Abstract
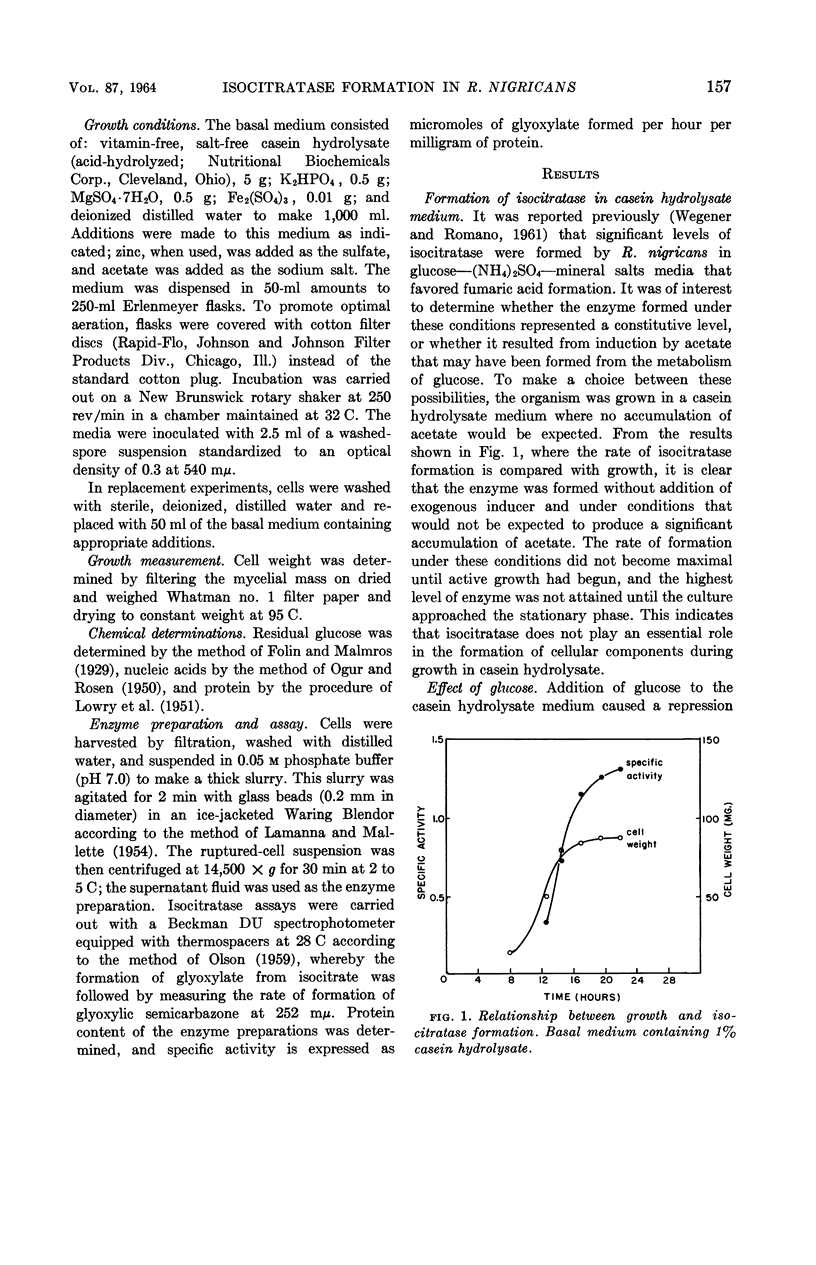
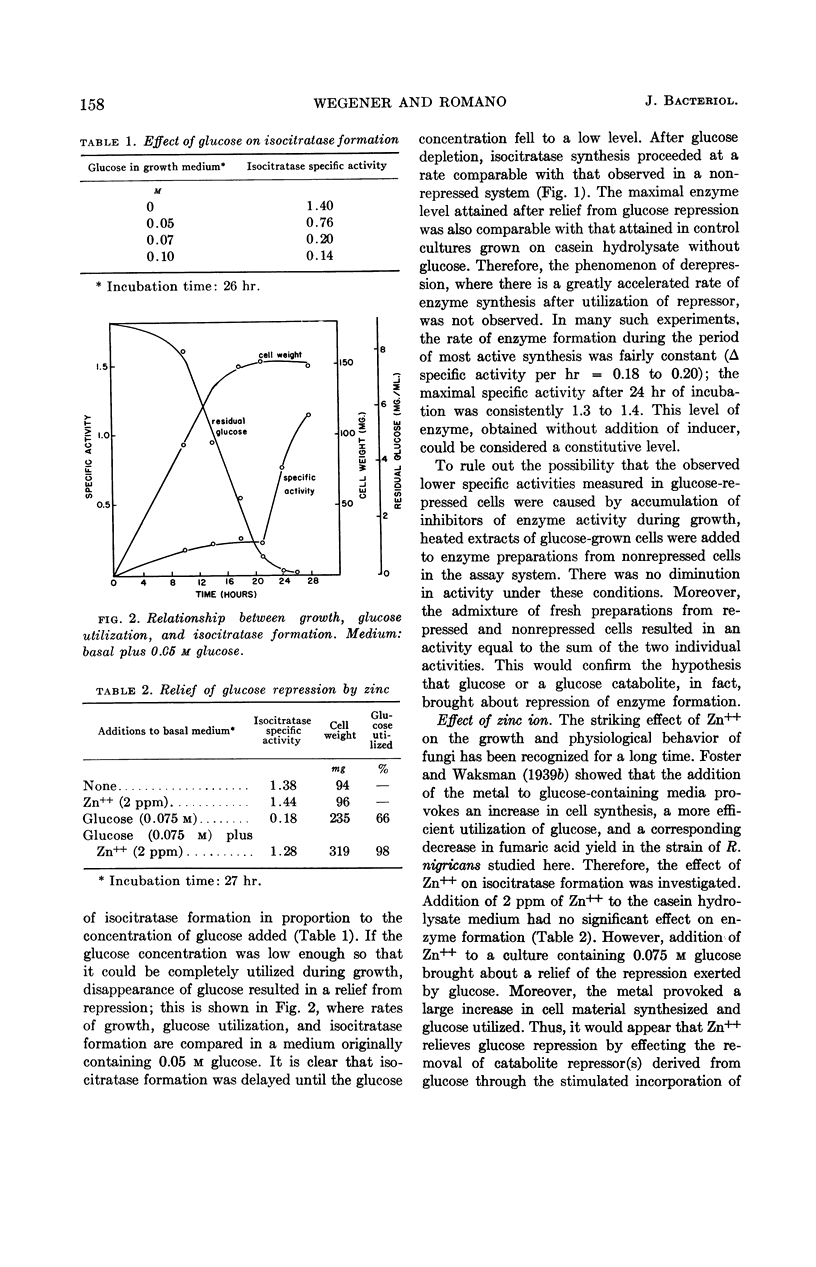
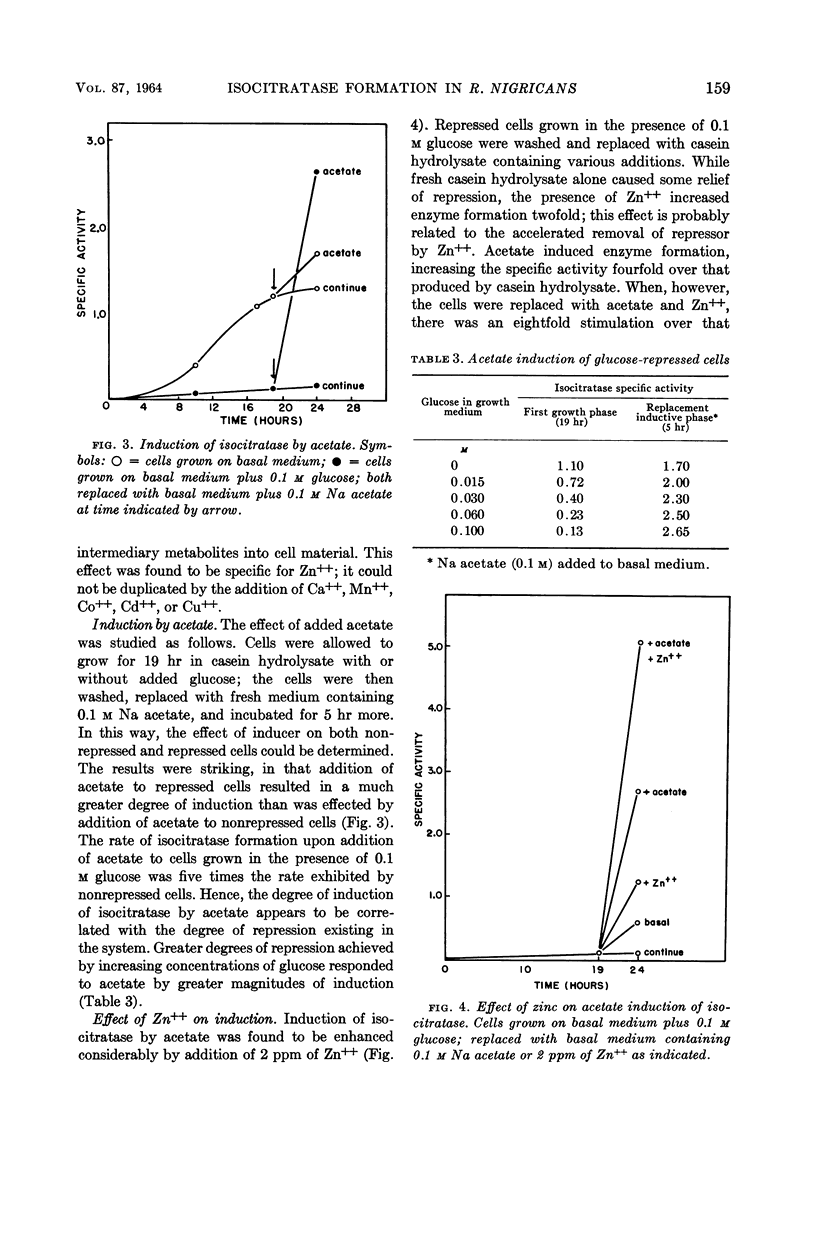
Wegener, Warner S. (University of Cincinnati, Cincinnati, Ohio), and Antonio H. Romano. Control of isocitratase formation in Rhizopus nigricans. J. Bacteriol. 87:156–161. 1964.—A fumaric acid-producing strain of Rhizopus nigricans was found to produce a fair level of isocitratase in a casein hydrolysate medium. Glucose repressed enzyme formation. When glucose was utilized during growth, there was a relief of repression, and enzyme synthesis was resumed at a rate equivalent to that found in nonrepressed cells. Zinc stimulated isocitratase formation in glucose-repressed cultures by stimulating growth and glucose utilization, thereby decreasing accumulation of repressor metabolites derived from glucose. The effectiveness of acetate as an inducer was greater on glucose-repressed cells than on nonrepressed cells; cells grown in the presence of glucose formed higher levels of isocitratase when subsequently replaced with an acetate-containing inductive medium than did cells grown without glucose. Moreover, addition of 2 ppm of Zn++ during the inductive replacement phase resulted in a twofold increase in isocitratase formation. The hypothesis is submitted that Zn++ exerts its action by stimulating ribonucleic acid (RNA) synthesis, thereby facilitating the formation of a specific RNA during induction. Preliminary evidence implicating Zn++ in the stimulation of RNA synthesis in this organism is presented.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLLINS J. F., KORNBERG H. L. The metabolism of C2 compounds in micro-organisms. 4. Synthesis of cell materials from acetate by Aspergillus niger. Biochem J. 1960 Dec;77:430–438. doi: 10.1042/bj0770430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOSTER J. W. An evaluation of the role of molds in the comparative biochemistry of carbohydrate oxidation. Tex Rep Biol Med. 1958;16(1):79–100. [PubMed] [Google Scholar]
- FOSTER J. W., CARSON S. F. Aerobic formation of fumaric acid in the mold Rhizopus nigricans, synthesis by direct C2 condensation. Proc Natl Acad Sci U S A. 1949 Dec;35(12):663–672. doi: 10.1073/pnas.35.12.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Waksman S. A. The Specific Effect of Zinc and Other Heavy Metals on the Growth and Nutrition of Rhizopus. J Bacteriol. 1939 Jun;37(6):599–617. doi: 10.1128/jb.37.6.599-617.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWES W. V., McFADDEN B. A. Isocitrate lyase and malate synthase in Pseudomonas indigofera. I. Suppression and stimulation during growth. J Bacteriol. 1962 Dec;84:1216–1221. doi: 10.1128/jb.84.6.1216-1221.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., COLLINS J. F., BIGLEY D. The influence of growth substrates on metabolic pathways in Micrococcus denitrificans. Biochim Biophys Acta. 1960 Mar 25;39:9–24. doi: 10.1016/0006-3002(60)90117-7. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., ELSDEN S. R. The metabolism of 2-carbon compounds by microorganisms. Adv Enzymol Relat Subj Biochem. 1961;23:401–470. doi: 10.1002/9780470122686.ch8. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., GOTTO A. M., LUND P. Effect of growth substrates on isocitratase formation by Pseudomonas ovalis Chester. Nature. 1958 Nov 22;182(4647):1430–1431. doi: 10.1038/1821430a0. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., KREBS H. A. Synthesis of cell constituents from C2-units by a modified tricarboxylic acid cycle. Nature. 1957 May 18;179(4568):988–991. doi: 10.1038/179988a0. [DOI] [PubMed] [Google Scholar]
- LAMANNA C., MALLETTE M. F. Use of glass beads for the mechanical rupture of microorganisms in concentrated suspensions. J Bacteriol. 1954 Apr;67(4):503–504. doi: 10.1128/jb.67.4.503-504.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACQUILLAN A. M., HALVORSON H. O. Metabolic control of beta-glucosidase synthesis in yeast. J Bacteriol. 1962 Jul;84:23–30. doi: 10.1128/jb.84.1.23-30.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- OGUR M., ROSEN G. The nucleic acids of plant tissues; the extraction and estimation of desoxypentose nucleic acid and pentose nucleic acid. Arch Biochem. 1950 Feb;25(2):262–276. [PubMed] [Google Scholar]
- OLSON J. A. The purification and properties of yeast isocitric lyase. J Biol Chem. 1959 Jan;234(1):5–10. [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. The initial kinetics of enzyme induction. Biochim Biophys Acta. 1961 Apr 29;49:77–88. doi: 10.1016/0006-3002(61)90871-x. [DOI] [PubMed] [Google Scholar]
- REEVES H. C., AJL S. J. Function of malate synthetase and isocitritase in the growth of bacteria on two-carbon compounds. J Bacteriol. 1962 Mar;83:597–601. doi: 10.1002/path.1700830243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACKER W. E. Nucleic acids and metals. III. Changes in nucleic acid, protein, and metal content as a consequence of zinc deficiency in Euglena gracilis. Biochemistry. 1962 Sep;1:859–865. doi: 10.1021/bi00911a019. [DOI] [PubMed] [Google Scholar]
- WEBLEY D. M., DUFF R. B., ANDERSON G. The metabolism of iron-, zinc- and manganese-deficient Nocardia opaca. J Gen Microbiol. 1962 Sep;29:179–187. doi: 10.1099/00221287-29-1-179. [DOI] [PubMed] [Google Scholar]
- WETTER L. R., CORRIGAL J. J. Detection of carbohydrase in paper electrophoresis. Nature. 1954 Oct 9;174(4432):695–695. doi: 10.1038/174695a0. [DOI] [PubMed] [Google Scholar]


