Abstract
The concentration of essential mineral nutrients in the edible portion of plants such as grains may affect the nutritional value of these foods, while concentrations of toxic minerals in the plant are matter of food safety. Minerals taken up by the roots from soils are normally redirected at plant nodes before they are finally transported into developing seeds. However, the molecular mechanisms involved in this process have not been identified so far. Herein, we report on a transporter (Lsi6) responsible for the redirection of a plant nutrient at the node. Lsi6 is a silicon transporter in rice (Oryza sativa), and its expression in node I below the panicles is greatly enhanced when the panicle is completely emerged. Lsi6 is mainly localized at the xylem transfer cells located at the outer boundary region of the enlarged large vascular bundles in node I. Knockout of Lsi6 decreased Si accumulation in the panicles but increased Si accumulation in the flag leaf. These results suggest that Lsi6 is a transporter involved in intervascular transfer (i.e., transfer of silicon from the large vascular bundles coming from the roots to the diffuse vascular bundles connected to the panicles). These findings will be useful for selectively enhancing the accumulation of essential nutrients and reducing toxic minerals in the edible portion of cereals.
INTRODUCTION
We obtain minerals, including essential nutrients and toxic elements, directly or indirectly from plants, while plants take up these minerals from the soil. Therefore, the mineral contents in crops can affect our health both positively and negatively. Mineral accumulation in the edible portion of plants is determined by many factors such as soil mobilization, uptake capacity by the roots, efficiency of xylem loading and translocation, and distribution among tissues (Epstein and Bloom, 2005). In gramineous plants, minerals taken up by the roots are not directly transported to the grains but are redirected at plant nodes (Kawahara et al., 1974; Chonan et al., 1985; Hoshikawa, 1989). Therefore, the distribution of minerals at the nodes is considered to be a key step in the selective control of mineral accumulation in the panicles. At node I beneath the panicles of rice (Oryza sativa), there are enlarged large and small vascular bundles and diffuse vascular bundles. Large and small vascular bundles come from the lower nodes and connect to the flag leaf and are markedly enlarged at the node. Diffuse vascular bundles are parallel to and surround the enlarged large vascular bundles. They also are assembled in the upper internode (peduncle) to form regular bundles, which connect toward the panicle tissues (Kawahara et al., 1974; Chonan et al., 1985; Hoshikawa, 1989). Therefore, intervascular transfer of minerals from the enlarged vascular bundles to diffuse vascular bundles is required to deliver minerals taken up by the roots to developing seeds. However, the molecular basis of this process has not been elucidated. Herein, we report on a transporter responsible for intervascular transfer in the nodes of rice.
RESULTS
Tissue-Specific and Spatial Expression of Lsi6
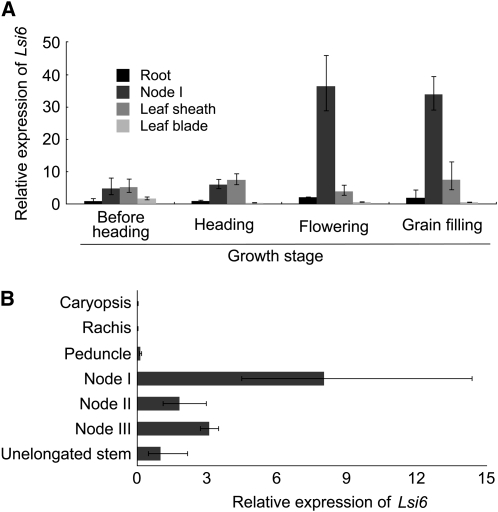
Lsi6 is a homolog of the silicon influx transporter Lsi1 in rice (Ma et al., 2006), which belongs to the nodulin-26-like intrinsic protein III (NIP III) subgroup of aquaporins. Lsi6 is permeable to silicic acid when expressed in Xenopus laevis oocytes (Mitani et al., 2008) and is a plasma membrane protein (Yamaji et al., 2008). At developmental stages before heading, Lsi6 is mainly localized at the xylem parenchyma cells of the leaf sheathes and blades and is responsible for the release of silicic acid from the xylem (Yamaji et al., 2008). However, we found that at the reproductive stage, Lsi6 is highly expressed in node I, which is connected to the flag leaf and panicle. Temporal analysis showed that the expression level of Lsi6 is greatly enhanced after the panicle is completely emerged (Figure 1A). Furthermore, the expression is much higher in node I than in other tissues, including root, leaf blade, and sheath. Spatial analysis of Lsi6 expression at the flowering stage showed that Lsi6 was expressed more in node I than in the other nodes (Figure 1B). Expression of Lsi6 was not observed in the panicle tissues, including caryopsis, rachis, and peduncle (Figure 1B). This expression pattern was not changed using either Histone H3 (Figure 1) or Ubiquitin (see Supplemental Figure 1 online) as an internal standard.
Figure 1.
Expression of Lsi6 in Different Tissues.
(A) Temporal variation of Lsi6 expression in different rice tissues at the reproductive stages from before heading to grain filling.
(B) Spatial expression of Lsi6 in different rice tissues at flowering stage. The expression was determined by quantitative RT-PCR, and expression level of Histone H3 was used as an internal control. Expression relative to the root expression before heading (A) or unelongated stem (B) is shown. Data are means ± sd of three biological replicates (n = 3).
Cell-Type Specificity of Lsi6 Localization
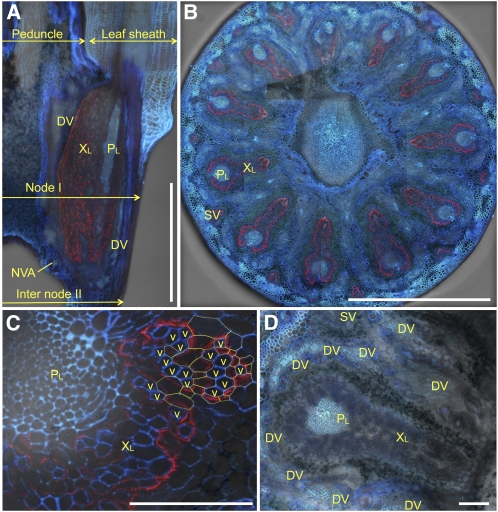
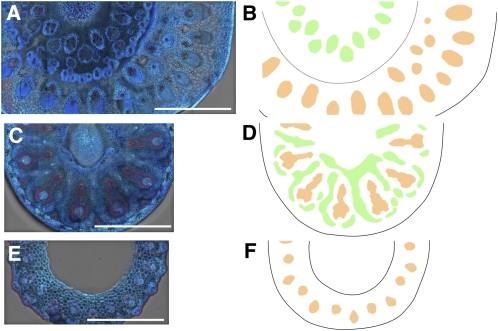
We examined the localization of Lsi6 by immunohistological staining in node I at flowering using a polyclonal antibody specific for Lsi6 (Yamaji et al., 2008). Lsi6 protein is detected mainly in the xylem part of enlarged–large vascular bundles, but not the phloem, in node I (Figure 2A). Lsi6 was localized at the outer boundary of the enlarged region of large and small vascular bundles in node I (Figure 2B), which are connected to the leaf sheath (Kawahara et al., 1974; Chonan et al., 1985; Hoshikawa, 1989) (Figures 2A and 3). Furthermore, observation with larger magnification revealed polar localization of Lsi6, facing toward the numerous xylem vessels in the xylem parenchyma cells of the bundles (Figure 2C). No fluorescence signals of Lsi6 protein were detected in node I of the T-DNA insertion knockout line (Yamaji et al., 2008) (Figure 2D), indicating high specificity of the antibody. In addition, we also conducted an immunohistological staining with neighboring tissues of node I. At the peduncle/leaf sheath above node I, Lsi6 protein was detected in the xylem parenchyma cells of large vascular bundles in flag leaf sheath and not in the vascular bundles of peduncle (Figure 3A). At internode II directly below node I, Lsi6 was also detected in the xylem parenchyma cells (Figure 3E). Unlike the vascular bundles in node I, these vascular bundles are not enlarged; therefore, the role of Lsi6 in these tissues is to release Si from the xylem for local distribution of Si in leaf sheath and internode.
Figure 2.
Localization of Lsi6 Protein in Node I.
Immunostaining of Lsi6 (red) and cell wall UV autofluorescence (blue and cyan) in node I of the wild type ([A] to [C]) and a Lsi6 T-DNA insertion line (D). Xylem and phloem region of large vascular bundles (XL and PL), small vascular bundle (SV), diffuse vascular bundle (DV), and nodal vascular anastomoses (NVA) are shown. Bars = 1 mm in (A) and (B) and 100 μm in (C) and (D).
(A) Longitudinal section of node I.
(B) Cross section at the center of node I.
(C) Enlarged image of cross section of a large vascular bundle. Outlines of some xylem transfer cells are highlighted by yellow broken lines, and the adjacent xylem vessels (v) are indicated.
(D) Xylem and phloem region of large vascular bundles and diffuse vascular bundles in a cross section of the T-DNA line.
Figure 3.
Localization of Lsi6 and Vascular Systems through Node I.
Immunostaining of Lsi6 at a cross section of peduncle (inside)/flag leaf sheath (outside) (A), node I (C), and internode II (E) of the wild type and corresponding schematic diagram of vascular systems ([B], [D], and [F]). Large vascular bundles originating from lower node to flag leaf (orange), diffuse vascular bundles in node I, and derived large vascular bundles in peduncle (green) are indicated. Bars = 1 mm.
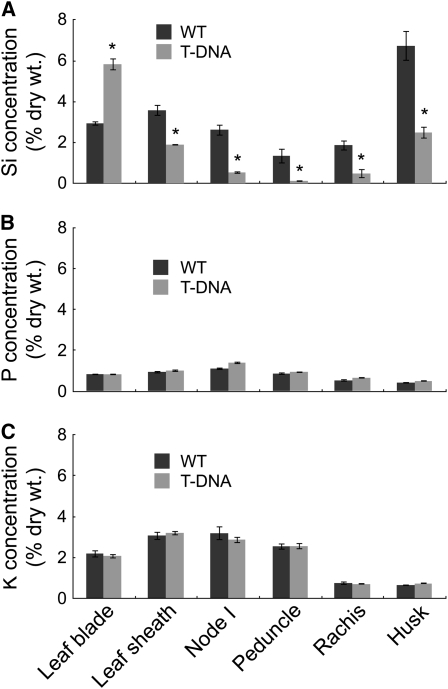
Altered Accumulation of Si
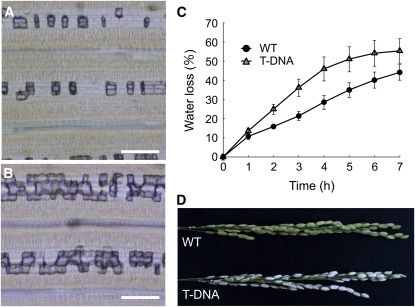
To examine the function of Lsi6 in the distribution of silicon at node I, we compared silicon accumulation from different tissues between the wild-type rice and a T-DNA insertion knockout line (Yamaji et al., 2008). The silicon concentration in the leaf blades was two times higher in the knockout line than in the wild-type rice (Figure 4A), resulting in heavy deposition of Si as silica bodies in the motor cells of the flag leaf blade, known as plant opal (Figures 5A and 5B). By contrast, a significant decrease in silicon concentration was found in the husk, rachis, peduncle, node I, and flag leaf sheath of the T-DNA line compared with the wild-type rice (Figure 4A). The silicon concentration in the husk of the knockout line was less than half that in the wild-type rice, resulting in increased water loss from the panicles and subsequent white head (Figures 5C and 5D). The husk accumulates the most silicon among all tissues in the wild-type plant (Figure 4A), reaching >6% of the dry weight. This high accumulation of silicon is required for optimal and sustainable production of rice (Savant et al., 1997) because silicon deposited in the husk prevents excess water loss from the panicles and reduces infection by fungal pathogens (Ma and Takahashi, 2002). Lower silicon deposition in the husk results in very low fertility and subsequent low yield (Tamai and Ma, 2008). In contrast with silicon, there is no difference in the tissue concentration of potassium and phosphorus between the two lines (Figures 4B and 4C). Because silicon uptake was similar between the wild-type rice and the knockout lines (Yamaji et al., 2008), our results indicate that knockout of Lsi6 selectively alters the pathway of silicon distribution between the panicles and flag leaf.
Figure 4.
Concentration of Si, P, and K in Different Rice Tissues.
Concentration of Si (A), phosphorus (B), and potassium (C) of the wild type and the Lsi6 T-DNA line. Both lines were grown in soil until ripening. Data are means ± sd of three biological replicates. Asterisks above bars indicate significant differences (P < 0.01) between the wild type and the T-DNA by the Student's t test.
Figure 5.
Phenotype of Lsi6 Knockout Line.
(A) and (B) Silica bodies in the flag leaf blade of the wild type (A) and the Lsi6 T-DNA line (B). Bars = 100 μm.
(C) Water loss from excised panicles at grain filling stage. Data are means ± sd of five biological replicates.
(D) Excised panicles after air-drying for 3 h.
DISCUSSION
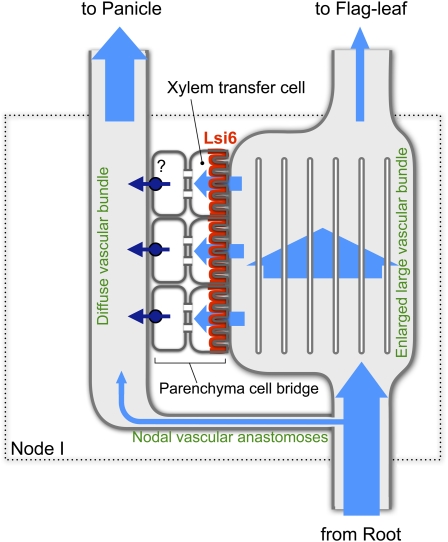
Silicon is taken up by rice roots in the form of silicic acid via two transporters, Lsi1 and Lsi2 (Ma et al., 2006, 2007). Lsi1 functions as an influx transporter of silicon, while Lsi2 as an efflux transporter (Ma et al., 2006, 2007). Both Lsi1 and Lsi2 are localized at the exodermis and endodermis but with different polarities at distal and proximal sides of the cells, respectively (Ma et al., 2006, 2007; Ma and Yamaji, 2008). Silicon transported into the root stele is then translocated to the shoot by transpiration stream through the culm in the form of silicic acid (Casey et al., 2003; Mitani et al., 2005) and then silicic acid is finally delivered to the husk. Vascular bundles that reach to the panicle tissues originate from diffuse vascular bundles in the node (Figures 2A, 3, and 6). Diffuse vascular bundles and large vascular bundles are linked via nodal vascular anastomoses, which are a cyclic vascular system in the basal region of the nodes (Figures 2A and 6) (Kawahara et al., 1974; Hoshikawa, 1989). If silicic acid from large vascular bundles is transferred to the diffuse vascular bundles only via nodal vascular anastomoses, the accumulation of silicon in the panicles (mainly husk) will be very low. This is because the distribution ratio of the water flow for each vasculature depends on transpiration rate of each peripheral organ. The husk has a much smaller surface area than the expanded leaf blades. Furthermore, the husk does not have stomata on the surface; therefore, the transpiration from the husk is much lower than that from the expanded leaf blades. In addition, the husk accumulates more silicon than the leaf blade in the wild-type rice (Figure 4A), indicating that silicon deposition in the tissues does not follow the transpiration rate. The preferential allocation of silicon to the panicle is achieved by an enlarged area of vascular tissues in the node and xylem transfer cells. The area of the large vascular bundles in the node is almost 10 times larger than that of the internode below the node, and the number of both xylem and phloem vessels is also increased (Figures 2A and 3) (Kawahara et al., 1974; Chonan et al., 1985; Hoshikawa, 1989). This enlargement slows down the velocity of the transpiration steam in the xylem vessels of the large vascular bundles at the nodes, allowing the xylem transfer cells surrounding the xylem to take up silicon very efficiently. Transfer cells are specialized parenchyma cells for efficient exchange of nutrients that have an increased surface area due to cell wall ingrowth and infoldings of the plasma membrane (Offler et al., 2002; McCurdy et al., 2008). Xylem transfer cells are formed at xylem parenchyma cells surrounding xylem vessels in the outer boundary region of the enlarged–large vascular bundles in the node of gramineous plants and are proposed to be involved in efficient intervascular transfer of nutrients (Kawahara et al., 1974; Chonan et al., 1985; Offler et al., 2002; McCurdy et al., 2008). Higher expression of Lsi6 at the xylem transfer cells (Figure 2C) suggests that Lsi6 is a silicon transporter that is responsible for intervascular transfer of silicon from large vascular bundles to the diffuse vascular bundles by unloading silicic acid from the transpiration stream (Figure 6).
Figure 6.
Schematic Diagram of Intervascular Transport of Si in Node I.
Flow of silicic acid is indicated by pale-blue arrows. Transpirational flow (containing silicic acid) from the root through large vascular bundles is partially diverted to diffuse vascular bundles via nodal vascular anastomoses following the transpirational rate. Most silicic acid enters into the enlarged–large vascular bundles, where the area and number of vessels are markedly increased, resulting in a slowing down of the transpirational flow. Silicic acid in the decelerated flow is then specifically unloaded by Lsi6 (red) localized at the proximal side of xylem transfer cells, which have unusually expanded surfaces due to cell wall ingrowth facing the vessels. Silicic acid then moves symplastically across the parenchyma cell bridge and finally is reloaded to the diffuse vascular bundles by an unidentified efflux transporter (dark blue).
There are a few layers of parenchyma cells that intervene between the xylem transfer cells surrounding the enlarged–large vascular bundles and the diffuse vascular bundles (Figure 2D). These parenchyma cells are referred to as the parenchyma cell bridge and are connected by large numbers of plasmodesmata (Kawahara et al., 1974; Chonan et al., 1985; Hoshikawa, 1989). Therefore, silicic acid unloaded by Lsi6 at the xylem transfer cells is probably trafficked by the symplastic pathway before entering the diffuse vascular bundles (Figure 6). Since Lsi6 is a channel-type passive transporter belonging to NIP subfamily of plant aquaporins (Mitani et al., 2008), an efflux-type active transporter(s) will be required to reload the silicic acid to the diffuse vascular bundles. This type of transporter may be similar to the silicon efflux transporter Lsi2 expressed in the roots (Ma et al., 2007) and will be identified in the future (Figure 6). Furthermore, after redirection at the node, silicic acid will be transported to the panicles and finally to the husk, in which most silicon is accumulated (Ma and Takahashi, 2002). Since Lsi6 is not expressed in the panicle organs (Figure 1B), other transporters, as yet unidentified, might be required to mediate the further distribution of Si between the husk and other parts.
Another mineral translocation pathway has been proposed in which minerals from the roots are transferred from the xylem to the phloem in the enlarged–large vascular bundles and then are translocated to the grains following source-to-sink phloem flow (Kawahara et al., 1974; Chonan et al., 1985; Hoshikawa, 1989). However, localization of Lsi6 at the distal side of the enlarged–large vascular bundles shows that the major pathway of silicon to the grains is via inter-vascular transfer from the enlarged–large vascular bundles to diffuse vascular bundles, rather than from the xylem-to-phloem transfer. This is also supported by the fact that when Lsi6 was knocked out, the translocation of silicon to the panicles was greatly reduced (Figure 4A). Recently, a boric acid channel from Arabidopsis thaliana (NIP6;1) was reported to be involved in the preferential transport of boron to growing shoot tissues (Tanaka et al., 2008). However, NIP6;1 is located at the phloem especially at the nodal region of the stem and petioles, suggesting that NIP6;1 is responsible for the xylem-phloem transfer of boric acid (Tanaka et al., 2008) but not for the intervascular transfer. This difference between rice Lsi6 and Arabidopsis NIP6;1 may reflect different transport plans of two major groups of vascular plants: monocots and dicots.
Our results demonstrate that Lsi6 is a transporter responsible for intervascular transfer in the node. Intervascular transfer is also required for other minerals to be selectively delivered to the grains. For example, redirection at the node from the xylem stream to newly developing organs, apical meristems, young leaves, or inflorescences, independent of the transpiration stream, has been observed for Zn (Obata and Kitagishi, 1980a, 1980b), Fe (Tsukamoto et al., 2009), and Cd (Kashiwagi et al., 2009). However, the transporters involved in these processes have not been identified.
The identification of the nodal transporter Lsi6 suggests the importance of intervascular transfer in the node of monocot plants. Highly organized vascular systems and the extensive surface area of xylem transfer cells in the node function like a mineral exchange column. Our findings on this nodal transporter may be useful in future studies to selectively enhance essential mineral nutrients in cereal grains and reduce toxic minerals in plants in our food chain.
METHODS
Growth Conditions
Wild-type rice (Oryza sativa cv Dongjin) and the T3 homozygous progeny of T-DNA insertion line (4A-01373; POSTECH RISD) (Jeong et al., 2006; Yamaji et al., 2008) were used. Three-week-old seedlings grown hydroponically were transplanted to a 3.5-liter plastic pot filled with rice paddy soils previously amended with coating fertilizer (14-12-14 for nitrogen-phosphorus-potassium) at a rate of 1 g/kg soil and cultured in a closed green house with natural light. Tap water was supplied daily.
Expression level of Lsi6
Different tissues, including root, nodes, leaf sheath, leaf blade, caryopsis, rachis, and peduncle, were sampled at various growth stages from before heading to the grain filling stage of the soil-cultured wild-type rice. Total RNA was extracted using an RNeasy plant extraction mini kit (Qiagen) according to the manufacturer's instructions. First-strand cDNA was synthesized from 1 μg of total RNA using oligo dT(18) primer and the SuperScript first-strand synthesis system for RT-PCR (Invitrogen). Histone H3 or Ubiquitin was used as an internal standard. Quantitative real-time PCR was performed in 20-μL reaction volume containing 2 μL of 1:5 diluted cDNA and 200 nM each gene-specific primers and SYBR Premix Ex Taq (Takara Bio) using Mastercycler ep realplex (Eppendorf). Primer sequences used are Lsi6, 5′-GAGTTCGACAACGTCTAATCGC-3′ (forward) and 5′-AGTACACGGTACATGTATACACG-3′ (reverse); Histone H3, 5′-AGTTTGGTCGCTCTCGATTTCG-3′ (forward) and 5′-TCAACAAGTTGACCACGTCACG-3′ (reverse); and Ubiquitin, 5′-AGAAGGAGTCCACCCTCCACC-3′ (forward) and 5′-GCATCCAGCACAGTAAAACACG-3′ (reverse).
Measurement of Panicle Transpiration
Transpirational water loss from panicles at the grain-filling stage was measured by weighing the excised panicles under natural air-drying conditions (20°C, 55% relative humidity) periodically for up to 7 h. Dry weight was finally measured after drying in a 70°C oven for 2 d. Panicle water loss was calculated based on the water content difference after drying.
Determination of Si, K, and P
Different plant tissues were harvested and dried at 70°C in an oven for at least 2 d and then ground to a powder. The sample was microwave digested in a mixture of 3 mL of HNO3 (62%), 3 mL of hydrogen peroxide (30%), and 2 mL of hydrofluoric acid (46%) (Microwave Closed System 850; Hansen Co.), and the digested sample was diluted to 50 mL with 4% boric acid. The Si concentration in the digest solution was determined by the colorimetric molybdenum blue method at 600 nm as described earlier (Tamai and Ma, 2008). The concentrations of P and K in the digested solution were determined by flame atomic absorption spectrometry for K (Z-5000; Hitachi) and by the colorimetric molybdenum blue method at a wavelength 882 nm for P.
Detection of Silicified Cells
The flag leaf was decolorized with 70% ethanol at room temperature for 3 d and subsequently stained in a boiled phenol solution containing 0.001% safranin for 5 min (Yamaji et al., 2008). Photographs of stained leafs were taken under optical microscopy (CKX41; Olympus).
Immunostaining of Lsi6 Protein
The synthetic peptide C-SMAADEFDNV (positions 289 to 298 of Lsi6) was used to immunize rabbits to obtain antibodies against Lsi6 (Yamaji et al., 2008). Immunostaining of Lsi6 in node I of the wild type and the T-DNA insertion line was performed as described previously (Yamaji et al., 2008). Fluorescence from secondary antibodies (Alexa Fluor 555 goat anti-rabbit IgG; Molecular Probes) was observed with a fluorescence microscope (Axio Imager with Apotome; Carl Zeiss).
Accession Number
Sequence data from this article can be found in the GenBank/EMBL/DDBJ data libraries under accession number AB253627 (Lsi6).
Supplemental Data
The following material is available in the online version of this article.
Supplemental Figure 1. Expression of Lsi6 in Different Tissues Normalized by Ubiquitin Expression.
Supplementary Material
Acknowledgments
This research was supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Genomics for Agricultural Innovation IPG-006) and a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (17078008 to J.F.M.). We thank Gynheung An at POSTECH Biotech Center for providing T-DNA rice seeds.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Jian Feng Ma (maj@rib.okayama-u.ac.jp).
Online version contains Web-only data.
References
- Casey, W.H., Kinrade, S.D., Knight, C.T.G., Rains, D.W., and Epstein, E. (2003). Aqueous silicate complexes in wheat, Triticum aestivam L. Plant Cell Environ. 27 51–54. [Google Scholar]
- Chonan, N., Kawahara, H., and Matsuda, T. (1985). Ultrastructure of elliptical and diffuse bundles in the vegetative nodes of rice. Jpn. J. Crop. Sci. 54 393–402. [Google Scholar]
- Epstein, E., and Bloom, A.J. (2005). Mineral Nutrition of Plants: Principles and Perspectives. (Sunderland, MA: Sinauer Associates).
- Hoshikawa, K. (1989). The Growing Rice Plant. (Tokyo: Nobunkyo).
- Jeong, D.H., et al. (2006). Generation of a flanking sequence-tag database for activation-tagging lines in japonica rice. Plant J. 45 123–132. [DOI] [PubMed] [Google Scholar]
- Kashiwagi, T., Shindoh, K., Hirotsu, N., and Ishimaru, K. (2009). Evidence for separate translocation pathways in determining cadmium accumulation in grain and aerial plants in rice. BMC Plant Biol. 9 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara, H., Chonan, N., and Matsuda, T. (1974). Studies on morphogenesis in rice plants 7. The morphology of vascular bundles in the vegetative nodes of the culm. Jpn. J. Crop. Sci. 43 389–401. [Google Scholar]
- Ma, J.F., and Takahashi, E. (2002). Soil, Fertilizer, and Plant Silicon Research in Japan. (Amsterdam: Elsevier Science).
- Ma, J.F., Tamai, K., Yamaji, N., Mitani, N., Konishi, S., Katsuhara, M., Ishiguro, M., Murata, Y., and Yano, M. (2006). A silicon transporter in rice. Nature 440 688–691. [DOI] [PubMed] [Google Scholar]
- Ma, J.F., and Yamaji, N. (2008). Functions and transport of silicon in plants. Cell. Mol. Life Sci. 65 3049–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J.F., Yamaji, N., Mitani, N., Tamai, K., Konishi, S., Fujiwara, T., Katsuhara, M., and Yano, M. (2007). An efflux transporter of silicon in rice. Nature 448 209–212. [DOI] [PubMed] [Google Scholar]
- McCurdy, D.W., Patrick, J.W., and Offler, C.E. (2008). Wall ingrowth formation in transfer cells: Novel examples of localized wall deposition in plant cells. Curr. Opin. Plant Biol. 11 653–661. [DOI] [PubMed] [Google Scholar]
- Mitani, N., Ma, J.F., and Iwashita, T. (2005). Identification of the silicon form in xylem sap of rice (Oryza sativa L.). Plant Cell Physiol. 46 279–283. [DOI] [PubMed] [Google Scholar]
- Mitani, N., Yamaji, N., and Ma, J.F. (2008). Characterization of substrate specificity of a rice silicon transporter, Lsi1. Pflugers Arch. 456 679–686. [DOI] [PubMed] [Google Scholar]
- Obata, H., and Kitagishi, K. (1980. a). Time course of zinc or manganese accumulation within individual leaves. Jpn. J. Soil Sci. Plant Nutri. 51 292–296. [Google Scholar]
- Obata, H., and Kitagishi, K. (1980. b). Investigation on pathway of zinc transport in vegetative node of rice plant by autoradiography. Jpn. J. Soil Sci. Plant Nutri.
- Offler, C.E., McCurdy, D.W., Patrick, J.W., and Talbot, M.J. (2002). Transfer cells: Cells specialized for a special purpose. Annu. Rev. Plant Biol. 54 431–454. [DOI] [PubMed] [Google Scholar]
- Savant, N.K., Snyder, G.H., and Datnoff, L.E. (1997). Silicon management and sustainable rice production. Adv. Agron. 58 151–199. [Google Scholar]
- Tamai, K., and Ma, J.F. (2008). Reexamination of silicon effects on rice growth and production under field conditions using a low silicon mutant. Plant Soil 307 21–27. [Google Scholar]
- Tanaka, M., Wallace, I.S., Takano, J., Roberts, D.M., and Fujiwara, T. (2008). NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 20 2860–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto, T., Nakanishi, H., Uchida, H., Watanabe, S., Matsuhashi, S., Mori, S., and Nishizawa, N.K. (2009). 52Fe translocation in barley as monitored by a positron-emitting tracer imaging system (PETIS): Evidence for the direct translocation of Fe from roots to young leaves via phloem. Plant Cell Physiol. 50 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji, N., Mitani, N., and Ma, J.F. (2008). A transporter regulating silicon distribution in rice shoots. Plant Cell 20 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.