Abstract
In superior cervical ganglion (SCG) neurons, stimulation of M1 receptors (M1Rs) produces a distinct pattern of modulation of N-type calcium (N-) channel activity, enhancing currents elicited with negative test potentials and inhibiting currents elicited with positive test potentials. Exogenously applied arachidonic acid (AA) reproduces this profile of modulation, suggesting AA functions as a downstream messenger of M1Rs. In addition, techniques that diminish AA's concentration during M1R stimulation minimize N-current modulation. However, other studies suggest depletion of phosphatidylinositol-4,5-bisphosphate during M1R stimulation suffices to elicit modulation. In this study, we used an expression system to examine the physiological mechanisms regulating modulation. We found the β subunit (CaVβ) acts as a molecular switch regulating whether modulation results in enhancement or inhibition. In human embryonic kidney 293 cells, stimulation of M1Rs or neurokinin-1 receptors (NK-1Rs) inhibited activity of N channels formed by CaV2.2 and coexpressed with CaVβ1b, CaVβ3, or CaVβ4 but enhanced activity of N channels containing CaVβ2a. Exogenously applied AA produced the same pattern of modulation. Coexpression of CaVβ2a, CaVβ3, and CaVβ4 recapitulated the modulatory response previously seen in SCG neurons, implying heterogeneous association of CaVβ with CaV2.2. Further experiments with mutated, chimeric CaVβ subunits and free palmitic acid revealed that palmitoylation of CaVβ2a is essential for loss of inhibition. The data presented here fit a model in which CaVβ2a blocks inhibition, thus unmasking enhancement. Our discovery that the presence or absence of palmitoylated CaVβ2a toggles M1R- or NK-1R–mediated modulation of N current between enhancement and inhibition identifies a novel role for palmitoylation. Moreover, these findings predict that at synapses, modulation of N-channel activity by M1Rs or NK-1Rs will fluctuate between enhancement and inhibition based on the presence of palmitoylated CaVβ2a.
INTRODUCTION
All neural function results from a series of electrical and chemical signals. The two realms of signaling are often bridged within neurons by voltage-gated Ca2+ channels, such as N channels (West et al., 2001). With depolarization of postsynaptic sites, N-channel activity (N current) triggers biochemical changes, including modulation of certain ion channels (Wisgirda and Dryer, 1994), enzyme activation (Rittenhouse and Zigmond, 1999), and gene transcription (Brosenitsch and Katz, 2001; West et al., 2001; Zhao et al., 2007). In turn, Gq protein-coupled receptors (PCRs) converge on several signal transduction cascades to modulate the N channel's response to changing membrane potential. Some of these pathways are well described, so both signaling molecules and sites of modulation on N channels are known (Suh and Hille, 2005).
A notable exception is the M1 receptor (M1R), a GqPCR that modulates postsynaptic N current by an incompletely described signaling cascade referred to as the slow pathway (Beech et al., 1992; Mathie et al., 1992). Other transmitters such as substance P (SP), the natural ligand for the neurokinin-1 receptor (NK-1R), also modulate N current by a slow pathway (Shapiro and Hille, 1993; Kammermeier et al., 2000) that couples to Gq (Macdonald et al., 1996), suggesting that multiple GqPCRs converge on this pathway (Suh and Hille, 2005). Recent studies propose that a reduction in phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) levels during GqPCR stimulation suffices for inhibition (Wu et al., 2002; Gamper et al., 2004; Michailidis et al., 2007). In this model, channels are available to open when PtdIns(4,5)P2 associates with them. During muscarinic stimulation, PtdIns(4,5)P2 is depleted from the membrane, creating a concentration gradient that favors PtdIns(4,5)P2 dissociating and diffusing away from channels. This model appeals to many in part because PtdIns(4,5)P2 dissociation and depletion have been established as the mechanism that inhibits M current, a voltage-gated K+ current (Suh and Hille, 2002, 2007; Ford et al., 2003; Suh et al., 2006).
However, our previous studies with superior cervical ganglion (SCG) neurons indicate that modulation of native N current by M1Rs requires events downstream of PtdIns(4,5)P2 hydrolysis (Liu and Rittenhouse, 2003a; Liu et al., 2004, 2008). We found that the muscarinic agonist oxotremorine-M (Oxo-M) and arachidonic acid (AA) elicit the same distinct pattern of modulation (Liu et al., 2001; Liu and Rittenhouse, 2003a), not only inhibiting N current at positive test potentials but also enhancing N current at negative test potentials (Fig. 1 A). Enhancement and inhibition by AA occur at distinct sites and exhibit different biophysical characteristics (Barrett et al., 2001; Liu et al., 2001). Moreover, M1R stimulation releases AA in central and SCG neurons (Tencé et al., 1994; Liu et al., 2006). In contrast, only minimal fatty acid release occurs after muscarinic stimulation in SCG neurons lacking group IVa PLA2(Liu et al., 2006). Consistent with the need for PtdIns(4,5)P2metabolites, inhibiting AA release from phospholipids by antagonizing PLA2 activity or including BSA, a scavenger of AA (Spector, 1975), in the bath solution minimizes N-current modulation by Oxo-M (Liu and Rittenhouse, 2003a; Liu et al., 2003, 2004), suggesting that AA may be a second messenger of the slow pathway.
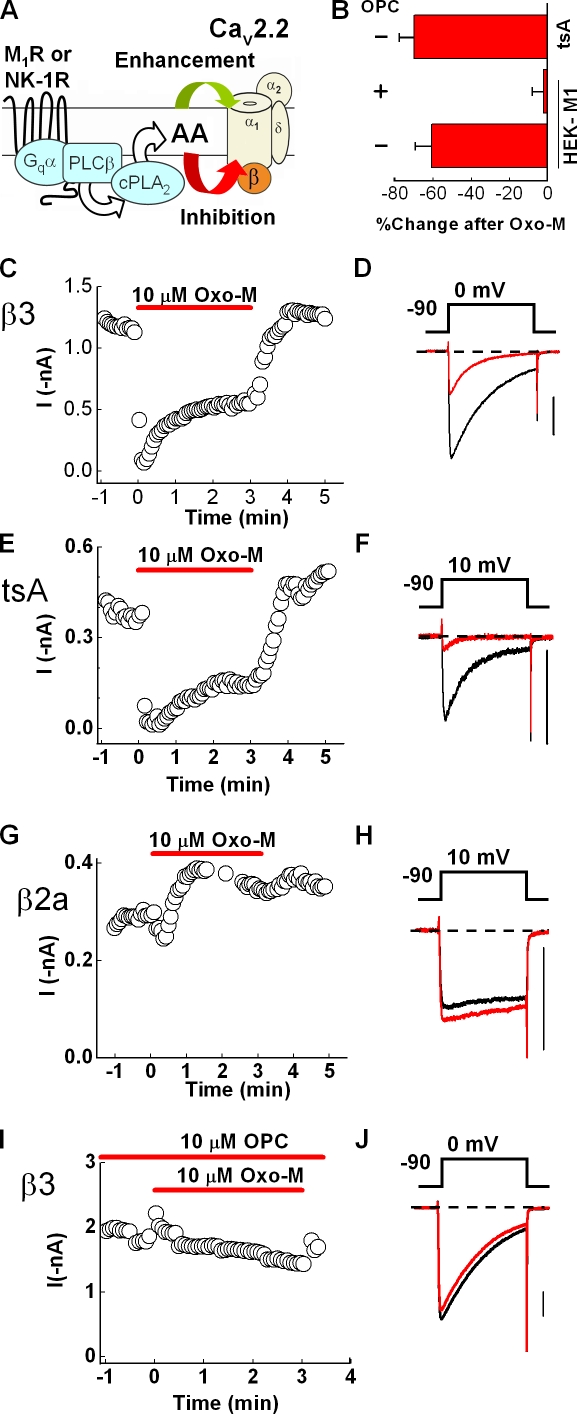
Figure 1.
M1R-induced inhibition of recombinant N current is blocked by a PLA2 antagonist. HEK-M1 cells were transiently transfected with N-channel subunits CaV2.2, α2δ -1, and CaVβ3. (A) Working model of the slow pathway in which AA release, catalyzed by PLA2, is a necessary component to elicit N-current modulation. AA enhances current (green arrow) by acting at a distinct site that may be extracellular or in the outer leaflet of the membrane (Barrett et al., 2001). AA inhibits N current by acting at an intracellular site or a site within the inner leaflet (Barrett et al., 2001; Liu et al., 2001). (B) Summary of the percent change in mean current amplitude in tsA 201 cells after 1-min exposure to 10 µM Oxo-M or in HEK-M1 cells in the presence or absence of 10 µM OPC, a PLA2 antagonist. Error bars represent SEM. (C) Time course of peak inward current using 5 mM Ba2+ as charge carrier before, during, and after bath application of 10 µM Oxo-M. (D) Representative whole-cell current traces selected from the respective time course before (black) and 1 min after (red) Oxo-M application. Currents were elicited every 4 s by stepping to a test potential of 0 mV for 100 ms. (E and F) Modulation of N current from tsA 201 cells stably transfected with CaV2.2, α2δ-1, and CaVβ3 and transiently transfected with 500–1,000 ng/well of M1Rs. eGFP is shown in the time course and representative sweeps. The charge carrier was increased to 20 mM Ba2+ to improve the signal to noise ratio, and accordingly the test potential was adjusted to 10 mV. (G and H) HEK-M1 cells were transiently transfected with N-channel subunits CaV2.2, α2δ-1, and CaVβ2a. Modulation of N current by M1R stimulation illustrated in a time course (G) and current traces (H). (I and J) N-current modulation from cells expressing HEK-M1 cells transfected as for C. Cells were exposed to OPC for at least 3 min before application of Oxo-M. Bars, 0.4 nA.
At present, no binding site for either PtdIns(4,5)P2 or AA on N channels has been determined. Moreover, whether the sites that confer AA sensitivity reside in the same channel or whether two distinct channel populations mediate enhancement and inhibition remains untested. Therefore, we attempted to use the power of mutagenesis to separate enhancement from inhibition in human embryonic kidney 293 (HEK-293) cells expressing recombinant M1Rs and N channels. N channels are multimeric complexes defined by a large pore-forming subunit (CaV2.2) that associates with ancillary α2δ and CaVβ subunits (Catterall, 2000). We made the serendipitous discovery that enhancement and inhibition separate without mutagenesis simply by expressing different wild-type CaVβs.
MATERIALS AND METHODS
Transfection of HEK-M1 cells
HEK-293 cells with a stably transfected M1R (HEK-M1; Peralta et al., 1988), a gift from E. Liman (University of Southern California, Los Angeles, CA), were cultured in Dulbecco's modified Eagle's medium/F12 (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 1% G418 (geneticin-selective antibiotic, nonsterile; Invitrogen), and 1% HT supplement (Invitrogen). Cells were transferred into 12-well plates for transfection. Recombinant cells at 50–80% confluence were transfected for 1 h with CaV2.2 e(Δ24a, +31a, +37b), the N-channel variant found in SCG neurons (Lin et al., 1997); α2δ-1, which is also expressed in SCG (Lin et al., 2004); and various CaVβs at a 1:1:1 or 1:1:2 molar ratio along with enhanced green fluorescent protein (eGFP) at ∼10% of the total DNA using Lipofectamine and PLUS reagent (Invitrogen) as per the manufacturer's instructions. Where noted, the transfection mixture contained expression plasmids encoding 0.028 µg/well NK-1R (UMR cDNA Resource Center, University of Missouri, Rolla, MO). 500–1,000 ng total DNA was transfected per well. 24–48 h after transfection, cells were replated on poly-l-lysine–coated coverslips and allowed to settle for at least 1 h before recording. tsA 201 cells stably transfected with CaV2.2, α2δ1, and CaVβ3 were provided by D. Lipscombe (Brown University, Providence, RI). These cells were transfected with 500–1,000 ng per well of the M1R (a gift from N. Nathanson, University of Washington, Seattle, WA) along with eGFP at 10% of the total DNA and processed as described above for current recordings.
CaVβ1b (GenBank/EMBL/DDBJ accession no. X61394), CaVβ2a (GenBank accession no. M80545), and CaVβ4 (GenBank accession no. L02315) were provided by E. Perez-Reyes (University of Virginia, Charlottesville, VA); CaV2.2 (GenBank accession no. AF055477), α2δ-1 (GenBank accession no. AF286488), and CaVβ3 (GenBank accession no. M88751) were provided by D. Lipscombe. The CaVβ2a(C3,4S) mutant was provided by A. Fox (University of Chicago, Chicago, IL); CaVβ2aβ3 and CaVβ2aβ1b chimeras were provided by R. Ten Eick (Northwestern University, Evanston, IL). The mutant and chimeric CaVβs were originally made in M. Hosey's laboratory (Northwestern University; Chien et al., 1996, 1998; Chien and Hosey, 1998). Recordings from untransfected HEK-M1 cells yielded whole-cell currents of 9.0 ± 1.3 pA in 20 mM Ba2+ (Roberts-Crowley, M., personal communication). To avoid spurious results from endogenous currents, any transfected cells having less than −150 pA were discarded.
Preparation of neonatal rat SCG neurons for electrophysiology
Dissociated sympathetic neurons were obtained from SCG of 1–4-d-old Sprague-Dawley rats (Charles River) according to the methods of Liu and co-workers (Liu et al., 2001; Liu and Rittenhouse, 2003a). To isolate the actions of the slow pathway on N-type current, cells were incubated for at least 5 h with 500 ng/ml pertussis toxin (Biological Laboratories, Inc.) before initiating whole-cell recording experiments (Bernheim et al., 1992). Also, bath solutions included 1 µM of the L-type Ca2+ channel antagonist nimodipine to minimize the small amount of L-type current present in SCG neurons. Cells were used within 12 h to avoid recording from cells with processes.
Electrophysiology
Ba2+ currents were recorded at room temperature (20–24°C) using the whole-cell configuration of a model 3900a (Dagan), Axon 200A, or Axon 200B (MDS Analytical Technologies) patch-clamp amplifier using methods that were described previously (Barrett et al., 2001; Liu et al., 2001). Currents were filtered at 5 kHz using the amplifier's four-pole low-pass Bessel filter and were digitized at 20 kHz with a micro1401 interface (Cambridge Electronic Design [CED]). Data were collected using Patch 6.4 or Signal 2.15 software suites (CED) and stored on a personal computer. Before analysis, capacitive and leak currents were subtracted using a scaled-up current elicited with a test pulse to −100 mV. Pipette resistance ranged from 2.5 to 5 MΩ. Ba2+ currents were elicited every 4 s by stepping from −90 to 0 mV for 100 ms unless otherwise noted. For cells expressing CaVβ2a, 20 mM Ba2+ was used as the charge carrier to improve the signal to noise ratio. The internal solution contained 135 mM Cs-Asp, 10 mM HEPES, 0.1 mM 1,2-bis(O-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid (BAPTA), 5 mM MgCl2, 4 mM ATP, and 0.4 mM GTP, adjusted to pH 7.5 with CsOH. The external solution contained 135 mM N-methyl-d-glutamine-aspartate, 10 mM HEPES, and 5 or 20 mM Ba2+ (as indicated), adjusted to pH 7.5 with CsOH. 135 mM N-methyl-d-glutamine-aspartate was substituted for Ba2+ when its concentration was lowered from 20 to 5 mM.
Pharmacological agents
Oxo-M (Tocris Biosciences, Inc.) was prepared as a stock solution in double-distilled water and was diluted 1,000 times with bath solution. SP was prepared as a stock solution in 0.05 M acetic acid and diluted at least 2,000 times with bath solution. AA (Nu-Chek Prep), oleyloxyethyl phosphorylcholine (OPC; EMD), and nimodipine were prepared as stock solutions made up in 100% ethanol and diluted 1,000 times with bath solution. All chemicals were obtained from Sigma-Aldrich except where noted.
Data analysis
The Patch 6.4 and Signal 2.15 software were used to measure peak inward current of whole-cell traces. A trough-seeking function was used to determine peak current where indicated. Data were further analyzed using Excel (Microsoft) and Origin 7.0 (OriginLab). Percent inhibition was calculated as [(I − I’)/I] × 100, where I is the control current amplitude determined by a mean of five whole-cell current measurements before application of a particular agent and I’ is the mean of five current measurements at the time specified for the determination. Conductance was calculated from a modified Ohm's law equation, G = I/(Vm− Vrev), where I is the peak current at each test potential, Vm is the test potential, and Vrev is the apparent reversal potential. Relative conductance curves, (G/Gmax) − voltage (Vm), were plotted for whole-cell currents before and after AA. Data were curve fit using the Boltzmann equation function in Origin 7.0, yielding a curve fitting the equation G/Gmax = Gmax + (Gmin − Gmax)/(1 + exp[(Vm − Vm1/2)/k)], where Gmax is the maximal conductance, Gmin is the minimal conductance, Vm is the test potential, Vm1/2 is the voltage at half-maximal conductance, and k is the slope factor.
Immunocytochemistry of CaVβ subunits
Adult SCG neurons were plated on chamber slides (Nagle Nune International), treated with poly-l-lysine, and cultured overnight. Cells were washed with PBS twice for 5 min and fixed with 100% acetone for 10 min. The fixative was washed away with PBS at room temperature three times for 5 min. Cells were then exposed to PBS containing 10% normal goat serum for 60 min at room temperature followed by incubation with primary antibody for 60 min at room temperature. Mouse anti-CaVβ2 (NeuroMab), rabbit anti-Cavβ3 (Sigma-Aldrich), and mouse anti-Cavβ4 (NeuroMab) were diluted 1:1,000 in Antibody Diluent (Dako). Thereafter, cells were washed with PBS three times for 5 min and were incubated for 60 min in the dark at room temperature with Alexa Fluor 488 anti–mouse antibody or Alexa Fluor 488 anti–rabbit antibody (Invitrogen) diluted 1:200 in Antibody Diluent. After incubation with secondary antibodies, cells were washed with PBS three times for 5 min. A second fixation was performed. Slides were then washed with distilled water and covered with the aqueous mounting medium Prolong Gold Antifade reagent (Invitrogen). Images of immunofluorescence were obtained at room temperature using a custom-built, video-rate confocal microscope (Sanderson and Parker, 2003) with a 40× objective lens. An excitation wavelength of 488 nm was used, and emission spectra were collected with long-pass filters (OGSIS) at 515 nm (Perez and Sanderson, 2005).
RT-PCR of CaVβ subunits
Homogenized tissue samples were lysed in 0.8 ml TRIZOL reagent (Invitrogen), and DNA was removed from RNA using phenol-chloroform phase separation. Next, RNA was precipitated using isopropyl alcohol and washed with 75% ethanol. After drying, the RNA pellet was dissolved in RNase-free water. Subsequently, reverse transcription was performed using 1.0 µl of 10× buffer reverse transcriptase, 1.0 µl deoxyribonucleotide triphosphate (dNTP) mix (5 mM each of dNTP), 1.0 µl oligo-dT primer (0.5 mg/ml), 0.125 µl RNase inhibitor (40 U/µl; Promega), 0.5 µl Omniscript Reverse transcription, and RNase-free water to make up a total volume of 10 µl (all reagents were obtained from QIAGEN unless otherwise noted). The mixture was incubated at 37°C for 1 h and heated at 93°C for 5 min followed by cooling on ice to rapidly inactivate the transcriptase. Aliquots of reverse transcription PCR product were amplified by PCR using the primers CaVβ2a (5′-ATAACCACAGAGAGGAGAGCCACA-3′ and 5′-TATACATCCCTGTTCCACTCGCCA-3′; 258-bp product) and CaVβ3 (5′-TCCCTGGACTTCAGAACCAGCAG-3′ and 5′-TTGTGGTCATGCTCCGAGTCCTG-3′; 368 bp; Lin et al., 1996). Reaction mixtures for PCR contained 2 µl of template cDNA, 2.5 µl of 10× buffer, 0.5 µl dNTP mix, and 2 µl each of forward and reverse primer and were made up in distilled water to a final volume of 25 µl. The protocol for amplification was 94°C for 4 min followed by 33 cycles of 94°C for 45 s, 60°C for 45 s, 72°C for 1 min, and holding at 72°C for 10 min for last extension. PCR products were run on 1.8% agarose gel stained with ethidium bromide along with a 1,000-bp DNA ladder (Fermentas Life Sciences).
Statistical analysis
Summary data are presented as means ± SEM. A two-tailed paired t test was used to determine differences between control and agonist. For comparisons of two different groups, data were analyzed by a two-tailed Student's t test for two means. For multiple comparisons, statistical significance was determined using Origin 7.0 by a one-way ANOVA followed by a Tukey multiple comparison posthoc test. Statistical significance was set at P ≤ 0.05.
Online supplemental material
Fig. S1 shows that exogenous application of AA inhibits N-type current in SCG neurons. The L-type channel agonist FPL 64176 was used to distinguish effects of AA on N current from L current. The AA analogue ETYA (5,8,11,14-eicosatetraynoic acid) was used to block metabolism of AA. Fig. S2 demonstrates that the enhancing and inhibiting effects of AA are reversible using an application of fatty acid–free BSA subsequent to AA application. Fig. S3 shows that in HEK-M1 cells transfected with CaV2.2, α2δ-1, CaVβ2a, and CaVβ3, both enhancement and inhibition of N current occurs during application of AA. Online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.200910203/DC1.
RESULTS
To test whether a homogeneous population of N channels recapitulates both enhancement and inhibition, N currents were screened for modulation by Oxo-M. Recombinant currents were evoked from HEK-M1 cells transiently transfected with N-channel subunits CaV2.2, α2δ-1, and CaVβ3, which most commonly associates with CaV2.2 (Witcher et al., 1993). Oxo-M rapidly inhibited peak inward current, reaching a stable inhibition within 90 s (Fig. 1, B–D). Because N-current enhancement is voltage dependent (Liu and Rittenhouse, 2003a), we compared current–voltage (I-V) plots measured before and after Oxo-M, expecting to observe current enhancement at negative test potentials. However, Oxo-M inhibited current at virtually all voltages (Fig. 2 C). tsA 201 cells stably transfected with the same subunits exhibited a similar profile of inhibition after exposure to Oxo-M (Fig. 1, B, E, and F), ruling out a system-specific effect.
Figure 2.
CaVβ determines N-current modulation by GqPCRs and AA. (A–D) HEK-M1 cells were transiently transfected with CaV2.2, α2δ-1, and various CaVβ subunits. Currents, measured in 5 mM [Ba2+], were elicited every 4 s by stepping from −90 to 0 mV for 100 ms unless otherwise noted. Representative whole-cell current traces were always taken before (black) and 3 min after (red) drug application. Averaged normalized I-V plots were taken before (black closed circles) and 90 s after (red open circles) agonist application. n = 3–6 cells per group. For cells expressing CaVβ2a, 20 mM [Ba2+] was used as charge carrier to improve the signal to noise ratio. The test potential was adjusted to 10 mV to correct for the shift in peak inward current. (E) Histogram summarizing N-current modulation after 3 min of 10 µM Oxo-M (gray bars) and 5 nM SP (black bars). Percent change in current amplitude was highly significant between CaVβ2a and CaVβ1b, CaVβ3, or CaVβ4 irrespective of whether Oxo-M or SP was applied (**, P < 0.005; one-way ANOVA). (F–I) Modulation of N current by 10 µM AA shown as current traces and averaged I-V plots (n = 4–5 cells per group). (J) Histogram summarizing N-current modulation after 3 min of AA (**, P < 0.005; one-way ANOVA). Error bars represent SEM. Bars, 0.4 nA.
CaVβ subunit controls N-current modulation by GqPCRs
Lack of enhancement at any potential indicated the recombinant system was fundamentally different from N-current modulation observed in SCG neurons. In addition to incomplete recapitulation of GqPCR modulation, recombinant N current exhibited robust, fast inactivation (Fig. 1, D and F, and Fig. 2 C) as previously observed with channels containing CaVβ3 (Olcese et al., 1994). In contrast, native N current in SCG neurons exhibits kinetics with little inactivation (Plummer et al., 1989), similar to recombinant N current from channels containing CaVβ2a (Hurley et al., 2000; Yasuda et al., 2004). Moreover, AA robustly inhibits noninactivating current in SCG neurons evoked with a test pulse duration ranging from 20 (Fig. S1) to 700 ms (Liu and Rittenhouse, 2000; Liu et al., 2001). Because SCG neurons express CaVβ2 mRNA (Lin et al., 1996), we hypothesized that the majority of N current arises from CaVβ2a-containing channels. If true, these channels might exhibit both inhibition and enhancement. We found that Oxo-M rapidly inhibited N current from cells expressing CaVβ2a. However, unlike CaVβ3, initial inhibition gave way to stable enhancement (Fig. 1, G and H). Moreover, comparison of I-V plots revealed that Oxo-M no longer inhibited current at any potential but enhanced current at negative test potentials (Fig. 2 B). Although we hypothesized that expression of CaVβ2a would elicit enhancement at negative potentials, separation of enhancement from inhibition based on CaVβ expression was unanticipated. Therefore, we tested whether other CaVβs would dually modulate N current by repeating the aforementioned experiments with two other neuronal CaVβs: CaVβ1b and CaVβ4. As with CaVβ3, when either CaVβ1b (Fig. 2 A) or CaVβ4 (Fig. 2 D) was expressed, Oxo-M inhibited current at all test potentials, indicating that channels containing CaVβ2a exhibit unique N-current modulation by M1Rs.
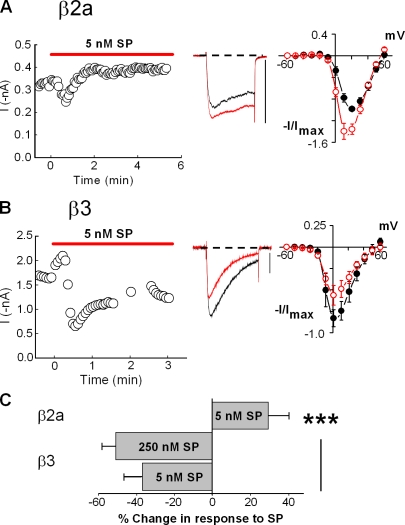
To determine whether M1Rs uniquely influence the profile of modulation, we examined whether N-current modulation by another GqPCR depends on CaVβ. We tested NK-1Rs because in SCG neurons, stimulation of M1Rs and NK-1Rs inhibits N current through converging signaling pathways that require Gαq and downstream activation of PLC (Shapiro et al., 1994; Kammermeier et al., 2000; Liu and Rittenhouse, 2003a). We examined current modulation that occurred after exposure to SP. As with Oxo-M, an initial transient inhibition progressed to a stable current enhancement when CaVβ2a was expressed along with CaV2.2 and α2δ-1 (Fig. 3, A and C). In contrast, SP elicited sustained inhibition with CaVβ3 expression (Fig. 2 E and Fig. 3, B and C). These data indicate that control of modulation by CaVβ is a mechanism used by other GqPCRs rather than being unique to M1Rs (Fig. 1 A).
Figure 3.
NK-1R stimulation elicits a similar profile of N-current modulation as M1Rs. (A and B) HEK-M1 cells were transiently transfected with the NK-1R along with CaV2.2, α2δ-1, and either CaVβ2a (A) or CaVβ3 (B). Using 5 nM SP as the agonist, current modulation is shown in a time course of peak current (left), representative whole-cell current traces (middle), and averaged I-V plots (right; n = 4). Bars, 0.4 nA. (C) Summary of the modulatory effects of SP on currents from cells expressing either CaVβ2a or CaVβ3 (***, P < 0.005; one-way ANOVA). No significant difference in current inhibition was observed from cells expressing CaVβ3 with 5 and 250 nM SP (P > 0.26). Error bars represent SEM.
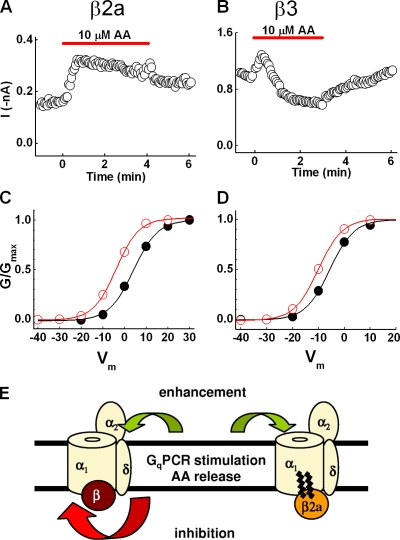
AA elicits a profile of N-current modulation similar to GqPCR stimulation
AA mimics N-current modulation by M1Rs in SCG neurons and may serve as a downstream messenger of this pathway (Liu and Rittenhouse, 2003a). If so, AA also should inhibit or enhance recombinant N current similarly to M1R or NK-1R stimulation. When this hypothesis was tested, AA's actions recapitulated the pattern of modulation observed with M1R or NK-1R stimulation: enhancement and inhibition separated based on CaVβ expression (Fig. 2, F–J). If elevated AA levels mediate enhancement and inhibition, both should reverse after washout of exogenous AA. As with SCG neurons, washing with copious amounts of bath solution or with 1 mg/ml BSA reversed modulation (Fig. S2, A–F). Moreover, enhancement recurred with a second application of AA after washing with BSA (Fig. S2 A). These findings corroborate our assertion that AA mediates N-current modulation by the slow pathway (Liu and Rittenhouse, 2003a,b; Liu et al., 2004).
As a final test of AA's role in the slow pathway, we antagonized endogenous AA's release from membrane phospholipids using the PLA2 antagonist OPC. Exposure to Oxo-M for 60 s significantly inhibited N current from CaVβ3-containing channels (P < 0.05; n = 16). In contrast, OPC eliminated significant current inhibition by Oxo-M (P > 0.05; n = 7; Fig. 1, B, I, and J). A similar profile was observed with SP (not depicted). The striking recapitulation by AA of GqPCR-induced N-current enhancement and inhibition, coupled with the loss of modulation when antagonizing AA release or sequestering free AA with BSA, advance our previous hypothesis that PLA2 and AA participate in the slow pathway (Liu and Rittenhouse, 2003a,b; Liu et al., 2004).
Expression of multiple CaVβ isoforms produces heterogeneous N currents
Our findings yield a possible explanation for why SCG neurons exhibit both enhancement and inhibition of whole-cell N current (Barrett et al., 2001; Liu and Rittenhouse, 2003a; Liu et al., 2004). N current's diverse biophysical properties in different neurons have been attributed to CaV2.2 coexpression with different CaVβ isoforms within individual neurons (Scott et al., 1996). Similarly, SCG neurons may exhibit both forms of modulation because CaV2.2 coassembles with different CaVβs. If this hypothesis is correct, the pattern of modulation observed in SCG neurons should recapitulate in HEK-M1 cells transfected with multiple CaVβs. When cells were transfected with CaVβ2a and CaVβ3, the I-V plots recapitulated I-V plots from SCG neurons (Liu and Rittenhouse, 2003a), with enhancement occurring at negative potentials and inhibition at positive potentials after exogenously applied AA (Fig. S3). However, unlike activity from SCG neurons (Liu et al., 2001; Liu and Rittenhouse, 2003a), the currents rapidly inactivated. It has been reported that SCG neurons from 4-d-old rat pups express CaVβ2a, CaVβ3, and CaVβ4 mRNA with trace amounts of CaVβ1b mRNA (Lin et al., 1996). We verified the presence of CaVβ2, CaVβ3, and CaVβ4 protein in freshly dissociated SCG neurons by immunocytochemistry (Fig. 4 A). Therefore, we retested for the modulatory response in HEK-M1 cells transfected with varying ratios of CaVβ2a-, CaVβ3-, and CaVβ4-transformed plasmid. Transfection with a mixture of cDNAs in the molar ratio of 10:1:1 for CaVβ2a/CaVβ3/CaVβ4 in a HEK-M1 cell closely recapitulated the N-current kinetics we had previously observed in SCG neurons (Barrett and Rittenhouse, 2000; Liu and Rittenhouse, 2003a). Application of 10 µM Oxo-M resulted in significant inhibition of currents elicited at test potentials 10–50 mV, with no significant inhibition at lower potentials (Fig. 4 B). To ensure the transfection mixture is an accurate recapitulation of the SCG neuron, we harvested fresh SCG neurons from neonatal rat pups and tested them using the same experimental conditions as we used for the HEK-M1 cells. We found that application of 10 µM Oxo-M also resulted in significant inhibition of native currents elicited at test potentials 10–50 mV, with no significant change at lower potentials (Fig. 4 C). These findings suggest that the N current observed in SCG neurons results from heterogeneous expression of different CaVβ isoforms with CaV2.2.
Figure 4.
Expression of multiple CaVβ subunits in SCG neurons. (A, top) Confocal micrographs of individual SCG neurons. Starting from the left, the neurons were exposed to no antibodies, secondary antibody (anti–mouse Alexa Fluor 488) only, mouse anti-CaVβ2 followed by secondary rabbit anti–mouse Alexa Fluor 488, anti-CaVβ3 followed by anti–rabbit Alexa Fluor 488, and mouse anti-CaVβ2 followed by secondary antibody. (bottom) Corresponding view shown in bright field. Bar, 10 µm. (B) Representative current traces and mean I-V plots taken from HEK-M1 cells transiently transfected with a 12:12:10:1:1 ratio of CaV2.2/α2δ-1/CaVβ2a/CaVβ3/CaVβ4; taken before (black) and after (red) 10-µM Oxo-M application (n = 6 cells). All currents for the figure were recorded using 20 mM Ba2+. *, P < 0.05; paired t test at each test potential. (C) Current traces and mean I-V plots taken from freshly dissociated SCG neurons before (black) and after (red) application of 10 µM Oxo-M (n = 4 neurons). Error bars represent SEM. Bars, 0.4 nA. (D) RT-PCR of SCG and hippocampal (HC) homogenates from three different 7-d-old rats comparing CaVβ2a and CaVβ3 expression. RNA from animal #1 generated lanes 1, 4, 7, and 10. RNA from animal #2 generated lanes 2, 5, 8, and 11. RNA from animal #3 generated lanes 3, 6, 9, and 12. Lane 13 represents a negative control: RT-PCR reaction without isolated RNA.
The antibody used in the immunocytochemistry experiments recognizes the peptide sequence RSPKPSANSVTSPHSKE (NeuroMab data sheet N8b_1). This sequence exists in CaVβ2a, 2b, 2c, 2d, and 2e but not 2f, 2g, or 2h. Because the presence of CaVβ2a variant has never been confirmed in rat neonatal SCG neurons, we looked for its presence by RT-PCR using primers designed specifically for CaVβ2a. Using tissue from three different animals, we assessed the presence of CaVβ2a and CaVβ3 in SCG. As a positive control, we used the same three animals to run parallel experiments of CaVβ isoform expression in the hippocampus, a region reported to be enriched in CaVβ2 (Day et al., 1998). We found CaVβ2a expression in three of three hippocampal samples and in three of three SCG samples. We also found CaVβ3 expression in two of three SCG samples but found negligible CaVβ3 expression in hippocampal samples.
A model for dual modulation of N current
We established a working model for how CaVβ2a controls modulation based on previous studies that examined AA's mechanism of inhibition of different Ca2+ channels (Barrett et al., 2001; Liu et al., 2001). Our experiments with SCG neurons demonstrated that current inhibition by AA occurs intracellularly or within the inner leaflet of the membrane. In contrast, enhancement appears located extracellularly or within the outer leaflet of the bilayer (Barrett et al., 2001; Liu et al., 2001). CaVβ subunits bind to cytoplasmic loops of CaV2.2 (Pragnell et al., 1994). Thus, we hypothesized that the unique interactions of CaVβ2a with CaV2.2 somehow attenuate inhibition. In SCG neurons (Barrett et al., 2001; Liu et al., 2001) and with recombinant channels containing either CaVβ2a or CaVβ3 (Fig. 5, A and B), AA induces initial N-current enhancement, which is sustained with CaVβ2a but for CaVβ3 is followed by a more slowly progressing inhibition. This observation supports the model. Thus, we postulated that every CaV2.2 exhibits current enhancement that becomes masked by subsequent, more dominant inhibition.
Figure 5.
CaVβ2a blocks N-current inhibition, revealing latent enhancement. (A and B) Time courses of cells expressing either CaVβ2a (A) or CaVβ3 (B) exposed to AA both exhibit enhancement during the initial phase of time course. However, when CaVβ3 is present, inhibition eventually dominates, indicating that both inhibitory and enhancement sites are available for AA to bind. When CaVβ2a is present, inhibition no longer occurs, suggesting the palmitoylated CaVβ2a occupies the inhibitory site without conferring inhibition. (C and D) Normalized conductance–voltage plots generated from I-V data show a negative shift in G/Gmax for cells expressing either CaVβ2a (C) or CaVβ3 (D), indicating that an increased sensitivity to voltage is masked by a dominating inhibition in cells expressing CaVβ3. (E) Schematic representation of working model. Upon GqPCR stimulation, the released AA binds to both inhibitory and enhancement sites on the channel. When CaVβ3 is present, both inhibitory and enhancement sites are available for AA to bind. However, because inhibition dominates, the resultant modulation observed is current inhibition. When CaVβ2a is present, inhibition no longer occurs because the palmitoylated CaVβ2a occupies the inhibitory site without conferring inhibition. With inhibition antagonized, sustained current enhancement is observed.
Because our previous work indicated that enhancement by AA arises from increased voltage sensitivity (Barrett et al., 2001), we compared normalized conductance–voltage (G-V) curves to determine whether increased voltage sensitivity occurs independently of CaVβ2a expression. Both CaVβ2a- and CaVβ3-containing cells exhibited a negative shift in conductance in response to AA (Fig. 5, C and D), demonstrating that AA increases N-channel voltage sensitivity independently of CaVβ subunit expression. These data suggest that CaVβ2a's unique ability to attenuate inhibition reveals sustained enhancement. In contrast, inhibition results from increased stability of a closed state (Liu and Rittenhouse, 2000), essentially reducing the number of channels available to open. This type of inhibition should dominate an increase in voltage sensitivity; if channels will not open at any voltage, increasing their voltage sensitivity will have little effect on currents. Thus, by blocking a dominating inhibition, CaVβ2a unmasks latent enhancement.
Loss of CaVβ2a palmitoylation restores partial inhibition of N current by Oxo-M or AA
Having established that CaVβ2a uniquely blocks N-current inhibition by GqPCR stimulation or exogenous AA, we investigated the structural aspects of CaVβ2a to determine its mechanism of action. A conspicuous feature of CaVβ2a is its unique palmitoylation of two cysteine residues near the N terminus (Chien et al., 1996). Our observation that palmitoylated CaVβ2a interferes with a fatty acid–mediated inhibition of CaV2.2 raises the possibility of direct antagonism between palmitic acid and AA (Fig. 5 E). Therefore, we tested a depalmitoylated mutant to determine whether CaVβ2a must be palmitoylated to minimize inhibition and reveal enhancement. Currents from channels containing depalmitoylated CaVβ2a (CaVβ2a[C3,4S]; Chien et al., 1996) exhibited an initial transient inhibition after Oxo-M that relaxed, yielding a small but insignificant inhibition (Fig. 6, A and G). AA initially enhanced current amplitude, followed by significant inhibition (Fig. 6, B and G). The loss of enhancement and appearance of inhibition, although differing in magnitude with Oxo-M or AA, support the idea that palmitoylation blocks inhibition.
Figure 6.
Palmitoylation determines which form of modulation is observed. (A–F) Using 20 mM Ba2+, modulation by Oxo-M or AA of cells expressing mutant CaVβ2a(C3,4S) (A and B), CaVβ2aβ1b (C and D), or CaVβ2aβ3 (E and F) is shown in current traces and I-V plots. Bars, 0.4 nA. (G) Summary of the modulatory effects on currents after application of Oxo-M or AA for 3 min (n = 4–7). Error bars represent SEM. (H) Schematic representation of working model.
If palmitoylation is necessary for antagonizing inhibition, palmitoylating another CaVβ should convert current inhibition to enhancement. Therefore, we tested a chimera in which the variable N terminus of CaVβ1b was replaced with the palmitoylated 16–amino acid N terminus of CaVβ2a (CaVβ2aβ1b; Chien et al., 1998). In cells expressing CaVβ2aβ1b, application of Oxo-M enhanced current with no inhibition (Fig. 6 G). Moreover, AA no longer inhibited these currents significantly (Fig. 6 G) compared with CaVβ1b (n = 4; Fig. 2 J). The difference in modulation by AA and Oxo-M may be attributed to an exaggerated response to exogenous application of AA compared with the response to physiological concentrations of AA released upon M1R stimulation. I-V plots after either Oxo-M or AA exhibited enhancement of current at negative test potentials and no inhibition at positive test potentials (Fig. 6, C and D), suggesting that the palmitoylated N terminus is both necessary and sufficient to block inhibition.
However, a second chimera in which the N terminus of CaVβ3 was replaced with the N terminus of CaVβ2a (CaVβ2aβ3; Chien et al., 1998) did not block inhibition as effectively. In cells expressing CaVβ2aβ3, inhibition by Oxo-M was diminished compared with wild-type CaVβ3, whereas AA reversibly inhibited currents similarly to wild-type levels (Fig. 6, E–G). I-V plots taken before and 90 s after application of Oxo-M or AA exhibited inhibition at positive test potentials (Fig. 6, E and F). These results indicate that addition of CaVβ2a's palmitoylated N terminus to CaVβ3 only partially reproduced the stable enhancement by Oxo-M or AA normally observed with wild-type CaVβ2a. Because CaVβ2a protein shares highest sequence homology to CaVβ1b (Birnbaumer et al., 1998), the simplest interpretation for the varied results with the different chimeras is that that CaVβ2aβ1b positions the palmitoylated N terminus so that it minimizes N-current inhibition similarly to wild-type CaVβ2a. In contrast, the more divergent CaVβ2aβ3 positions the palmitoylated N terminus in a different position relative to CaV2.2 where it is less effective in minimizing inhibition (Fig. 6 H). In support of this notion, CaVβ2aβ3-containing cells exhibited varied kinetics, from noninactivating currents resembling CaVβ2a-containing channel activity (Fig. 2 B) to rapidly inactivating currents resembling depalmitoylated CaVβ2a-containing channel activity (Fig. 6, A and B). Moreover, the kinetics varied within individual recordings and from cell to cell from a rapidly inactivating to a noninactivating kinetic profile (unpublished data). The unstable inactivation kinetics suggests that CaVβ2aβ3 cannot dock properly to CaV2.2, thus destabilizing and changing the location of the chimera's palmitoyl groups.
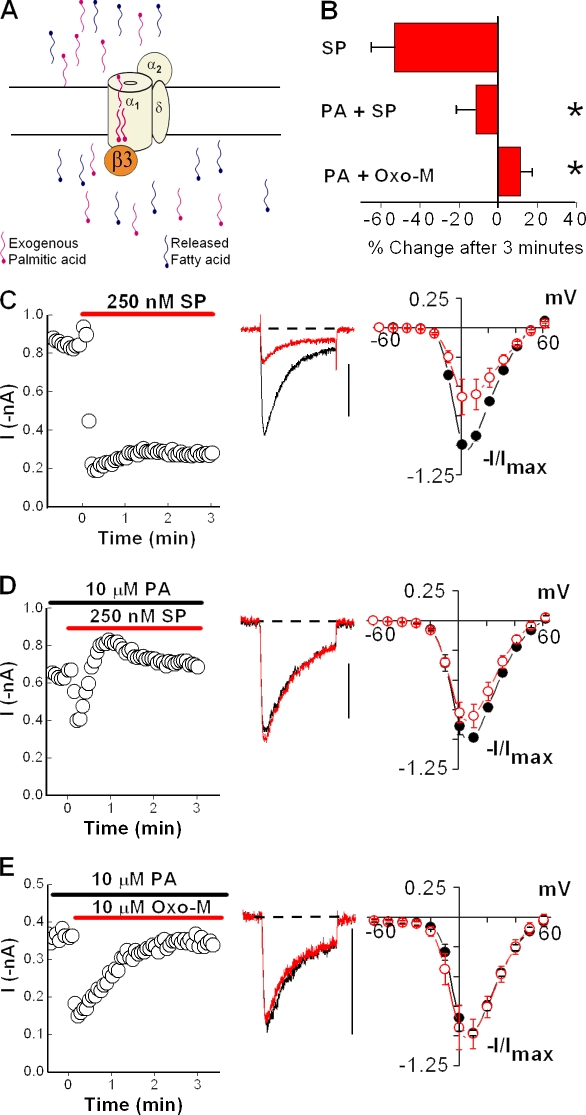
Free palmitic acid minimizes N-current inhibition
If our interpretation that the palmitoyl groups antagonize inhibition is correct, freeing the palmitoyl groups from protein constraints might allow the fatty acids to find and assume their optimal position for blocking inhibition (Fig. 7 A). Alternatively, if the palmitic acids do not interact directly with CaV2.2, introducing free palmitic acid should not alter N-channel modulation by agonist. We tested this prediction first by preincubating cells expressing NK-1Rs and CaVβ3-containing channels with free palmitic acid for at least 8 min. Under these conditions, the sustained inhibition normally observed with SP (Fig. 7, B and C) was replaced by an initial enhancement that relaxed, resulting in no significant change (P > 0.24) in current amplitude over time (Fig. 7, B and D). Moreover, compared with control conditions, little inhibition by SP was detected in I-V plots from cells preincubated with 10 µM palmitic acid (Fig. 7, C and D). To ensure the block of inhibition was not specific to NK-1Rs, we tested N-current modulation of CaVβ3-containing channels in HEK-M1 cells by Oxo-M after a 10-min preincubation with 10 µM palmitic acid. As with SP, free palmitic acid prevented the sustained inhibition observed in its absence, as demonstrated by time courses and I-V plots (compare Fig. 7, B and E with Fig. 1, B–F and Fig. 2, C and E). Bath application of palmitic acid for 8 min did not significantly affect N current (not depicted) when compared with currents recorded under control conditions (Fig. 7 C), indicating that palmitic acid itself has no modulatory effect. These findings demonstrate that exogenous application of free palmitic acid suffices to block current inhibition of CaVβ3-containing N channels. More importantly, these results indicate palmitic acid is both necessary and sufficient to antagonize N-current inhibition during GqPCR stimulation (Fig. 8).
Figure 7.
Palmitic acid antagonizes inhibition of N current by GqPCR stimulation. HEK-M1 cells were transiently transfected with NK-1R, CaV2.2, α2δ-1, and CaVβ3. (A) Diagram showing that preincubation of HEK-M1 cells with exogenously applied palmitic acid blocks inhibition of N channels by fatty acids, such as AA, released after NK-1R or M1R activation. (B) Summary showing preincubation of cells with 10 µM palmitic acid (PA) significantly attenuates inhibition by SP or Oxo-M (*, P < 0.05 compared with percent change in the absence of palmitic acid). Error bars represent SEM. (C) Modulation of N current by SP from cells expressing CaVβ3 is shown in a representative time course, current traces taken before and 3 min after application of SP, and I-V plots (n = 5). (D) Preincubation of cells with palmitic acid causes loss of inhibition by SP as shown in a time course, current traces, and I-V plot (n = 6). (E) Preincubation of HEK-M1 cells with palmitic acid also causes loss of inhibition by Oxo-M, as shown in a representative time course, current traces, and I-V plots (n = 4). Bars, 0.4 nA.
Figure 8.
Model of interference of inhibition by palmitoylated CaVβ2a. (A) As presented, our data support a model in which upon GqPCR stimulation, release of AA initially enhances N current by acting at a site distinct from inhibition. However, N-current inhibition by AA of channels containing CaVβ1b, CaVβ3, or CaVβ4 dominates modulation of CaV2.2 and obscures enhancement. (B) In the presence of CaVβ2a, the palmitoylated N terminus of the CaVβ subunit blocks N-current inhibition by endogenously released AA as a result of the two palmitic acids competitively interacting with CaV2.2, thus revealing latent enhancement.
DISCUSSION
In this study, we used a recombinant system to investigate the enhancement and inhibition of N current that occurs during M1R stimulation and AA application. In so doing, we made the discovery that the N-channel's β subunit directs opposite effects of N-current modulation. Specifically, M1R stimulation or exogenous AA uniquely enhanced whole-cell N current from cells expressing CaVβ2a but inhibited N current from cells expressing CaVβ1b, CaVβ3, or CaVβ4. This finding allowed us to further examine the modulation of N current and reach several additional conclusions. First, the striking recapitulation of M1R-induced modulation by AA, coupled with the loss of modulation when AA release by OPC is blocked, is strong evidence that AA is an integral component of the slow pathway. Second, NK-1R stimulation exhibited a similar dual effect, indicating this unique profile of modulation may be conserved among GqPCRs. Third, cells expressing multiple forms of CaVβ subunits matched the modulatory profile of SCG neurons under the same recording conditions, implying that the characteristic pattern of modulation observed in SCG neurons results from CaV2.2 coupling with different CaVβ isoforms. This finding provides an explanation of how stimulation of M1Rs or exogenous AA can elicit both enhancement and inhibition of N current in individual sympathetic neurons (Liu and Rittenhouse, 2003a). Fourth, using mutated and chimeric CaVβ constructs, we demonstrated that palmitoylation of CaVβ2a is a key feature in its capacity to toggle modulation from inhibition to enhancement. Finally, exogenous application of palmitic acid prevented the characteristic N-current modulation by GqPCRs, suggesting that CaVβ2a's palmitoyl groups interfere with a mechanism or mechanisms within the slow pathway.
Increased free AA appears necessary for N-current inhibition by the slow pathway
It is well documented that PtdIns(4,5)P2 breakdown by PLC is necessary for slow pathway inhibition (Wu et al., 2002; Liu and Rittenhouse, 2003a; Gamper et al., 2004; Liu et al., 2004; Delmas et al., 2005; Suh and Hille, 2005); however, whether its breakdown is sufficient for N-current inhibition remains controversial (Wu et al., 2002; Gamper et al., 2004; Suh and Hille, 2005). Our data indicate that fatty acid liberation is both necessary and sufficient to elicit inhibition of CaV2.2 (Barrett et al., 2001; Liu and Rittenhouse, 2003a; Liu et al., 2004, 2007). To demonstrate a role for free AA in N-current inhibition, we used pharmacological agents, the AA scavenger BSA, PLA2 antibodies dialyzed internally, and neurons harvested from PLA2−/− knockout mice (Liu and Rittenhouse, 2003a; Liu et al., 2004, 2006). Here we have taken a molecular approach in which expression of palmitoylated CaVβ2a provides a highly specific agent to block inhibition by AA, whereas nonpalmitoylated wild-type CaVβ subunits conveniently act as negative controls. It must be stressed that none of the findings reported here minimizes PtdIns(4,5)P2's role but rather documents a requirement for AA in N-current inhibition.
A resolution to the paradox would entail a role for both PtdIns(4,5)P2 and AA acting either directly or indirectly on the channel. A model for PtdIns(4,5)P2 and AA opposing each other in modulation of K+ channels has been published (Oliver et al., 2004) in which PtdIns(4,5)P2 and AA act at different sites of the channel. Direct competition is possible because AA is an integral piece of the PtdIns(4,5)P2 molecule, normally residing in the sn-2 position, and thus could compete for binding to CaV2.2. Although indirect actions of PtdIns(4,5)P2 and AA must be considered, as both are bioactive molecules with multiple downstream effects, no requirement for downstream enzymes such as phosphatases or kinases has been found. Moreover, antagonizing enzymes that further metabolize AA have no effect on modulation (Liu et al., 2001; Liu and Rittenhouse, 2003a). Nevertheless, increased levels of PtdIns(4,5)P2 increase N-channel availability to open, whereas increased levels of AA stabilize N channels in closed and/or inactivated states (Liu and Rittenhouse, 2000; Gamper et al., 2004). These opposing effects suggest competition.
A working model for enhancement and inhibition
By exposing SCG neurons to exogenous AA, we characterized N-current enhancement and inhibition as distinct molecular events acting at different sites on N channels. Restriction of AA movement across the cell membrane suggested that enhancement requires AA on the extracellular side or within the outer leaflet, whereas inhibition requires AA on the intracellular side or within the inner leaflet (Barrett et al., 2001). These two sites presumably would be equally accessible to AA generated internally because movement of long-chain fatty acids across cell membranes occurs in <1 s (Hamilton et al., 2002). Because CaVβ subunits are cytoplasmic, we hypothesized that CaVβ2a attenuates inhibition without affecting enhancement. In dissociated neurons, AA induces an initial current enhancement followed by a slower progressing inhibition (Barrett et al., 2001; Liu et al., 2001). The simplest explanation for these data is that the initial enhancement becomes masked by the subsequent, more dominant, inhibition. Because previous work indicated that enhancement stems from increased voltage sensitivity (Barrett et al., 2001), we used normalized G-V curves (Fig. 5) to show that an increase in voltage sensitivity occurs independently of CaVβ2a expression. In contrast, inhibition results from an increase in a slow form of inactivation most likely as a result of increased dwell time in one or more intermediate closed states (Liu and Rittenhouse, 2000). This type of inhibition would essentially reduce the available number of channels providing calcium influx, dominating an enhancement produced by a shift in voltage sensitivity. Thus, by blocking a dominant inhibition, CaVβ2a unmasks a latent enhancement. The masking of enhancement offers some explanation for the finding that the majority of studies on N-current modulation have observed inhibition (Elmslie, 2003).
Nevertheless, enhancement and inhibition have been reported from experimental systems similar to the one used here. For example, AA both shifts activation toward negative potentials and simultaneously increases steady-state inactivation of T channels (Talavera et al., 2004). Also, in SCG neurons, the amide of AA, anandamide, elicited enhancement at negative potentials and inhibition at positive potentials in I-V plots (Guo and Ikeda, 2004). Enhancement marked by a leftward shift in voltage sensitivity was reported for R-type channels in the hippocampus (Tai et al., 2006), a region in which CaVβ2 is highly expressed (Day et al., 1998). Finally, using HEK-293 cells transiently transfected with recombinant M1Rs and N channels containing CaVβ3, an earlier study showed that carbachol inhibited Ca2+ current with no obvious enhancement (Melliti et al., 2001). However, I-V curves showed a discernible leftward shift in voltage sensitivity after carbachol.
A new role for palmitoyl groups of palmitoylated proteins
Our data identify a previously unrecognized role for protein palmitoylation in which it serves as the key feature of CaVβ2a's capacity to toggle CaV2.2 modulation from inhibition to enhancement after stimulation of GqPCRs. Here and in other systems, palmitoylation targets or anchors proteins to specific membrane domains (Chien et al., 1996; Resh, 2006). Although dynamic palmitoylation of CaVβ2a has been shown to alter the inactivation kinetics of voltage-gated Ca2+ channels in bovine chromaffin cells, the assumed mechanism of action is restriction of movement of channel domains by anchoring the CaVβ2a subunit to the membrane (Hurley et al., 2000). This anchoring in some way is thought to impede current inactivation (Restituito et al., 2001). Thus, palmitoylation increases protein stability and efficiency of action but rarely alters the functional properties of proteins. One exception to this generalization is retinal epithelial protein 65 (RPE65), a chaperone protein for all-trans-retinal esters. In this case, palmitoylation not only targets RPE65 to the plasma membrane but also reverses RPE65's binding specificity for vitamin A to all-trans-retinyl ester, consequently affecting how rapidly photoreceptors respond to light (Xue et al., 2004). Thus, palmitoylation qualitatively alters the substrate specificity of the same protein that is reversibly palmitoylated. Here, our data extend the functions of palmitoylation in a new direction by revealing that after stimulation of GqPCRs, the palmitoyl groups of one protein, CaVβ2a, block inhibition of a second protein, CaV2.2.
Our finding that palmitoylation antagonizes an inhibition mediated by PtdIns(4,5)P2 breakdown and increased free AA (Liu and Rittenhouse, 2003a; Gamper et al., 2004) raises the possibility that dual palmitoyl groups interact directly with CaV2.2 to antagonize AA's interaction with CaV2.2 and subsequent inhibition of N-channel activity. The idea that AA may interact directly with Ca2+ channels is supported by the finding that M1R stimulation or application of exogenous AA also inhibits recombinant CaV3 currents in whole-cell (Zhang et al., 2000; Talavera et al., 2004; Hildebrand et al., 2007) and ripped-off patch configurations (Chemin et al., 2007). Because CaV3 (T type) channels do not require coexpression of CaVβ or α2δ to open, T-channel inhibition must occur at a site on the pore-forming subunit. Moreover, AA inhibits T current with a Hill coefficient of 1.6, indicating cooperative binding of at least two AA molecules to T channels (Talavera et al., 2004). Collectively, these findings are consistent with a direct interaction between AA and T channels. AA likely confers N-current inhibition by acting at a homologous site found on many CaV channels because native and recombinant CaV1, CaV2, and CaV 3 channels exhibit similar changes in gating after AA (Shimada and Somlyo, 1992; Petit-Jacques and Hartzell, 1996; Liu and Rittenhouse, 2000; Vellani et al., 2000; Talavera et al., 2004; Chemin et al., 2007; Liu, 2007; Roberts-Crowley and Rittenhouse, 2009).
Palmitoylated CaVβ2a may serve as a phospholipid mimic by competing with PtdIns(4,5)P2 and free AA for interaction with CaV2.2
Under basal conditions, AA normally resides in the sn-2 position of PtdIns(4,5)P2. Because palmitoylated CaVβ2a appears to antagonize the actions of free AA on CaV2.2, it may also compete with PtdIns(4,5)P2 for interaction with CaV2.2, the two palmitic acids of CaVβ2a residing in sites normally occupied by the two fatty acid tails of PtdIns(4,5)P2. Consistent with its ability to block N-current inhibition by free AA, palmitoylated CaVβ2a acts as a phospholipid mimic to maintain normal channel activity (Fig. 8). This model is attractive in that it incorporates previous models, which propose that PtdIns(4,5)P2 associates with channels, increasing their availability to open (Wu et al., 2002; Gamper et al., 2004). The model also supports our previous findings (Liu and Rittenhouse, 2003a) that increased free AA confers current inhibition either by displacing PtdIns(4,5)P2 or by remaining associated with channels after phospholipid breakdown (Roberts-Crowley et al., 2009).
Our findings that free palmitic acid blocks inhibition by SP of CaVβ3-containing channels or depalmitoylated CaVβ2a-containing channels (see Mitra-Ganguli et al. in this issue) is consistent with the idea that the palmitic acids occupy the same sites recognized by AA. Whether PtdIns(4,5)P2 as well as AA compete for the same site of interaction with CaV2.2 as the palmitoyl groups of CaVβ2a and where the location of that site is awaits future investigation. However, we have observed that whole-cell recombinant current from CaVβ2a-containing channels, in contrast to CaVβ3-containing channels (Gamper et al., 2004), run down only minimally over time (unpublished data), consistent with palmitoylated CaVβ2a functionally substituting for PtdIns(4,5)P2. Thus, when taken together, our findings and the results of other laboratories best fit a model in which palmitoylated CaVβ2a, PtdIns(4,5)P2, and free AA compete for an overlapping interaction site on CaV2.2. Given the extent that lipids such as PtdIns(4,5)P2 and AA associate with membrane protein complexes (Piomelli, 1993; Doughman et al., 2003), the interference of such interactions by palmitoylated proteins is predicted to occur in other protein complexes, yielding broad importance beyond ion channel functioning.
Expression of palmitoylated CaVβ2a may underlie novel forms of synaptic plasticity
In particular, the presence or absence of palmitoylated CaVβ2a may have far-reaching consequences for synaptic plasticity. CaVβ2 expression displays an overlapping distribution with GqPCRs (Tencé et al., 1994; Liu and Rittenhouse, 2003a) throughout the brain and is primarily localized postsynaptically in dendrites and cell bodies (Lie et al., 1999), indicating that enhancement of Ca2+ channel activity may specifically affect postsynaptic membrane excitability. In support of this notion, increased current amplitude and/or kinetic changes associated with Ca2+ current enhancement (Zhang et al., 2000; Bannister et al., 2004; Guo and Ikeda, 2004; Talavera et al., 2004; Tai et al., 2006; Chemin et al., 2007; Mitra-Ganguli et al., 2009) occur in different neurons and recombinant channels from the CaV2 and CaV3 families (Keyser and Alger, 1990; Melliti et al., 2001; Chemin et al., 2007; Meza et al., 2007). In contrast, native or recombinant channels associated with CaVβ1b, CaVβ3, or CaVβ4 exhibit inhibition after similar stimulation (Keyser and Alger, 1990; Liu and Rittenhouse, 2003a; Gamper et al., 2004; Guo and Ikeda, 2004; Meza et al., 2007). Because CaVβ2 expression changes developmentally (Tanaka et al., 1995) and with activity (Lie et al., 1999), postsynaptic responses also may change over time. Patients with temporal lobe epilepsy exhibit increased postsynaptic CaVβ2 expression in damaged hippocampal regions. In contrast, patients that underwent surgical lesioning exhibit hippocampal CaVβ levels indistinguishable from control patients (Lie et al., 1999), suggesting CaVβ2 expression is dynamically regulated by electrical activity. Moreover, it has been reported that although CaVβ expression appears necessary for surface expression of the N channel, once the channel reaches the cell surface, the CaVβ subunit can be exchanged (Restituito et al., 2001; Hidalgo et al., 2006). Thus, changes in current modulation caused by changes in CaVβ expression should not require a turnover of the entire channel complex.
Whether up-regulation of CaVβ2 is a response to counteract hyperexcitability or whether increased CaVβ2 levels contribute to excitotoxic neurodegeneration has not been determined. Nevertheless, these findings document in vivo plasticity of CaVβ2 expression. In the short term, palmitoyl acyl transferases dynamically regulate protein palmitoylation in hippocampal dendrites and cell bodies to control synaptic function (El-Husseini et al., 2002; Fukata et al., 2004). Pulse–chase experiments using tritiated palmitate indicate that palmitate turns over multiple times during the lifetime of most palmitoylated proteins, and recent experiments suggest that the pulse–chase experiments may underestimate the turnover rate by 15–20-fold as a result of reacylation of proteins by the radiolabeled palmitate within a cell (for review see Baekkeskov and Kanaani, 2009). Thus, expression of CaVβ2a in postsynaptic regions should create a previously unrealized level of plasticity in which the response to a transmitter, acting on its GqPCR from moment to moment, may switch from inhibitory to excitatory depending on whether CaVβ2a's palmitoyl groups interact with CaV2.2.
A role for multiple CaVβ isoforms in neurons
In summary, we have shown that multiple CaVβ isoforms expressed in a recombinant system can recapitulate native N-current modulation by the slow pathway. Given the diversity of neuronal functions for calcium influx, there must be localized control of a channel's response to modulation. Our findings provide a previously unrecognized role for CaVβ subunits in the control of modulation and indicate a means by which GqPCR stimulation can simultaneously up- and down-regulate calcium flux at distinct sites within a single cell. Moreover, our experiments and analyses identify the sites that confer sensitivity to inhibition by AA as a likely site of plasticity.
Acknowledgments
We thank E. Liman for HEK-M1 cells, D. Lipscombe for tsA cells and CaV2.2, α2δ-1, and CaVβ3 plasmids, N. Nathanson for M1R plasmid, E. Perez-Reyes for CaVβ1b, CaVβ2a, and CaVβ4 plasmids, A. Fox for CaVβ2a(C3,4S) plasmid, and R. Ten Eick for CaVβ2aβ3 and CaVβ2aβ1b plasmids. We thank Y. Bai and M. Sanderson for helping capture fluorescent images of SCG neurons. We also thank M.L. Roberts-Crowley for help with transfection and sharing unpublished data. We thank A. Carruthers, P. Charnet, H. Colecraft, H. Florman, J. Glaven, L. Hayward, J.R. Lemos, J. Marshall, W.R. Kobertz, H.-S. Li, S.R. Ikeda, J. Jonassen, and M.L. Roberts-Crowley for reading various versions of the manuscript. We thank T-F. Chung (University of Massachusetts Medical School [UMMS] tissue culture facility) for help growing HEK-M1 cells.
This project was funded by National Institutes of Health grant NS34195 (to A.R. Rittenhouse) and support from UMMS.
Edward N. Pugh, Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- AA
- arachidonic acid
- dNTP
- deoxyribonucleotide triphosphate
- eGFP
- enhanced green fluorescent protein
- HEK
- human embryonic kidney
- M1R
- M1 receptor
- NK-1R
- neurokinin-1 receptor
- OPC
- oleyloxyethyl phosphorylcholine
- Oxo-M
- oxotremorine-M
- PCR
- protein-coupled receptor
- SCG
- superior cervical ganglion
- SP
- substance P
References
- Baekkeskov S., Kanaani J. 2009. Palmitoylation cycles and regulation of protein function (Review). Mol. Membr. Biol. 26:42–54 10.1080/09687680802680108 [DOI] [PubMed] [Google Scholar]
- Bannister R.A., Melliti K., Adams B.A. 2004. Differential modulation of CaV2.3 Ca2+ channels by Galphaq/11-coupled muscarinic receptors. Mol. Pharmacol. 65:381–388 10.1124/mol.65.2.381 [DOI] [PubMed] [Google Scholar]
- Barrett C.F., Rittenhouse A.R. 2000. Modulation of N-type calcium channel activity by G-proteins and protein kinase C. J. Gen. Physiol. 115:277–286 10.1085/jgp.115.3.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett C.F., Liu L., Rittenhouse A.R. 2001. Arachidonic acid reversibly enhances N-type calcium current at an extracellular site. Am. J. Physiol. Cell Physiol. 280:C1306–C1318 [DOI] [PubMed] [Google Scholar]
- Beech D.J., Bernheim L., Hille B. 1992. Pertussis toxin and voltage dependence distinguish multiple pathways modulating calcium channels of rat sympathetic neurons. Neuron. 8:97–106 10.1016/0896-6273(92)90111-P [DOI] [PubMed] [Google Scholar]
- Bernheim L., Mathie A., Hille B. 1992. Characterization of muscarinic receptor subtypes inhibiting Ca2+ current and M current in rat sympathetic neurons. Proc. Natl. Acad. Sci. USA. 89:9544–9548 10.1073/pnas.89.20.9544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Qin N., Olcese R., Tareilus E., Platano D., Costantin J., Stefani E. 1998. Structures and functions of calcium channel beta subunits. J. Bioenerg. Biomembr. 30:357–375 10.1023/A:1021989622656 [DOI] [PubMed] [Google Scholar]
- Brosenitsch T.A., Katz D.M. 2001. Physiological patterns of electrical stimulation can induce neuronal gene expression by activating N-type calcium channels. J. Neurosci. 21:2571–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W.A. 2000. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16:521–555 10.1146/annurev.cellbio.16.1.521 [DOI] [PubMed] [Google Scholar]
- Chemin J., Nargeot J., Lory P. 2007. Chemical determinants involved in anandamide-induced inhibition of T-type calcium channels. J. Biol. Chem. 282:2314–2323 10.1074/jbc.M610033200 [DOI] [PubMed] [Google Scholar]
- Chien A.J., Hosey M.M. 1998. Post-translational modifications of beta subunits of voltage-dependent calcium channels. J. Bioenerg. Biomembr. 30:377–386 10.1023/A:1021941706726 [DOI] [PubMed] [Google Scholar]
- Chien A.J., Carr K.M., Shirokov R.E., Rios E., Hosey M.M. 1996. Identification of palmitoylation sites within the L-type calcium channel beta2a subunit and effects on channel function. J. Biol. Chem. 271:26465–26468 10.1074/jbc.271.43.26465 [DOI] [PubMed] [Google Scholar]
- Chien A.J., Gao T., Perez-Reyes E., Hosey M.M. 1998. Membrane targeting of L-type calcium channels. Role of palmitoylation in the subcellular localization of the beta2a subunit. J. Biol. Chem. 273:23590–23597 10.1074/jbc.273.36.23590 [DOI] [PubMed] [Google Scholar]
- Day N.C., Volsen S.G., McCormack A.L., Craig P.J., Smith W., Beattie R.E., Shaw P.J., Ellis S.B., Harpold M.M., Ince P.G. 1998. The expression of voltage-dependent calcium channel beta subunits in human hippocampus. Brain Res. Mol. Brain Res. 60:259–269 10.1016/S0169-328X(98)00186-7 [DOI] [PubMed] [Google Scholar]
- Delmas P., Coste B., Gamper N., Shapiro M.S. 2005. Phosphoinositide lipid second messengers: new paradigms for calcium channel modulation. Neuron. 47:179–182 10.1016/j.neuron.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Doughman R.L., Firestone A.J., Anderson R.A. 2003. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J. Membr. Biol. 194:77–89 10.1007/s00232-003-2027-7 [DOI] [PubMed] [Google Scholar]
- El-Husseini A.E.-D., Schnell E., Dakoji S., Sweeney N., Zhou Q., Prange O., Gauthier-Campbell C., Aguilera-Moreno A., Nicoll R.A., Bredt D.S. 2002. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 108:849–863 10.1016/S0092-8674(02)00683-9 [DOI] [PubMed] [Google Scholar]
- Elmslie K.S. 2003. Neurotransmitter modulation of neuronal calcium channels. J. Bioenerg. Biomembr. 35:477–489 10.1023/B:JOBB.0000008021.55853.18 [DOI] [PubMed] [Google Scholar]
- Ford C.P., Stemkowski P.L., Light P.E., Smith P.A. 2003. Experiments to test the role of phosphatidylinositol 4,5-bisphosphate in neurotransmitter-induced M-channel closure in bullfrog sympathetic neurons. J. Neurosci. 23:4931–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M., Fukata Y., Adesnik H., Nicoll R.A., Bredt D.S. 2004. Identification of PSD-95 palmitoylating enzymes. Neuron. 44:987–996 10.1016/j.neuron.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Gamper N., Reznikov V., Yamada Y., Yang J., Shapiro M.S. 2004. Phosphatidylinositol [correction] 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J. Neurosci. 24:10980–10992 10.1523/JNEUROSCI.3869-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.S., Ikeda S.R. 2004. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol. Pharmacol. 65:665–674 10.1124/mol.65.3.665 [DOI] [PubMed] [Google Scholar]
- Hamilton J.A., Guo W., Kamp F. 2002. Mechanism of cellular uptake of long-chain fatty acids: do we need cellular proteins? Mol. Cell. Biochem. 239:17–23 10.1023/A:1020542220599 [DOI] [PubMed] [Google Scholar]
- Hidalgo P., Gonzalez-Gutierrez G., Garcia-Olivares J., Neely A. 2006. The alpha1-beta-subunit interaction that modulates calcium channel activity is reversible and requires a competent alpha-interaction domain. J. Biol. Chem. 281:24104–24110 10.1074/jbc.M605930200 [DOI] [PubMed] [Google Scholar]
- Hildebrand M.E., David L.S., Hamid J., Mulatz K., Garcia E., Zamponi G.W., Snutch T.P. 2007. Selective inhibition of Cav3.3 T-type calcium channels by Galphaq/11-coupled muscarinic acetylcholine receptors. J. Biol. Chem. 282:21043–21055 10.1074/jbc.M611809200 [DOI] [PubMed] [Google Scholar]
- Hurley J.H., Cahill A.L., Currie K.P., Fox A.P. 2000. The role of dynamic palmitoylation in Ca2+ channel inactivation. Proc. Natl. Acad. Sci. USA. 97:9293–9298 10.1073/pnas.160589697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier P.J., Ruiz-Velasco V., Ikeda S.R. 2000. A voltage-independent calcium current inhibitory pathway activated by muscarinic agonists in rat sympathetic neurons requires both Galpha q/11 and Gbeta gamma. J. Neurosci. 20:5623–5629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser D.O., Alger B.E. 1990. Arachidonic acid modulates hippocampal calcium current via protein kinase C and oxygen radicals. Neuron. 5:545–553 10.1016/0896-6273(90)90092-T [DOI] [PubMed] [Google Scholar]
- Lie A.A., Blümcke I., Volsen S.G., Wiestler O.D., Elger C.E., Beck H. 1999. Distribution of voltage-dependent calcium channel beta subunits in the hippocampus of patients with temporal lobe epilepsy. Neuroscience. 93:449–456 10.1016/S0306-4522(99)00162-1 [DOI] [PubMed] [Google Scholar]
- Lin Z., Harris C., Lipscombe D. 1996. The molecular identity of Ca channel alpha 1-subunits expressed in rat sympathetic neurons. J. Mol. Neurosci. 7:257–267 10.1007/BF02737063 [DOI] [PubMed] [Google Scholar]
- Lin Z., Haus S., Edgerton J., Lipscombe D. 1997. Identification of functionally distinct isoforms of the N-type Ca2+ channel in rat sympathetic ganglia and brain. Neuron. 18:153–166 10.1016/S0896-6273(01)80054-4 [DOI] [PubMed] [Google Scholar]
- Lin Y.S., McDonough S.I., Lipscombe D. 2004. Alternative splicing in the voltage-sensing region of N-type CaV2.2 channels modulates channel kinetics. J. Neurophysiol. 92:2820–2830 10.1152/jn.00048.2004 [DOI] [PubMed] [Google Scholar]
- Liu L., Rittenhouse A.R. 2000. Effects of arachidonic acid on unitary calcium currents in rat sympathetic neurons. J. Physiol. 525:391–404 10.1111/j.1469-7793.2000.00391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Rittenhouse A.R. 2003a. Arachidonic acid mediates muscarinic inhibition and enhancement of N-type Ca2+ current in sympathetic neurons. Proc. Natl. Acad. Sci. USA. 100:295–300 10.1073/pnas.0136826100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Rittenhouse A.R. 2003b. Pharmacological discrimination between muscarinic receptor signal transduction cascades with bethanechol chloride. Br. J. Pharmacol. 138:1259–1270 10.1038/sj.bjp.0705157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.C., Barrett C.F., Rittenhouse A.R. 2001. Arachidonic acid both inhibits and enhances whole cell calcium currents in rat sympathetic neurons. Am. J. Physiol. Cell Physiol. 280:C1293–C1305 [DOI] [PubMed] [Google Scholar]
- Liu L.P., Gonzalez P.K., Barrett C.F., Rittenhouse A.R. 2003. The calcium channel ligand FPL 64176 enhances L-type but inhibits N-type neuronal calcium currents. Neuropharmacology. 45:281–292 10.1016/S0028-3908(03)00153-9 [DOI] [PubMed] [Google Scholar]
- Liu L., Roberts M.L., Rittenhouse A.R. 2004. Phospholipid metabolism is required for M1 muscarinic inhibition of N-type calcium current in sympathetic neurons. Eur. Biophys. J. 33:255–264 10.1007/s00249-003-0387-7 [DOI] [PubMed] [Google Scholar]
- Liu L., Zhao R., Bai Y., Stanish L.F., Evans J.E., Sanderson M.J., Bonventre J.V., Rittenhouse A.R. 2006. M1 muscarinic receptors inhibit L-type Ca2+ current and M-current by divergent signal transduction cascades. J. Neurosci. 26:11588–11598 10.1523/JNEUROSCI.2102-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.J., Heneghan J.F., Mitra-Ganguli T., Roberts-Crowley M.L., Rittenhouse A.R. 2007. Role of PIP2 in regulating versus modulating Ca2+ channel activity. J. Physiol. 583:1165–1166; author reply 1167 10.1113/jphysiol.2007.141424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.J., Heneghan J.F., Michael G.J., Stanish L.F., Egertová M., Rittenhouse A.R. 2008. L- and N-current but not M-current inhibition by M1 muscarinic receptors requires DAG lipase activity. J. Cell. Physiol. 216:91–100 10.1002/jcp.21378 [DOI] [PubMed] [Google Scholar]
- Liu S.J. 2007. Inhibition of L-type Ca2+ channel current and negative inotropy induced by arachidonic acid in adult rat ventricular myocytes. Am. J. Physiol. Cell Physiol. 293:C1594–C1604 10.1152/ajpcell.00284.2007 [DOI] [PubMed] [Google Scholar]
- Macdonald S.G., Dumas J.J., Boyd N.D. 1996. Chemical cross-linking of the substance P (NK-1) receptor to the alpha subunits of the G proteins Gq and G11. Biochemistry. 35:2909–2916 10.1021/bi952351+ [DOI] [PubMed] [Google Scholar]
- Mathie A., Bernheim L., Hille B. 1992. Inhibition of N- and L-type calcium channels by muscarinic receptor activation in rat sympathetic neurons. Neuron. 8:907–914 10.1016/0896-6273(92)90205-R [DOI] [PubMed] [Google Scholar]
- Melliti K., Meza U., Adams B.A. 2001. RGS2 blocks slow muscarinic inhibition of N-type Ca(2+) channels reconstituted in a human cell line. J. Physiol. 532:337–347 10.1111/j.1469-7793.2001.0337f.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza U., Thapliyal A., Bannister R.A., Adams B.A. 2007. Neurokinin 1 receptors trigger overlapping stimulation and inhibition of CaV2.3 (R-type) calcium channels. Mol. Pharmacol. 71:284–293 10.1124/mol.106.028530 [DOI] [PubMed] [Google Scholar]
- Michailidis I.E., Zhang Y., Yang J. 2007. The lipid connection-regulation of voltage-gated Ca(2+) channels by phosphoinositides. Pflugers Arch. 455:147–155 10.1007/s00424-007-0272-9 [DOI] [PubMed] [Google Scholar]
- Mitra-Ganguli T., Vitko I., Perez-Reyes E., Rittenhouse A.R. 2009. Orientation of palmitoylated CaVβ2a relative to CaV2.2 is critical for slow pathway modulation of N-type Ca2+ current by tachykinin receptor activation. J. Gen. Physiol. 134:385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcese R., Qin N., Schneider T., Neely A., Wei X., Stefani E., Birnbaumer L. 1994. The amino terminus of a calcium channel beta subunit sets rates of channel inactivation independently of the subunit's effect on activation. Neuron. 13:1433–1438 10.1016/0896-6273(94)90428-6 [DOI] [PubMed] [Google Scholar]
- Oliver D.C., Lien C.C., Soom M., Baukrowitz T., Jonas P., Fakler B. 2004. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 304:265–270 10.1126/science.1094113 [DOI] [PubMed] [Google Scholar]
- Peralta E.G., Ashkenazi A., Winslow J.W., Ramachandran J., Capon D.J. 1988. Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature. 334:434–437 10.1038/334434a0 [DOI] [PubMed] [Google Scholar]
- Perez J.F., Sanderson M.J. 2005. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J. Gen. Physiol. 125:535–553 10.1085/jgp.200409216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Jacques J.H., Hartzell H.C. 1996. Effect of arachidonic acid on the L-type calcium current in frog cardiac myocytes. J. Physiol. 493:67–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. 1993. Arachidonic acid in cell signaling. Curr. Opin. Cell Biol. 5:274–280 10.1016/0955-0674(93)90116-8 [DOI] [PubMed] [Google Scholar]
- Plummer M.R., Logothetis D.E., Hess P. 1989. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 2:1453–1463 10.1016/0896-6273(89)90191-8 [DOI] [PubMed] [Google Scholar]
- Pragnell M., De Waard M., Mori Y., Tanabe T., Snutch T.P., Campbell K.P. 1994. Calcium channel beta-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature. 368:67–70 10.1038/368067a0 [DOI] [PubMed] [Google Scholar]
- Resh M.D. 2006. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci. STKE. 10.1126/stke.3592006re14 [DOI] [PubMed] [Google Scholar]
- Restituito S., Cens T., Rousset M., Charnet P. 2001. Ca(2+) channel inactivation heterogeneity reveals physiological unbinding of auxiliary beta subunits. Biophys. J. 81:89–96 10.1016/S0006-3495(01)75682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse A.R., Zigmond R.E. 1999. Role of N- and L-type calcium channels in depolarization-induced activation of tyrosine hydroxylase and release of norepinephrine by sympathetic cell bodies and nerve terminals. J. Neurobiol. 40:137–148 [DOI] [PubMed] [Google Scholar]
- Roberts-Crowley M.L., Rittenhouse A.R. 2009. Arachidonic acid inhibition of L-type calcium (CaV1.3b) channels varies with accessory CaVβ subunits. J. Gen. Physiol. 133:387–403 10.1085/jgp.200810047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Crowley M.L., Mitra-Ganguli T., Liu L., Rittenhouse A.R. 2009. Regulation of voltage-gated Ca2+ channels by lipids. Cell Calcium. 45:589–601 10.1016/j.ceca.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson M.J., Parker I. 2003. Video-rate confocal microscopy. Methods Enzymol. 360:447–481 10.1016/S0076-6879(03)60123-0 [DOI] [PubMed] [Google Scholar]
- Scott V.E., De Waard M., Liu H., Gurnett C.A., Venzke D.P., Lennon V.A., Campbell K.P. 1996. Beta subunit heterogeneity in N-type Ca2+ channels. J. Biol. Chem. 271:3207–3212 10.1074/jbc.271.6.3207 [DOI] [PubMed] [Google Scholar]
- Shapiro M.S., Hille B. 1993. Substance P and somatostatin inhibit calcium channels in rat sympathetic neurons via different G protein pathways. Neuron. 10:11–20 10.1016/0896-6273(93)90237-L [DOI] [PubMed] [Google Scholar]
- Shapiro M.S., Wollmuth L.P., Hille B. 1994. Modulation of Ca2+ channels by PTX-sensitive G-proteins is blocked by N-ethylmaleimide in rat sympathetic neurons. J. Neurosci. 14:7109–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T.A., Somlyo A.P. 1992. Modulation of voltage-dependent Ca channel current by arachidonic acid and other long-chain fatty acids in rabbit intestinal smooth muscle. J. Gen. Physiol. 100:27–44 10.1085/jgp.100.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A.A. 1975. Fatty acid binding to plasma albumin. J. Lipid Res. 16:165–179 [PubMed] [Google Scholar]
- Suh B.C., Hille B. 2002. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 35:507–520 10.1016/S0896-6273(02)00790-0 [DOI] [PubMed] [Google Scholar]
- Suh B.C., Hille B. 2005. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 15:370–378 10.1016/j.conb.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Suh B.C., Hille B. 2007. Regulation of KCNQ channels by manipulation of phosphoinositides. J. Physiol. 582:911–916 10.1113/jphysiol.2007.132647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh B.C., Inoue T., Meyer T., Hille B. 2006. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 314:1454–1457 10.1126/science.1131163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C., Kuzmiski J.B., MacVicar B.A. 2006. Muscarinic enhancement of R-type calcium currents in hippocampal CA1 pyramidal neurons. J. Neurosci. 26:6249–6258 10.1523/JNEUROSCI.1009-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K., Staes M., Janssens A., Droogmans G., Nilius B. 2004. Mechanism of arachidonic acid modulation of the T-type Ca2+ channel α1G. J. Gen. Physiol. 124:225–238 10.1085/jgp.200409050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka O., Sakagami H., Kondo H. 1995. Localization of mRNAs of voltage-dependent Ca(2+)-channels: four subtypes of alpha 1- and beta-subunits in developing and mature rat brain. Brain Res. Mol. Brain Res. 30:1–16 10.1016/0169-328X(94)00265-G [DOI] [PubMed] [Google Scholar]
- Tencé M., Cordier J., Premont J., Glowinski J. 1994. Muscarinic cholinergic agonists stimulate arachidonic acid release from mouse striatal neurons in primary culture. J. Pharmacol. Exp. Ther. 269:646–653 [PubMed] [Google Scholar]
- Vellani V.A., Reynolds A.M., McNaughton P.A. 2000. Modulation of the synaptic Ca2+ current in salamander photoreceptors by polyunsaturated fatty acids and retinoids. J. Physiol. 529:333–344 10.1111/j.1469-7793.2000.00333.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A.E., Chen W.G., Dalva M.B., Dolmetsch R.E., Kornhauser J.M., Shaywitz A.J., Takasu M.A., Tao X., Greenberg M.E. 2001. Calcium regulation of neuronal gene expression. Proc. Natl. Acad. Sci. USA. 98:11024–11031 10.1073/pnas.191352298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisgirda M.E., Dryer S.E. 1994. Functional dependence of Ca(2+)-activated K+ current on L- and N-type Ca2+ channels: differences between chicken sympathetic and parasympathetic neurons suggest different regulatory mechanisms. Proc. Natl. Acad. Sci. USA. 91:2858–2862 10.1073/pnas.91.7.2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher D.R., De Waard M., Sakamoto J., Franzini-Armstrong C., Pragnell M., Kahl S.D., Campbell K.P. 1993. Subunit identification and reconstitution of the N-type Ca2+ channel complex purified from brain. Science. 261:486–489 10.1126/science.8392754 [DOI] [PubMed] [Google Scholar]
- Wu L.C., Bauer C.S., Zhen X.G., Xie C., Yang J. 2002. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature. 419:947–952 10.1038/nature01118 [DOI] [PubMed] [Google Scholar]
- Xue L.D., Gollapalli D.R., Maiti P., Jahng W.J., Rando R.R. 2004. A palmitoylation switch mechanism in the regulation of the visual cycle. Cell. 117:761–771 10.1016/j.cell.2004.05.016 [DOI] [PubMed] [Google Scholar]
- Yasuda T., Chen L., Barr W., McRory J.E., Lewis R.J., Adams D.J., Zamponi G.W. 2004. Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur. J. Neurosci. 20:1–13 10.1111/j.1460-9568.2004.03434.x [DOI] [PubMed] [Google Scholar]
- Zhang Y.L., Cribbs L.L., Satin J. 2000. Arachidonic acid modulation of alpha1H, a cloned human T-type calcium channel. Am. J. Physiol. Heart Circ. Physiol. 278:H184–H193 [DOI] [PubMed] [Google Scholar]
- Zhao R., Liu L., Rittenhouse A.R. 2007. Ca2+ influx through both L- and N-type Ca2+ channels increases c-fos expression by electrical stimulation of sympathetic neurons. Eur. J. Neurosci. 25:1127–1135 10.1111/j.1460-9568.2007.05359.x [DOI] [PubMed] [Google Scholar]