Abstract
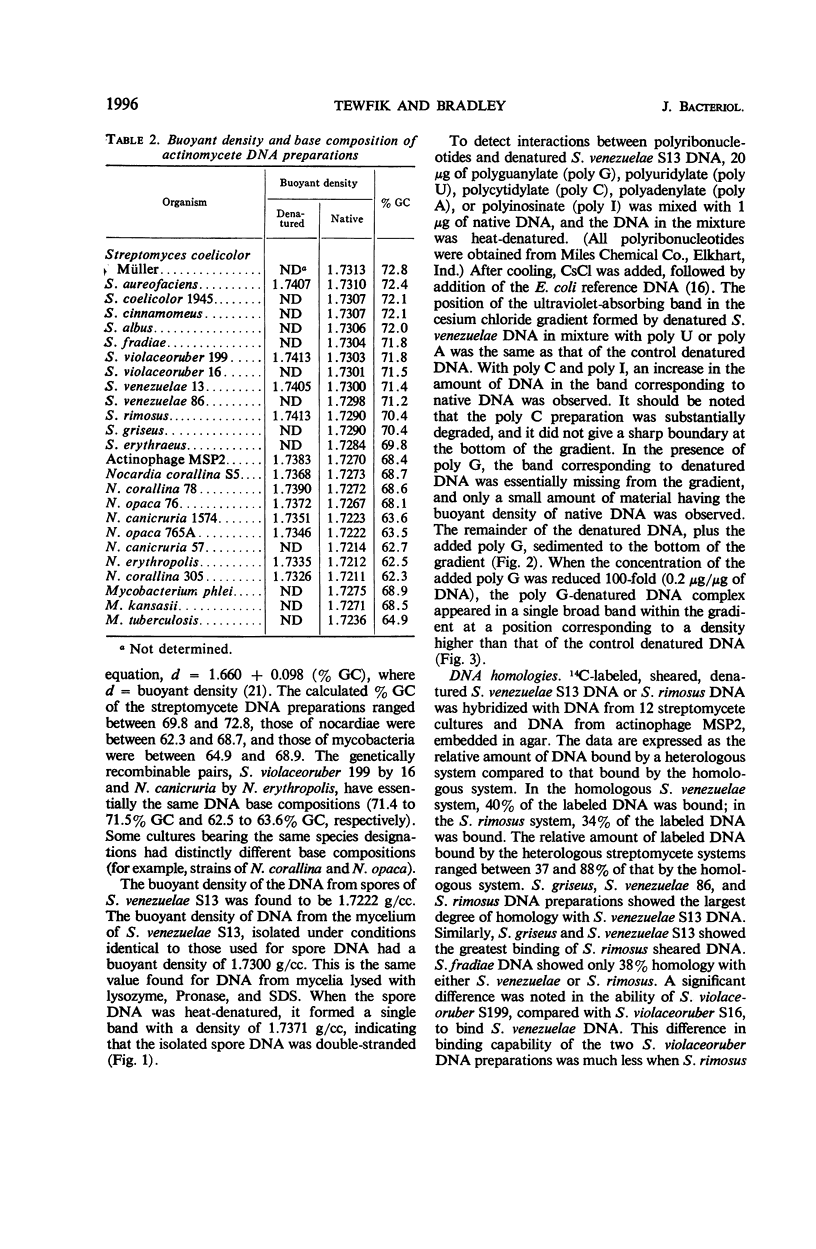
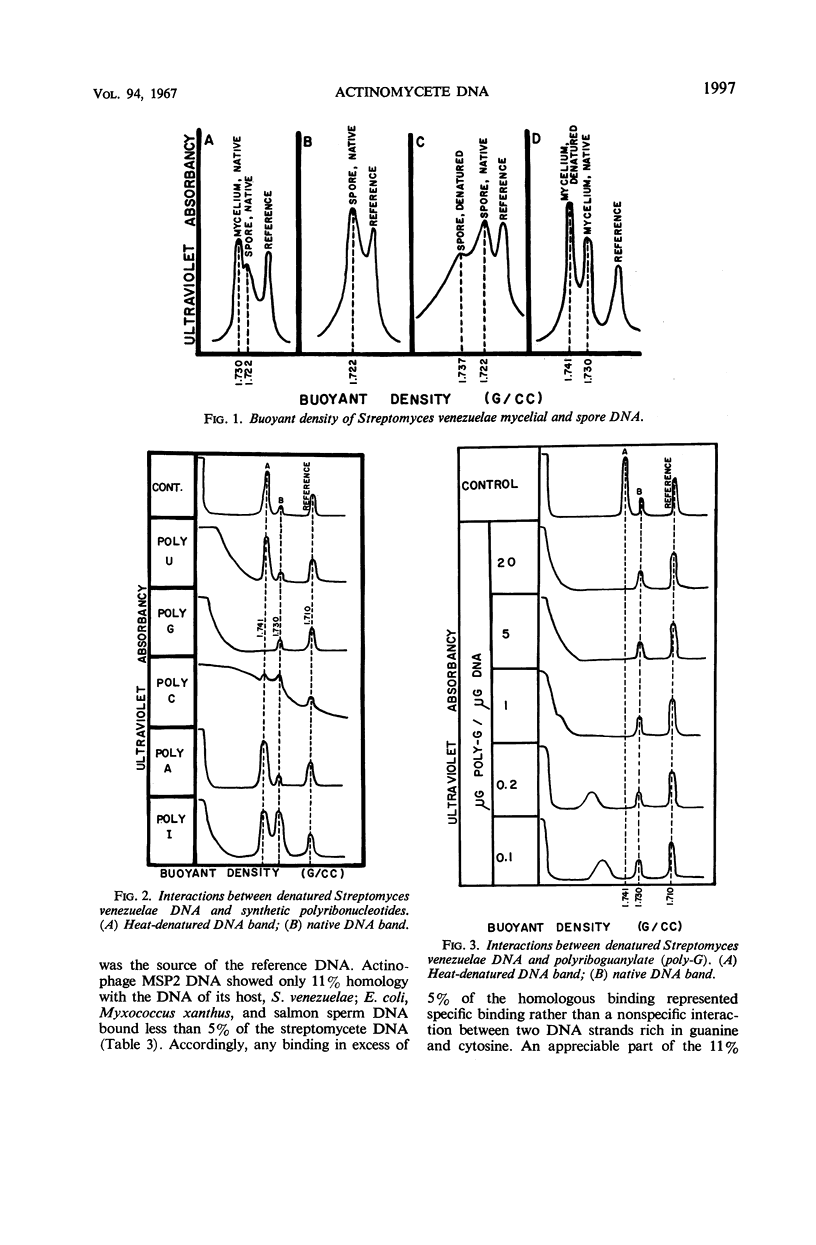
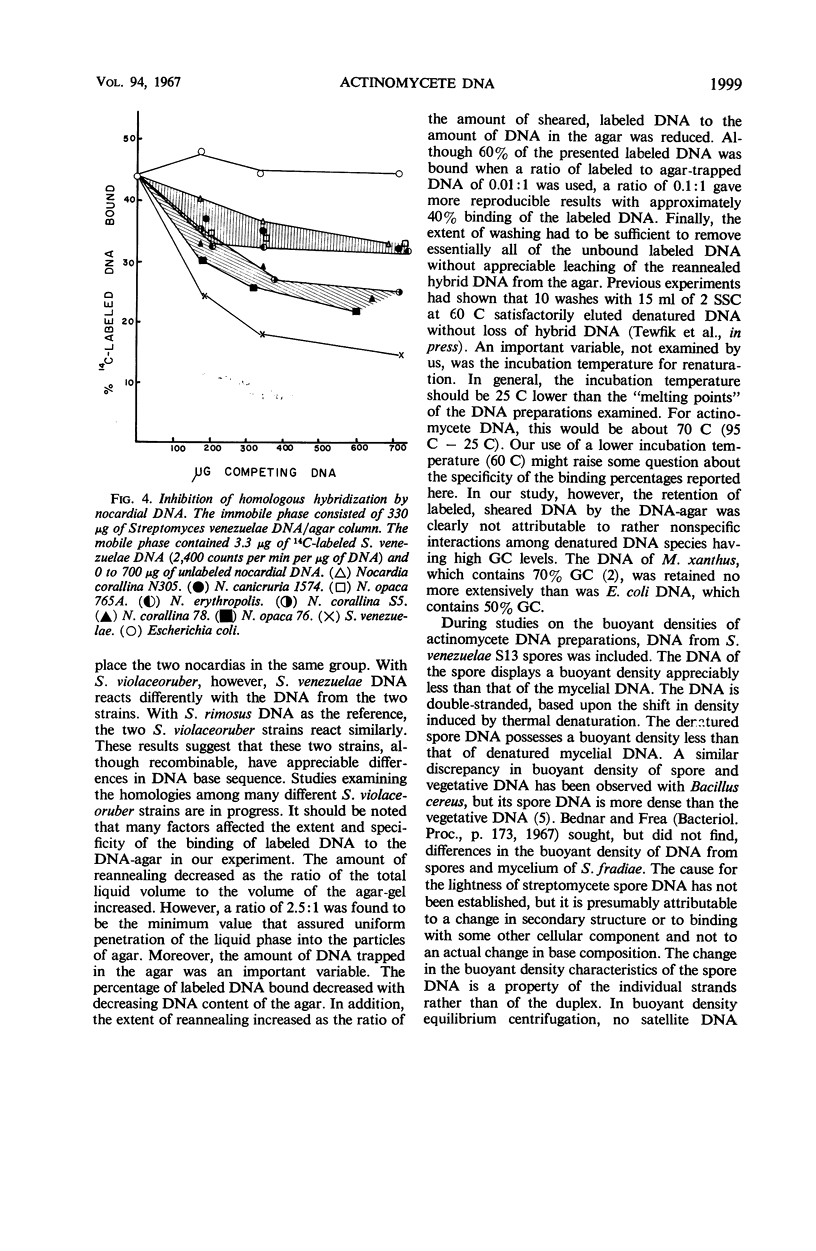
The relationships among selected streptomycetes, nocardiae, and mycobacteria have been determined, based upon the base composition of their deoxyribonucleic acid (DNA) and upon the ability of their denatured DNA to anneal with single-stranded reference DNA. The streptomycetes constituted a homogeneous group whose DNA contained between 69 and 73 mole% guanine + cytosine (% GC). Moreover, the streptomycetes examined showed 37 to 88% homology with the Streptomyces venezuelae and S. rimosus reference DNA. The nocardial and mycobacterial DNA both contained 62 to 69% GC. The nocardial strains studied fell into either a 62 to 64% GC group or a 68 to 69% GC group, indicating that they should not be assigned to a single species. The nocardiae having 68 to 69% GC showed 24 to 44% homology with S. venezuelae reference DNA. In competition experiments, wherein unlabeled heterologous DNA interfered with binding of labeled homologous DNA, the nocardial DNA with 68 to 69% GC showed a greater degree of homology with the streptomycetes than did the nocardial DNA with 62 to 64% GC. In addition, the DNA from spores of S. venezuelae was cursorily examined, and interactions between S. venezuelae denatured DNA and polyribonucleotides were sought. The buoyant density of the DNA from S. venezuelae spores was distinctly less than that from mycelia. Moreover, denatured S. venezuelae DNA formed a dense complex with polyriboguanylate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY S. G., ANDERSON D. L. Compatibility system controlling heterokaryon formation in Streptomyces coelicolor. Proc Soc Exp Biol Med. 1958 Nov;99(2):476–478. doi: 10.3181/00379727-99-24389. [DOI] [PubMed] [Google Scholar]
- Bradley S. G. Genetics in applied microbiology. Adv Appl Microbiol. 1966;8:29–59. doi: 10.1016/s0065-2164(08)70491-x. [DOI] [PubMed] [Google Scholar]
- Brownell G. H., Adams J. N., Bradley S. G. Growth and characterization of nocardiophages for Nocardia canicruria and Nocardia erythropolis mating types. J Gen Microbiol. 1967 May;47(2):247–256. doi: 10.1099/00221287-47-2-247. [DOI] [PubMed] [Google Scholar]
- Douthit H. A., Halvorson H. O. Satellite deoxyribonucleic acid from Bacillus cereus strain T. Science. 1966 Jul 8;153(3732):182–183. doi: 10.1126/science.153.3732.182. [DOI] [PubMed] [Google Scholar]
- FALKOW S., WOHLHIETER J. A., CITARELLA R. V., BARON L. S. TRANSFER OF EPISOMIC ELEMENTS TO PROTEUS. II. NATURE OF LAC+ PROTEUS STRAINS ISOLATED FROM CLINICAL SPECIMENS. J Bacteriol. 1964 Dec;88:1598–1601. doi: 10.1128/jb.88.6.1598-1601.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRONTALI C., HILL L. R., SILVESTRI L. G. THE BASE COMPOSITION OF DEOXYRIBONUCLEIC ACIDS OF STREPTOMYCES. J Gen Microbiol. 1965 Feb;38:243–250. doi: 10.1099/00221287-38-2-243. [DOI] [PubMed] [Google Scholar]
- GORDON R. E., MIHM J. M. A comparative study of some strains received as nocardiae. J Bacteriol. 1957 Jan;73(1):15–27. doi: 10.1128/jb.73.1.15-27.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON R. E., MIHM J. M. A comparison of four species of mycobacteria. J Gen Microbiol. 1959 Dec;21:736–748. doi: 10.1099/00221287-21-3-736. [DOI] [PubMed] [Google Scholar]
- Gordon R. E. Some strains in search of a genus--Corynebacterium, Mycobacterium, Nocardia or what? J Gen Microbiol. 1966 Jun;43(3):329–343. doi: 10.1099/00221287-43-3-329. [DOI] [PubMed] [Google Scholar]
- Hill L. R. An index to deoxyribonucleic acid base compositions of bacterial species. J Gen Microbiol. 1966 Sep;44(3):419–437. doi: 10.1099/00221287-44-3-419. [DOI] [PubMed] [Google Scholar]
- KUTZNER H. J., WAKSMAN S. A. Streptomyces coelicolor Mueller and Streptomyces violaceoruber Waksman and Curtis, two distinctly different organisms. J Bacteriol. 1959 Oct;78:528–538. doi: 10.1128/jb.78.4.528-538.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolstad R. A., Bradley S. G. Purification of Streptomyces venezuelae phage. J Bacteriol. 1964 May;87(5):1157–1161. doi: 10.1128/jb.87.5.1157-1161.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubinski H., Opara-Kubinska Z., Szybalski W. Patterns of interaction between polyribonucleotides and individual DNA strands derived from several vertebrates, bacteria and bacteriophages. J Mol Biol. 1966 Sep;20(2):313–329. doi: 10.1016/0022-2836(66)90067-2. [DOI] [PubMed] [Google Scholar]
- MCCARTHY B. J., BOLTON E. T. An approach to the measurement of genetic relatedness among organisms. Proc Natl Acad Sci U S A. 1963 Jul;50:156–164. doi: 10.1073/pnas.50.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]


