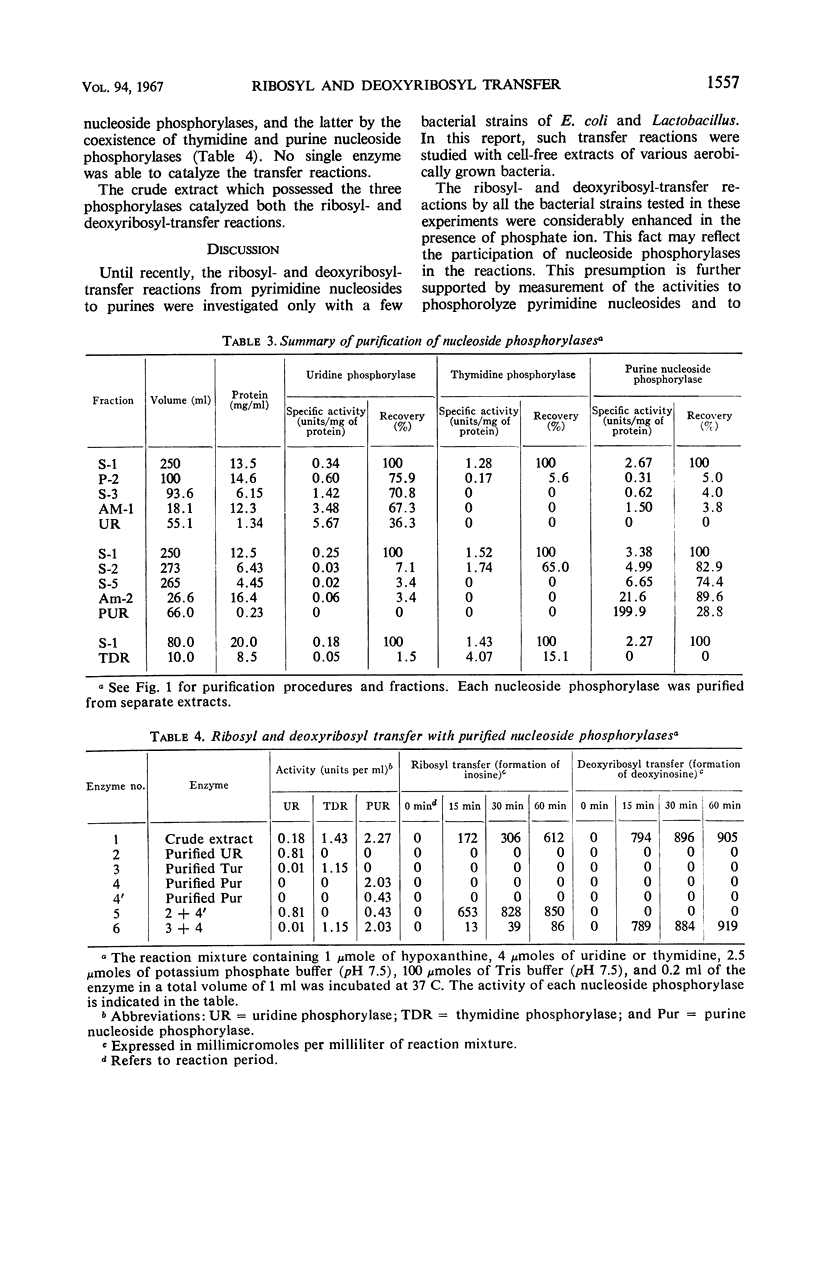
The enzymatic transfer of ribose and deoxyribose residues in pyrimidine nucleosides to purines was catalyzed by cell-free extracts of various bacteria. Almost all the strains belonging to Enterobacteriaceae were capable of catalyzing the transfer reactions. The transfer activities were also detected among some bacterial strains of other families: Pseudomonadaceae, Corynebacteriaceae, Micrococcaceae, Bacteriaceae, and Bacillaceae. The rates of the transfer reactions were greatly enhanced in the presence of phosphate ion, and the participation of nucleoside phosphorylases in the reactions was suggested. Uridine phosphorylase, thymidine phosphorylase, and purine nucleoside phosphorylase were purified from cell-free extract of Aerobacter aerogenes IFO 3321. The ribosyl transfer from uridine to hypoxanthine was found to be catalyzed by the coupled reactions of uridine and purine nucleoside phosphorylases and the deoxyribosyl transfer from thymidine to hypoxanthine by the coupled reactions of thymidine and purine nucleoside phosphorylases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams R., Edmonds M., Libenson L. Deoxyribosyl exchange activity associated with nucleoside phosphorylase. Biochem Biophys Res Commun. 1965 Jul 26;20(3):310–314. doi: 10.1016/0006-291x(65)90365-7. [DOI] [PubMed] [Google Scholar]
- BECK W. S., LEVIN M. Effect of pool size of acid-soluble deoxyribosyl compounds upon trans-N-deoxyribosidase activity in Lactobacillus leichmannii. Biochim Biophys Acta. 1962 Jan 22;55:245–247. doi: 10.1016/0006-3002(62)90962-9. [DOI] [PubMed] [Google Scholar]
- BECK W. S., LEVIN M. Purification, kinetics, and repression control of bacterial trans-N-deoxyribosylase. J Biol Chem. 1963 Feb;238:702–709. [PubMed] [Google Scholar]
- KALCKAR H. M., MACNUTT W. S., HOFF-JØRGENSEN E. Trans-N-glycosidase studied with radioactive adenine. Biochem J. 1952 Jan;50(3):397–400. doi: 10.1042/bj0500397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH A. L. Some enzymes of nucleoside metabolism of Escherichia coli. J Biol Chem. 1956 Nov;223(1):535–549. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACNUTT W. S. The enzymically catalysed transfer of the deoxyribosyl group from one purine or pyrimidine to another. Biochem J. 1952 Jan;50(3):384–397. doi: 10.1042/bj0500384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSON L. A., LAMPEN J. O. The metabolism of desoxyribose nucleosides in Escherichia coli. J Biol Chem. 1951 Dec;193(2):539–547. [PubMed] [Google Scholar]
- MARSH J. C., KING M. E. Purification of trans-N-glycosidase of Thermobacter acidophilus: inhibition of enzyme by 6-azathymidine. Biochem Pharmacol. 1959 Aug;2:146–153. doi: 10.1016/0006-2952(59)90081-4. [DOI] [PubMed] [Google Scholar]
- OTT J. L., WERKMAN C. H. Coupled nucleoside phosphorylase reactions in Escherichia coli. Arch Biochem Biophys. 1957 Jul;69:264–276. doi: 10.1016/0003-9861(57)90491-5. [DOI] [PubMed] [Google Scholar]
- OTT J. L., WERKMAN C. H. Enzymic transfer of the ribosyl group from inosine to adenine. Biochem J. 1957 Apr;65(4):609–611. doi: 10.1042/bj0650609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTT J. L., WERKMAN C. H. Formation of adenosine by cell-free extracts of Escherichia coli. Arch Biochem Biophys. 1954 Feb;48(2):483–484. doi: 10.1016/0003-9861(54)90366-5. [DOI] [PubMed] [Google Scholar]
- PAEGE L. M., SCHLENK F. Bacterial uracil riboside phosphorylase. Arch Biochem Biophys. 1952 Sep;40(1):42–49. doi: 10.1016/0003-9861(52)90071-4. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E., KHORANA H. G. Purification and properties of a pyrimidine deoxyriboside phosphorylase from Escherichia coli. Biochim Biophys Acta. 1958 Jun;28(3):562–566. doi: 10.1016/0006-3002(58)90519-5. [DOI] [PubMed] [Google Scholar]
- ROUSH A. H., BETZ R. F. Purification and properties of trans-N-deoxyribosylase. J Biol Chem. 1958 Aug;233(2):261–266. [PubMed] [Google Scholar]
- ZIMMERMAN M. DEOXYRIBOSYL TRANSFER. II. NUCLEOSIDE:PYRIMIDINE DEOXYRIBOSYLTRANSFERASE ACTIVITY OF THREE PARTIALLY PURIFIED THYMIDINE PHOSPHORYLASES. J Biol Chem. 1964 Aug;239:2622–2627. [PubMed] [Google Scholar]
- ZIMMERMAN M., SEIDENBERG J. DEOXYRIBOSYL TRANSFER. I. THYMIDINE PHOSPHORYLASE AND NUCLEOSIDE DEOXYRIBOSYLTRANSFERASE IN NORMAL AND MALIGNANT TISSUES. J Biol Chem. 1964 Aug;239:2618–2621. [PubMed] [Google Scholar]


