Abstract
Although lipid peroxidation in the subendothelial space has been hypothesized to play a central role in atherogenesis, the role of vitamin E in preventing lipid peroxidation and lesion development remains uncertain. Here we show that in atherosclerosis-susceptible apolipoprotein E knockout mice, vitamin E deficiency caused by disruption of the α-tocopherol transfer protein gene (Ttpa) increased the severity of atherosclerotic lesions in the proximal aorta. The increase was associated with increased levels of isoprostanes, a marker of lipid peroxidation, in aortic tissue. These results show that vitamin E deficiency promotes atherosclerosis in a susceptible setting and support the hypothesis that lipid peroxidation contributes to lesion development. Ttpa−/− mice are a genetic model of vitamin E deficiency and should be valuable for studying other diseases in which oxidative stress is thought to play a role.
Keywords: antioxidants
Oxidative modification of lipoproteins (e.g., low-density lipoproteins) has been hypothesized to play a key role in the pathogenesis of atherosclerosis (1, 2). Because vitamin E is the most potent lipid-soluble antioxidant normally found on lipoproteins in the plasma, there is strong interest in the relationship between vitamin E levels and the development of atherosclerosis. In animal models and human clinical trials, studies of the effects of vitamin E supplementation on atherosclerosis have yielded conflicting results (3–8), and little is known about the effects of vitamin E deficiency on atherosclerosis development (9).
The major form of vitamin E in human plasma and tissues is α-tocopherol (10). α-Tocopherol enrichment of plasma and tissues is mediated by the α-tocopherol transfer protein (α-TTP), a cytosolic lipid-transfer protein expressed in the liver (11–14). Although the mechanism is unknown (15), α-TTP is believed to selectively transfer α-tocopherol from lipoproteins taken up by hepatocytes via the endocytic pathway to newly secreted lipoproteins, which facilitate its delivery to peripheral tissues (12). Humans with α-TTP gene defects have extremely low plasma α-tocopherol concentrations and develop severe neurodegenerative disease unless they are treated with high doses of vitamin E (16–18).
To investigate the relationship between vitamin E deficiency and atherosclerosis, we used gene targeting to disrupt the mouse α-TTP gene (Ttpa) and generate a genetic model of vitamin E deficiency. We then crossed the α-TTP-deficient mice (Ttpa−/−) mice with apolipoprotein (apo) E knockout (Apoe−/−) mice (19), which spontaneously develop atherosclerosis on a chow diet (20, 21). This enabled us to generate Apoe−/− mice with different Ttpa genotypes (+/+, +/−, and −/−) to test the hypothesis that α-TTP and vitamin E deficiency increase atherosclerosis in a susceptible setting.
Materials and Methods
Generation of α-TTP Knockout Mice.
A 14-kb 129/Sv genomic λ clone containing the Ttpa gene was isolated and subcloned into pBSSKII. A sequence replacement vector was constructed by PCR amplification and subcloning of the short (≈1.1-kb) and long (≈9.5-kb) arms of homologous α-TTP sequence into a modified version of pKSloxPNT [a gift from A. Joyner, New York University, New York (22)]. A lacZ expression cassette (a gift from A. Joyner) was also cloned into the 5′ untranslated region of the Ttpa gene. The vector was used to generate targeted embryonic stem cells and mice (23). Heterozygous mice (Ttpa+/−) were intercrossed to generate Ttpa−/− mice. Wild-type (16-kb) and disrupted (7-kb) HindIII fragments were identified by hybridizing a 32P-labeled 450-bp probe (located 5′ of the short arm of homology) synthesized by PCR amplification with sense (5′-AGCCAGAGGCAGACACATTTAGG-3′) and antisense (5′-GCTTTGAATTC TATACTGAGGAAGG-3′) primers. Subsequent genotyping in mice was performed by PCR with primers A (5′-TGAGTGTGCGTGGGGCGGCGTCC-3′), B (5′-CTGTTTCCCAACCAATGGCCCC-3′), and C (5′-CATTCAGGCTGCGCAACT GTTGGG-3′) at 95°C for 10 min, followed by 30 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min. A and B amplify a ≈138-bp fragment from the wild-type allele, and A and C amplify a ≈266-bp fragment from the knockout allele. Mice initially studied were of a mixed (50% C57BL/6 and 50% 129/SvJae) genetic background. Immunoblots were performed with a polyclonal antiserum as described (24).
Atherosclerosis Study Mice.
Ttpa−/− mice were crossed with Apoe−/− mice (≈100% C57BL/6) to generate Ttpa+/+Apoe−/−, Ttpa+/−Apoe−/−, and Ttpa−/−Apoe−/− mice (≈75% C57BL/6 and ≈25% 129Sv/Jae background). Only females were used in this study. We selected n = 20 per genotype set on the basis of power calculations, which assumed standard deviations approximately equal to the mean (found in many atherosclerosis studies with apoE knockout mice) and a power of 80% in detecting a 75% difference between the means at P = 0.05 confidence levels. Mice were housed in a pathogen-free barrier facility (12 h/12 h light/dark cycle) and were fed chow (Picolab Mouse Chow 20, Purina) containing ≈99 units of vitamin E per kilogram.
Blood and Tissue Biochemical Analysis.
When the mice were 30 weeks of age, blood was collected by cardiac puncture, the mice were perfused with PBS, and tissues were harvested and frozen in liquid nitrogen. Cholesterol levels were measured by colorimetric assay (Spectrum, Abbott). High density lipoprotein cholesterol was quantified after the precipitation of the apoB-containing lipoproteins with polyethylene glycol-8000 (25).
Vitamin E was measured in plasma after extraction without saponification, a modified method of Lang et al. (26). Tissue vitamin E was extracted by a modified alcoholic potassium hydroxide saponification procedure described by Podda et al. (27). The HPLC system consisted of a Shimadzu pump (LC-10ADVP), controller (SCL-10AVP), an autoinjector (SIL-10ADVP), and a Waters Spherisorb ODS2 C-18 column (4.6 mm i.d., 100 mm, 3-μm particle size) and Spherisorb ODS precolumn (5 μm, 1 cm × 4.6 mm). In addition, a LC-4C amperometric electrochemical detector (Bioanalytical Systems, West Lafayette, IN) with a glassy carbon working electrode and a silver chloride reference electrode was used with an isocratic system. The electrochemical detector was in the oxidizing mode, potential 500 mV, full recorder scale at 500 numerical aperture. Shimadzu Scientific 4.2 Class-VP software was used to integrate peak areas. Ascorbate and urate were measured by paired-ion reversed-phase HPLC coupled with electrochemical detection (28).
Atherosclerotic Lesion Analysis.
Female mice were killed at 30 weeks of age after 27 weeks of chow feeding. Blood was collected by cardiac puncture. Tissues were fixed by perfusion with 3% paraformaldehyde in phosphate buffer (pH 7.3), and aortas were removed, opened longitudinally from the heart to the iliac bifurcation, and pinned out flat (29). Aortic images were captured with a Polaroid digital camera (DMC1) mounted on a Leica MZ6 dissection microscope and analyzed with Adobe photoshop 5.0.1 software (Adobe Systems, Mountain View, CA) and Image Processing Tool Kit (Reindeer Games, Gainesville, FL) plug ins. An image of each aorta was captured and divided into three regions (arch, thorax, and abdomen), from which both surface and lesion areas were quantified. Percent lesion area results were calculated from lesion area and total surface area.
Aortic root morphology was examined in three Ttpa+/+Apoe−/− and three Ttpa−/−Apoe−/− mice, which had total aortic lesion areas representative of the means of each genotype. Aortic roots were fixed by perfusion with 3% paraformaldehyde in phosphate buffer (pH 7.3), embedded in OCT compound, frozen, sectioned, and stained with Movat's pentachrome.
Aortic α-Tocopherol and F2-Isoprostane Measurements.
Mice were killed at age 30 weeks and perfused with PBS. Whole aortas were dissected and divided into two portions (proximal 2/3 and distal 1/3) for measurements of total F2-isoprostane and α-tocopherol levels. Aortas were immediately frozen in liquid nitrogen until analysis. Total F2-isoprostanes were measured as described (30), and α-tocopherol was measured as described above.
Results
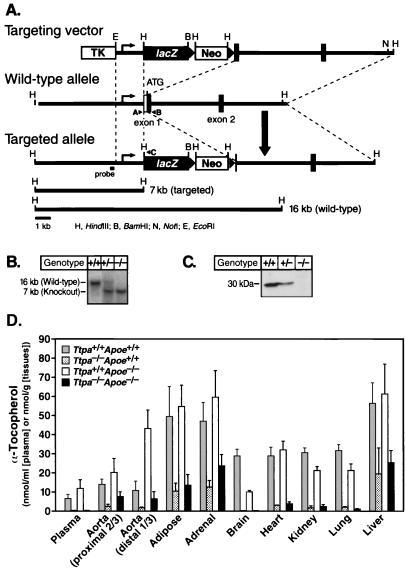
We created a genetic model of vitamin E deficiency by disrupting the mouse α-TTP gene (Ttpa) (Fig. 1 A and B). Immunoblotting of liver homogenates showed no α-TTP in Ttpa−/− mice and decreased amounts in Ttpa+/− mice (Fig. 1C). In chow-fed mice, α-tocopherol levels in plasma and most tissues were reduced by ≈50% in Ttpa+/− mice (not shown) and by more than 90% in Ttpa−/− mice (Fig. 1D). In Ttpa−/− liver, adipose tissue, adrenal gland, aorta, α-tocopherol levels were 15–35% of those of wild-type mice. The reason for the higher α-tocopherol levels in these tissues is uncertain but may reflect the delivery and accumulation of dietary α-tocopherol from chylomicrons and their remnants.
Figure 1.
Generation of Ttpa−/− mice. (A) Strategy for disrupting the Ttpa gene. On homologous recombination of the targeting vector with the Ttpa locus, lacZ (β-galactosidase) and neo genes are inserted into the 5′ untranslated sequences of exon 1, resulting in the deletion of the Ttpa translational start codon. A, B, and C represent primers used for PCR genotyping. (B) Southern blot analysis of genomic DNA from offspring of heterozygous intercrosses. (C) Absence of α-TTP protein in liver homogenates of Ttpa−/− mice. α-TTP protein levels were reduced by ≈50% in Ttpa+/− mice. (D) α-Tocopherol levels in Ttpa+/+Apoe+/+ and Ttpa−/−Apoe+/+ mice and in Ttpa+/+Apoe−/− and Ttpa−/−Apoe−/− mice, of 7–12 months of age. Aortic γ-tocopherol levels were similarly low in both Ttpa+/+Apoe−/− and Ttpa−/−Apoe−/− mice (0.60 ± 0.64 vs. 0.77 ± 0.68 nmol/g, P = 0.55). Data are expressed as mean ± SD.
Ttpa−/− mice were generally healthy. Offspring from heterozygous intercrosses were born with the expected Mendelian distribution. Ttpa−/− mice at 18 months of age had no obvious signs of neurological disease. In contrast, humans with α-TTP gene defects develop ataxia with vitamin E deficiency by the first decade of life (16–18). This discrepancy may reflect species differences in the susceptibility of the nervous system to vitamin E deficiency (31). Ttpa−/− females were, however, infertile. This fertility defect presumably resulted from vitamin E deficiency. Vitamin E is required to prevent fetal resorption in rodents (32, 33), and vitamin E supplementation (1,000 units/kg of diet) completely reversed the fertility defect (not shown). Ttpa−/− males had no obvious impairment in fertility.
To investigate whether deficiency of α-TTP and α-tocopherol increased atherosclerosis, we crossed Ttpa−/− mice with Apoe−/− mice (19). In Ttpa−/−Apoe−/− and Ttpa+/−Apoe−/− mice, plasma α-tocopherol levels were 1.4 and 76%, respectively, of those in Ttpa+/+Apoe−/− mice (Fig. 1D and Table 1). In most tissues of Ttpa−/−Apoe−/− mice, including the proximal aorta, α-tocopherol levels were more than 85% lower than those in Ttpa+/+Apoe−/− mice (Fig. 1D). However, the reduction in α-tocopherol levels was not as marked (60–75%) in Ttpa−/−Apoe−/− liver, adipose tissue, adrenal gland, and distal aorta. Plasma levels of γ-tocopherol were low in all groups, probably reflecting the small amounts of γ-tocopherol in the diet.
Table 1.
Plasma levels of cholesterol and antioxidants
| Genotypes | Total cholesterol, mg/dl | α-Tocopherol, μM | γ-Tocopherol, μM | Ascorbate, μM | Urate, μM |
|---|---|---|---|---|---|
| Ttpa+/+Apoe−/− | 427.4 ± 143.7 | 11.9 ± 4.5 | 0.15 ± 0.06 | 71.3 ± 16.0 | 68.7 ± 25.7 |
| Ttpa+/−Apoe−/− | 442.0 ± 91.3 | 9.0 ± 2.3 | 0.11 ± 0.04† | ND | ND |
| Ttpa−/−Apoe−/− | 433.1 ± 113.9 | 0.17 ± 0.09* | 0.01 ± 0.00‡ | 81.3 ± 24.4 | 56.5 ± 10.1 |
Data are presented as mean ± SD. Plasma cholesterol levels were measured at the time the mice were killed for atherosclerotic lesion quantitation (20 Ttpa+/+Apoe−/−, 19 Ttpa+/−Apoe−/−, and 21 Ttpa−/−Apoe−/− female mice). Plasma α-tocopherol and γ-tocopherol levels were measured in these study mice and others for a total of 30 Ttpa+/+Apoe−/−, 19 Ttpa+/−Apoe−/−, and 32 Ttpa−/−Apoe−/− mice. Plasma α-tocopherol levels for Ttpa+/+Apoe and Ttpa−/−Apoe−/− mice are also shown in Fig. 1D. Ascorbate and urate levels were measured from a subset of mice (eight Ttpa+/+Apoe−/− and nine Ttpa−/−Apoe−/− mice).
, P < 0.05 vs. Ttpa+/+Apoe−/− or Ttpa+/−Apoe−/−, ANOVA with Dunn's test;
, P = 0.004 vs. Ttpa+/+Apoe−/−, ANOVA with Tukey test;
P < 0.001 vs. Ttpa+/+Apoe−/− or Ttpa+/−Apoe−/−, ANOVA with Tukey test. ND, not determined.
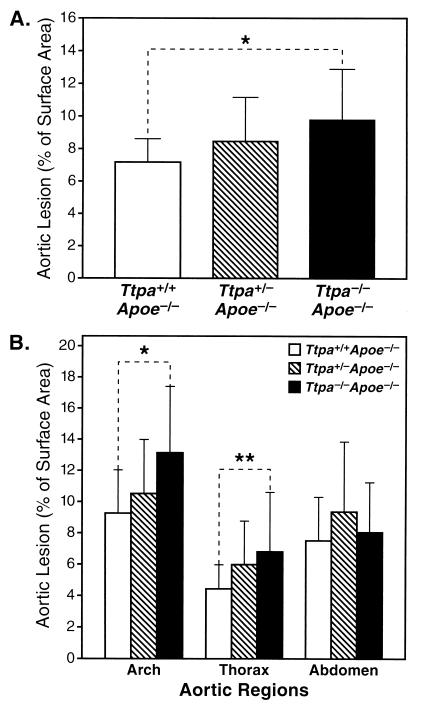
Aortic atherosclerotic lesions were quantified in chow-fed Ttpa+/+Apoe−/−, Ttpa+/−Apoe−/−, and Ttpa−/−Apoe−/− mice at 30 weeks of age. Total aortic lesion area was ≈36% greater in Ttpa−/−Apoe−/− mice than in Ttpa+/+Apoe−/− controls (9.77 ± 3.12 vs. 7.17 ± 1.43% of surface area, P = 0.005) (Fig. 2A). Aortic lesions in all groups were most severe in the aortic arch region (proximal 1/3 of aorta) (Fig. 2B). Compared with Ttpa+/+Apoe−/− controls, Ttpa−/−Apoe−/− had 42% larger lesions (P = 0.002), and Ttpa+/−Apoe−/− mice had 13% larger lesions (P = 0.054) in the aortic arch. In the thorax region (middle 1/3 of aorta), Ttpa−/−Apoe−/− mice had 53% more lesion area than Ttpa+/+Apoe−/− mice (P = 0.03). α-TTP deficiency did not affect lesion size in the abdominal (distal 1/3) aorta.
Figure 2.
Atherosclerotic lesion area in aortas. Ttpa+/+Apoe−/− (n = 20), Ttpa+/−Apoe−/− (n = 19), and Ttpa−/−Apoe−/− (n = 21) mice were killed at age 30 weeks. (A) Total aortic lesion area (mean ± SD). *, P = 0.005, ANOVA with Tukey test. (B) Regional aortic lesion area (mean ± SD). *, P = 0.002, **, P = 0.03, ANOVA with Tukey test.
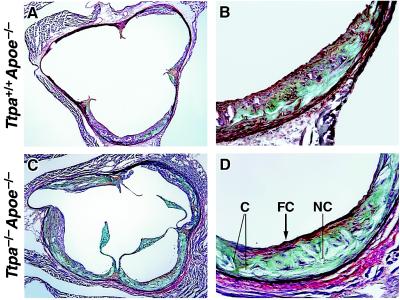
In a subset of mice, we examined the morphology of the aortic root lesions. Lesions of Ttpa−/−Apoe−/− mice consistently appeared more complex than those of Ttpa+/+Apoe−/− controls, with more area occupied by necrotic core and cholesterol crystals and some lesions having fibrous caps (Fig. 3). Macrophage immunostaining appeared similar in aortic root sections of the two groups of mice (not shown).
Figure 3.
Morphology of aortic lesions from proximal aortic roots. Representative section from the aortic root (at the level of the first coronary) showing lesions in Ttpa+/+Apoe−/− mouse at low- (×4) (A) and high-magnification detail of lower right profile in A (×20). (B) Representative section at the aortic root lesion from Ttpa−/−Apoe−/− mouse at low- (×4) (C) and high-magnification detail of lower right profile in C (×20). (D) Lesions in Ttpa−/−Apoe−/− mice show more complex features, including a large necrotic core (NC), numerous needle-shaped lucencies indicative of cholesterol crystals (C), and fibrous capping (FC) from smooth muscle cells (stained red). Within the lesion core, greenish-blue staining represents proteoglycan, and yellow staining represents collagen.
To establish that the differences in atherosclerotic lesion development did not result from differences in plasma levels of cholesterol or antioxidants other than vitamin E, we measured total cholesterol, high density lipoprotein cholesterol, ascorbate, and urate in the plasma. Total plasma cholesterol levels were similar in mice of all genotypes (Table 1), as were HDL cholesterol levels (22.5 ± 5.8 vs. 22.8 ± 7.5 mg/dl for Ttpa+/+Apoe−/− and Ttpa−/−Apoe−/− mice, respectively). In addition, cholesterol distribution in the lipoprotein fractions assessed by fast protein liquid chromatography was similar for Ttpa+/+Apoe−/− and Ttpa−/−Apoe−/− mice (not shown). Plasma ascorbate and urate levels were also similar in both groups (Table 1).
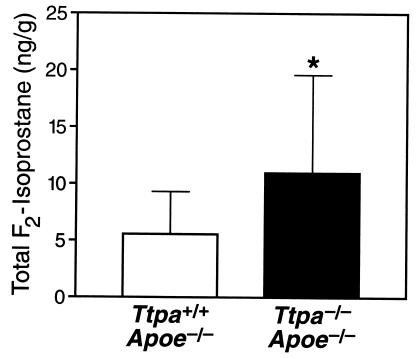
To analyze the relationship between lesion development and lipid peroxidation, we measured aortic levels of total F2-isoprostanes, a marker of lipid peroxidation (34), in separate groups of Ttpa+/+Apoe−/− and Ttpa−/−Apoe−/− mice. Total F2-isoprostanes in the proximal aorta, where α-tocopherol levels in Ttpa−/−Apoe−/− mice were <15% of those in Ttpa+/+ Apoe−/+controls (Fig. 1D), were nearly 2-fold higher in Ttpa−/−Apoe−/− mice than in Ttpa+/+Apoe−/− controls (11.32 ± 8.78 vs. 5.93 ± 3.71 ng/g, n = 10 for each genotype, P = 0.03) (Fig. 4). Total F2-isoprostane levels were also nearly 2-fold higher in distal aortas of Ttpa−/−Apoe−/− mice than Ttpa+/+Apoe−/− controls (27.3 ± 2.1 vs. 14.8 ± 3.5 ng/g, n = 6 for each genotype, P = 0.002), despite the less pronounced difference between α-tocopherol levels.
Figure 4.
Aortic F2-isoprostane levels in proximal aortas of Ttpa+/+Apoe−/− and Ttpa−/−Apoe−/− mice (n = 10 and 11 females, respectively). Data are expressed as mean ± SD. *, P = 0.03, Mann–Whitney rank sum test.
Discussion
In human and animal studies, the ability of vitamin E supplementation to prevent atherosclerosis (3–8) has varied, possibly because of differences in vitamin E supplementation regimens, other dietary factors, or the degree of preexisting atherosclerosis. Our study examines the effect of vitamin E deficiency on atherogenesis as a single modifying factor present before lesion development. Our results indicate that α-TTP deficiency and associated vitamin E deficiency promote lesion formation in the proximal aorta in the setting of increased susceptibility to atherosclerosis, in this case caused by apoE deficiency. Thus, vitamin E deficiency appears to modulate rather than cause atherosclerosis. Supporting this, we have not observed spontaneous atherosclerosis in normolipidemic α-TTP-deficient mice that have apoE (Y.T. and R.F., unpublished observations). Similarly, early-onset atherosclerosis has not been reported in humans with α-TTP gene defects (35).
The increase in atherosclerotic lesion area in aortas was significant (increased by 35–40% in α-tocopherol-deficient mice) but not dramatic. Several factors may have accounted for this. First, although α-tocopherol levels were reduced in Ttpa−/−Apoe−/− aortas, substantial amounts of α-tocopherol were present in this tissue, possibly because of the delivery of α-tocopherol from dietary lipoproteins, which circulate at high levels in apoE-deficient mice (19). Lesion area might have been greater if α-tocopherol levels had been more severely reduced. Second, the lesion analysis method we used (whole aorta analysis) may have minimized differences. ApoE-deficient mice tend to develop prominent lesions in the aortic root (36). Cross-sectional analysis of lesion area in aortic roots therefore might have resulted in greater lesion areas and might have amplified any differences because of α-tocopherol deficiency. Finally, compensatory changes in other antioxidant systems might have mitigated the effects of α-tocopherol deficiency on lesion development. For example, deficiency of paraoxonase, a high density lipoprotein-associated enzyme with antioxidant properties, results in increased atherosclerosis in apoE-deficient mice and is associated with up-regulation of hepatic expression of heme oxygenase-1, possibly to compensate for the increase in oxidative stress (37).
The major effects of α-TTP and α-tocopherol deficiency on lesion formation were observed in the proximal two-thirds of the aorta. In this region, a >85% reduction in α-tocopherol levels was associated with a 35–40% increase in lesion areas. These results are consistent with those of Praticò et al. (7), who showed that vitamin E supplementation of Apoe−/− mice fed a chow diet resulted in decreased levels of atherosclerosis. Why α-tocopherol deficiency had no effect on atherosclerosis in the distal aortas of our study mice is unknown. α-Tocopherol levels in the distal aortas of Ttpa−/−Apoe−/− mice were reduced only by ≈60% compared with Ttpa+/+Apoe−/− controls, perhaps accounting for the lack of effect. Another possibility is that the effects of vitamin E on lesion development may vary with anatomical location. It is noteworthy that probucol, a potent lipid-soluble antioxidant, did not prevent progression of femoral artery lesions in a human clinical trial (38) or of lesion development in abdominal aortas or iliac arteries of nonhuman primates (39).
Decreased lipid peroxidation is a likely mechanism by which vitamin E prevents atherosclerotic lesion formation. We therefore examined the relationship between aortic α-tocopherol and F2-isoprostane levels. In the proximal aorta, reduced tissue α-tocopherol levels were associated with a 2-fold increase in F2-isoprostanes. These results are consistent with those of Praticò et al. (7), who found that Apoe−/− mice fed a chow diet had higher levels of a subset of F2-isoprostanes (iPF2α-VI) in their aortas than did Apoe−/− mice fed a diet supplemented with vitamin E. In our study, the increase in F2-isoprostane levels in the proximal aorta was associated with increased lesion areas. However, we did not find increased lesion areas in the distal aortas of Ttpa−/−Apoe−/− mice, despite a 2-fold increase in aortic F2-isoprostanes. This suggests either that factors other than lipid peroxidation contribute to lesion formation in this region or that the content of F2-isoprostanes in this tissue at the end of the study period may not accurately reflect the oxidant status during lesion formation. Although our data generally support the hypothesis that vitamin E reduces atherosclerosis through its antioxidant properties, the mechanism by which vitamin E affects atherosclerosis development was not directly addressed by our study, and other mechanisms (reviewed in refs. 5, 6, 40) might have contributed. We also cannot exclude the possibility that the increased atherosclerosis in Ttpa−/−Apoe−/− mice resulted from an effect of α-TTP deficiency other than reduced α-tocopherol levels. We believe this is unlikely, however, because the only known function of α-TTP is in vitamin E metabolism.
The Ttpa−/− mice provide an exciting genetic model of vitamin E deficiency. Plasma and tissue α-tocopherol levels are reduced in a stepwise and consistent manner in Ttpa+/− and Ttpa−/− mice. In the present study, we used this model to address the role of vitamin E and oxidative stress on atherosclerosis, but Ttpa−/− mice will likely prove valuable for studying other diseases in which lipid peroxidation or antioxidants may play a role.
Acknowledgments
We thank Sylvaine Cases (Gladstone Institute of Cardiovascular Disease, San Francisco, CA) for providing the λ clone-containing mouse Ttpa; Tina Yu for blastocyst injections; David Liang for assistance in mouse breeding; Shelley Mettler for assistance in histology; Hubert Chen, David Dichek, Andrew Plump, Stephen Young, and Karl Weisgraber for comments on the manuscript; John Carroll and Stephen Gonzales for graphics; Stephen Ordway and Gary Howard for editorial assistance; and Bethany Taylor for manuscript preparation. This work was supported by an Established Investigator Award from the American Heart Association and National Institutes of Health (NIH) Grant HL41633 (to R.F.); by a grant from the California Dairy Research Foundation (to M.T.); by NIH Grants DK48831, GM15431, CA77839, and DK26657; a Burroughs Wellcome Clinical Scientist Award (to J.M.); and by the J. David Gladstone Institutes.
Abbreviations
- apo
apolipoprotein
- α-TTP
α-tocopherol transfer protein
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240462697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240462697
References
- 1.Steinberg D, Parthasarathy S, Carew T E, Khoo J C, Witztum J L. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg D. Circulation. 1997;95:1062–1071. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 3.Upston J M, Terentis A C, Stocker R. FASEB J. 1999;13:977–994. doi: 10.1096/fasebj.13.9.977. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 5.Chan A C. J Nutr. 1998;128:1593–1596. doi: 10.1093/jn/128.10.1593. [DOI] [PubMed] [Google Scholar]
- 6.Keaney J F, Jr, Simon D I, Freedman J E. FASEB J. 1999;13:965–976. doi: 10.1096/fasebj.13.9.965. [DOI] [PubMed] [Google Scholar]
- 7.Praticò D, Tangirala R K, Rader D J, Rokach J, FitzGerald G A. Nat Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- 8.Shaish A, George J, Gilburd B, Keren P, Levkovitz H, Harats D. Arterioscler Thromb Vasc Biol. 1999;19:1470–1475. doi: 10.1161/01.atv.19.6.1470. [DOI] [PubMed] [Google Scholar]
- 9.Sulkin N M, Sulkin D F. Proc Soc Exp Biol Med. 1960;103:111–115. doi: 10.3181/00379727-103-25429. [DOI] [PubMed] [Google Scholar]
- 10.Burton G W, Traber M G, Acuff R V, Walters D N, Kayden H, Hughes L, Ingold K U. Am J Clin Nutr. 1998;67:669–684. doi: 10.1093/ajcn/67.4.669. [DOI] [PubMed] [Google Scholar]
- 11.Catignani G L, Bieri J G. Biochim Biophys Acta. 1977;497:349–357. doi: 10.1016/0304-4165(77)90192-1. [DOI] [PubMed] [Google Scholar]
- 12.Traber M G, Arai H. Annu Rev Nutr. 1999;19:343–355. doi: 10.1146/annurev.nutr.19.1.343. [DOI] [PubMed] [Google Scholar]
- 13.Sato Y, Arai H, Miyata A, Tokita S, Yamamoto K, Tanabe T, Inoue K. J Biol Chem. 1993;268:17705–17710. [PubMed] [Google Scholar]
- 14.Arita M, Sato Y, Miyata A, Tanabe T, Takahashi E, Kayden H J, Arai H, Inoue K. Biochem J. 1995;306:437–443. doi: 10.1042/bj3060437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arita M, Nomura K, Arai H, Inoue K. Proc Natl Acad Sci USA. 1997;94:12437–12441. doi: 10.1073/pnas.94.23.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokol R J, Kayden H J, Bettis D B, Traber M G, Neville H, Ringel S, Wilson W B, Stumpf D A. J Lab Clin Med. 1988;111:548–559. [PubMed] [Google Scholar]
- 17.Ouahchi K, Arita M, Kayden H, Hentati F, Hamida M B, Sokol R, Arai H, Inoue K, Mandel J-L, Koenig M. Nat Genet. 1995;9:141–145. doi: 10.1038/ng0295-141. [DOI] [PubMed] [Google Scholar]
- 18.Hentati A, Deng H-X, Hung W-Y, Nayer M, Ahmed M S, He X, Tim R, Stumpf D A, Siddique T. Ann Neurol. 1996;39:295–300. doi: 10.1002/ana.410390305. [DOI] [PubMed] [Google Scholar]
- 19.Piedrahita J A, Zhang S H, Hagaman J R, Oliver P M, Maeda N. Proc Natl Acad Sci USA. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plump A S, Smith J D, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft J G, Rubin E M, Breslow J L. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S H, Reddick R L, Piedrahita J A, Maeda N. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 22.Hanks M, Wurst W, Anson-Cartwright L, Auerbach A B, Joyner A L. Science. 1995;269:679–682. doi: 10.1126/science.7624797. [DOI] [PubMed] [Google Scholar]
- 23.Meiner V L, Cases S, Myers H M, Sande E R, Bellosta S, Schambelan M, Pitas R E, McGuire J, Herz J, Farese R V., Jr Proc Natl Acad Sci USA. 1996;93:14041–14046. doi: 10.1073/pnas.93.24.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terasawa Y, Cases S J, Wong J S, Jamil H, Jothi S, Traber M G, Packer L, Gordon D A, Hamilton R L, Farese R V., Jr J Lipid Res. 1999;40:1967–1977. [PubMed] [Google Scholar]
- 25.Purcell-Huynh D A, Farese R V, Jr, Johnson D F, Flynn L M, Pierotti V, Newland D L, Linton M F, Sanan D A, Young S G. J Clin Invest. 1995;95:2246–2257. doi: 10.1172/JCI117915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang J K, Gohil K, Packer L. Anal Biochem. 1986;157:106–116. doi: 10.1016/0003-2697(86)90203-4. [DOI] [PubMed] [Google Scholar]
- 27.Podda M, Weber C, Traber M G, Packer L. J Lipid Res. 1996;37:893–901. [PubMed] [Google Scholar]
- 28.Kutnink M A, Hawkes W C, Schaus E E, Omaye S T. Anal Chem. 1987;166:424–430. doi: 10.1016/0003-2697(87)90594-x. [DOI] [PubMed] [Google Scholar]
- 29.Palinski W, Tangirala R K, Miller E, Young S G, Witztum J L. Arterioscler Thromb Vasc Biol. 1995;15:1569–1576. doi: 10.1161/01.atv.15.10.1569. [DOI] [PubMed] [Google Scholar]
- 30.Awad J A, Morrow J D, Hill K E, Roberts L J, II, Burk R E. J Nutr. 1994;124:810–816. doi: 10.1093/jn/124.6.810. [DOI] [PubMed] [Google Scholar]
- 31.Follis R H., Jr . Deficiency Disease: Functional and Structural Changes in Mammalia Which Result from Exogenous or Endogenous Lack of One or More Essential Nutrients. Springfield, IL: Thomas; 1958. pp. 159–170. [Google Scholar]
- 32.Evans H M, Bishop K S. Science. 1922;56:650–651. doi: 10.1126/science.56.1458.650. [DOI] [PubMed] [Google Scholar]
- 33.Urner J A. Anat Rec. 1931;50:175–187. [Google Scholar]
- 34.Morrow J D, Roberts L J. Prog Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 35.Cavalier L, Ouahchi K, Kayden H J, Di Donato S, Reutenauer L, Mandel J-L, Koenig M. Am J Hum Genet. 1998;62:301–310. doi: 10.1086/301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakashima Y, Plump A S, Raines E W, Breslow J L, Ross R. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 37.Shih D M, Xia Y-R, Wang X-P, Miller E, Castellani L W, Subbanagounder G, Cheroutre H, Faull K F, Berliner J A, Witztum J L, Lusis A J. J Biol Chem. 2000;275:17527–17535. doi: 10.1074/jbc.M910376199. [DOI] [PubMed] [Google Scholar]
- 38.Walldius G, Erikson U, Olsson A G, Bergstrand L, Hådell K, Johansson J, Kaijser L, Lassvik C, Mölgaard J, Nilsson S, Schäfer-Elinder L, Stenport G, Holme I. Am J Cardiol. 1994;74:875–883. doi: 10.1016/0002-9149(94)90579-7. [DOI] [PubMed] [Google Scholar]
- 39.Sasahara M, Raines E W, Chait A, Carew T E, Steinberg D, Wahl P W, Ross R. J Clin Invest. 1994;94:155–164. doi: 10.1172/JCI117301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traber M G, Packer L. Am J Clin Nutr. 1995;62:1501S–1509S. doi: 10.1093/ajcn/62.6.1501S. [DOI] [PubMed] [Google Scholar]