Abstract
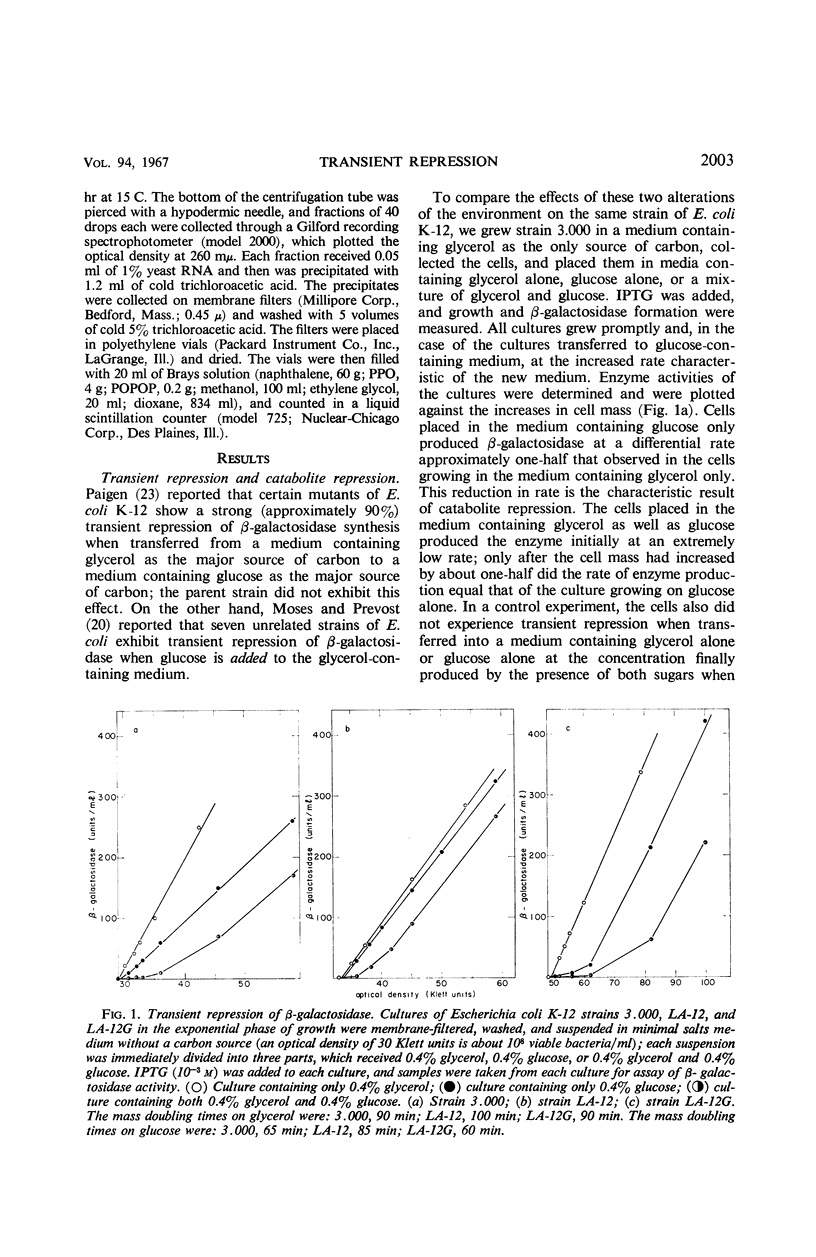
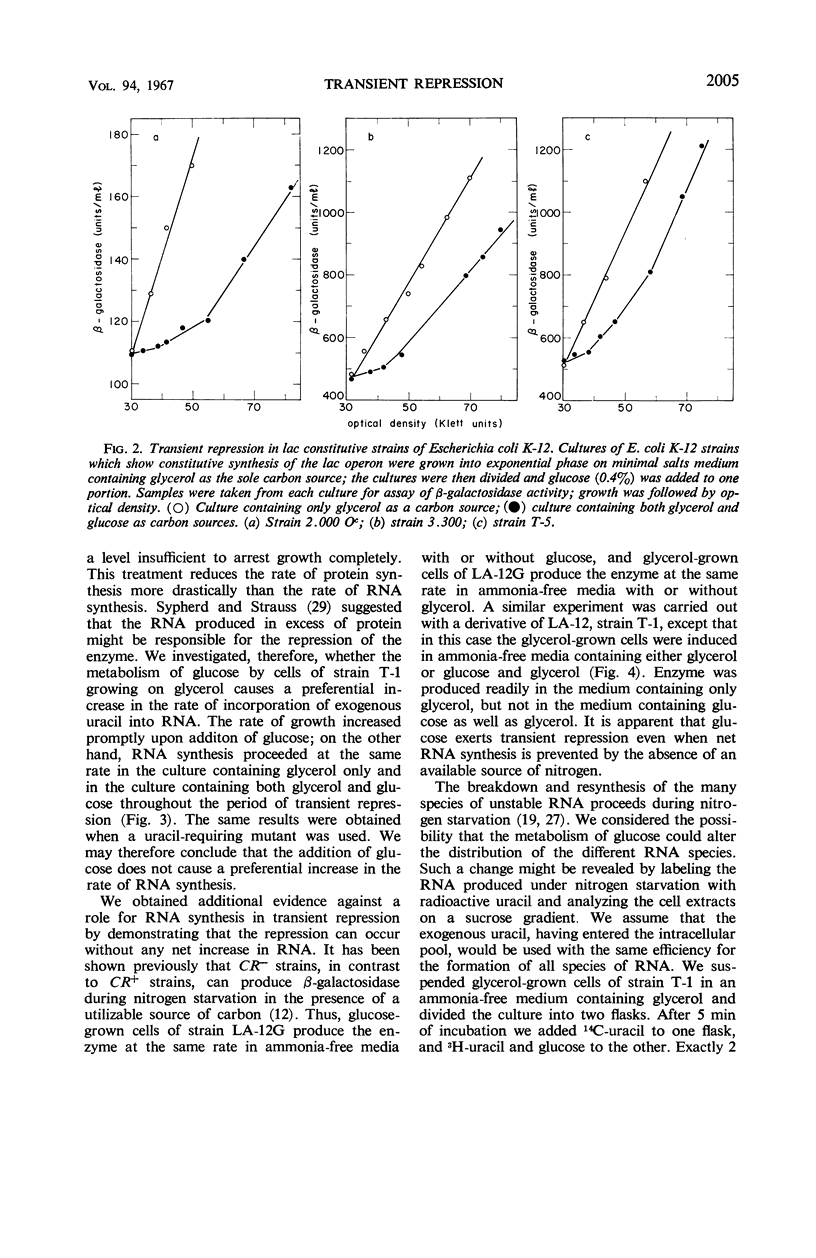
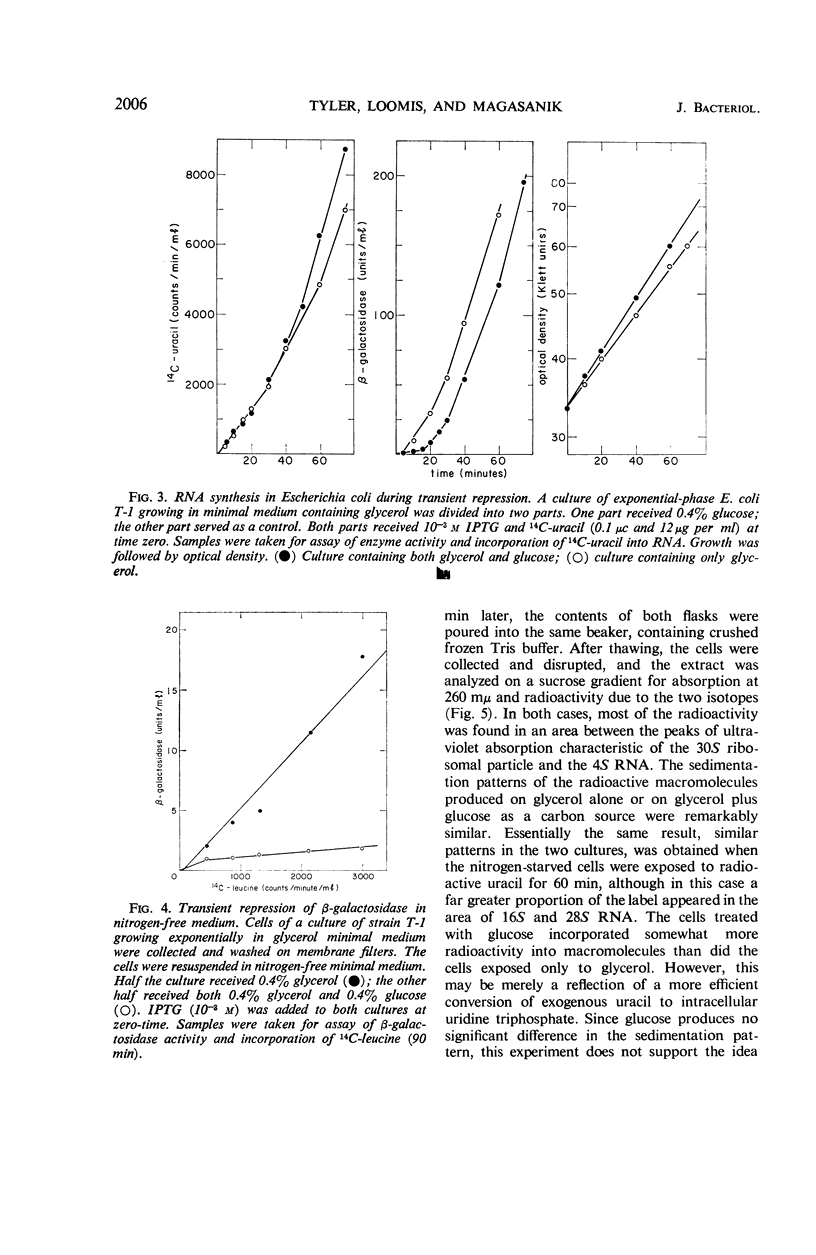
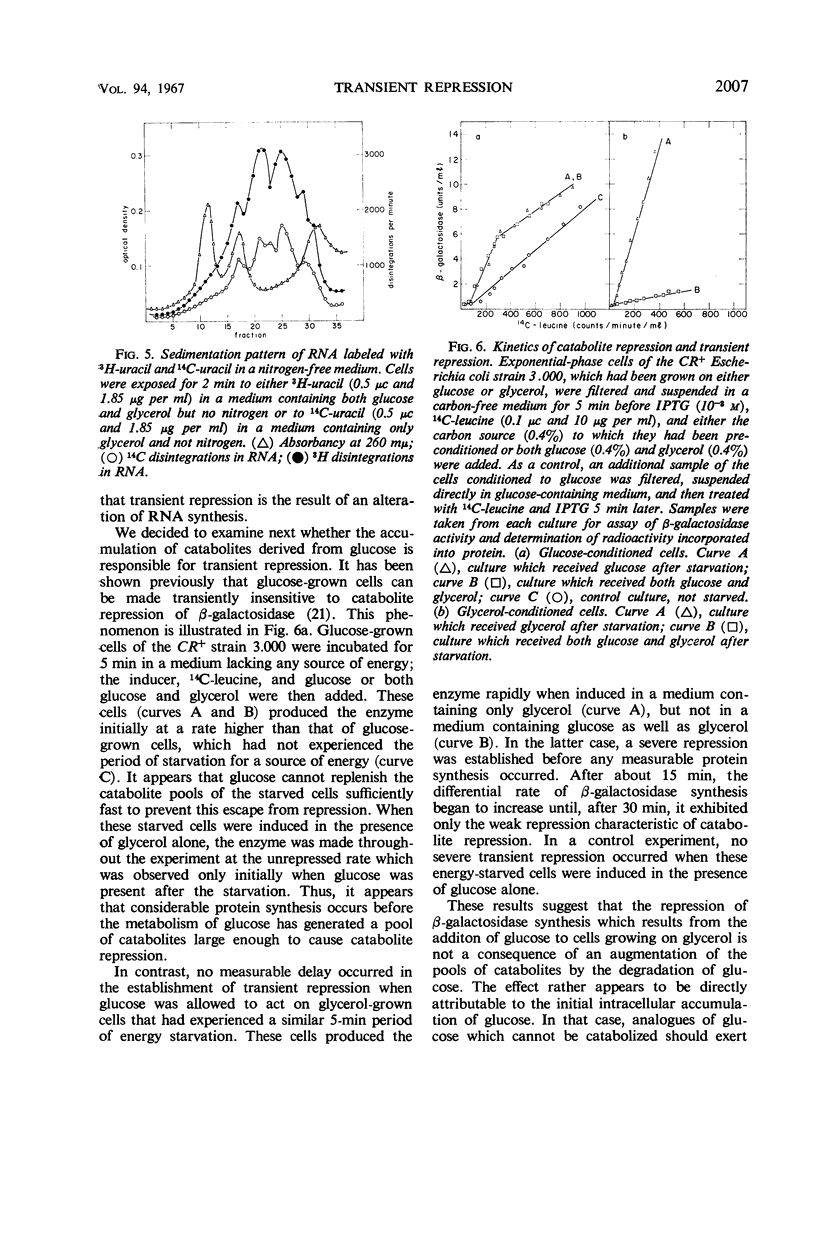
Severe transient repression of constitutive or induced β-galactosidase synthesis occurs upon the addition of glucose to cells of Escherichia coli growing on glycerol, succinic acid, or lactic acid. Only mutants particularily well adapted to growth on glucose exhibit this phenomenon when transferred to a glucose-containing medium. No change in ribonucleic acid (RNA) metabolism was observed during transient repression. We could show that transient repression is pleiotropic, affecting all products of the lac operon. It occurs in a mutant insensitive to catabolite repression. It is established much more rapidly than catabolite repression, and is elicited by glucose analogues that are phosphorylated but not further catabolized by the cell. Thus, transient repression is not a consequence of the exclusion of inducer from the cell, does not require catabolism of the added compound, and does not involve a gross change in RNA metabolism. We conclude that transient repression is distinct from catabolite repression.
Full text
PDF


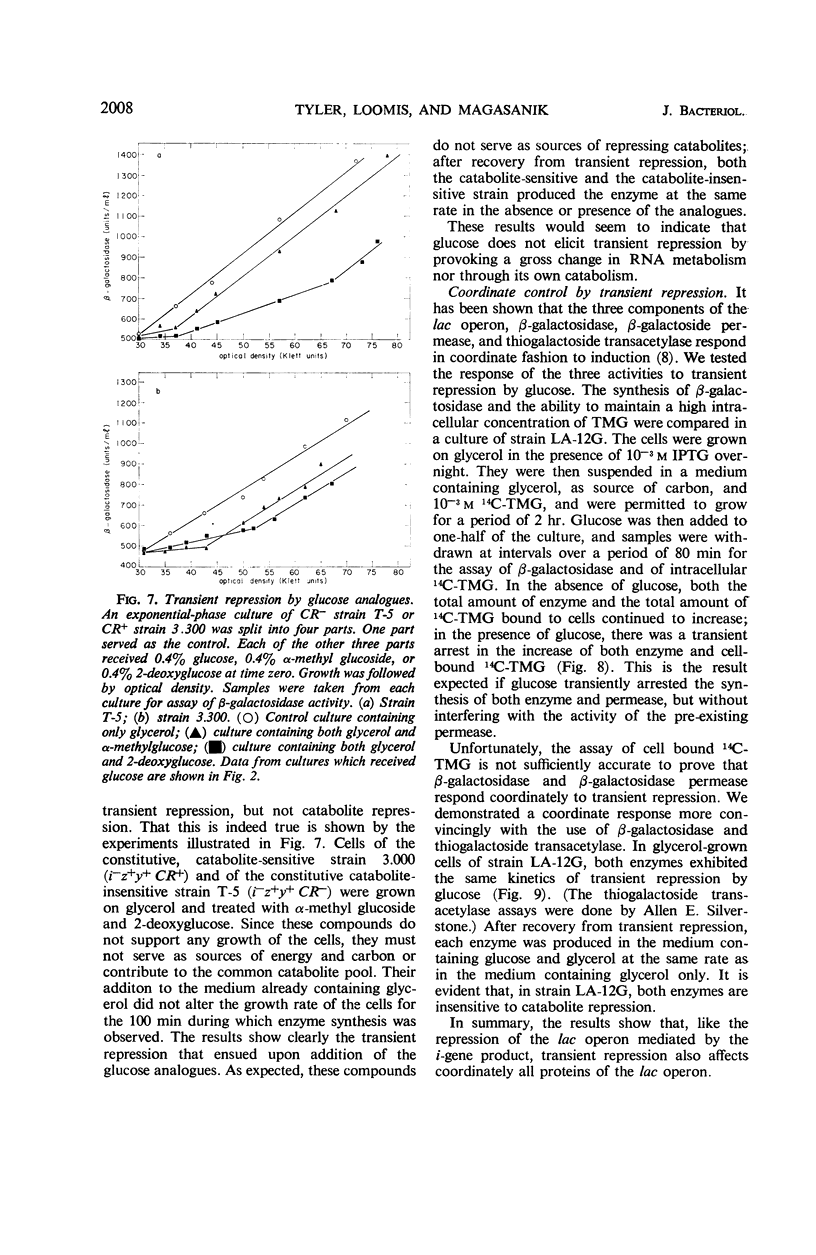
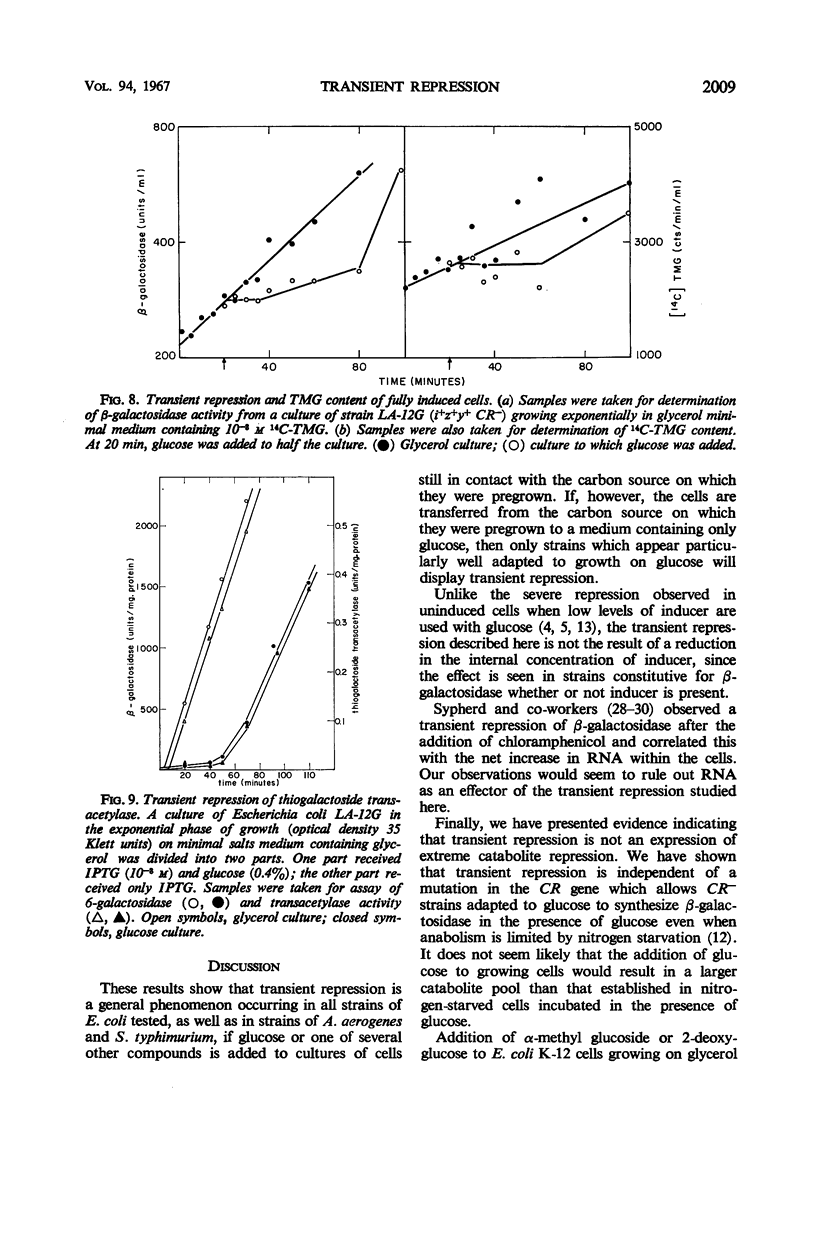


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPERS D. H., TOMKINS G. M. THE ORDER OF INDUCTION AND DEINDUCTION OF THE ENZYMES OF THE LACTOSE OPERON IN E. COLI. Proc Natl Acad Sci U S A. 1965 Apr;53:797–802. doi: 10.1073/pnas.53.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEZI J. A., COWIE D. B. Kinetic studies of beta-galactosidase induction. Biophys J. 1961 Nov;1:639–647. doi: 10.1016/s0006-3495(61)86913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK D. J., MARR A. G. STUDIES ON THE REPRESSION OF BETA-GALACTOSIDASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Oct 23;92:85–94. doi: 10.1016/0926-6569(64)90272-x. [DOI] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Inhibition by glucose of the induced synthesis of the beta-galactoside-enzyme system of Escherichia coli. Analysis of maintenance. J Bacteriol. 1959 Nov;78:601–612. doi: 10.1128/jb.78.5.601-612.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSHANOVICH V. N. [On the permeability of the bacterial cell of Escherichia coli to 2-D-deoxyglucose]. Biokhimiia. 1962 Nov-Dec;27:1023–1031. [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEPES A. KINETICS OF INDUCED ENZYME SYNTHESIS. DETERMINATION OF THE MEAN LIFE OF GALACTOSIDASE-SPECIFIC MESSENGER RNA. Biochim Biophys Acta. 1963 Oct 15;76:293–309. [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- LOOMIS W. F., Jr, MAGASANIK B. THE RELATION OF CATABOLITE REPRESSION TO THE INDUCTION SYSTEM FOR BETA-GALACTOSIDASE IN ESCHERICHIA COLI. J Mol Biol. 1964 Mar;8:417–426. doi: 10.1016/s0022-2836(64)80205-9. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. Genetic control of catabolite repression of the lac operon in Escherichia coli. Biochem Biophys Res Commun. 1965 Jul 12;20(2):230–234. doi: 10.1016/0006-291x(65)90351-7. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. Glucose-lactose diauxie in Escherichia coli. J Bacteriol. 1967 Apr;93(4):1397–1401. doi: 10.1128/jb.93.4.1397-1401.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. The catabolite repression gene of the lac operon in Escherichia coli. J Mol Biol. 1967 Feb 14;23(3):487–494. doi: 10.1016/s0022-2836(67)80120-7. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B., NEIDHARDT F. C. Inhibitory effect of glucose on enzyme formation. Nature. 1956 Oct 13;178(4537):801–802. doi: 10.1038/178801b0. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Induction and repression of beta-galactosidase in non-growing Escherichia coli. Biochem J. 1961 Jun;79:489–496. doi: 10.1042/bj0790489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. The repression of constitutive beta-galactosidase in Escherichia coli by glucose and other carbon sources. Biochem J. 1962 Mar;82:489–493. doi: 10.1042/bj0820489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. Turnover of protein in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):110–119. doi: 10.1042/bj0690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses V., Prevost C. Catabolite repression of beta-galactosidase synthesis in Escherichia coli. Biochem J. 1966 Aug;100(2):336–353. doi: 10.1042/bj1000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. THE ROLES OF INDUCER AND CATABOLITE REPRESSOR IN THE SYNTHESIS OF BETA-GALACTOSIDASE BY ESCHERICHIA COLI. J Mol Biol. 1964 Jan;8:105–127. doi: 10.1016/s0022-2836(64)80153-4. [DOI] [PubMed] [Google Scholar]
- Paigen K. Phenomenon of transient repression in Escherichia coli. J Bacteriol. 1966 Mar;91(3):1201–1209. doi: 10.1128/jb.91.3.1201-1209.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost C., Moses V. Pool sizes of metabolic intermediates and their relation to glucose repression of beta-galactosidase synthesis in Escherichia coli. Biochem J. 1967 May;103(2):349–357. doi: 10.1042/bj1030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REVEL H. R. SYNTHESIS OF BETA-D-GALACTOSIDASE AFTER F-DUCTION OF LAC+GENES INTO ESCHERICHIA COLI. J Mol Biol. 1965 Jan;11:23–34. doi: 10.1016/s0022-2836(65)80168-1. [DOI] [PubMed] [Google Scholar]
- SCHICK M., LANDAU B., TSCHUDY D. P. Effect of hexose analogues on the growth of Escherichia coli. J Bacteriol. 1958 Apr;75(4):414–416. doi: 10.1128/jb.75.4.414-416.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYPHERD P. S., DEMOSS J. A. THE STIMULATION BY CHLORAMPHENICOL OF "REPRESSOR" FORMATION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Dec 20;76:589–599. [PubMed] [Google Scholar]
- SYPHERD P. S., STRAUSS N. THE ROLE OF RNA IN REPRESSION OF ENZYME SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Dec;50:1059–1066. doi: 10.1073/pnas.50.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger D., Ben-Hamida F. Turnover of protein in Escherichia coli starving for nitrogen. Biochim Biophys Acta. 1966 Apr 18;119(1):171–182. doi: 10.1016/0005-2787(66)90048-7. [DOI] [PubMed] [Google Scholar]
- Sypherd P. S., Strauss N. CHLORAMPHENICOL-PROMOTED REPRESSION OF beta-GALACTOSIDASE SYNTHESIS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Mar;49(3):400–407. doi: 10.1073/pnas.49.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. A hexose-phosphate transport system in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):231–240. doi: 10.1016/0304-4165(66)90170-x. [DOI] [PubMed] [Google Scholar]


