Abstract
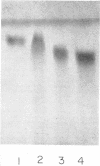
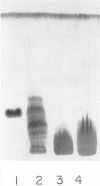
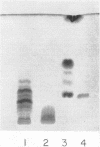
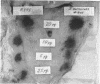
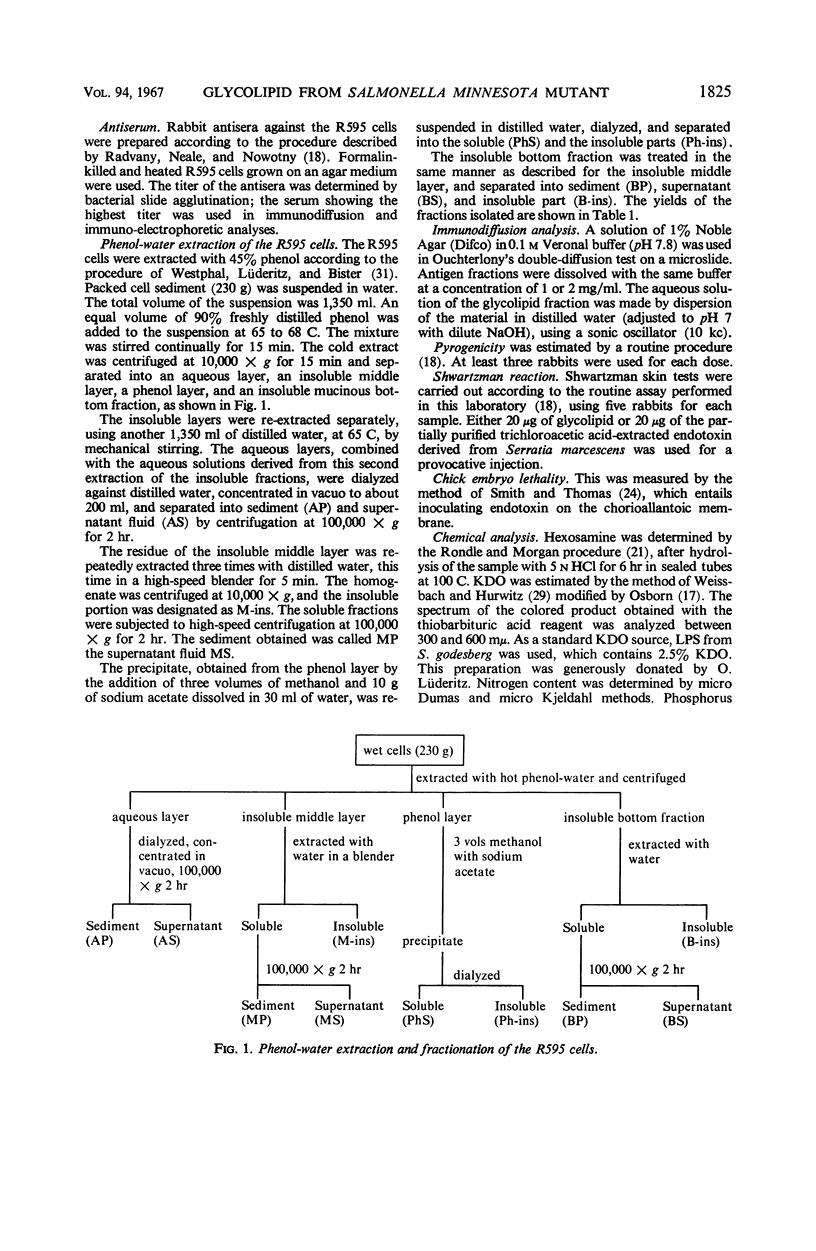
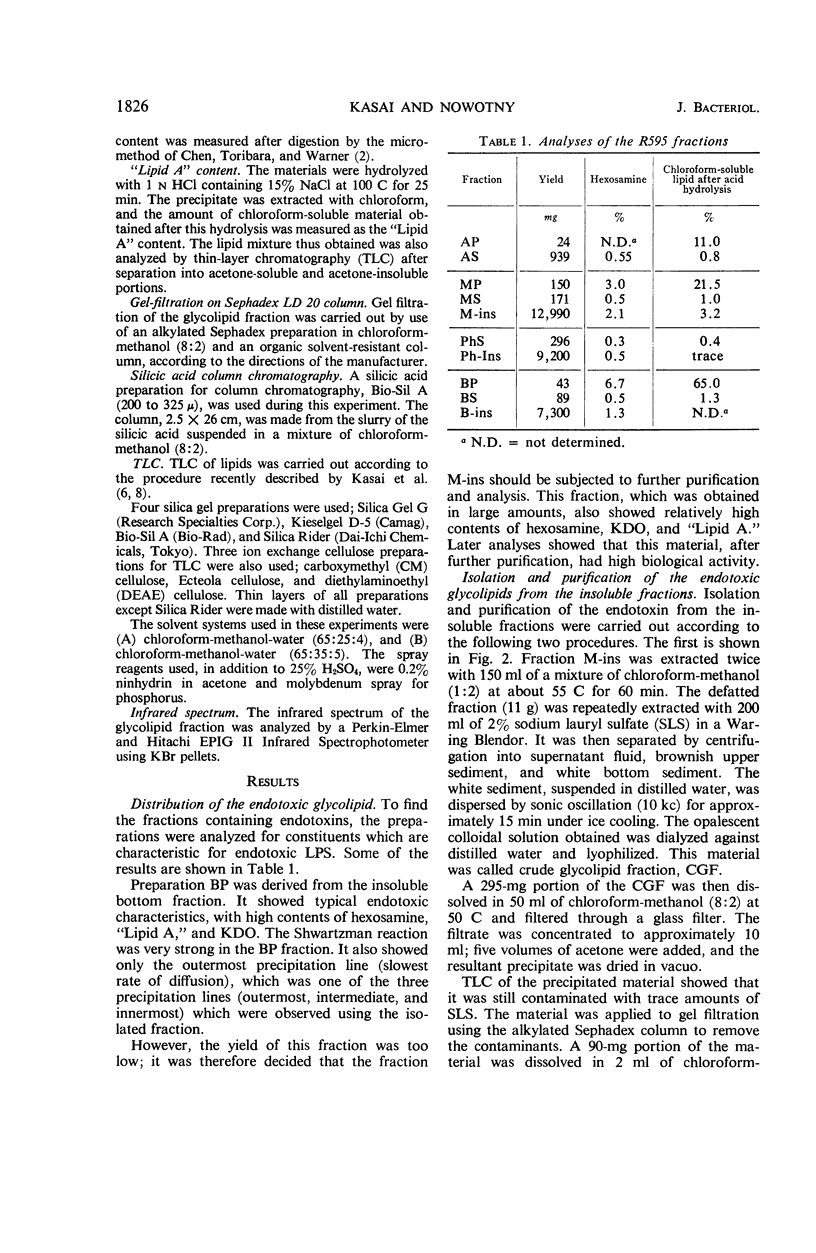
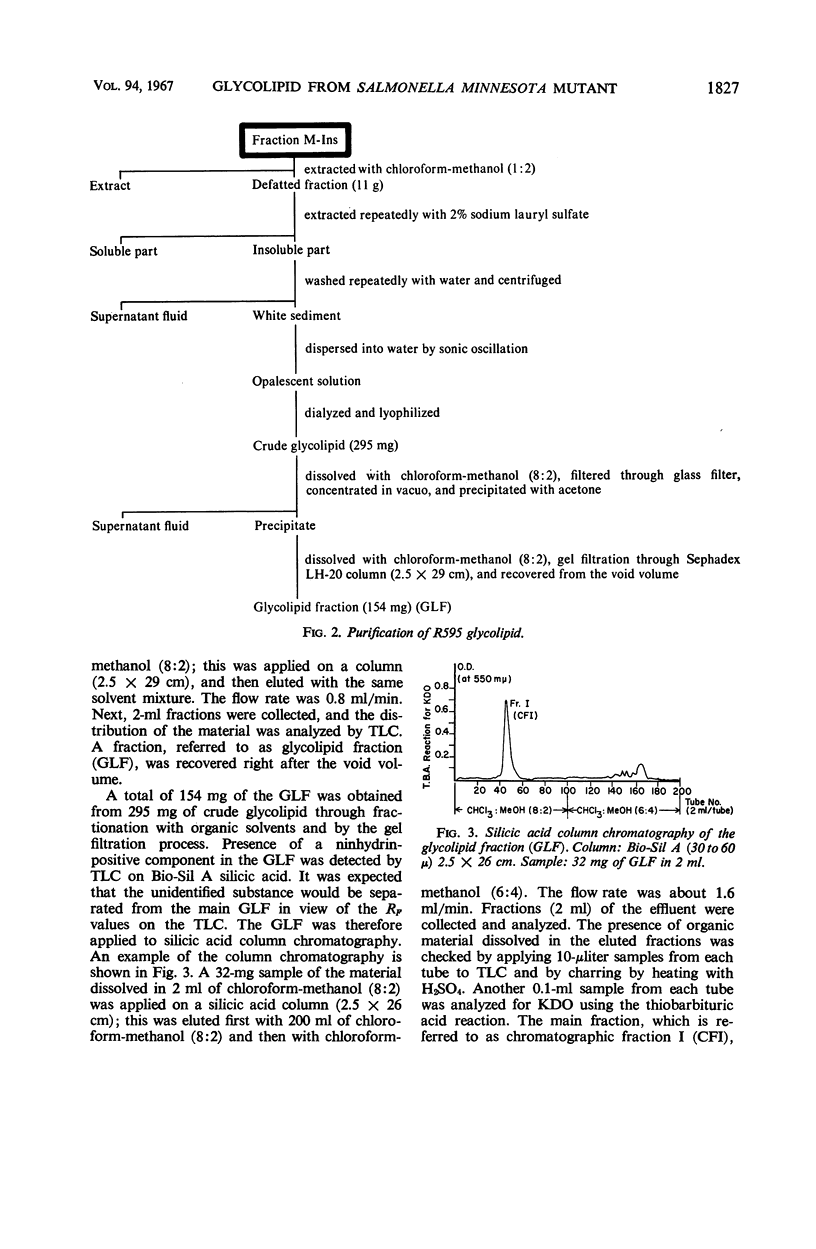
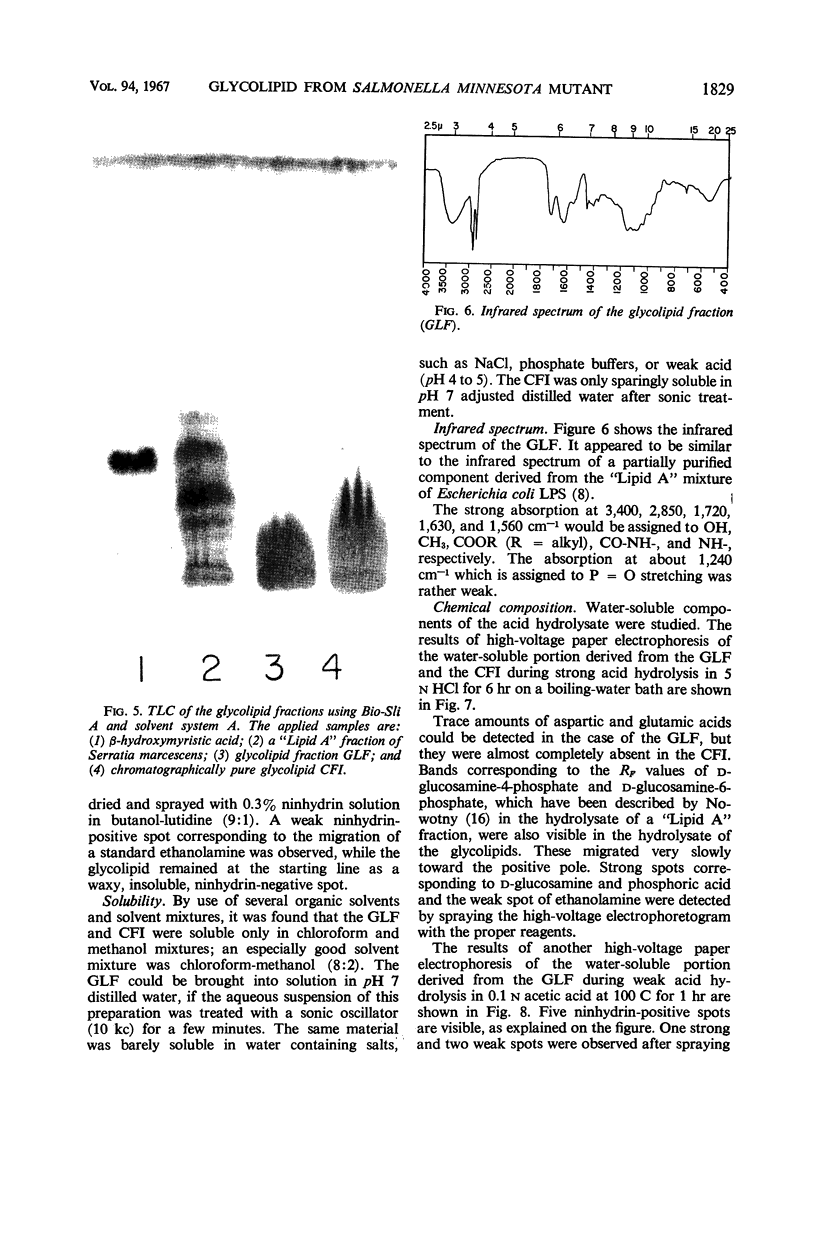
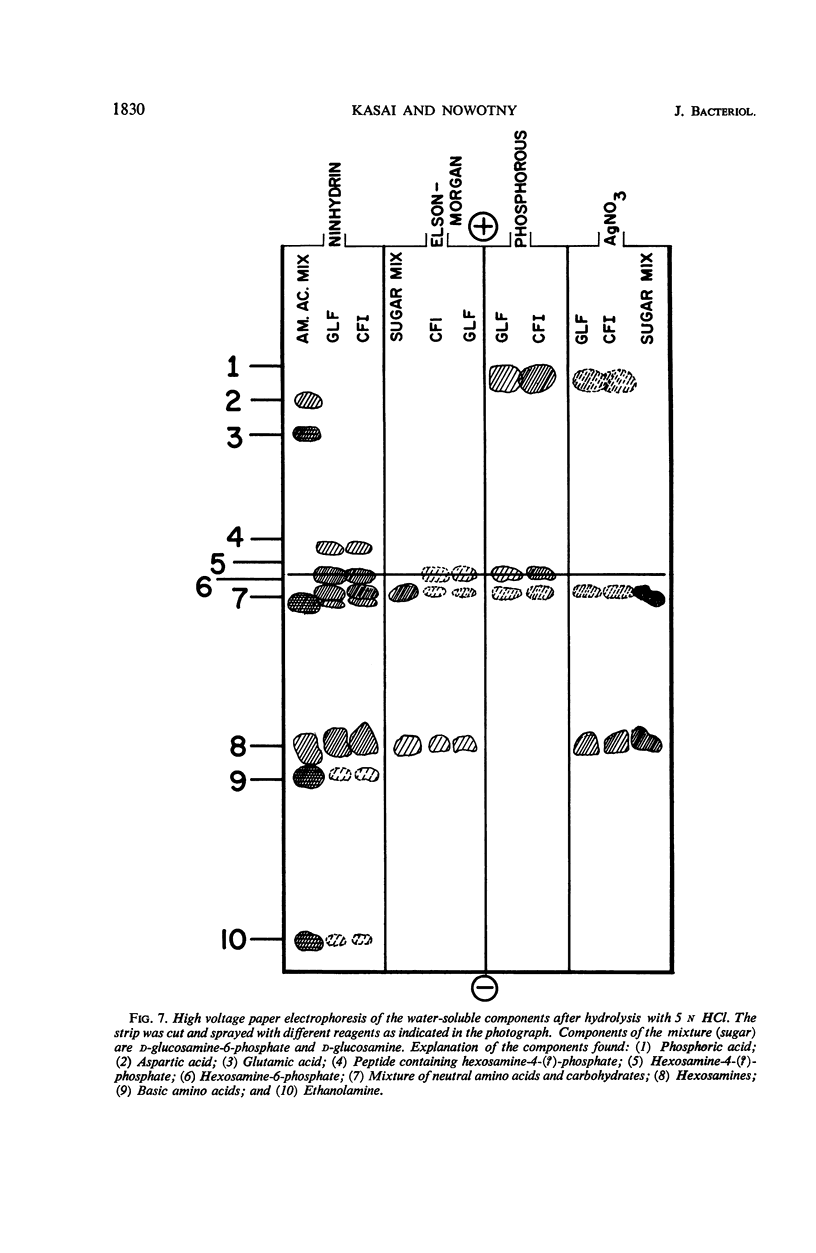
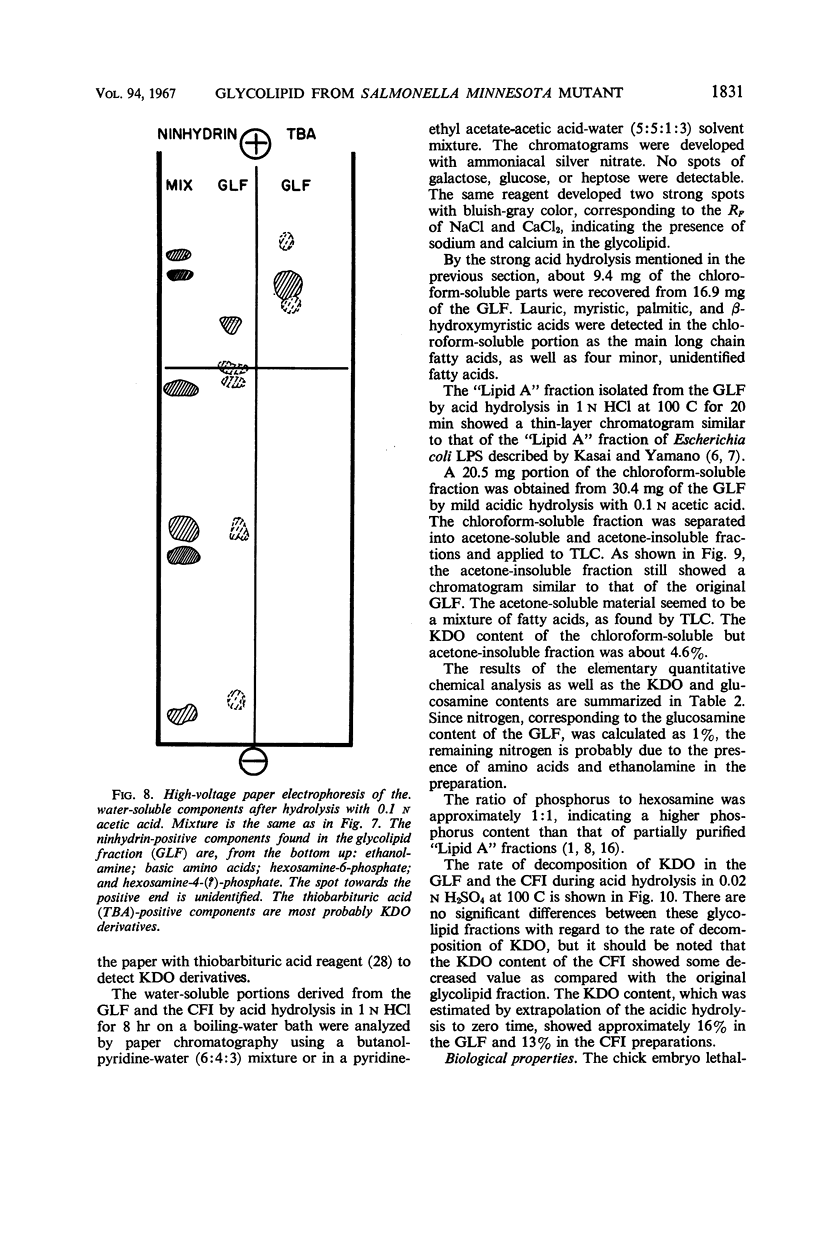
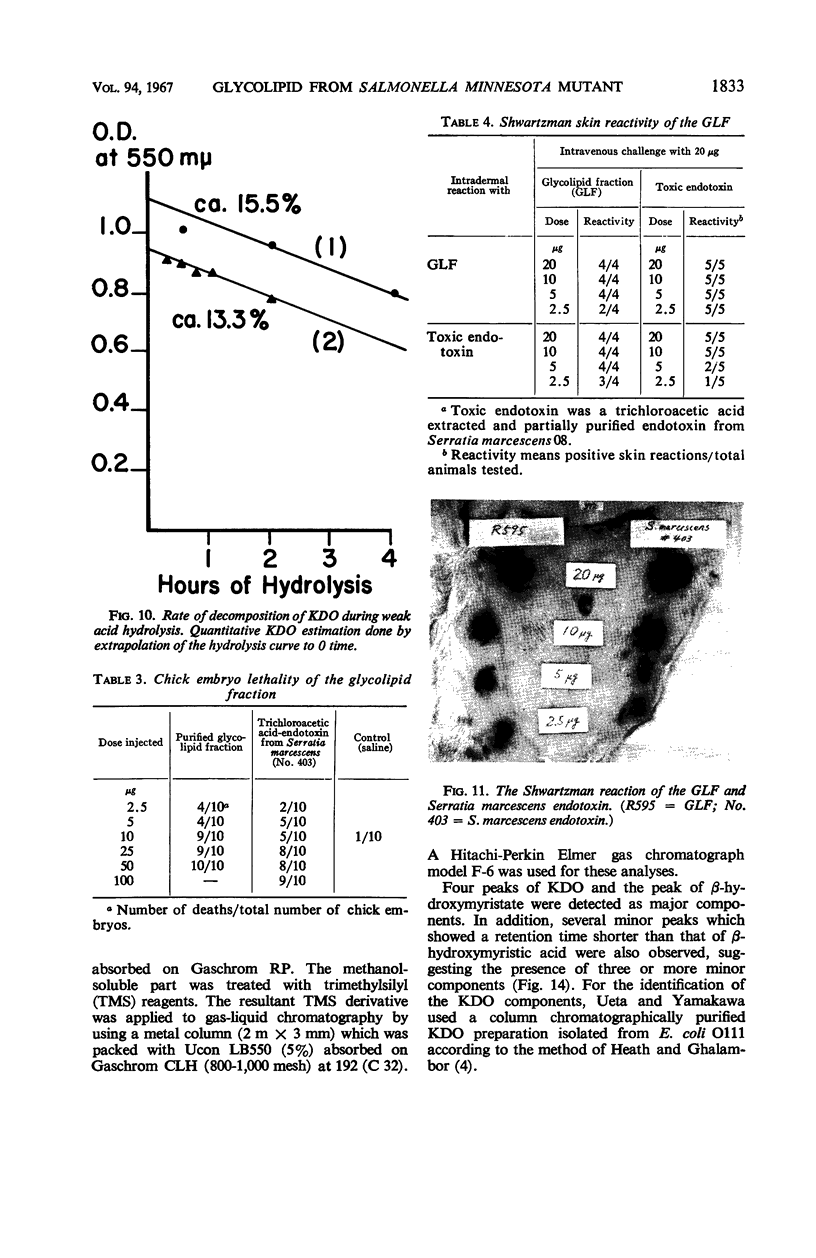
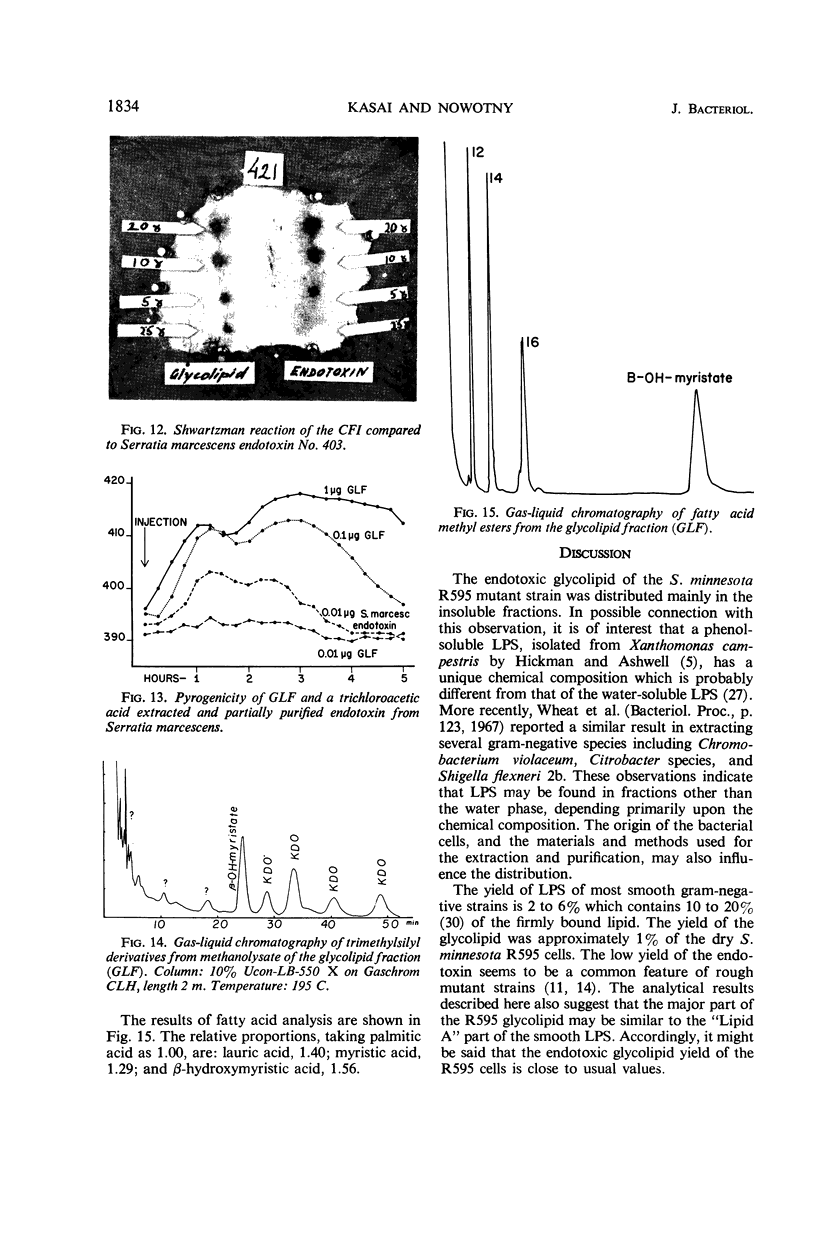
The endotoxin of a heptoseless mutant of Salmonella minnesota R595 was extracted with phenol-water. Most of this material was found distributed in the insoluble fraction of the extract. The results showed that the R595 endotoxin behaved as a lipid rather than as a lipopolysaccharide (LPS). The preparation, although it does not contain O-specific polysaccharides, does contain 2-keto-3-deoxyoctonic acid (KDO), hexosamine, and several other unidentified compounds. Therefore, the term “glycolipid” is used in this paper instead of lipopolysaccharide. The crude glycolipid fraction, which was soluble in a mixture of chloroform-methanol (8:2), was purified by a procedure including fractionation with organic solvents and by different-column chromatographic methods. Although a chromatographic fraction of the glycolipid showed homogeneity in most systems investigated, the presence of contaminants could not be excluded. Chemical analysis of the glycolipids showed the absence of hexoses and heptoses. Constituents which were found were hexosamine, KDO, fatty acids, and phosphorus, which showed a relatively simple chemical composition. Partial acidic hydrolysis of the glycolipid yielded hexosamine-phosphates, as described in “Lipid A” fractions of smooth LPS preparations. Thin-layer chromatography of the partially hydrolyzed glycolipid showed a pattern similar to “Lipid A” fractions of other strains. The biological activity of the glycolipid was at the same level as that of other gram-negative endotoxins. Pyrogenicity, Shwartzman reactivity, and chick embryo ld50 values were as high or higher than those of purified Serratia marcescens endotoxin preparations, but mouse ld50 measurements gave significantly lower results.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON A. J., CARTER H. E. PURIFICATION AND CHARACTERIZATION OF THE LIPID A COMPONENT OF THE LIPOPOLYSACCHARIDES FROM ESCHERICHIA COLI. Biochemistry. 1964 Mar;3:411–418. doi: 10.1021/bi00891a018. [DOI] [PubMed] [Google Scholar]
- DAVIES D. A. A specific polysaccharide of Pasteurella pestis. Biochem J. 1956 May;63(1):105–116. doi: 10.1042/bj0630105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman J., Ashwell G. Isolation of a bacterial lipopolysaccharide from Xanthomonas campestris containing 3-acetamido-3,6-dideoxy-D-galactose and D-rhamnose. J Biol Chem. 1966 Mar 25;241(6):1424–1428. [PubMed] [Google Scholar]
- KASAI N., YAMANO A. STUDIES ON THE LIPIDS OF ENDOTOXINS.(THIN-LAYER CHROMATOGRAPHY OF LIPID FRACTIONS). Jpn J Exp Med. 1964 Dec;34:329–344. [PubMed] [Google Scholar]
- Kessel R. W., Freedman H. H., Braun W. Relation of polysaccharide content to some biological properties of endotoxins from mutants of Salmonella typhimurium. J Bacteriol. 1966 Sep;92(3):592–596. doi: 10.1128/jb.92.3.592-596.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Risse H. J., Ruschmann E., Schlecht S., Schmidt G., Schulte-Holthausen H., Wheat R., Westphal O., Schlosshardt J. Structural relationship of Salmonella O and R antigens. Ann N Y Acad Sci. 1966 Jun 30;133(2):349–374. doi: 10.1111/j.1749-6632.1966.tb52376.x. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLENNAN A. P., RONDLE C. J. Pasteurella septica: the occurence of type-specific polysaccharides containing aldoheptose sugars. Nature. 1957 Nov 16;180(4594):1045–1046. doi: 10.1038/1801045a0. [DOI] [PubMed] [Google Scholar]
- MACLENNAN A. P. Specific lipopolysaccharides of Bordetella. Biochem J. 1960 Feb;74:398–409. doi: 10.1042/bj0740398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKANO M. Mutants of Salmonella with unusually low toxicity for mice. Nature. 1962 Dec 15;196:1118–1119. doi: 10.1038/1961118a0. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIBI E., HASKINS W. T., MILNER K. C., ANACKER R. L., RITTER D. B., GOODE G., TRAPANI R. J., LANDY M. Physicochemical changes in endotoxin associated with loss of biological potency. J Bacteriol. 1962 Oct;84:803–814. doi: 10.1128/jb.84.4.803-814.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radvany R., Neale N. L., Nowotny A. Relation of structure to function in bacterial O-antigens. VI. Neutralization of endotoxic O-antigens by homologous O-antibody. Ann N Y Acad Sci. 1966 Jun 30;133(2):763–786. doi: 10.1111/j.1749-6632.1966.tb52404.x. [DOI] [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Brown R., Haskins W. T., Malmgren B., Milner K. C., Rudbach J. A. Reaction of endotoxin and surfactants. I. Physical and biological properties of endotoxin treated with sodium deoxycholate. J Bacteriol. 1966 Nov;92(5):1493–1509. doi: 10.1128/jb.92.5.1493-1509.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH R. T., THOMAS L. The lethal effect of endotoxins on the chick embryo. J Exp Med. 1956 Aug 1;104(2):217–231. doi: 10.1084/jem.104.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Wachstum und Lipopolysaccharid (O-Antigen)-Gehalt von Salmonellen bei Züchtung auf Agarnährböden. Zentralbl Bakteriol Orig. 1966 Jun;200(2):241–259. [PubMed] [Google Scholar]
- TAUBER H., RUSSELL H. Amino compounds in lipopolysaccharides. J Biol Chem. 1960 Apr;235:961–964. [PubMed] [Google Scholar]
- Tripodi D., Nowotny A. Relation of structure to function in bacterial O-antigens. V. Nature of active sites in endotoxic lipopolysaccharides of Serratia marcescens. Ann N Y Acad Sci. 1966 Jun 30;133(2):604–621. doi: 10.1111/j.1749-6632.1966.tb52392.x. [DOI] [PubMed] [Google Scholar]
- Volk W. A. Cell wall lipopolysaccharides from xanthomonas species. J Bacteriol. 1966 Jan;91(1):39–42. doi: 10.1128/jb.91.1.39-42.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. Thiobarbituric acid spray reaction for deoxy sugars and sialic acids. Nature. 1960 Apr 16;186:237–237. doi: 10.1038/186237a0. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- YAMAKAWA T., UETA N. GASCHROMATOGRAPHIC STUDIES OF MICROBIAL COMPONENTS. I. CARBOHYDRATE AND FATTY ACID CONSTITUTION OF NEISSERIA. Jpn J Exp Med. 1964 Dec;34:361–374. [PubMed] [Google Scholar]