Abstract
Reactive oxygen species (ROS) exert pleiotropic effects on a wide array of signaling proteins that regulate cellular growth and apoptosis. This study shows that long-term treatment with a low concentration of H2O2 leads to the activation of signaling pathways involving extracellular signal-regulated kinase, ribosomal protein S6 kinase, and protein kinase D (PKD) that increase cAMP binding response element protein (CREB) phosphorylation at Ser133 in cardiomyocytes. Although CREB-Ser133 phosphorylation typically mediates cAMP-dependent increases in CREB target gene expression, the H2O2-dependent increase in CREB-Ser133 phosphorylation is accompanied by a decrease in CREB protein abundance and no change in Cre-luciferase reporter activity. Mutagenesis studies indicate that H2O2 decreases CREB protein abundance via a mechanism that does not require CREB-Ser133 phosphorylation. Rather, the H2O2-dependent decrease in CREB protein is prevented by the proteasome inhibitor lactacystin, by inhibitors of mitogen-activated protein kinase kinase or protein kinase C activity, or by adenoviral-mediated delivery of a small interfering RNA that decreases PKD1 expression. A PKD1-dependent mechanism that links oxidative stress to decreased CREB protein abundance is predicted to contribute to the pathogenesis of heart failure by influencing cardiac growth and apoptosis responses.
CREB is a bZip transcription factor that binds to specific DNA elements (termed cAMP-response elements or CREs) within the regulatory regions of CREB target genes; many genes with CREs in their promoters play key roles in cellular proliferation and apoptosis pathways. CREB is regulated via phosphorylation at Ser133, a modification variably attributed to protein kinase A (PKA), calcium/calmodulin-dependent kinase, p90 kDa ribosomal S6 kinase (RSK, an effector of the ERK-mitogen-activated protein kinase pathway), mitogen- and stress-activated protein kinase 1, protein kinase D (PKD), or AKT (depending upon the cell type or inciting stimulus) (Johannessen et al., 2004). CREB-Ser133 phosphorylation increases CREB-dependent gene transcription at least in part by recruiting coactivators (CREB binding protein or CBP) to the promoters of CREB target genes. In the heart, CREB is implicated in the maintenance of normal ventricular structure and function (Fentzke et al., 1998). CREB protein expression decreases in a pacing-induced cardiac memory model in dogs that is characterized by a specialized form of electrical remodeling involving alterations in the T-wave morphology during sinus rhythm (Patberg, 2005). There is recent evidence that pacing protocols that induce cardiac memory lead to enhanced tissue markers of oxidative stress (Özgen et al., 2007). It is tempting to speculate that this increase in ROS mediates the pacing-induced decrease CREB protein expression in cardiomyocytes, because oxidative stress decreases CREB protein abundance, depresses CRE-dependent gene transcription, and enhances apoptosis in neuronal and vascular smooth muscle cell models (Pugazhenthi et al., 2003; Reusch and Klemm, 2003; Tokunou et al., 2003). However, the ROS-activated pathways that regulate CREB protein abundance have not been examined. Moreover, although an ROS-dependent increase in CREB-Ser133 phosphorylation is detected in many cellular models, the role of CREB-Ser133 phosphorylation in the ROS-dependent decrease in CREB protein expression also has not been considered.
Reactive oxygen species (ROS) such as H2O2, superoxide, and hydroxy radicals contribute to the pathogenesis of ischemia-reperfusion injury, cardiac hypertrophy, heart failure, and other cardiovascular disorders associated with aging. ROS regulate the growth and survival of cardiomyocytes by modulating the activity of a wide array of signaling enzymes and transcription factors. However, cellular responses to oxidative stress can vary substantially, depending on the magnitude and duration of the ROS stimulus (Kwon et al., 2003). Low levels of ROS (typically associated with the activation of receptors that induce cardiac hypertrophy) act as second messengers to regulate signal transduction pathways that increase protein synthesis, promote cardiomyocyte growth, and/or induce ischemic preconditioning, typically without impairing cell survival. Higher levels of oxidative stress (that accompany cardiac ischemia and reperfusion injury) result in cell death, either through apoptosis and/or frank necrosis. Whereas oxidative stress has been linked to the activation of an array of signaling enzymes with CREB-Ser133 kinase activity, certain CRE-regulated genes (such as the cytoprotective Bcl-2 protein that inhibits the mitochondrial apoptosis pathway) are paradoxically down-regulated by oxidative stress (Pugazhenthi et al., 2003; Valks et al., 2003). This study examines the ROS-regulated pathways that regulate CREB protein abundance and function in cardiomyocytes.
Materials and Methods
Cardiomyocyte Culture and Adenoviral Infections.
Cardiomyocytes were isolated from the hearts of 2-day-old Wistar rats by a trypsin dispersion procedure using a differential attachment procedure to enrich for cardiomyocytes followed by irradiation as detailed in previous publications (Steinberg et al., 1991; Ozgen et al., 2008). The final yield of cells was typically 2.5 to 3 × 106 per neonatal heart. Cells were plated on protamine sulfate-coated culture dishes at a density of 5 × 106 cells/100-mm dish. Experiments were performed on cultures grown for 5 days in minimal essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum and then serum-deprived for a subsequent 24 h interval.
Cardiomyocytes were infected with adenoviral constructs that drive the expression of β-galactosidase (β-gal), wild-type CREB (WT-CREB), nonphosphorylatable CREB-Ser133A (a CREB construct with a serine 133 to alanine mutation, generously provided by Dr. Jane Reusch), or an adenovirus that silences PKD1 expression (Ad-PKD1-RNAi, generously provided by Dr. Metin Avkiran); in each case, multiplicity of infection was 20 plaque forming units per cell.
Western Blotting.
Western blotting was performed on cell extracts according to methods described previously or the manufacturer's instructions (Ozgen et al., 2008; Rybin et al., 2009). The anti-PKD1-pSer742 (numbering based on human sequence, corresponding to Ser748 in rodent PKD1) was from Abcam Inc. (Cambridge, MA). All other antibodies were from Cell Signaling Technology (Danvers, MA). Studies that validate the specificity of the phospho-site-specific antibodies (PSSAs) that recognize the phosphorylated forms of PKD1 have been published previously (Jacamo et al., 2008; Rybin et al., 2009). Results in each figure represent data from a single experiment, at a single gel exposure, with all results replicated in at least three experiments on separate culture preparations.
Luciferase Assays.
pCRE-luciferase (Stratagene, La Jolla, CA) and Renilla reniformis luciferase (Promega, Madison, WI) vectors were introduced into cardiomyocytes using a nucleofector kit (Amaxa Biosystems, Gaithersburg, MD) according to manufacturers' instructions. Cre-luciferase signals were normalized to luminescence from R. reniformis (to eliminate interassay variability and control for agonist-dependent changes in transfection efficiency or cell viability) using a dual-luciferase reporter assay system (Promega) according to methods described previously (Ozgen et al., 2008).
Statistical Analysis.
All results are expressed as means ± S.E.M. Comparisons were made using one-way analysis of variance or Student's t test. p < 0.05 was considered significant.
Results
H2O2 Decreases CREB Protein Content in Cardiomyocytes.
Initial studies examined the effects of a range of H2O2 concentrations on signaling pathways that are predicted to regulate CREB-Ser133 phosphorylation in cardiomyocytes. Stimulations were performed at 15 and 60 min to discriminate transient responses from the more long-term changes in signal transduction pathways that are more likely to regulate gene expression and growth responses.
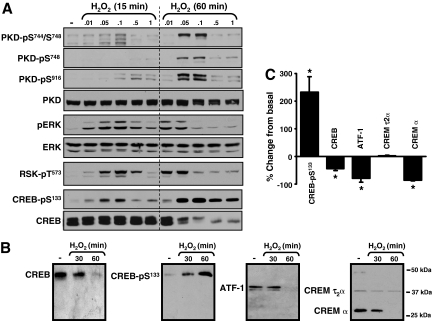
Figure 1A shows that H2O2 activates at least two enzymes with CREB-Ser133 kinase activity. H2O2 induces a rapid increase in the phosphorylation of ERK and its downstream target RSK, a known CREB-Ser133 kinase. All H2O2 concentrations increase ERK-RSK phosphorylation at the 15-min time point, although activation of the ERK-RSK cascade is most robust at 0.05 to 0.1 mM H2O2. The ERK-RSK pathway is activated by even lower H2O2 concentrations (0.01–0.05 mM) when the stimulation interval is prolonged to 60 min. H2O2 also activates PKD, a family of three structurally related serine/threonine kinases (PKD1, PKD2, and PKD3) that recently also have been implicated as CREB-Ser133 kinases (Johannessen et al., 2007; Ozgen et al., 2008). PKD activation is generally attributed to a phosphorylation-dependent mechanism involving protein kinase C (PKC). PKD activation is tracked by immunoblot analysis with PSSAs that specifically recognize the PKC-dependent phosphorylation of PKD at a pair of highly conserved serine residues in the activation loops of all three PKD isoforms (corresponding to Ser744 and Ser748 in rodent PKD1). PKD activation also is detected with a PSSA that recognizes an autophosphorylation at Ser916, a site conserved at the extreme C termini of PKD1 and PKD2, that is not conserved in PKD3; PKD1-Ser916 autophosphorylation is widely used as a surrogate marker of PKD activation. Figure 1A shows that H2O2 increases PKD phosphorylation at both the activation loop (at Ser744 and Ser748) and at the C-terminal autophosphorylation site (at Ser916). However, the effect of H2O2 to activate PKD is delayed, relative to H2O2-dependent ERK-RSK activation pathway. PKD activation is prominent in cardiomyocytes treated with 0.05 to 0.1 mM H2O2 for 60 min; little to no PKD activation is detected in cardiomyocytes treated with H2O2 for 15 min (or with H2O2 concentrations less than 0.05 mM or greater than 0.1 mM for 60 min).
Fig. 1.
H2O2-activated signaling pathways that regulate CREB protein abundance and CREB-Ser133 phosphorylation in neonatal rat cardiac myocytes. Cell extracts from cardiomyocytes treated with the indicated concentrations of H2O2 for 15 or 60 min were subjected to Western blotting with the indicated antibodies. Representative data are depicted in A and B with the results for CREB-Ser133 phosphorylation and CREB, CREMτ2α, CREMα, and ATF-1 protein content after a 60-min treatment with 0.05 mM H2O2 quantified in C. In B, the molecular mass markers are applied to all Western blots. *, p < 0.05 versus control (n = 5).
H2O2 also increases CREB-Ser133 phosphorylation at both 15 and 60 min. The CREB-Ser133 phosphorylation response at 15 min is greatest in cardiomyocytes treated with 0.05 to 0.1 mM H2O2; CREB-Ser133 phosphorylation is less robust in cardiomyocytes treated for 15 min with either higher or lower H2O2 concentrations. This concentration-response relationship for H2O2-dependent CREB-Ser133 phosphorylation parallels the concentration-response relationship for H2O2-dependent ERK-RSK activation. A 15-min treatment with H2O2 increases CREB-Ser133 phosphorylation without changing CREB protein abundance.
H2O2 increases CREB-Ser133 phosphorylation in association with a profound decrease in CREB protein abundance at 60 min. This is not due to a generalized decrease in protein recovery (and gross cytotoxicity), because the recovery of other proteins such as PKD and ERK is not impaired under these conditions. The antithetical effects of H2O2 on CREB protein abundance and CREB-Ser133 phosphorylation also can not be attributed to an effect of H2O2 to increase the phosphorylation of other CREB/ATF transcription factor family members such as CREM and ATF-1 that in theory could be recognized by the anti-CREB-pSer133 antibody. Figure 1B shows that CREM and ATF-1 are detected in neonatal rat cardiomyocytes, but their electrophoretic mobilities are considerably faster than the electrophoretic mobility of the CREB protein. CREM is detected as two isoforms, with mobilities corresponding to the CREM isoforms generated as a result of alternative splicing in uterine myometrium. H2O2 treatment leads to a decrease in abundance of the smaller CREMα isoform (molecular mass of 28 kDa, which has been implicated as a transcriptional repressor), but no change in the abundance of the larger CREMt2α isoform (molecular mass of 39 kDa, which has been implicated as a transcriptional activator). H2O2 treatment also leads to a decrease in the abundance of ATF-1. Additional studies showed that the anti-CREB-pSer133 antibody recognizes a band that comigrates with CREB but it does not detect bands that comigrate with CREM or ATF-1 in H2O2-treated cardiomyocytes. Effects of H2O2 on CREB, CREMτ2α, CREMα, and ATF-1 protein abundance, and CREB-Ser133 phosphorylation, are quantified in Fig. 1C.
Figure 1A shows that treatment with 0.1 mM H2O2 for 60 min leads to an increase in CREB-Ser133 phosphorylation in association with a marked increase in PKD phosphorylation and only a low level of ERK-RSK activation. Higher H2O2 concentrations (0.5–1 mM) increase CREB-Ser133 phosphorylation without increasing ERK-RSK or PKD phosphorylation, presumably through a mechanism that involves the activation of a different CREB-Ser133 kinase or the inhibition of a CREB-Ser133 phosphatase.
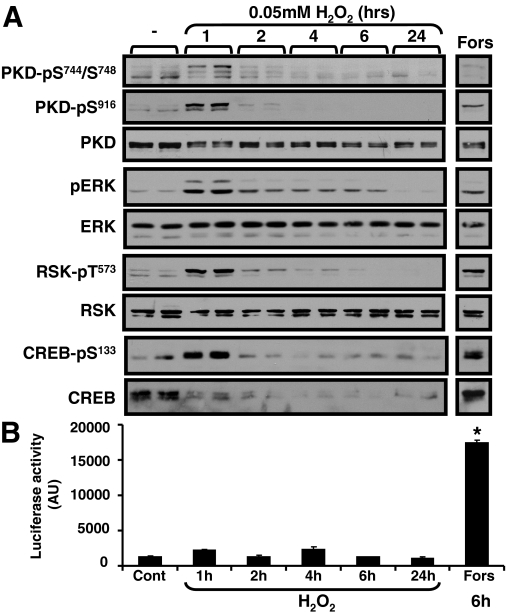
Figure 2 provides a more detailed analysis of H2O2 regulation of CREB, showing that 0.05 mM H2O2 induces a transient increase in CREB-Ser133 phosphorylation that is confined to the initial 60 min of stimulation; CREB-Ser133 phosphorylation wanes when the H2O2 stimulation interval is prolonged to 2 to 24 h. In contrast, treatment with 0.05 mM H2O2 leads to a decrease CREB protein abundance that is sustainable for up to 72 h. Control experiments show that forskolin (an adenylyl cyclase activator that increases PKA activity) increases CREB-Ser133 phosphorylation without decreasing CREB protein content. These results indicate that long-lasting changes in CREB-Ser133 phosphorylation do not necessarily lead to decreased CREB protein abundance in cardiomyocytes.
Fig. 2.
H2O2 increases CREB-Ser133 phosphorylation without increasing CRE-luciferase activity in cardiomyocytes. A, cardiomyocytes were treated with vehicle, H2O2 (50 μM), or forskolin (10 μM) for indicated time intervals, and cell extracts were subjected to Western blotting with indicated antibodies. B, the CRE-firefly luciferase reporter construct and a R. reniformis luciferase vector (included as an internal control to normalized for differences in transfection efficiency) were introduced into cardiomyocytes by electroporation. Cells were treated with vehicle, H2O2 (0.05 mM), or forskolin (Fors, 10 μM, as a positive control) for indicated intervals, and CRE-firefly luciferase activity was corrected for minor differences in R. reniformis luciferase activity (n = 4). *, p < 0.05 versus control.
Although CREB-Ser133 phosphorylation is sufficient to induce a CRE-dependent transcription response in cells treated with agonists that activate the cAMP-PKA pathway, the CREB transcriptional activity in cardiomyocytes treated with H2O2 is difficult to predict because CREB-Ser133 phosphorylation is generally not sufficient to induce transcription in response to agonist that activate other growth regulatory pathways and the stimulatory effect of increased CREB-Ser133 phosphorylation might be functionally offset by the decrease in CREB protein abundance. Therefore, effects of H2O2 on CRE-luciferase assay were directly examined. Figure 2B shows that treatment with 0.05 mM H2O2 does not lead to a detectable increase in CRE-luciferase activity (at any time point from 1–24 h). Control experiments show that a 6-h stimulation with forskolin leads to a robust increase in CRE-luciferase activity. These results indicate that the H2O2-dependent increase in CREB-Ser133 phosphorylation is not associated with a transcriptional response.
H2O2-Dependent Signaling Pathways That Regulate CREB Protein: The Role of Ser133 Phosphorylation.
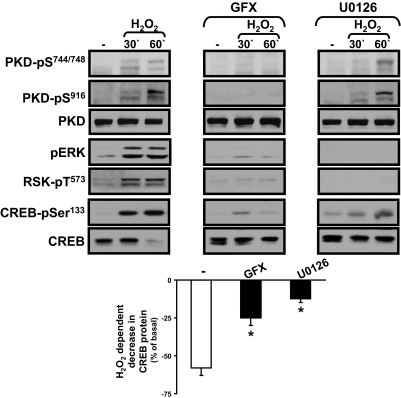
The kinetic studies suggest that H2O2 might promote CREB-Ser133 phosphorylation via a different signaling pathway at very short (15–30 min) and more protracted (60 min) stimulation intervals, because H2O2 increases CREB-Ser133 phosphorylation in association with a pronounced increase in ERK-RSK activity (and little to no increase in PKD activity) at 15–30 min, whereas 0.1 mM H2O2 increases CREB-Ser133 phosphorylation in association with a pronounced increase in PKD and only a low level of ERK-RSK activation at 60 min. We used pharmacological inhibitors as an initial strategy to examine the role of ERK-RSK and PKC-PKD pathways in the H2O2-dependent mechanism that regulates CREB.
Figure 3 shows that GF109203X (an inhibitor of PKC isoforms that also displays some inhibitory activity toward RSK) abrogates H2O2-dependent ERK-RSK and PKD activation and CREB-Ser133 phosphorylation at both 30 and 60 min; GF109203X also prevents the H2O2-dependent decrease in CREB protein content. In contrast, U0126 (an inhibitor of MEK, the upstream activation of ERK) blocks the rapid H2O2-dependent increase in ERK, RSK, and CREB-Ser133 phosphorylation at 30 min and the late H2O2-dependent decrease in CREB protein content at 60 min; U0126 does not interfere with the H2O2-dependent pathway that activates PKD, and it does not prevent the late H2O2-dependent increase in CREB-Ser133 phosphorylation at 60 min. The observation that U0126 prevents the H2O2-dependent decrease in CREB protein content, without fully blocking CREB-Ser133 phosphorylation, suggests that H2O2 decreases CREB protein content via a mechanism that does not require Ser133 phosphorylation. It is noteworthy that whereas H2O2 treatment leads to the activation of many other signaling pathways (Clerk et al., 1998; Guo et al., 2009), inhibitors that abrogate the H2O2-dependent increases in p38-mitogen-activated protein kinase, c-Jun NH2-terminal kinase, or AKT activity did not prevent the H2O2-dependent decrease in CREB protein expression (data not shown).
Fig. 3.
The role of ERK-RSK and PKD in H2O2-dependent regulation of CREB. Immunoblotting on cell extracts from cardiomyocytes pretreated for 45 min with vehicle, GF109203X (GFX, 10 μM), or U0126 (5 μM) and then challenged with vehicle or H2O2 (0.05 mM) for the indicated intervals. Representative results are depicted on the top, with the results quantified at the bottom. *, p < 0.05 versus control (n = 3).
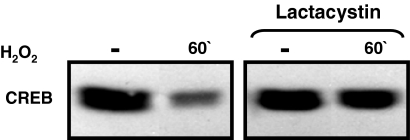
On the basis of previous studies showing that CREB is polyubiquitinated and targeted to the proteosome for degradation in pancreatic β-cells treated with high glucose (Costes, 2009), and that CREB is targeted for proteasomal degradation via a phosphorylation-dependent mechanism in hypoxic epithelial cells (Taylor, 2000), we examined whether the H2O2-dependent decreases in CREB protein abundance can be blocked by lactacystin (an inhibitor of protein degradation by the proteosome). Figure 4 shows that lactacystin prevents the H2O2-dependent decrease in CREB protein abundance, suggesting a role for the proteasomal degradation pathway.
Fig. 4.
Lactacystin prevents the H2O2-dependent decrease in CREB protein content. Extracts from cultures pretreated for 30 min with lactacystin (10 μM) and then challenged for 1 h with vehicle or H2O2 (0.05 mM) were subjected to Western blotting for CREB phosphorylation and CREB protein content. Similar results were obtained in two other experiments.
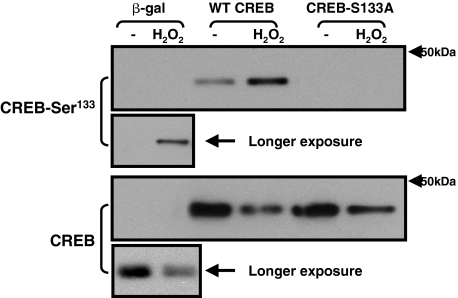
We then examined the role of CREB-Ser133 phosphorylation in the H2O2-dependent mechanism that regulates CREB protein abundance using a mutagenesis strategy. Cardiomyocytes were infected with adenoviruses that drive the overexpression of WT- and S133A-substituted forms of CREB and then treated with vehicle or 0.05 mM H2O2 for 60 min. Figure 5 shows that the effect of H2O2 to regulate CREB is retained in this overexpression model. H2O2 treatment leads to a similar increase in Ser133 phosphorylation and a decrease in CREB protein abundance for both the endogenous CREB protein (in β-gal infected cultures) and the heterologously overexpressed WT-CREB transgene. The CREB mutant harboring an S133A substitution (that is not regulated by H2O2 through Ser133 phosphorylation) also is down-regulated by the H2O2 treatment protocol, indicating that H2O2 down-regulates CREB protein content via a proteosomal mechanism that does not require Ser133 phosphorylation.
Fig. 5.
H2O2 decreases CREB protein content through a mechanism that does not require CREB-Ser133 phosphorylation. Adenoviral-mediated gene transfer was used to overexpress β-gal WT-CREB, or CREB-Ser133A. Cultures were treated with vehicle or H2O2 (0.05 mM) for 1 h, and cell extracts were subjected to Western blotting for CREB phosphorylation and CREB protein content. Similar results were obtained in two other experiments.
H2O2 Down-Regulates CREB via a PKD1-Dependent Mechanism.
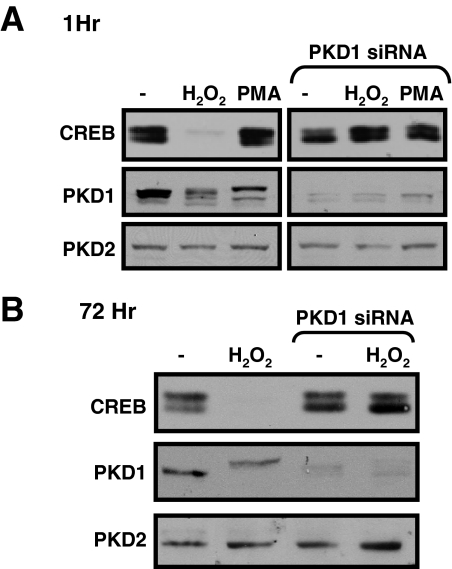
The observation that GF109203X prevents the H2O2-dependent decrease in CREB protein abundance suggests that CREB protein levels are regulated by PKC and/or a PKC-activated enzyme such as PKD. Because the specificity of most available pharmacological inhibitors is imperfect, we used a gene-silencing approach to examine the role of PKD1 in the H2O2-dependent mechanism that regulates CREB protein expression in cardiomyocytes. Figure 6 shows that an adenoviral vector designed to silence PKD1 expression (Ad-PKD1-RNAi) specifically decreases PKD1 expression (without any associated changes in PKD2 expression) and that PKD1 down-regulation also prevents the H2O2-dependent decrease in CREB protein expression (at both 60 min and 72 h). Collectively, these experiments implicate PKD1 as an H2O2-activated enzyme that regulates CREB protein abundance via a mechanism that does not require CREB-Ser133 phosphorylation.
Fig. 6.
PKD1 knockdown prevents the H2O2-dependent decrease in CREB protein content. Cardiomyocyte cultures were infected with the Ad-PKD1-RNAi or Ad-β-gal as a control. Cells were treated with vehicle or 0.05 mM H2O2 for 1 (A) or 72 h (B) (starting 3 days after adenoviral infections). Cell extracts were subjected to immunoblot analysis for PKD1, PKD2, or CREB protein content. Data were replicated in three separate experiments on separate culture preparations.
Discussion
The major findings of this study are the following: 1) low physiological concentrations of H2O2 activate the ERK-RSK and PKD pathways, promote CREB-Ser133 phosphorylation, and decrease CREB protein content in cultured neonatal rat cardiomyocytes; 2) the H2O2-dependent increase in CREB-Ser133 phosphorylation is not sufficient to induce a CRE-dependent transcriptional response; and 3) H2O2 decreases CREB protein content via a mechanism that requires PKD1 but does not require CREB phosphorylation at Ser133. Although CREB-Ser133 phosphorylation leads to increased CREB-mediated transcription in cardiomyocytes treated with forskolin (an agonist that increases cAMP), the H2O2-dependent pathway leading to CREB-Ser133 phosphorylation does not increase CRE-luciferase activity. Similar discrepancies between CREB-Ser133 phosphorylation and CRE-dependent gene transcription have been identified in studies of other agonists that act via cAMP-PKA-independent pathways. A high level of CREB-Ser133 phosphorylation that is not associated with a CRE-dependent transcriptional response has been attributed to inhibitory modifications elsewhere on CREB that destabilize the CREB-CBP complex (Wagner et al., 2000; Mayr et al., 2001) and/or the failure to recruit additional signal-regulated coactivators that cooperate with CBP/p300 to regulate CREB target gene expression (Ravnskjaer et al., 2007). The ROS-dependent decrease in CREB protein abundance identified in this study might constitute a third mechanism that would conspire to limit CREB target gene expression.
These studies provide novel evidence that H2O2 decreases CREB protein abundance via a mechanism involving PKD1. The initial focus on PKD as a potential mediator of ROS-dependent effects on CREB was based on recent studies implicating PKD as a CREB-Ser133 kinase. Although studies with pharmacological inhibitors suggest that PKD contributes to the H2O2-dependent CREB-Ser133 phosphorylation response under certain conditions, studies with the CREB-Ser133A mutant indicate that H2O2 regulates CREB protein levels via a PKD-dependent pathway that does not require Ser133 phosphorylation. Other mechanisms must be considered. In theory, PKD might regulate CREB protein levels by phosphorylating CREB at a site other than Ser133. In fact, Johannessen et al. (2007) identified Ser98 (which resides in a PKD consensus phosphorylation motif) as an alternative site on CREB that functions in vitro as a phosphoacceptor site for PKD. Although these investigators did not detect PKD-Ser98 phosphorylation in epidermoid carcinoma cells treated with PKD-activating stimuli or in human embryonic kidney 293 cells engineered to ectopically overexpress an activated form of the PKD1 enzyme, an H2O2-dependent pathway involving PKD (or a PKD-activated effector) that regulates CREB phosphorylation at a site other than Ser133 in cardiomyocytes remains possible and is the focus of ongoing studies. Alternatively, the H2O2-dependent decrease in CREB protein abundance might be due to the phosphorylation of a different cellular PKD substrate that regulates CREB protein levels indirectly.
The observation that H2O2 decreases CREB protein abundance via a mechanism that is specific for PKD1 is quite significant. In general, studies that have used RNAi or genetic knockout strategies to down-regulate PKD1 have exposed a high level of functional redundancy between PKD1 and other PKD isoforms that are coexpressed in most cells. For example, agonist-dependent pathways involving PKD that promote class II histone deacetylase phosphorylation in lymphocytes and cardiomyocytes are inhibited only when PKD1 and other PKD isoforms are silenced simultaneously (Matthews, 2006). The loss of PKD1 alone also does not prevent agonist-dependent CREB-Ser133 phosphorylation responses in epidermoid carcinoma cells (Johannessen et al., 2007). Preliminary studies show that the PKD1 down-regulation protocol (that abrogates the H2O2-dependent decrease in CREB protein abundance) also does not prevent CREB-Ser133 phosphorylation in cardiomyocytes (data not shown), providing further evidence that Ser133 phosphorylation is not required for H2O2-dependent regulation of CREB protein abundance.
Although the gene-silencing studies implicate PKD1 in the H2O2-dependent pathway that regulates CREB protein content, pharmacological studies with U0126 suggest that the MEK-ERK pathway also regulates this process. Although MEK-ERK and PKD1 pathways might act independently, PKD1 could regulate CREB protein abundance indirectly by controlling signaling via the ERK cascade, because PKD1 can phosphorylate RIN, a Ras binding protein that prevents Ras-Raf interactions; RIN dissociates from Ras when it is phosphorylated by PKD, leading to enhanced signaling via the Raf-ERK pathway (Wang, 2002).
Decreased CREB protein expression is implicated in the pathogenesis of certain rodent models of oxidative stress-induced vascular injury and atherosclerosis. This study implicates PKD1 in a similar ROS-regulated pathway that decreases CREB expression and disrupts CREB-mediated transcription in cardiomyocytes. This mechanism may contribute to the acquisition of cardiac memory, a specialized form of electrical remodeling that follows a period of altered ventricular activation and is characterized by increased tissue markers of oxidative stress, decreased CREB protein abundance, decreased expression of KChIP2 (an accessory subunit of the transient outward potassium current Ito that contains a cAMP response element in its promoter), and altered T-wave morphology (Patberg et al., 2005). An oxidative stress-dependent mechanism involving PKD1 that disrupts CREB responses also may contribute to the pathogenesis of age-related diseases such as diabetes and lipid disorders that are characterized by increased oxidative stress.
These studies were supported by United States Public Health Service and National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL67101, HL77860, and T32-HL076116].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- CREB
- cAMP binding response element protein
- β-gal
- β-galactosidase
- CRE
- cAMP response element
- GF109203X
- 3-[1-[3-(dimethylaminopropyl]-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione monohydrochloride
- ERK
- extracellular signal-regulated kinase
- MEK
- mitogen-activated protein kinase kinase
- PKA
- protein kinase A
- PKC
- protein kinase C
- PKD
- protein kinase D
- PSSA
- phospho-site-specific antibodies
- ROS
- reactive oxygen species
- RSK
- p90 kDa ribosomal S6 kinase
- WT
- wild type
- CBP
- cAMP binding response element protein binding protein
- ATF-1
- activating transcription factor 1
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene.
References
- Clerk A, Fuller SJ, Michael A, Sugden PH. (1998) Stimulation of “stress-regulated” mitogen-activated protein kinases (stress-activated protein kinases/c-Jun N-terminal kinases and p38-mitogen-activated protein kinases) in perfused rat hearts by oxidative and other stresses. J Biol Chem 273:7228–7234 [DOI] [PubMed] [Google Scholar]
- Costes S, Vandewalle B, Tourrel-Cuzin C, Broca C, Linck N, Bertrand G, Kerr-Conte J, Portha B, Pattou F, Bockaert J, et al. (2009) Degradation of cAMP-responsive element-binding protein by the ubiquitin-proteasome pathway contributes to glucotoxicity in beta-cells and human pancreatic islets. Diabetes 58:1105–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentzke RC, Korcarz CE, Lang RM, Lin H, Leiden JM. (1998) Dilated cardiomyopathy in transgenic mice expressing a dominant-negative CREB transcription factor in the heart. J Clin Invest 101:2415–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Gertsberg Z, Ozgen N, Steinberg SF. (2009) p66Shc links alpha1-adrenergic receptors to a reactive oxygen species-dependent AKT-FOXO3A phosphorylation pathway in cardiomyocytes. Circ Res 104:660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacamo R, Sinnett-Smith J, Rey O, Waldron RT, Rozengurt E. (2008) Sequential PKC-dependent and PKC-independent protein kinase D catalytic activation via Gq-coupled receptors: differential regulation of activation loop Ser744 and Ser748 phosphorylation. J Biol Chem 283:12877–12887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen M, Delghandi MP, Moens U. (2004) What turns CREB on? Cell Signal 16:1211–1227 [DOI] [PubMed] [Google Scholar]
- Johannessen M, Delghandi MP, Rykx A, Dragset M, Vandenheede JR, Van Lint J, Moens U. (2007) Protein kinase D induces transcription through direct phosphorylation of the cAMP-response element-binding protein. J Biol Chem 282:14777–14787 [DOI] [PubMed] [Google Scholar]
- Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. (2003) H2O2 regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol 35:615–621 [DOI] [PubMed] [Google Scholar]
- Matthews SA, Liu P, Spitaler M, Olson EN, McKinsey TA, Cantrell DA, Scharenberg AM. (2006) Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol Cell Biol 26:1569–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr BM, Canettieri G, Montminy MR. (2001) Distinct effects of cAMP and mitogenic signals on CREB-binding protein recruitment impart specificity to target gene activation via CREB. Proc Natl Acad Sci U S A 98:10936–10941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgen N, Obreztchikova M, Guo J, Elouardighi H, Dorn GW, 2nd, Wilson BA, Steinberg SF. (2008) Protein kinase D links Gq-coupled receptors to cAMP response element-binding protein (CREB)-Ser133 phosphorylation in the heart. J Biol Chem 283:17009–17019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özgen N, Plotnikov AN, Shlapakova I, Dankort D, Steinberg SF, Rosen MR. (2007) Abstract 508: a reactive oxygen species-mediated PKC-ERK-RSK pathway decreases the cyclic AMP response element binding protein and may initiate transcriptional changes in cardiac memory. Circulation 116:II-88 [Google Scholar]
- Patberg KW, Obreztchikova MN, Giardina SF, Symes AJ, Plotnikov AN, Qu J, Chandra P, McKinnon D, Liou SR, Rybin AV, et al. (2005) The cAMP response element binding protein modulates expression of the transient outward current: implications for cardiac memory. Cardiovasc Res 68:259–267 [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Jambal P, Audesirk G, Kern M, Cabell L, Eves E, Rosner MR, Boxer LM, Reusch JE. (2003) Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J Neurochem 84:982–996 [DOI] [PubMed] [Google Scholar]
- Ravnskjaer K, Kester H, Liu Y, Zhang X, Lee D, Yates JR, 3rd, Montminy M. (2007) Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. EMBO J 26:2880–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch JE, Klemm DJ. (2003) Cyclic AMP response element-binding protein in the vessel wall: good or bad? Circulation 108:1164–1166 [DOI] [PubMed] [Google Scholar]
- Rybin VO, Guo J, Steinberg SF. (2009) Protein kinase D1 autophosphorylation via distinct mechanisms at Ser744/Ser748 and Ser916. J Biol Chem 284:2332–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SF, Robinson RB, Lieberman HB, Stern DM, Rosen MR. (1991) Thrombin modulates phosphoinositide metabolism, cytosolic calcium, and impulse initiation in the heart. Circ Res 68:1216–1229 [DOI] [PubMed] [Google Scholar]
- Taylor CT, Furuta GT, Synnestvedt K, Colgan SP. (2000) Phosphorylation-dependent targeting of cAMP response element binding protein to the ubiquitin/proteasome pathway in hypoxia. Proc Natl Acad Sci USA 97:12091–12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunou T, Shibata R, Kai H, Ichiki T, Morisaki T, Fukuyama K, Ono H, Iino N, Masuda S, Shimokawa H, et al. (2003) Apoptosis induced by inhibition of cyclic AMP response element-binding protein in vascular smooth muscle cells. Circulation 108:1246–1252 [DOI] [PubMed] [Google Scholar]
- Valks DM, Kemp TJ, Clerk A. (2003) Regulation of Bcl-xL expression by H2O2 in cardiac myocytes. J Biol Chem 278:25542–25547 [DOI] [PubMed] [Google Scholar]
- Wagner BL, Bauer A, Schütz G, Montminy M. (2000) Stimulus-specific interaction between activator-coactivator cognates revealed with a novel complex-specific antiserum. J Biol Chem 275:8263–8266 [DOI] [PubMed] [Google Scholar]
- Wang Y, Waldron RT, Dhaka A, Patel A, Riley MM, Rozengurt E, Colicelli J. (2002) The RAS effector RIN1 directly competes with RAF and is regulated by 14-3-3 proteins. Mol Cell Biol 22:916–926 [DOI] [PMC free article] [PubMed] [Google Scholar]