Abstract
Allergic asthma is a complex immunologically mediated disease associated with increased oxidative stress and altered antioxidant defenses. It was hypothesized that α-tocopherol (α-T) decreases oxidative stress and therefore its absence may influence allergic inflammatory process, a pathobiology known to be accompanied by oxidative stress. Therefore, selected parameters of allergic asthma sensitization and inflammation were evaluated following ovalbumin sensitization and re-challenge of α-T transfer protein (TTP) knock-out mice (TTP–/–) that have greatly reduced lung α-T levels (e.g. < 5%) compared to their litter mate controls (TTP+/+). Results showed that severe α-T deficiency result in a blunted lung expression of IL-5 mRNA and IL-5 protein and plasma IgE levels compared with TTP+/+ mice following immune sensitization and rechallenge, although lung lavage eosinophil levels were comparable in both genomic strains. It is concluded that the initial stimulation of immune responses by the TTP–/– mice were generally blunted compared to the TTP+/+ mice, thus diminishing some aspects of subsequent allergic inflammatory processes.
Keywords: Eosinophils, allergic inflammation, asthma, cytokines, IgE, vitamin E
Introduction
Allergic sensitization in the respiratory tract and its accompanying inflammatory-immune mediated responses represent the proximate cause of the airflow limitation characterizing allergic asthma. The operative immunological pathways involve a complex interaction of mediators, signalling pathways and cellular responses including the proliferation and activation of sub-type Th2 CD4+ lymphocytes, IgE production and eosinophilic airway infiltration [1]. Most airway diseases, including asthma, are related to inflammatory processes and these processes have been shown to be accompanied by oxidative stress [2]. These evidences have thus given rise to proposals that antioxidants may represent a logical therapeutic approach [3–5].
Evidence of oxidative stress [3–9] and the reduction of protective nutritional [10,11] and some [12–14], but not all [15], enzymatic antioxidant defenses in asthmatics is particularly intriguing. There has been considerable interest in the possible modulation of asthma by antioxidants [3,8,16]. These and other observational studies suggesting possible associations between asthma incidence and severity and dietary intake of antioxidant micronutrients have given rise to clinical trials utilizing supplemental administration of the major hydrophilic and lipophilic antioxidants, vitamins C and E, respectively. However, these oral supplement trials have thus far failed to demonstrate overall convincing beneficial effects in asthma [8,17,18].
The experimental rodent model of allergic asthma, utilizing the surrogate allergen, ovalbumin (OVA), has been extensively studied [19,20]. Although several investigators have used this model to evaluate the effects of nutritional antioxidants to modulate the ensuing allergen-mediated airway immunological responses, the results have been variable [21–23]. A recent well conducted study utilizing the rodent animal model showed that vitamin E administration could ameliorate both the oxidative stress and immunophysiologic array of allergic respiratory tract responses, including most notably antigen-induced early IL-5 increases and airway hyper-reactivity [24]. In elderly human populations administrations of vitamin E have been reported to increase immune responses [25,26]. In another large random human study (n = 2633) dietary levels of vitamin E were inversely associated with serum IgE concentrations and the frequency of allergen sensitization [27].
In order to further test the hypothesis that allergic inflammatory processes can be modulated by vitamin E, we designed a study of select parameters of allergic sensitization and inflammation utilizing the standard OVA sensitized allergic mouse model in a strain with a genetic absence of the α-tocopherol (α-T) transfer protein (TTP–/–). These mice are unable to incorporate absorbed α-T into liver secreted VLDL [28] and exhibit less than 5% of plasma and lung tissue α-T levels compared to their wild-type littermate controls (TTP+/+) [29]. The results of this study have been partly presented in the form of an abstract [30].
Material and methods
Animals and diet
C57BL/6 male mice with a deletion of the TTP gene (TTP–/–) and their TTP+/+ littermates were generated from our own colony. Originally, the deletion of the TTP gene was performed in 129 Sv/Jae background mice [31]. One hundred and twenty-nine Sv/Jae mice with deletion of TTP were bred with C57BL/6 mice over 10 generations. Animals were randomly screened for the absence of viral and respiratory pathogens and housed in polycarbonate shoebox type cages with wire tops in a room maintained at 20°C and 60–70% humidity on a 12 h light/dark schedule and with ad libitum access to water and standard rodent chow diet (PicoLab® Mouse Diet 20, 5058, containing 66 IU dl α-tocopheryl acetate per kg diet, roughly equivalent to 30 mg 2R-α-tocopherol). TTP–/– mice were bred by mating male and female heterozygous TTP+/– mice for the gene deletion because α-T deficiency causes foetal resorption in female TTP–/– mice. The offspring were genotyped using a specific primer for TTP and the genotype of each animal was confirmed by liver TTP protein expression by Western Blot analysis using a specific antibody against the TTP protein [29]. Protocols for the care and use of animals were approved by the UC Davis Animal Use and Care Committee.
Ovalbumin exposure
Procedures for sensitization and exposure of mice to OVA aerosol have been described in detail elsewhere [20,32]. Briefly, mice, 6–8 weeks old were systemically sensitized to OVA (10 μg/0.1 ml chicken egg albumin, ovalbumin, grade V, 98% pure, Sigma, St. Louis, MO) by intraperitoneal (i.p.) injection on days 0 and 14. Exposures to aerosolized OVA (10 ml of 10 mg OVA/ml 1% PBS solution) were commenced on day 28 after the first i.p. OVA injection for 30 min per day for 3 consecutive days; 1% PBS solution was used for the control group. Nebulization was performed in a plastic chamber connected to a side-steam nebulizer (Invacare Corporation, Elyria, OH), ProNeb compressor nebulizer (Pari, Richmond) and passport Compressor (Invacare, Sanford, FL) [20,32].
Plasma, broncho-alveolar lavage (BAL) and tissue harvest
Immediately following the third exposure mice were euthanized with pentobarbital (i.p.) and blood was collected by cardiac puncture in heparinized tubes. Plasma was obtained by centrifugation and stored at –80°C. After blood collection, lungs were lavaged twice with 1 ml of PBS (pH 7.4) as previously described [29]. Collected BAL fluid was immediately placed on ice and centrifuged at 500 × g. For differential cell analysis, an aliquot (10 μl) of BAL cell suspension was cyto-centrifuged and stained with Wright-Giemsa stain (Diff-Quik, Baxter Scientific Products, McGrow Park, IL). The BAL supernatants were used for the measurement of cytokines.
Plasma and tissue levels of α-T and γ-tocopherol (γ-T)
Plasma, liver and lung α-T and γ-T concentrations were determined using high pressure liquid chromatography (HPLC) with electrochemical detection as previously described [33].
Plasma IgE levels
Mouse plasma IgE was measured using a standard sandwich ELISA kit purchased from BD Pharmingen (San Diego, CA). Briefly, 100 μl of purified anti-mouse IgE, diluted to 2 μg/ml in PBS, was used to coat a 96-well ELISA plate. Plates were coated overnight at 4°C and then washed three times with PBS/Tween (0.05%); 200 μl of 10% foetal calf serum (FCS)/PBS was added to each well for 1 h at room temperature. After three washes with PBS/Tween, 100 μl of sample or purified mouse IgE standard diluted in 10% FCS were added to each well and incubated for 1 h. After washing, biotinylated anti-mouse IgE (2 μg/ml) was added for 1 h; 100 μl of diluted strepavidin-horseradish peroxidase (1:1000 in 10% FCS) was then added for 30 min. After a final three washes, 100 μl of an equal mixture of 30% H2O2 and tetramethylbenzidine (TMB, BD Pharmingen) was added for 10–20 min. The reaction was stopped with 50 μl of 1 m H3PO4 and the plate was read immediately with a microplate reader at 450 nm.
Cytokine concentrations
Plasma and BAL concentrations of cytokines (IL-4, IL-5, TNF-α, IFN-γ and sICAM) were measured using commercially available ELISA assays, (Quantikine R&D system, MN).
Lung gene expression of inflammatory markers
These methods have been described in detail [29]. Briefly, RNA from lung was isolated by extraction into Trizol following the manufacturer's instructions and cDNA was synthesized from 5 μg total RNA as in reverse transcript PCR. Gene-specific primers were designed with use of Primer Express 1.0 software (Applied Biosystems) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels which were used to normalize the gene expression level. Real-time PCR was carried out with SYBR Green I Master Mix (Applied Biosystems) reagent. Real time PCR values were determined by reference to a standard curve that was generated by real time PCR amplification of serially diluted cDNAs for each genes. A duplicate reaction was carried out for each sample. Quantitative PCR assay was performed with gene-specific double-labelled fluorescent probes and sets of specific primers in an ABI PRISM 7700 Sequence detection system (PE Applied Biosystems). Reverse transcriptase reactions were incubated at 50°C for 30 min and after inactivation of reverse transcriptase at 95°C for 12 min, 40 cycles of amplification were carried out with denaturation at 95°C for 15 s each and both annealing and extension at 60°C for 1 min each. The sequences of primer pairs used in the experiments were as follows: TNF-α: (forward) GAAAAGCA AGCAGCCAACCA, (reverse) GGGCCAGTGAGTGAAAGGG, IFN-γ: (forward) AGC TCA TCC GAG TGG TCC AC, (reverse) GCT TCC TGA GGC TGG ATT CC, ICAM-1: (forward) TGC GTT TTG GAG CTA GCG GAC CA, (reverse) CGA GGA CCA TAC AGC ACG TGC AG, IL-4: (forward) CACGGATGCGACAAAAATCA, (reverse) CTCGTTCA AAATGCC GATGA, IL-5: (forward) ACGCAGGAGGAT CACATACC, (reverse) GGCTCTCATTCACACTGCAA.
Statistical analysis
Prior to making comparisons, we used the Shapiro-Wilk test for normalizing the responsible variables of interest and the results indicated that a number of responsible variables were departed from normality. Hence, we used non-parametric analyses. For each of the response variables including α-T and γ-T levels in plasma, liver and lung, we tested for a significant difference between PBS exposed TTP+/+ (PBS/TTP+/+) mice and that of OVA exposed TTP+/+ (OVA/TTP+/+) mice and between PBS exposed TTP–/– (PBS/TTP–/–) mice and that of OVA exposed TTP–/– (OVA/TTP–/–) mice by using the two-sided exact Wilcoxon-Mann-Whitney (W-M-W) test; the one-sided exact W-M-W test was used to determine whether the median of the response variable was higher in the PBS/TTP+/+ and OVA/TTP+/+ groups compared with the PBS/TTP–/– and OVA/TTP–/– groups, respectively. For the other response variables, the two-sided exact W-M-W test was used to assess whether there was a significant difference between the PBS/TTP+/+ and PBS/TTP–/– groups and between the OVA/TTP+/+ and OVA/TTP–/– groups; we used the one-sided exact W-M-W test to evaluate whether the median of the response variable in the OVA/TTP+/+ and OVA/TTP–/– groups exceeded that in the PBS/TTP+/+ and PBS/TTP–/– groups, respectively. A p-value ≤ 0.05 was considered statistically significant. Summary statistics were expressed as mean ± standard deviation (SD) and median (minimum, maximum).
Results
Plasma and tissue α-T and γ-T concentrations
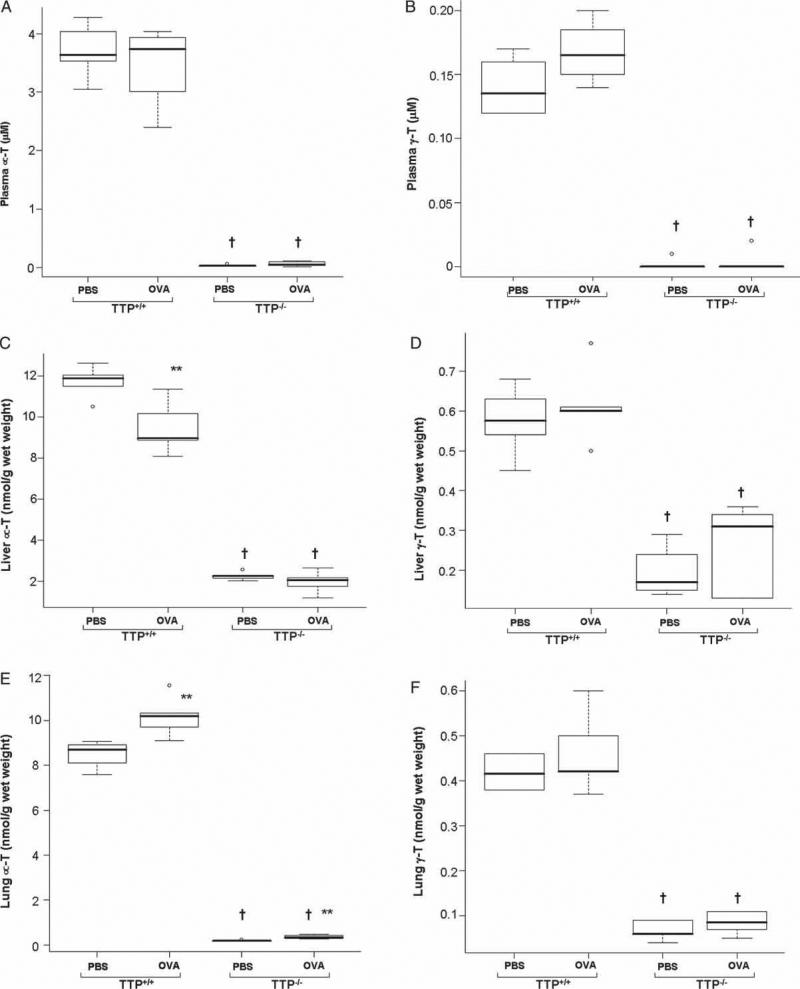
Plasma and tissue α-T and γ-T concentrations in TTP+/+ and TTP–/– mice exposed to aerosolized PBS or to OVA are shown in Figures 1(A–F), respectively. As expected, vitamin E concentrations were significantly lower in TTP–/– mice compared with TTP+/+ mice. Baseline plasma, lung and liver rα-T levels in TTP–/– mice were 0.8%, 2% and 19% of those of TTP+/+ mice, respectively, while γ-T levels were 0.7%, 16% and 34% of those of TTP+/+ mice (Figure 1A–F) as a result of TTP gene deletion. Regardless of genotype, after OVA exposure, plasma α-T and γ-T concentrations were not significantly different (Figure 1A and B). Liver α-T concentrations significantly decreased (p < 0.01) in TTP+/+ mice after OVA exposure as compared to respective PBS exposed TTP+/+ controls (Figure 1C), while lung α-T concentrations increased in both TTP+/+ and TTP–/– mice (p < 0.01) (Figure 1E) after OVA exposure as compared to its respective PBS treated controls. Both liver and lung γ-T concentrations in TTP+/+ and TTP–/– mice were unchanged after OVA challenge (Figure 1D and F).
Figure 1.
α-T and γ-T levels in plasma (A, B), liver (C, D) and lung (E, F) of TTP+/+ and TTP–/– mice after PBS or OVA exposure. Values are mean ± SD and median (minimum, maximum), n = 6–5 per group. TTP–/– mice have low Vitamin E tissue levels due to TTP gene deletion as compared to TTP+/+ mice († p < 0.001). Liver α-T levels were significantly decreased in OVA exposed TTP+/+ mice as compared to respective PBS treated controls (** p < 0.01). On the contrary, lung α-T levels were significantly increased (p < 0.01) after OVA exposure in both the genotypes as compared to PBS treated controls. Plasma, liver and lung γ-T levels remained unchanged after OVA exposure.
BAL cells
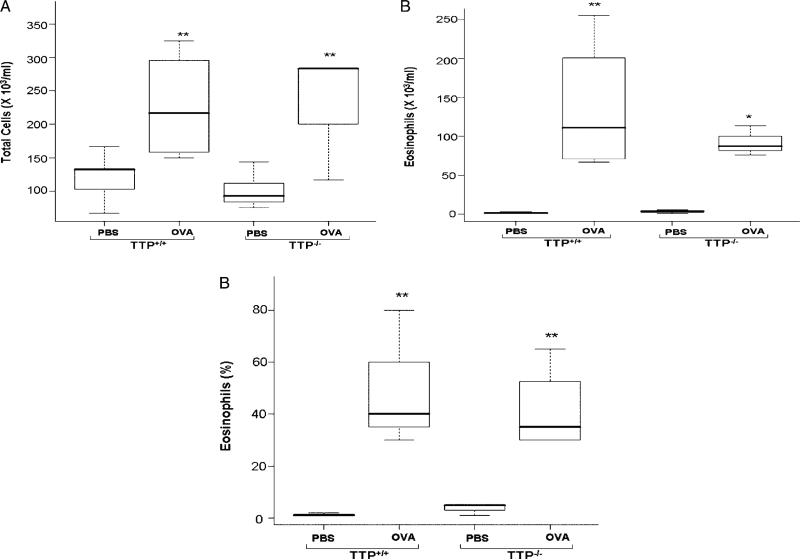
BAL cells were counted and differential cell analysis was performed. Total cell numbers were significantly increased after OVA exposure in both groups of TTP+/+ and TTP–/– mice (p < 0.01) (Figure 2A). Eosinophil counts were significantly increased after OVA exposure in TTP+/+ mice (p < 0.01) and TTP–/– mice (p < 0.05) as compared to respective PBS treated controls (Figure 2B). Eosinophil percentages of the BAL cells in the OVA exposed TTP+/+ and TTP–/– mice were significantly higher (p < 0.01), representing 50% and 41%, respectively, as compared to PBS treated mice (Figure 2C). For the three response variables, there were no significant differences between the two genotypes. The normal lung lavage from a healthy mouse contains more that 90% alveolar macrophages [34] and our observations in the air-exposed groups were consistent with this finding. The increase in inflammatory cells seen in the BAL from the OVA-exposed groups is predominantly accounted for by the increase in lung lavage eosinophils.
Figure 2.
Total number of cells (A), number of eosinophils (B) and% of eosinophils (C) in BAL fluid of TTP+/+ and TTP–/– mice after PBS or OVA exposure. Values are mean ± SD and median (minimum, maximum), n = 6–5 per group. As a result of OVA exposure, total BAL cells were significantly increased (** p < 0.01) in both the genotypes as compared to respective PBS controls. Eosinophil cells in BAL fluid also showed a significant increase in OVA exposed TTP+/+ (** p < 0.01) and TTP–/– (* p < 0.05) mice as compared to PBS treated TTP+/+ and TTP+/+ controls. Similar trend was also seen in eosinophil percentage in BAL cells after OVA exposure (** p < 0.01), but no significant differences were seen in both the genotypes in any of the parameters.
Plasma IgE levels
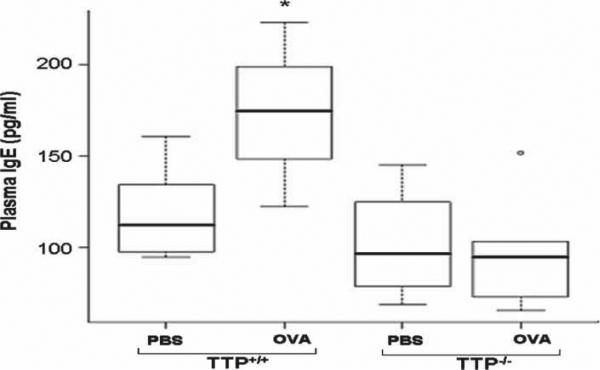
Plasma IgE levels were significantly increased after OVA exposure in TTP+/+ mice (p < 0.05) but unchanged in TTP–/– mice (Figure 3). There were no significant differences in plasma IgE level between the two genotypes.
Figure 3.
Plasma IgE levels in TTP+/+ and TTP–/– mice after PBS or OVA exposure. Values are mean ± SD and median (minimum, maximum), n = 6–5 per group. A significant increase in plasma IgE levels in TTP+/+ mice (* p < 0.05) was observed after OVA exposure as compared to PBS treated TTP+/+ controls. There was no significant difference in OVA exposed TTP–/– mice or between the two genotypes.
Inflammatory mediators
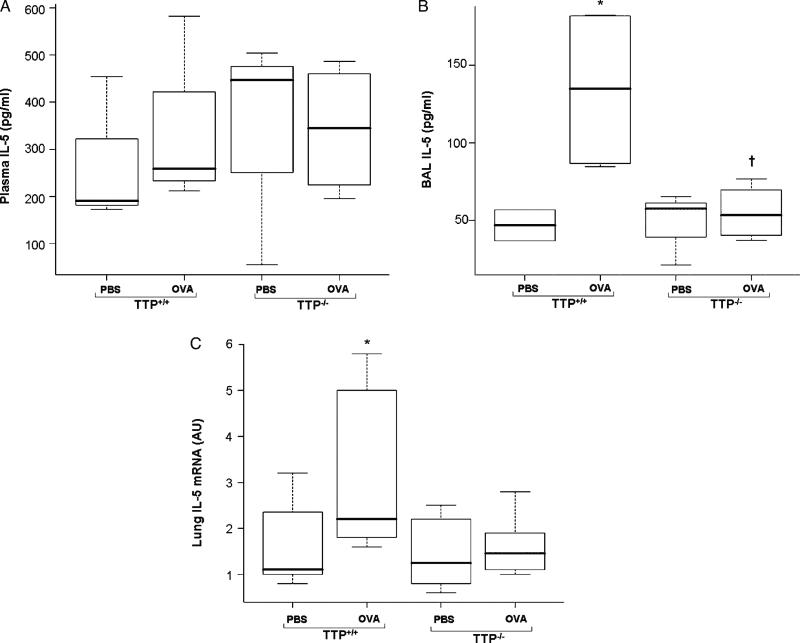
Chemokine, cytokine and adhesion molecules play important roles in the trafficking of activated immune cells into the airway during inflammatory responses and to a degree these processes are reflected in the levels of these species in both plasma and BAL fluids. Plasma IL-5 levels were not significantly increased in OVA exposed mice regardless of genotype (Figure 4A). IL-5, a cytokine related to eosinophil recruitment into airways, in BAL was significantly increased in OVA exposed TTP+/+ mice (p < 0.05) but not in TTP–/– mice as compared to respective PBS treated controls (Figure 4B). IL-5 mRNA levels in lung homogenates of OVA exposed TTP+/+ mice were significantly increased (p < 0.05); however, lung homogenate IL-5 mRNA levels were relatively unchanged in TTP–/– mice (Figure 4C). Plasma IL-4, IFN-γ and TNF- α concentrations were not different between PBS and OVA exposed mice regardless of genotype (data not shown).
Figure 4.
IL-5 protein levels in plasma (A) and BALF (B) and mRNA expression levels in lung tissues (C) of TTP+/+ and TTP–/– mice after PBS or OVA exposure. Values are mean ± SD and median (minimum, maximum), n = 6–5 per group. Plasma IL-5 protein levels were not significantly different irrespective of genotypes. BAL IL-5 protein levels and lung IL-5 mRNA expression were significantly increased (* p < 0.05) in OVA exposed TTP+/+ mice as compared to PBS treated TTP+/+ controls but no change were observed in TTP–/– mice.
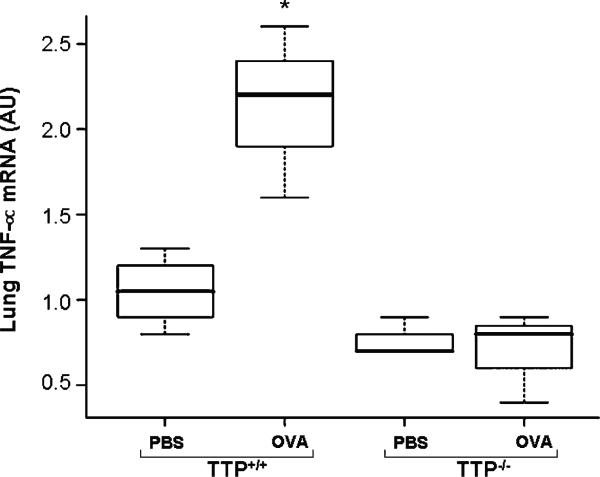
TNF-α mRNA levels were also significantly increased in lung homogenates of OVA exposed TTP+/+ mice (p < 0.01) (Figure 5), but BAL fluid was limited so it was not possible to determine BAL TNF-α protein levels (thus it is unknown if BAL TNF-α protein was also increased). Remarkably, lung TNF-α mRNA was essentially unchanged in TTP–/– mice, again demonstrating that the vitamin E deficient mice were relatively less responsive.
Figure 5.
TNF-α mRNA expression levels in lung tissues of TTP+/+ and TTP–/– mice after PBS or OVA exposure. Values are mean ± SD and median (minimum, maximum), n = 6–5 per group. Lung tissues of TTP+/+ mice exposed to OVA showed a significantly increased expression of TNF-α mRNA (** p < 0.05) as compared to PBS treated TTP+/+ controls. There was no significant difference in OVA exposed TTP–/– mice or between the two genotypes.
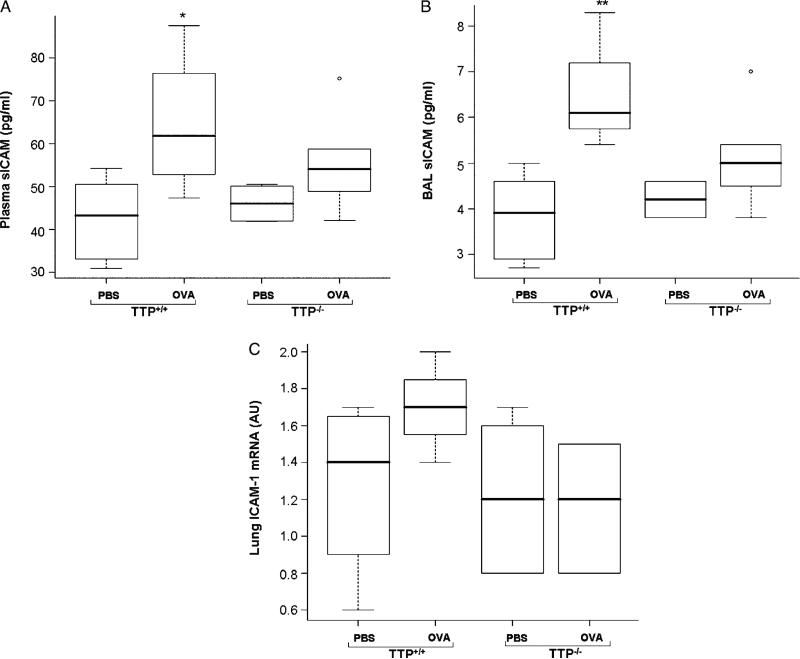
Plasma and BAL sICAM concentrations significantly increased after OVA exposure in TTP+/+ mice (p < 0.05, p < 0.01, respectively); however, no significant changes were seen in lung ICAM mRNA expression (Figure 6A–C). There was no significant change in plasma and BAL sICAM levels or lung ICAM mRNA expression in OVA exposed TTP–/–. There was no significant difference between the two genotypes for any of the three response variables (Figure 6A–C).
Figure 6.
sICAM protein levels in plasma (A) and BAL fluid (B) and ICAM-1 mRNA levels (C) in lung tissues of TTP+/+ and TTP–/– mice after PBS or OVA exposure. Values are mean ± SD and median (minimum, maximum), n = 6–5 per group. Plasma and BAL sICAM levels were significantly increased (* p < 0.05, ** p < 0.01, respectively) in TTP+/+ mice after OVA exposure as compared to PBS treated TTP+/+ controls. There was no significant difference in OVA exposed TTP–/– mice or between the two genotypes. The changes in lung mRNA ICAM expression was not statistically different in OVA treated TTP+/+ or TTP–/– mice or between the two genotypes.
Discussion
The present studies have demonstrated that TTP–/– mice with greatly reduced lung α-T levels exhibit a very significant modulation of allergic airway inflammation, as scored by both IL-5 and IgE responses. It is likely that the initial stimulation to generate allergic responses in the TTP–/– mice were somewhat blunted compared to the TTP+/+ mice.
Allergic inflammation and vitamin E
It is generally accepted that Th2 CD4+ cells and their cytokine profiles, IgE and eosinophils play important roles in the pathogenesis of allergic inflammatory processes including allergic airway inflammation [1] and to a large extent these findings are well demonstrated in the OVA sensitization mouse model [19,20,24,32] including those of the present report. α-T has been reported to influence a large and diverse number of pathways that could contribute to immunologically activated inflammatory processes. These include NF-κB [35,36] and cytokine [24,29,37] pathways, phospholipase and arachidonic pathways [38,39], mast cell activation [40,41] and, importantly, in the context of the present studies, cell-mediated immunity [26,42,43] and antibody production [44] including that of IgE [22]. Central to the pathogenesis of allergic inflammation are antigen-specific, memory T-cell responses and antigen-specific IgE responses [1]. The present findings of α-T related modulations of these pathways are thus compatible with numerous studies showing that α-T plays a demonstrable role in modulating immune functions.
IgE hyperproduction to antigen sensitization has long been recognized to play a key role in the immunopathology of allergic airway inflammation [1,22,45]. Although the precise mechanisms of action are incompletely characterized, IgE has a wide spectrum of abilities to induce the production and secretion of inflammatory cytokines such as TNF-α and IL-6 [46]. Moreover, plasma IgE levels have been associated in some studies with the severity of allergic airway inflammation [47].
Our findings of low IgE concentrations in TTP–/– mice compared to TTP+/+ mice are at variance with reports of an inverse relationship between α-T levels and IgE production in allergic patients including asthmatics [11,47] and in an allergic asthma rodent model with a much lower range of vitamin E levels (e.g. ~4:1 vs the current study of 100:1) [24], but are in concordance with reports of α-T induced enhanced responses to immunologic stimuli in aged human populations [26,48]. Given the complexities of antigen-induced immune responses and the variability of experimental methodologies concerning dose–time relationships and endpoint scoring methodologies, it can be concluded that although evidence points to a role of α-T in regulating IgE concentrations, the mechanisms remain obscure. Finally, the current study is compatible with those of Bando et al. [22] who have demonstrated a biphasic effect of α-T in an allergic OVA sensitization model of systemic allergy.
Accumulation of eosinophils in the airways is multifunctional, involving eosinopoiesis, migration and diapedesis and facilitation by numerous cytokines including IL-5, chemotactic factors, adhesion molecules and proteolytic enzymes [1,49,50]. IL-5 has been implicated in the development of allergic inflammation and reportedly is important in mobilizing eosinophils from the bone marrow, whereas chemokines such as eotaxin-1 may be largely responsible for the recruitment of eosinophils into respiratory tract tissues [51]. In the current study, at the time interval studied, the important Th 2 cytokine, IL-5 was significantly lower in TTP–/– mice than in TTP+/+ mice, but the reductions of airway lumen eosinophil recruitment did not reach statistical significance. Although the present studies showed greatly diminished augmentations of IL-5 in the BAL of TTP–/– mice compared to TTP+/+ mice, the results suggest that IL-5 is not the only mechanism for attracting eosinophils into airway lumens, perhaps in keeping with the limited effectiveness of anti-IL-5 therapeutic strategies in human allergy [52]. In addition, TNF-α and ICAM levels were significantly decreased in TTP–/– mice as compared with TTP+/+ mice, thus showing some consistency with the IL-5 and IgE determinations in that these pathways are also activated in allergic inflammatory responses [1,49].
Pathobiological significance
Although still incompletely understood, the mechanisms underlying the development of allergic airways inflammation, a key clinical feature of allergic asthma, have been associated with augmented Th2 CD4+ cytokine production and IgE elevations and have involved both sensitization and subsequent rechallenge inflammatory-immune responses. The same therapeutic intervention would thus not necessarily be expected to both of these complex processes in the same manner. For example, markedly differently effects of iNOS inhibitors have been demonstrated on allergic airway inflammation in an similar experimental murine asthma model (e.g. iNOS inhibitors showed opposite effects depending on their schedule of administration in the overall sensitization-re-challenge process) [53].
Allergic airway inflammation is a complex process involving interactions among airway epithelium, inflammatory cells including T-cells, macrophages, mast cells and eosinophils and their mediators. Vitamin E potentially influences many constituents of these complex integrated pathways of allergen sensitization and subsequent inflammatory-immune processes. In various studies vitamin E has been touted to have immunostimulatory properties [48], anti-inflammatory [54,55] and/or both properties [29]. In clinical studies, α-T appeared to both decrease IgE levels and clinical manifestations of atopy in patients with allergic dermatitis [56] and appeared to have had a small clinical benefit in allergic rhinitis [57]. Of some relevance to the current study is a recent report of dramatic inhibition of murine allergic sensitization responses by the administration of pharmacological doses of γ-T [58,59].
The following limitations of the present study should be mentioned. These include the limited range of sensitizing and challenging antigenic doses and α-T levels utilized, the limited post-challenge period examined, the limited range of mediators scored for endpoint analysis and the lack of patho-physiological correlations. A temporal analysis of sensitization effectiveness and post-challenge spectrum of chemokine and cytokine profiles, including such counter-regulatory mediators as INF-γ and IL-10, would have more completely characterized α-T effect on allergen-induced airway inflammatory-immune processes in this model [24,60], but was beyond the scope of the present project designed to show allergic inflammatory responses to be modulated in the TTP–/– mouse compared to their TTP+/+ littermates. Finally, it is likely that the administration schedules of α-T, like another pleuropotential biological metabolic modular, nitric oxide [53,61], effect overall allergic reactions differently at various stages of the sensitization-rechallenge cycle. The finding that γ-T administration appear to dramatically ameliorate allergic inflammation when given after antigen stimulation is relevant in this regard [58,59], possibly via mechanisms interacting with prostaglandin metabolic pathways [62–64]. Although it has been shown that vitamin E administration can modulate biomarkers of oxidative stress in this model [24], it is possible that non-antioxidant vitamin E functions [65] contribute to the vitamins’ modulation of allergic responses.
In summary, results from this animal study demonstrate that α-T has complex interactions with allergic inflammatory processes. In at least the current model, TTP–/– mice with severe α-T deficiency appear to dampen selected parameters of the early sensitization phase as compared to their TTP+/+ littermates, supporting the concept that inadequate vitamin E levels leads to reduced immune responses.
Acknowledgements
We would like to thank Jennifer Jew for her assistance with IgE ELISA determinations. Support for this work was received from NIH Grant #ES011985 and USDA grant # 35200–13456 to CEC and MGT and NIH grant # K08-HL76415 to NJK.
References
- 1.Umetsu DT, Dekruyff RH. Immune dysregulation in asthma. Curr Opin Immunol. 2006;18:727–732. doi: 10.1016/j.coi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Cross CE, Vandervliet A, O'Neill CA, Eiserich JP. Reactive oxygen species and the lung. Lancet. 1994;344:930–933. doi: 10.1016/s0140-6736(94)92275-6. [DOI] [PubMed] [Google Scholar]
- 3.Bowler RP, Crapo JD. Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol. 2002;110:349–356. doi: 10.1067/mai.2002.126780. [DOI] [PubMed] [Google Scholar]
- 4.Grimble RF. Anitioxidant modulation in immune function. In: Rimbach G, Fuchs J, Packer L, editors. Nutrigenomics. Taylor & Francis; New York: 2005. pp. 97–122. [Google Scholar]
- 5.Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;11:476–494. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Dworski R, Roberts LJ, 2nd, Murray JJ, Morrow JD, Hartert TV, Sheller JR. Assessment of oxidant stress in allergic asthma by measurement of the major urinary metabolite of F2-isoprostane, 15-F2t-IsoP (8-iso-PGF2alpha). Clin Exp Allergy. 2001;31:387–390. doi: 10.1046/j.1365-2222.2001.01055.x. [DOI] [PubMed] [Google Scholar]
- 7.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 8.Caramori G, Papi A. Oxidants and asthma. Thorax. 2004;59:170–173. doi: 10.1136/thorax.2002.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujisawa T. Role of oxygen radicals on bronchial asthma. Curr Drug Targets Inflamm Allergy. 2005;4:505–509. doi: 10.2174/1568010054526304. [DOI] [PubMed] [Google Scholar]
- 10.Rubin RN, Navon L, Cassano PA. Relationship of serum antioxidants to asthma prevalence in youth. Am J Respir Crit Care Med. 2004;169:393–398. doi: 10.1164/rccm.200301-055OC. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Mannino DM, Redd SC. Serum antioxidant concentrations among U.S. adults with self-reported asthma. J Asthma. 2004;41:179–187. doi: 10.1081/jas-120026075. [DOI] [PubMed] [Google Scholar]
- 12.De Raeve HR, Thunnissen FB, Kaneko FT, Guo FH, Lewis M, Kavuru MS, Secic M, Thomassen MJ, Erzurum SC. Decreased Cu, Zn-SOD activity in asthmatic airway epithelium: correction by inhaled corticosteroid in vivo. Am J Physiol. 1997;272:L148–L154. doi: 10.1152/ajplung.1997.272.1.L148. [DOI] [PubMed] [Google Scholar]
- 13.Comhair SA, Bhathena PR, Dweik RA, Kavuru M, Erzurum SC. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet. 2000;355:624. doi: 10.1016/S0140-6736(99)04736-4. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, Janocha AJ, Aronica MA, Swaidani S, Comhair SA, Xu W, Zheng L, Kaveti S, Kinter M, Hazen SL, Erzurum SC. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J Immunol. 2006;176:5587–5897. doi: 10.4049/jimmunol.176.9.5587. [DOI] [PubMed] [Google Scholar]
- 15.Comhair SA, Bhathena PR, Farver C, Thunnissen FB, Erzurum SC. Extracellular glutathione peroxidase induction in asthmatic lungs: evidence for redox regulation of expression in human airway epithelial cells. Faseb J. 2001;15:70–78. doi: 10.1096/fj.00-0085com. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard R, Fogarty A. The developing story of antioxidants and asthma. Thorax. 2004;59:3–4. [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax. 2004;59:652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riccioni G, Barbara M, Bucciarelli T, di Ilio C, D'Orazio N. Antioxidant vitamin supplementation in asthma. Ann Clin Lab Sci. 2007;37:96–101. [PubMed] [Google Scholar]
- 19.Hellings PW, Ceuppens JL. Mouse models of global airway allergy: what have we learned and what should we do next? Allergy. 2004;59:914–919. doi: 10.1111/j.1398-9995.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 20.Kenyon NJ, Last JA. Reversible and irreversible airway inflammation and fibrosis in mice exposed to inhaled ovalbumin. Inflamm Res. 2005;54:57–65. doi: 10.1007/s00011-004-1325-6. [DOI] [PubMed] [Google Scholar]
- 21.Kilic FS, Erol K. The effects of vitamin E in ovalbumin-sensitized guinea pigs. Methods Find Exp Clin Pharmacol. 2003;25:27–31. doi: 10.1358/mf.2003.25.1.772544. [DOI] [PubMed] [Google Scholar]
- 22.Bando N, Yamanishi R, Terao J. Inhibition of immunoglobulin E production in allergic model mice by supplementation with vitamin E and beta-carotene. Biosci Biotechnol Biochem. 2003;67:2176–2182. doi: 10.1271/bbb.67.2176. [DOI] [PubMed] [Google Scholar]
- 23.Suchankova J, Voprsalova M, Kottova M, Semecky V, Visnovsky P. Effects of oral alpha-tocopherol on lung response in rat model of allergic asthma. Respirology. 2006;11:414–421. doi: 10.1111/j.1440-1843.2006.00864.x. [DOI] [PubMed] [Google Scholar]
- 24.Talati M, Meyrick B, Peebles RS, Jr, Davies SS, Dworski R, Mernaugh R, Mitchell D, Boothby M, Roberts LJ, 2nd, Sheller JR. Oxidant stress modulates murine allergic airway responses. Free Radic Biol Med. 2006;40:1210–1219. doi: 10.1016/j.freeradbiomed.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA. 2004;292:828–836. doi: 10.1001/jama.292.7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meydani SN, Han SN, Wu D. Vitamin E and immune response in the aged: molecular mechanisms and clinical implications. Immunol Rev. 2005;205:269–284. doi: 10.1111/j.0105-2896.2005.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fogarty A, Lewis S, Weiss S, Britton J. Dietary vitamin E, IgE concentrations, and atopy. Lancet. 2000;356:1573–1574. doi: 10.1016/S0140-6736(00)03132-9. [DOI] [PubMed] [Google Scholar]
- 28.Traber MG, Burton GW, Hamilton RL. Vitamin E trafficking. Ann NY Acad Sci. 2004;1031:1–12. doi: 10.1196/annals.1331.001. [DOI] [PubMed] [Google Scholar]
- 29.Schock BC, Van der Vliet A, Corbacho AM, Leonard SW, Finkelstein E, Valacchi G, Obermueller-Jevic U, Cross CE, Traber MG. Enhanced inflammatory responses in alpha-tocopherol transfer protein null mice. Arch Biochem Biophys. 2004;423:162–169. doi: 10.1016/j.abb.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Lim Y, Vasu VT, Gohil K, Corbacho AM, Aung HH, Valacchi G, Traber MG, Kenyon NJ, Cross CE. Role of vitamin E in allergic responses induced by inhaled ovalbumin exposure in α-tocopherol transfer protein (TTP) null mice. FASEB J. 2005;9:A443. [Google Scholar]
- 31.Terasawa Y, Ladha Z, Leonard SW, Morrow JD, Newland D, Sanan D, Packer L, Traber MG, Farese RV., Jr Increased atherosclerosis in hyperlipidemic mice deficient in alpha-tocopherol transfer protein and vitamin E. Proc Natl Acad Sci USA. 2000;97:13830–13834. doi: 10.1073/pnas.240462697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenyon NJ, Ward RW, Last JA. Airway fibrosis in a mouse model of airway inflammation. Toxicol Appl Pharmacol. 2003;186:90–100. doi: 10.1016/s0041-008x(02)00025-x. [DOI] [PubMed] [Google Scholar]
- 33.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res. 1996;37:893–901. [PubMed] [Google Scholar]
- 34.Hamada K, Goldsmith CA, Goldman A, Kobzik L. Resistance of very young mice to inhaled allergen sensitization is overcome by coexposure to an air-pollutant aerosol. Am J Respir Crit Care Med. 2000;161:1285–1293. doi: 10.1164/ajrccm.161.4.9906137. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki YJ, Packer L. Inhibition of NF-kappa B activation by vitamin E derivatives. Biochem Biophys Res Commun. 1993;193:277–283. doi: 10.1006/bbrc.1993.1620. [DOI] [PubMed] [Google Scholar]
- 36.Morante M, Sandoval J, Gomez-Cabrera MC, Rodriguez JL, Pallardo FV, Vina JR, Torres L, Barber T. Vitamin E deficiency induces liver nuclear factor-kappaB DNA-binding activity and changes in related genes. Free Radic Res. 2005;39:1127–1138. doi: 10.1080/10715760500193820. [DOI] [PubMed] [Google Scholar]
- 37.Hybertson BM, Chung JH, Fini MA, Lee YM, Allard JD, Hansen BN, Cho OJ, Shibao GN, Repine JE. Aerosol-administered alpha-tocopherol attenuates lung inflammation in rats given lipopolysaccharide intratracheally. Exp Lung Res. 2005;31:283–294. doi: 10.1080/01902140590918560. [DOI] [PubMed] [Google Scholar]
- 38.Wu D, Liu L, Meydani M, Meydani SN. Vitamin E increases production of vasodilator prostanoids in human aortic endothelial cells through opposing effects on cyclooxygenase-2 and phospholipase A2. J Nutr. 2005;135:1847–1853. doi: 10.1093/jn/135.8.1847. [DOI] [PubMed] [Google Scholar]
- 39.Khanna S, Roy S, Ryu H, Bahadduri P, Swaan PW, Ratan RR, Sen CK. Molecular basis of vitamin E action: tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J Biol Chem. 2003;278:43508–43515. doi: 10.1074/jbc.M307075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gueck T, Aschenbach JR, Fuhrmann H. Influence of vitamin E on mast cell mediator release. Vet Dermatol. 2002;13:301–305. doi: 10.1046/j.1365-3164.2002.00307.x. [DOI] [PubMed] [Google Scholar]
- 41.Kempna P, Reiter E, Arock M, Azzi A, Zingg JM. Inhibition of HMC-1 mast cell proliferation by vitamin E: involvement of the protein kinase B pathway. J Biol Chem. 2004;279:50700–50709. doi: 10.1074/jbc.M410800200. [DOI] [PubMed] [Google Scholar]
- 42.Li-Weber M, Giaisi M, Treiber MK, Krammer PH. Vitamin E inhibits IL-4 gene expression in peripheral blood T cells. Eur J Immunol. 2002;32:2401–2408. doi: 10.1002/1521-4141(200209)32:9<2401::AID-IMMU2401>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 43.Han SN, Adolfsson O, Lee CK, Prolla TA, Ordovas J, Meydani SN. Age and vitamin E-induced changes in gene expression profiles of T cells. J Immunol. 2006;177:6052–6061. doi: 10.4049/jimmunol.177.9.6052. [DOI] [PubMed] [Google Scholar]
- 44.Mahabir S, Frenkel K, Brady MS, Coit D, Leibes L, Karkoszka J, Berwick M. Randomized, placebo-controlled pilot trial of the effects of alpha-tocopherol supplementation on levels of autoantibodies against 5-hydroxymethyl-2-deoxyuridine in melanoma patients. Melanoma Res. 2004;14:49–56. doi: 10.1097/00008390-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Schramm CM, Puddington L, Wu C, Guernsey L, Gharaee-Kermani M, Phan SH, Thrall RS. Chronic inhaled ovalbumin exposure induces antigen-dependent but not antigen-specific inhalational tolerance in a murine model of allergic airway disease. Am J Pathol. 2004;164:295–304. doi: 10.1016/S0002-9440(10)63119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis BJ, Flanagan BF, Gilfillan AM, Metcalfe DD, Coleman JW. Nitric oxide inhibits IgE-dependent cytokine production and Fos and Jun activation in mast cells. J Immunol. 2004;173:6914–6920. doi: 10.4049/jimmunol.173.11.6914. [DOI] [PubMed] [Google Scholar]
- 47.Borish L, Chipps B, Deniz Y, Gujrathi S, Zheng B, Dolan CM. Total serum IgE levels in a large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2005;95:247–253. doi: 10.1016/S1081-1206(10)61221-5. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed T, Marko M, Wu D, Chung H, Huber B, Meydani SN. Vitamin E supplementation reverses the age-associated decrease in effective immune synapse formation in CD4+ T cells. Ann NY Acad Sci. 2004;1031:412–414. doi: 10.1196/annals.1331.059. [DOI] [PubMed] [Google Scholar]
- 49.Wegner CD, Gundel RH, Reilly P, Haynes N, Letts LG, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990;247:456–459. doi: 10.1126/science.1967851. [DOI] [PubMed] [Google Scholar]
- 50.Lampinen M, Carlson M, Hakansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy. 2004;59:793–805. doi: 10.1111/j.1398-9995.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 51.Stirling RG, van Rensen EL, Barnes PJ, Chung KF. Interleukin-5 induces CD34(+) eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am J Respir Crit Care Med. 2001;164:1403–1409. doi: 10.1164/ajrccm.164.8.2010002. [DOI] [PubMed] [Google Scholar]
- 52.Oldhoff JM, Darsow U, Werfel T, Katzer K, Wulf A, Laifaoui J, Hijnen DJ, Plotz S, Knol EF, Kapp A, Bruijnzeel-Koomen CA, Ring J, de Bruin-Weller MS. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60:693–696. doi: 10.1111/j.1398-9995.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 53.Abe M, Hayashi Y, Murai A, Shibata K, Sakata N, Igarashi R, Katsuragi T, Tanaka K. Effects of inducible nitric oxide synthase inhibitors on asthma depending on administration schedule. Free Radic Biol Med. 2006;40:1083–1095. doi: 10.1016/j.freeradbiomed.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 54.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151–174. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 55.Desideri G, Marinucci MC, Tomassoni G, Masci PG, Santucci A, Ferri C. Vitamin E supplementation reduces plasma vascular cell adhesion molecule-1 and von Willebrand factor levels and increases nitric oxide concentrations in hypercholesterolemic patients. J Clin Endocrinol Metab. 2002;87:2940–2945. doi: 10.1210/jcem.87.6.8545. [DOI] [PubMed] [Google Scholar]
- 56.Tsoureli-Nikita E, Hercogova J, Lotti T, Menchini G. Evaluation of dietary intake of vitamin E in the treatment of atopic dermatitis: a study of the clinical course and evaluation of the immunoglobulin E serum levels. Int J Dermatol. 2002;41:146–150. doi: 10.1046/j.1365-4362.2002.01423.x. [DOI] [PubMed] [Google Scholar]
- 57.Shahar E, Hassoun G, Pollack S. Effect of vitamin E supplementation on the regular treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2004;92:654–658. doi: 10.1016/S1081-1206(10)61432-9. [DOI] [PubMed] [Google Scholar]
- 58.Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, Roubey RA, Peden DB. Gamma-Tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin Exp Allergy. 2007 doi: 10.1111/j.1365-2222.2007.02855.x. [DOI] [PubMed] [Google Scholar]
- 59.Wagner JG, Jiang Q, Harkema JR, Illek B, Patel DD, Ames BN, Peden DB. Ozone enhancement of lower airway allergic inflammation is prevented by [gamma]-tocopherol. Free Radic Biol Med. 2007;43:1176–1188. doi: 10.1016/j.freeradbiomed.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gajewska BU, Swirski FK, Alvarez D, Ritz SA, Goncharova S, Cundall M, Snider DP, Coyle AJ, Gutierrez-Ramos JC, Stampfli MR, Jordana M. Temporal-spatial analysis of the immune response in a murine model of ovalbumin-induced airways inflammation. Am J Respir Cell Mol Biol. 2001;25:326–334. doi: 10.1165/ajrcmb.25.3.4482. [DOI] [PubMed] [Google Scholar]
- 61.Mathrani VC, Kenyon NJ, Zeki A, Last JA. Mouse models of asthma: can they give us mechanistic insights into the role of nitric oxide? Curr Med Chem. 2007;14:2204–2213. doi: 10.2174/092986707781389628. [DOI] [PubMed] [Google Scholar]
- 62.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. Gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. Faseb J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 64.Jaffar Z, Ferrini ME, Buford MC, Fitzgerald GA, Roberts K. Prostaglandin I2-IP signaling blocks allergic pulmonary inflammation by preventing recruitment of CD4+ Th2 cells into the airways in a mouse model of asthma. J Immunol. 2007;179:6193–6203. doi: 10.4049/jimmunol.179.9.6193. [DOI] [PubMed] [Google Scholar]
- 65.Azzi A, Gysin R, Kempna P, Ricciarelli R, Villacorta L, Visarius T, Zingg JM. Regulation of gene and protein expression by vitamin E. Free Radic Res. 2002;36(Suppl 2):30–35. [Google Scholar]