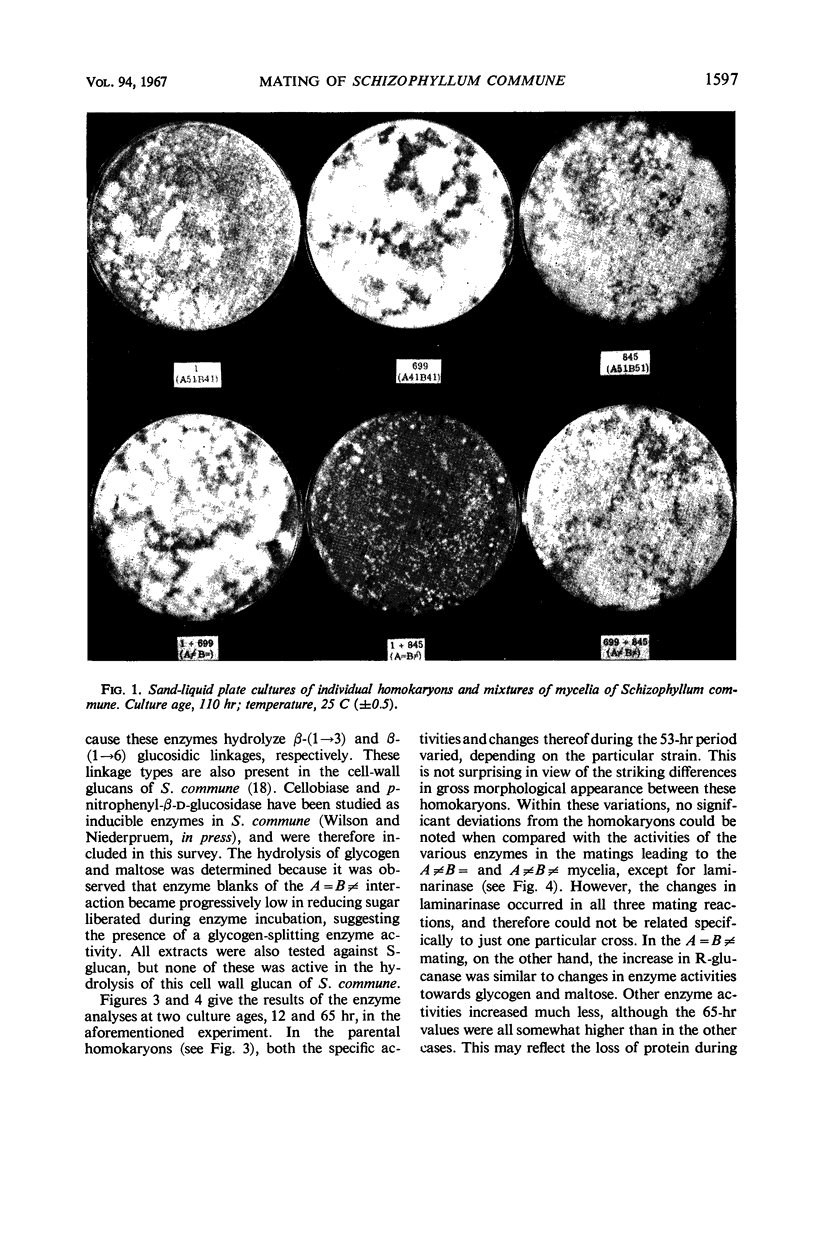
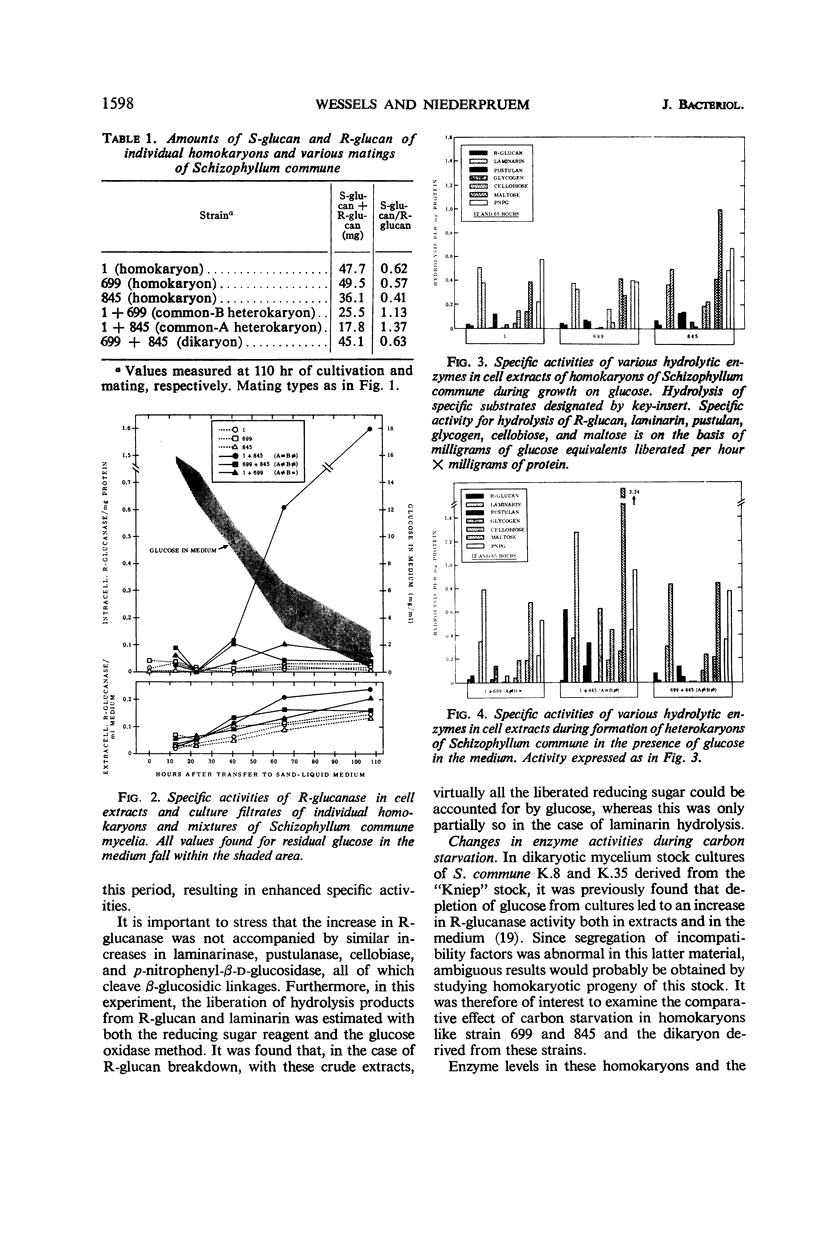
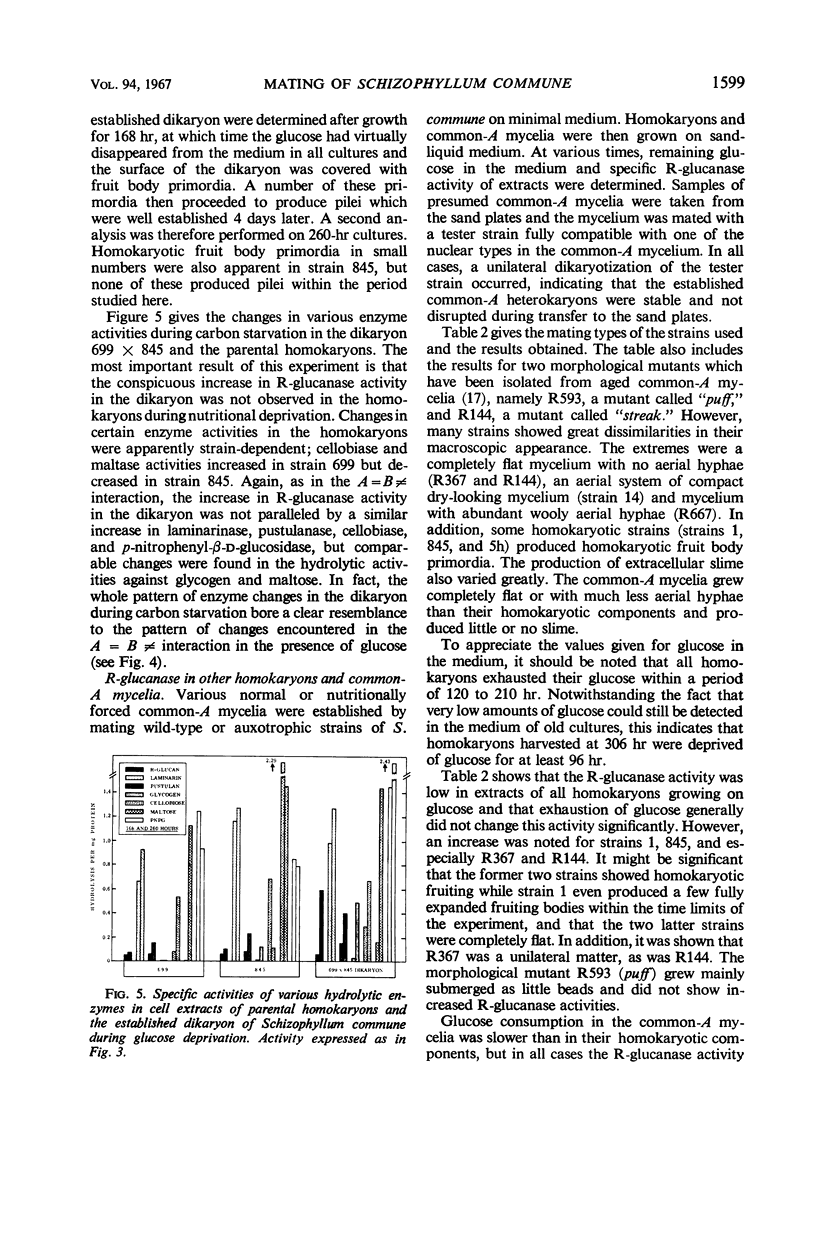
Abstract
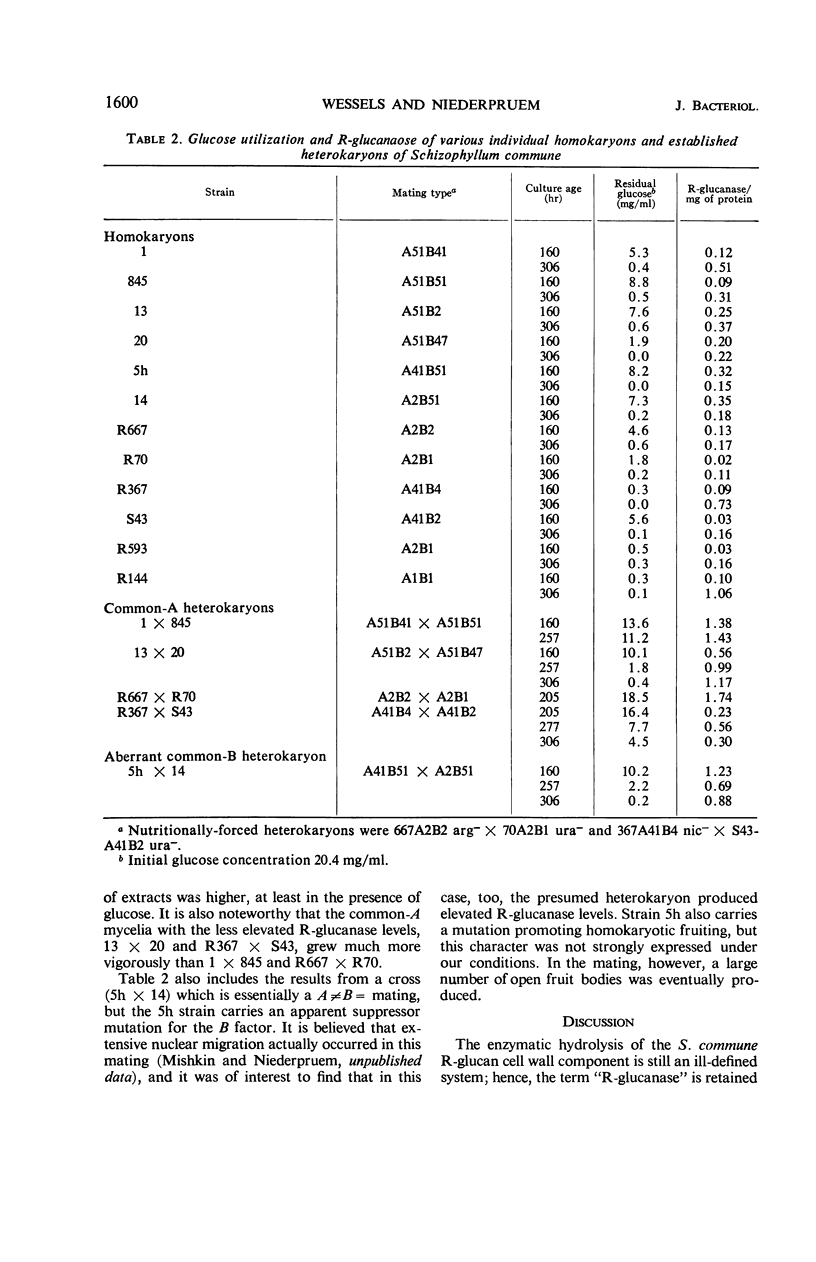
Mycelial enzyme extracts of Schizophyllum commune were prepared during vegetative growth matings leading to common-A and common-B heterokaryons and the dikaryon, and were examined for hydrolytic activity against an alkaliinsoluble cell-wall glucan (R-glucan) isolated from this mushroom. In extracts from several individual homokaryotic mycelia the R-glucanase activity was low and did not increase when the cultures exhausted glucose in the medium. In common-A matings, a 30-fold increase in specific activity of intracellular R-glucanase was found even in the presence of glucose in the broth. An increase of this magnitude was not observed in the common-B mating nor in the fully compatible cross leading to the dikaryon. Extracts of the dikaryon did show elevated R-glucanase activity after exogenous glucose disappearance and subsequent fruiting. In none of these situations was an enzyme activity detected towards an alkali-soluble cell-wall glucan (S-glucan) prepared from S. commune. Changes in R-glucanase were not parallelled by identical changes in laminarinase, pustulanase, cellobiase, and p-nitrophenyl-β-d-glucosidase, but comparable increases in specific activities were found for hydrolysis of glycogen and maltose. After interaction of the various mycelia in mating combinations, the S-glucan/R-glucan ratio of the cell wall of the dikaryon was found to be similar to that of the homokaryons, but increased in the common-B interaction and was elevated almost threefold in the common-A heterokaryon.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D. Biochemical and cellular changes occuring during conjugation in Hansenula wingei. J Bacteriol. 1965 Oct;90(4):1019–1025. doi: 10.1128/jb.90.4.1019-1025.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull A. T., Chesters C. G. The biochemistry of laminarin and the nature of laminarinase. Adv Enzymol Relat Areas Mol Biol. 1966;28:325–364. doi: 10.1002/9780470122730.ch5. [DOI] [PubMed] [Google Scholar]
- Dygert S., Li L. H., Florida D., Thoma J. A. Determination of reducing sugar with improved precision. Anal Biochem. 1965 Dec;13(3):367–374. doi: 10.1016/0003-2697(65)90327-1. [DOI] [PubMed] [Google Scholar]
- Koltin Y., Raper J. R. Schizophyllum commune: new mutations in the B incompatibility factor. Science. 1966 Oct 28;154(3748):510–511. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PARAG Y. Mutations in the B incompatibility factor of Schizophyllum commune. Proc Natl Acad Sci U S A. 1962 May 15;48:743–750. doi: 10.1073/pnas.48.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPER J. R., ESSER K. Antigenic differences due to the incompatibility factors in Schizophyllum commune. Z Vererbungsl. 1961;92:439–444. doi: 10.1007/BF00890065. [DOI] [PubMed] [Google Scholar]
- Raper C. A., Raper J. R. Mutations modifying sexual morphogenesis in schizophyllum. Genetics. 1966 Nov;54(5):1151–1168. doi: 10.1093/genetics/54.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J. R., Boyd D. H., Raper C. A. Primary and secondary mutations at the incompatibility loci in Schizophyllum. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1324–1332. doi: 10.1073/pnas.53.6.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels J. G. Control of cell-wall glucan degradation during development in Schizophyllum commune. Antonie Van Leeuwenhoek. 1966;32(4):341–355. doi: 10.1007/BF02097484. [DOI] [PubMed] [Google Scholar]