Abstract
Microscopic colitis (MC) is an inflammatory condition of the colon distinct from Crohn disease or ulcerative colitis that can cause chronic diarrhea as well as cramping and bloating. Although it was first described 30 years ago, awareness of this entity as a cause of diarrhea has only become more widespread recently. Up to 20% of adults with chronic diarrhea who have an endoscopically normal colonoscopy may have MC. Endoscopic and radiological examinations are usually normal, but histology reveals increased lymphocytes in the colonic mucosa, which typically cause watery nonbloody diarrhea. Treatment is initially supportive but can include corticosteroids and immunomodulatory therapy for resistant cases. Since surgeons perform a large number of colonoscopies and sigmoidoscopies to assess diarrhea, it is important to be aware of this disease and to look for it with mucosal biopsy in appropriate patients.
Abstract
La colite microscopique (CM) est une inflammation du côlon différente de la maladie de Crohn ou de la colite ulcéreuse, et qui peut causer une diarrhée chronique, des crampes et du ballonnement. Même si on l’a décrite pour la première fois il y a 30 ans, la connaissance de cette entité comme cause de diarrhée ne s’est généralisée que récemment. Jusqu’à 20 % des adultes présentant une diarrhée chronique et dont la coloscopie est normale sur le plan endoscopique peuvent être atteints de CM. L’endoscopie et la radiologie donnent habituellement des résultats normaux, mais l’histologie révèle une élévation des lymphocytes dans la muqueuse du côlon, ce qui cause typiquement une diarrhée aqueuse non sanglante. Le traitement initial consiste à donner du soutien, mais peut inclure l’administration de corticostéroïdes et d’immunomodulateurs dans les cas résistants. Comme les chirurgiens pratiquent de nombreuses coloscopies et sigmoïdoscopies pour évaluer la diarrhée, il importe d’être conscient de cette maladie et de la rechercher par biopsie de la muqueuse chez les patients qui semblent présenter ce profil.
Microscopic colitis (MC) is a common and previously under-recognized cause of chronic diarrhea. In 1 study, MC was found in 10% of all patients with nonbloody diarrhea referred for colonoscopy and in almost 20% of those older than 70 years.1 Collagenous colitis (CC) and lymphocytic colitis (LC) are 2 morphologically distinct entities of MC. They are similar in presentation but differ histologically. The hallmark of diagnosis in MC is specific histological changes in the setting of colonic mucosa that appear to be endoscopically normal. Because these entities were only first described in the 1970s2,3 and because the main reports on incidence have only surfaced in the last few years, there is a concern that MC is not a commonly noted diagnosis. In addition, at least 1 study has shown that MC is diagnosed less commonly in smaller nonacademic centres.4 Consequently, the purpose of our review is to highlight the epidemiology, etiology, diagnosis and management of MC for the surgical endoscopist.
Epidemiology
The incidence of MC has been estimated to be 4.2–10.0 per 100 0001,5–8 (Table 1). Notably, 2 North American studies have incidence rates of 8.6 and 10.0 per 100 000, respectively, which may reflect a more accurate estimate for Canadian populations. The condition classically presents in adulthood, with the peak age of onset being in the sixth to seventh decades of life.6,10,13 A female predominance has been described in several studies,6,10,14 and this appears to be stronger in CC than LC. Rarely, MC can present in childhood.15–17
Table 1.
Incidence rates of microscopic colitis reported in the literature
| Country | Time period | No. | Incidence per 100 000 | ||
|---|---|---|---|---|---|
| CC | LC | MC | |||
| France9 | 1987–1992 | 22 | 0.6 | — | — |
| Sweden10 | 1984–1993 | 30 | 1.8 | — | — |
| Sweden1 | 1993–1998 | 97 | 4.9 | 4.4 | 9.3 |
| Spain6 | 1993–1997 | 60 | 1.1 | 3.1 | 4.2 |
| Iceland11 | 1995–1999 | 125 | 5.2 | 4.0 | 9.2 |
| United States8 | 1985–2001 | 130 | 3.1 | 5.5 | 8.6 |
| Canada12 | 2002–2004 | 164 | 4.6 | 5.4 | 10.0 |
CC = collagenous colitis; LC = lymphocytic colitis; MC = microscopic colitis.
Studies from both Europe and North America have shown an apparent increase in the incidence in MC over time.1,8 However, it is not clear whether this represents an escalating awareness of the disease or intensified diagnostic efforts.
Etiology
The mechanisms involved in the development of MC are unknown. However, there seems to be an association with bile acid malabsorption, infectious agents, nonsteroidal anti-inflammatory drugs (NSAIDs), other drugs, smoking and autoimmune conditions. It has been hypothesized that bile salts play a role in the development of MC. This was based on some studies that suggested an increase in bile malabsorption and that some patients report symptomatic improvement with bile acid binding agents.18,19 Bile acid malabsorption can occur following cholecystectomy,19,20 and thus it has been hypothesized that it may be a risk factor for MC. However, it is now evident that prior cholecystectomy is not associated with MC. A recent case–control study compared 130 patients with MC and 130 matched controls. The MC group had 12 patients with prior cholecystectomy compared with 17 in the control group (p = 0.32).20 In the same study, there was no link found between previous appendectomies and MC.20 Furthermore, the degree of bile salt malabsorption does not appear to correlate well with the incidence of diarrhea postcholecystectomy.21
An infectious etiology has also been proposed for MC. Historically, some patients report a preceding infectious enteropathy. Furthermore, some studies have reported a substantial clinical response to antibiotics.13 No specific infectious agent has been identified in patients with MC.
Some studies have reported a significant association between the use of NSAIDs and MC. One such study showed 60% of patients with CC had substantial NSAID use compared with less than 15% of matched controls.22 A more recent study showed that those with CC more commonly consumed NSAIDs (46.2%v 23%, odds ratio [OR] 2.9, 95% confidence interval [CI] 1.3–6.4) and selective serotonin reuptake inhibitors (SSRIs; 18%v. 1%, OR 21, 95% CI 2.5–177), than controls, whereas those with LC more commonly consumed SSRIs (28%v. 1%, OR 37.7, 95% CI 4.7–304), β-blockers (13 vs. 3%, OR 4.79, 95% CI 1.04–20), statins (13%vs 3%, OR 4.6, 95% CI 1.04–20) and biphosphonates (8%v. 0%).23 Other agents, including some proton pump inhibitors,24 ticlopidine14,25,26 and flutamide,14,26 have been linked with MC in case studies (Box 1). Although there are a few reports of symptom improvement with cessation of NSAIDs,13,27,28 this has not been adequately studied with the other agents mentioned.
Box 1.
Drugs that have been associated with microsopic colitis
Acarbose
Acetylsalicylic acid (ASA)
Cirkan
Lansoprazole
Nonsteroidal anti-inflammatory drugs (NSAIDs)
Ranitidine
Sertraline
Ticlopidine
Microscopic colitis is commonly seen in patients with underlying autoimmune disease. Celiac disease, asthma, diabetes, thyroiditis and arthritis have all been associated with MC.29–34 In 1 study, one-third of celiac disease patients have histological changes compatible with MC,35 and thus MC should be suspected in patients with celiac disease who are not responding to a gluten-free diet. Those with Hashimoto thyroiditis also have a marked increase risk of MC, with one study reporting that 40% (20/50) of those with Hashimoto thyroiditis had histologic findings consistent with LC.33
Natural History
Microscopic colitis has a variable course. Symptoms of diarrhea and abdominal pain may precede diagnosis for months. These symptoms are generally mild, and most patients’ symptoms resolve spontaneously or with medication.
Although controversial, there does not appear to be an increased risk of progression to other inflammatory bowel diseases or colorectal cancers in patients with MC.36,37 A small study of 24 patients showed no evidence of ulcerative colitis or Crohn disease at a minimum follow-up of 6 years.37 Chan and colleagues38 identified 117 patients with CC through the Johns Hopkins Registry and looked specifically at the possible risk of associated colorectal cancer. No cases of colorectal cancer were identified, with follow-up ranging from 2 to 12 years.38 However, it is difficult to draw definitive conclusions with these small sample sizes, and larger population-based studies are warranted to further evaluate these questions.
Diagnosis
Diagnosis of MC relies on a thorough history and adequate sampling of mucosa through colonic biopsy. A comprehensive history should begin with onset type and duration of symptoms. Features that could suggest celiac disease or inflammatory bowel disease should be detailed. Other causes of chronic diarrhea such as infections, inflammatory bowel disease, neuroendocrine or autoimmune disorders as well as drug-induced diarrhea must be ruled out. In general, radiological investigations, including barium studies, are not useful.7
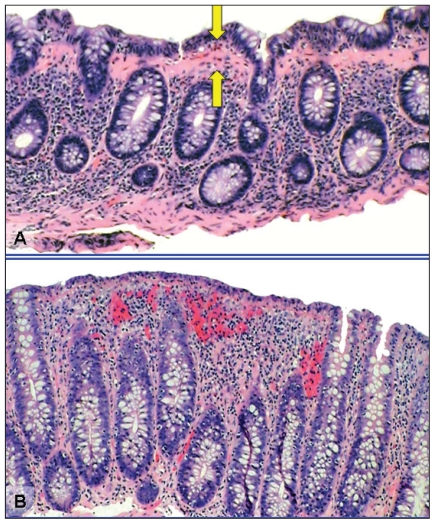
Endoscopic biopsy is required for the diagnosis of MC. As there are no mucosal abnormalities in MC, the biopsies taken must be random. On histology, both CC and LC demonstrate lymphocytic infiltration of the lamina propria and epithelium. Collagenous colitis is differentiated from LC by the presence of marked thickening of the subepithelial collagen layer (Fig. 1).7 There is currently no consensus on whether flexible sigmoidoscopy or colonoscopy is the best first-line treatment.39 The benefits of flexible sigmoidoscopy include time efficiency, cost savings, easier bowel preparation and no sedation. Some studies state that biopsies of the left colon alone may be sufficient.40 Fine and colleagues40 reported on 809 patients evaluated for chronic diarrhea who had no endoscopic abnormalities on colonoscopy. Eighty (10%) were found to have MC, all of whom had evidence of disease in the left colon. Furthermore, 99.7% of patients with any histopathological abnormalities had evidence of disease in the left colon.40 The benefits of flexible sigmoidoscopy must be weighed against the potentially increased diagnostic yield of colonoscopy. Tanaka and colleagues22 reported 10% of all patients with MC had isolated right-sided disease. Thijs and colleagues41 demonstrated isolated histopathological evidence of MC in the ascending or transverse colon in 3 of 13 patients. Furthermore, a full colonoscopy is often indicated to rule out other differential diagnoses, including inflammatory bowel disease and colorectal cancer. If a flexible sigmoidoscopy is chosen as the initial investigation and is negative, a colonoscopy should be completed. Since MC is a relatively newly recognized entity, we recommend the biopsies be reviewed by a pathologist with a special interest in gastroenterology.
Fig. 1.
Histological features of microscopic colitis. (A) Collagenous colitis is associated with a increased lymphocytic infiltrate of the lamina propria and intraepithelial lymphocytosis. There is a markedly thickened (> 10 μm) collagen band underlying the colonic epithelial cells (arrows). (B) Lymphocytic colitis is associated with an increased lymphocytic infiltrate of the lamina propria and intraepithelial lymphocytosis. The collagen band underlying the colonic epithelial cells is normal in width (< 10 μm). (Hematoxylin and eosin stain, original magnification × 100.)
Treatment
General approach
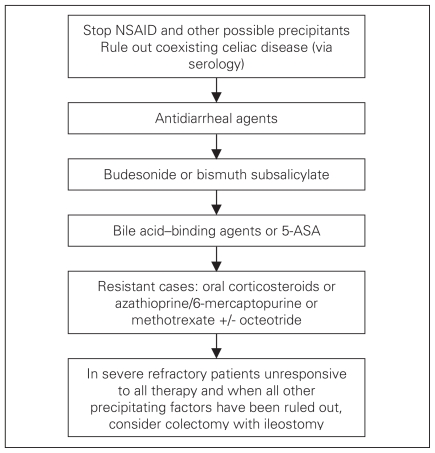
Most have adopted treating the individual patient in a stepwise approach, balancing potential side effects of the agent with the severity and chronicity of symptoms. A suggested treatment algorithm is presented in Figure 2. Treatment approaches with the exception of budesonide are based on little or low-quality evidence. It should be noted that most therapies have been tested in either CC or LC patients, but not both. However, it is reasonable to apply these therapies in both conditions. Prior to starting medical therapy, medications such as NSAIDs that may aggravate symptoms or even contribute in pathogenesis should be stopped if possible. Since celiac disease is a common condition in North America affecting up to 1 in 300–500 people and since there is a described association between celiac disease and MC, we suggest that all MC patients undergo serologic testing for celiac disease (i.e., antiendomysial antibody or tissue transglutaminase with an IgA level).
Fig. 2.
Suggested algorithm for the treatment of microscopic colitis. 5-ASA = 5-aminosalicylic acid; NSAID = nonsteroidal anti-inflammatory drugs.
Antidiarrheal medications
Nonspecific antidiarrheal therapy such as loperamide has been commonly used in the management of MC. Retrospective studies have suggested benefit with doses ranging from 2–16 mg per day.30,42 Owing to the relative safety of this agent and the possibility of spontaneous remission, we suggest loperamide as a first-line therapy for MC. In our experience, however, those with moderate to severe diarrhea or associated substantial abdominal pain often fail to respond to antidiarrheal therapy alone.
Budesonide
Budesonide is the most thoroughly studied agent in the treatment of MC. Three randomized studies have been undertaken with budesonide in patients with CC, all of which were positive.43 Significant clinical and histological improvements were seen in all treated groups (OR 12.32, 95% CI 5.53–27.46 for clinical response), with a number needed to treat of 2 patients. Dosage was 9 mg orally taken daily for 6 weeks, and the medication was generally well tolerated with only a small fraction of the typical side effects of systemic glucocorticoids. Based on the results of these studies, we recommend budesonide therapy in patients who do not respond to loperimide, and generally we have found that patients with severe diarrhea and/or substantial abdominal cramping usually do not respond well to antidiarrhea therapy alone. We have found that patients appear to have fewer relapses when the budesonide is tapered slowly, and commonly we prescribe 9 mg per day for 1 month, followed by 6 mg per day for 1 month, followed by 3 mg per day for 1 month. Often after putting the patient into remission with this 3-month approach, they can use loperimide and/or bismuth subsalicylate even if they failed to respond to these agents in the past, suggesting that milder MC is more responsive to these agents. If patients experience a relapse of their symptoms that is not responsive to loperimide and/or bismuth subsalicylate, often a shorter course of entocort is effective (9 mg/d for 7 d, followed by 6 mg/d for 7 d, followed by 3 mg/d for 7 d).
Bismuth subsalicylate
There is only 1 published study assessing the use bismuth subsalicylate in patients with MC. Fine and Lee44 performed a prospective open-label study in which 13 patients with MC (7 with CC) were treated with 8 chewable 262-mg tablets per day for 8 weeks. Eleven of 12 patients who completed the trial had resolution of their diarrhea, with histological improvement noted in 9 of 12 patients. After a follow-up period of 7–28 months, 9 patients remained well and 2 required retreatment. A subsequent randomized placebo-controlled trial of 9 patients published in abstract form by the same group45 had similar positive findings with a dosage of 3 tablets of 262 mg taken 3 times daily for 8 weeks. In our experience, many patients do not tolerate the substantial amount of bismuth required. For this reason, we recommend bismuth subsalicylate as second-line therapy and generally use it in patients with milder disease. We have also used it with success in patients who responded to budesonide but who had a mild flare-up when treatment was stopped.
Bile acid–binding agents
Bile-acid binders have been used as treatment for MC. Bohr and colleagues13 reported a 59% response rate to cholestyramine in a retrospective series. Subsequently, Ung and colleagues18 found that cholestyramine at a dose of 4 g per day or colestipol at a dose of 5 g per day resulted in a rapid, marked or complete improvement in 78% of patients and in 92% of the patients with bile-acid malabsorption. A recent study also suggests that cholestyramine may have some incremental benefit in CC patients treated with mesalamine.46 The use of bile acid–binding agents is therefore reasonable, especially in those with known bile-acid malabsorption and among those in whom MC developed following cholecystectomy.
5-aminosalicylic acid
The use of 5-aminosalicylic acid (5-ASA) products is well established in other inflammatory bowel diseases, although, to our knowledge, no placebo-controlled studies with mesalamine or sulfasalazine in the treatment of MC have been described. A recent study of 64 MC patients tested mesalamine (800 mg 3 times daily) against mesalamine plus cholestyramine, with 84% of patients overall achieving resolution of diarrhea within 2 weeks and 85% of LC and 91% of CC patients in clinical remission at 6 months.46 Since these agents are generally less expensive than budesonide, have few side effects and have some evidence of efficacy, we still recommend these agents in the management of MC, but only if treatment with more potent agents such as budesonide has not been successful.
Resistant cases
In most settings, patients respond to budesonide therapy. Occasionally other corticosteroids can be tried. An open-label series of prednisolone (50 mg daily) in 6 CC patients showed a substantial decrease in stool frequency and a trend toward reduction of inflammatory histology, but relapse was rapid when therapy was stopped.47 A subsequent randomized placebo-controlled trial of 12 MC patients using 50 mg of prednisolone daily for 2 weeks showed a trend toward improvement in stool frequency in the treated group, but it was not statistically significant.48 Those with frequent relapses or those who cannot be tapered off budesonide may respond to oral immune modulators. Pardi and colleagues49 reported retrospective analyses of all the patients treated at the Mayo clinic for MC with azathioprine and 6-mercaptopurine. All patients were either refractory to steroids or dependent on steroids. A total of 9 patients with MC were identified (6 with CC). Four of 8 patients who were on steroids had resolution of diarrhea and withdrawal of steroids with the introduction of either azathioprine or 6-mercaptopurine. The other patients were able to taper or discontinue steroids but had continued mild diarrhea. Severe diarrhea persisted in 1 patient, requiring an ileostomy. Methotrexate has also been shown to be of benefit in a small number of patients.50 A single case report also suggested that octreotide may be of benefit in controlling diarrhea in severe refractory cases.51 A randomized placebo-controlled trial of probiotics (Lactobacillus acidophilus and Bifidobacterium animalis subsp. Lactis) was not effective in patients with CC.52
Surgical management
In rare cases when all medical options have been exhausted unsuccessfully, MC can be treated surgically. One study involved 9 female patients who underwent ileostomy creation for MC.53 Medical management with sulfasalazine, mepacrine, corticosteroids, mesalamine and antibiotics in these patients was unsuccessful.53 In all patients, diversion of the fecal stream resulted in resolution of symptoms. Three patients underwent ileostomy closure and experienced a relapse of symptoms. In a histopathologic study, Munch and colleagues54 found that fecal stream diversion via temporary loop ileostomy resulted in clinical remission and histopathologic resolution. However, subsequent restoration of bowel continuity resulted in a relapse of symptoms and histopathologic recurrence of CC. Varghese and colleagues55 noted a case of a single patient with LC treated initially with fecal diversion through an end ileostomy. Six months later, she underwent a restorative proctocolectomy (without temporary ileostomy). Of note, the colon specimen still had microscopic changes consistent with LC despite 6 months of fecal diversion. Although a definite conclusion cannot be drawn, these reports suggest a potential benefit from restorative proctocolectomy in patients with MC refractory to medical management. Restorative proctocolectomy has been performed in rare cases.56,57 Again, most patients will respond to medical management of MC and such surgical approaches should only be considered for patients in whom all other forms of therapy have been unsuccessful, in whom all other possible precipitating factors have been ruled out and only in those with debilitating diarrhea.
Conclusion
Microscopic colitis is a common cause of diarrhea in the general population and more so in elderly patients. It can be associated with autoimmune diseases such as celiac disease, thyroid problems and diabetes. Since MC is a relatively recently described entity, it likely was not adequately covered in medical school and residency curricula before 1995–2000. Since most patients have excellent response to therapy, we feel it is critical for the surgeon involved in the assessment of patients with diarrhea to be well aware of the approach to diagnose and manage MC.
Footnotes
Competing interests: None declared.
Contributors: Drs. Datta, Brar, Andrews, Dupre, Buie and Beck designed the study. Drs. Datta, Dupre, Ball and Beck acquired and analyzed the data. Drs. Datta, Brar, Andrews, Ball and Beck wrote the article, which Drs. Datta, Andrews, Dupre, Ball, Buie and Beck reviewed. All authors approved final publication.
References
- 1.Olesen M, Eriksson S, Bohr J, et al. Microscopic colitis: a common diarrhoeal disease. An epidemiological study in Orebro, Sweden, 1993–1998. Gut. 2004;53:346–50. doi: 10.1136/gut.2003.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman HJ, Weinstein WM, Shnitka TK, et al. Watery diarrhea syndrome associated with a lesion of the colonic basement membrane-lamina propria interface. Ann R Coll Phys Surg Can. 1976;9:45. [Google Scholar]
- 3.Lindstrom CG. ‘Collagenous colitis’ with watery diarrhoea — A new entity. Pathol Eur. 1976;11:87–9. [PubMed] [Google Scholar]
- 4.Harewood GC, Olson JS, Mattek NC, et al. Colonic biopsy practice for evaluation of diarrhea in patients with normal endoscopic findings: results from a national endoscopic database. Gastrointest Endosc. 2005;61:371–5. doi: 10.1016/s0016-5107(04)02594-5. [DOI] [PubMed] [Google Scholar]
- 5.Olesen M, Eriksson S, Bohr J, et al. Lymphocytic colitis: a retrospective clinical study of 199 Swedish patients. Gut. 2004;53:536–41. doi: 10.1136/gut.2003.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Banares F, Salas A, Forne M, et al. Incidence of collagenous and lymphocytic colitis: a 5-year population-based study. Am J Gastroenterol. 1999;94:418–23. doi: 10.1111/j.1572-0241.1999.00870.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdo AA, Urbanski SJ, Beck PL. Lymphocytic and collagenous colitis: the emerging entity of microscopic colitis. An update on pathophysiology, diagnosis and management. Can J Gastroenterol. 2003;17:425–32. doi: 10.1155/2003/404857. [DOI] [PubMed] [Google Scholar]
- 8.Pardi DS, Loftus EVJ, Smyrk TC, et al. The epidemiology of microscopic colitis: a population-based study in Olmsted County, Minnesota. Gut. 2007;56:504–8. doi: 10.1136/gut.2006.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raclot G, Queneau P, Ottignon Y. Incidence of collagenous colitis. A retrospective study in the east of France. Gastroenterology. 1994;106:A23. [Google Scholar]
- 10.Bohr J, Tysk C, Eriksson S, et al. Collagenous colitis in Orebro, Sweden, an epidemiological study 1984–1993. Gut. 1995;37:394–7. doi: 10.1136/gut.37.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agnarsdottir M, Gunnlaugsson O, Orvar KB, et al. Collagenous and lymphocytic colitis in Iceland. Dig Dis Sci. 2002;47:1122–8. doi: 10.1023/a:1015058611858. [DOI] [PubMed] [Google Scholar]
- 12.Williams JJ, Kaplan GG, Makhija S, et al. Microscopic colitis-defining incidence rates and risk factors: a population-based study. Clin Gastroenterol Hepatol. 2008;6:35–40. doi: 10.1016/j.cgh.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Bohr J, Tysk C, Eriksson S, et al. Collagenous colitis: a retrospective study of clinical presentation and treatment in 163 patients. Gut. 1996;39:846–51. doi: 10.1136/gut.39.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baert F, Wouters K, D’Haens G, et al. Lymphocytic colitis: A distinct clinical entity? A clinicopathological confrontation of lymphocytic and collagenous colitis. Gut. 1999;45:375–81. doi: 10.1136/gut.45.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gremse DA, Boudreaux CW, Manci EA. Collagenous colitis in children. Gastroenterology. 1993;104:906–9. doi: 10.1016/0016-5085(93)91030-l. [DOI] [PubMed] [Google Scholar]
- 16.Deslandres C, Moussavou-Kombilia JB, Russo P, et al. Steroid-resistant lymphocytic enterocolitis and bronchitis responsive to 6-mercaptop-urine in an adolescent. J Pediatr Gastroenterol Nutr. 1997;25:341–6. doi: 10.1097/00005176-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Mahajan L, Wyllie R, Goldblum J. Lymphocytic colitis in a pediatric patient: a possible adverse reaction to carbamazepine. Am J Gastroenterol. 1997;92:2126–7. [PubMed] [Google Scholar]
- 18.Ung KA, Gillberg R, Kilander A, et al. Role of bile acids and bile acid binding agents in patients with collagenous colitis. Gut. 2000;46:170–5. doi: 10.1136/gut.46.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Banares F, Esteve M, Salas A, et al. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci. 2001;46:2231–8. doi: 10.1023/a:1011927302076. [DOI] [PubMed] [Google Scholar]
- 20.Laing AW, Pardi DS, Loftus EV, Jr, et al. Microscopic colitis is not associated with cholecystectomy or appendectomy. Inflamm Bowel Dis. 2006;12:708–11. doi: 10.1097/00054725-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Sauter GH, Moussavian AC, Meyer G, et al. Bowel habits and bile acid malabsorption in the months after cholecystectomy. Am J Gastroenterol. 2002;97:1732–5. doi: 10.1111/j.1572-0241.2002.05779.x. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Mazzoleni G, Riddell RH. Distribution of collagenous colitis: utility of flexible sigmoidoscopy. Gut. 1992;33:65–70. doi: 10.1136/gut.33.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Banares F, Esteve M, Espinós JC, et al. Drug consumption and the risk of microscopic colitis. Am J Gastroenterol. 2007;102:324–30. doi: 10.1111/j.1572-0241.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 24.Hilmer SN, Heap TR, Eckstein RP, et al. Microscopic colitis associated with exposure to lansoprazole. Med J Aust. 2006;184:185–6. doi: 10.5694/j.1326-5377.2006.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 25.Chande N, Driman DK, Reynolds RP. Collagenous colitis and lymphocytic colitis: patient characteristics and clinical presentation. Scand J Gastroenterol. 2005;40:343–7. doi: 10.1080/00365520510011623. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Banares F, Salas A, Esteve M, et al. Collagenous and lymphocytic colitis. Evaluation of clinical and histological features, response to treatment, and long-term follow-up. Am J Gastroenterol. 2003;98:340–7. doi: 10.1111/j.1572-0241.2003.07225.x. [DOI] [PubMed] [Google Scholar]
- 27.Miquel Plaza J, Lopez SanRoman A, del Pozo D, et al. Evolution and treatment response in microscopic colitis. Gastroenterol Hepatol. 2001;24:433–9. [PubMed] [Google Scholar]
- 28.Goff JS, Barnett JL, Pelke T, et al. Collagenous colitis: histopathology and clinical course. Am J Gastroenterol. 1997;92:57–60. [PubMed] [Google Scholar]
- 29.Jean R, Durand JM, Cretel E, et al. Lymphocytic colitis and Gougerot-Sjogren syndrome. Report of two cases. Rev Med Interne. 1999;20:923–5. doi: 10.1016/s0248-8663(00)80098-3. [DOI] [PubMed] [Google Scholar]
- 30.Bohr J, Tysk C, Yang P, et al. Autoantibodies and immunoglobulins in collagenous colitis. Gut. 1996;39:73–6. doi: 10.1136/gut.39.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kandemir O, Utas C, Gonen O, et al. Colonic subepithelial collagenous thickening in diabetic patients. Dis Colon Rectum. 1995;38:1097–100. doi: 10.1007/BF02133986. [DOI] [PubMed] [Google Scholar]
- 32.Taccari E, Spada S, Giuliani A, et al. Co-occurrence of psoriatic arthritis with collagenous colitis: clinicopathologic findings of a case. Clin Rheumatol. 2002;21:335–8. doi: 10.1007/s100670200088. [DOI] [PubMed] [Google Scholar]
- 33.Cindoruk M, Tuncer C, Dursun A, et al. Increased colonic intraepithelial lymphocytes in patients with Hashimoto’s thyroiditis. J Clin Gastroenterol. 2002;34:237–9. doi: 10.1097/00004836-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Pariente EA, Chaumette MT, Maitre F, et al. Collagenous colitis, IgA deficiency, Basedow’s disease and atrophic gastritis. Gastroenterol Clin Biol. 1985;9:738–41. [PubMed] [Google Scholar]
- 35.Breen EG, Farren C, Connolly CE, et al. Collagenous colitis and coeliac disease. Gut. 1987;28:364. doi: 10.1136/gut.28.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pokorny CS, Kneale KL, Henderson CJ. Progression of collagenous colitis to ulcerative colitis. J Clin Gastroenterol. 2001;32:435–8. doi: 10.1097/00004836-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Bonderup OK, Folkersen BH, Gjersoe P, et al. Collagenous colitis: a long-term follow-up study. Eur J Gastroenterol Hepatol. 1999;11:493–5. [PubMed] [Google Scholar]
- 38.Chan JL, Tersmette AC, Offerhaus GJ, et al. Cancer risk in collagenous colitis. Inflamm Bowel Dis. 1999;5:40–3. doi: 10.1097/00054725-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Eisen GM, Dominitz JA, Faigel DO, et al. Use of endoscopy in diarrheal illnesses. Gastrointest Endosc. 2001;54:821–3. doi: 10.1016/s0016-5107(01)70085-5. [DOI] [PubMed] [Google Scholar]
- 40.Fine KD, Seidel RH, Do K. The prevalence, anatomic distribution, and diagnosis of colonic causes of chronic diarrhea. Gastrointest Endosc. 2000;51:318–26. doi: 10.1016/s0016-5107(00)70362-2. [DOI] [PubMed] [Google Scholar]
- 41.Thijs WJ, van Baarlen J, Kleibeuker JH, et al. Microscopic colitis: prevalence and distribution throughout the colon in patients with chronic diarrhoea. Neth J Med. 2005;63:137–40. [PubMed] [Google Scholar]
- 42.Zins BJ, Tremaine WJ, Carpenter HA. Collagenous colitis: mucosal biopsies and association with fecal leukocytes. Mayo Clin Proc. 1995;70:430–3. doi: 10.4065/70.5.430. [DOI] [PubMed] [Google Scholar]
- 43.Chande N, McDonald JW, MacDonald JK. Interventions for treating collagenous colitis. Cochrane Database Syst Rev. 2006;(4):CD003575. doi: 10.1002/14651858.CD003575.pub4. [DOI] [PubMed] [Google Scholar]
- 44.Fine KD, Lee EL. Efficacy of open-label bismuth subsalicylate for the treatment of microscopic colitis. Gastroenterology. 1998;114:29–36. doi: 10.1016/s0016-5085(98)70629-8. [DOI] [PubMed] [Google Scholar]
- 45.Fine K, Ogunji F, Lee E, et al. Randomized, double-blind, placebo-controlled trial of bismuth subsalicylate for microscopic colitis [abstract] Gastroenterology. 1999;116:880. [Google Scholar]
- 46.Calabrese C, Fabbri A, Areni A, et al. Mesalazine with or without cholestyramine in the treatment of microscopic colitis: randomized controlled trial. J Gastroenterol Hepatol. 2007;22:809–14. doi: 10.1111/j.1440-1746.2006.04511.x. [DOI] [PubMed] [Google Scholar]
- 47.Sloth H, Bisgaard C, Grove A. Collagenous colitis: a prospective trial of prednisolone in six patients. J Intern Med. 1991;229:443–6. doi: 10.1111/j.1365-2796.1991.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 48.Munck LK, Kjeldsen J, Philipsen E, et al. Incomplete remission with short-term prednisolone treatment in collagenous colitis: a randomized study. Scand J Gastroenterol. 2003;38:606–10. doi: 10.1080/00365520310002210. [DOI] [PubMed] [Google Scholar]
- 49.Pardi DS, Loftus EV, Jr, Tremaine WJ, et al. Treatment of refractory microscopic colitis with azathioprine and 6- mercaptopurine. Gastroenterology. 2001;120:1483–4. doi: 10.1053/gast.2001.23976. [DOI] [PubMed] [Google Scholar]
- 50.Benucci M, Bardazzi G, Magaro L, et al. A case report of a man with rheumatoid factor positive rheumatoid arthritis associated with collagenous colitis. Clin Exp Rheumatol. 2001;19:475. [PubMed] [Google Scholar]
- 51.Fisher NC, Tutt A, Sim E, et al. Collagenous colitis responsive to octreotide therapy. J Clin Gastroenterol. 1996;23:300–1. doi: 10.1097/00004836-199612000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Wildt S, Munck LK, Vinter-Jensen L, et al. Probiotic treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial with Lactobacillus acidophilus and Bifidobacterium animalis subsp. Lactis. Inflamm Bowel Dis. 2006;12:395–401. doi: 10.1097/01.MIB.0000218763.99334.49. [DOI] [PubMed] [Google Scholar]
- 53.Jarnerot G, Tysk C, Bohr J, et al. Collagenous colitis and fecal stream diversion. Gastroenterology. 1995;109:449–55. doi: 10.1016/0016-5085(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 54.Munch A, Soderholm JD, Wallon C, et al. Dynamics of mucosal permeability and inflammation in collagenous colitis before, during, and after loop ileostomy. Gut. 2005;54:1126–8. doi: 10.1136/gut.2004.058750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varghese L, Galandiuk S, Tremaine WJ, et al. Lymphocytic colitis treated with proctocolectomy and ileal J-pouch-anal anastomosis: report of a case. Dis Colon Rectum. 2002;45:123–6. doi: 10.1007/s10350-004-6126-z. [DOI] [PubMed] [Google Scholar]
- 56.Williams RA, Gelfand DV. Total proctocolectomy and ileal pouch anal anastomosis to successfully treat a patient with collagenous colitis. Am J Gastroenterol. 2000;95:2147. doi: 10.1111/j.1572-0241.2000.02225.x. [DOI] [PubMed] [Google Scholar]
- 57.Riaz AA, Pitt J, Stirling RW, et al. Restorative proctocolectomy for collagenous colitis. J R Soc Med. 2000;93:261. doi: 10.1177/014107680009300513. [DOI] [PMC free article] [PubMed] [Google Scholar]