Abstract
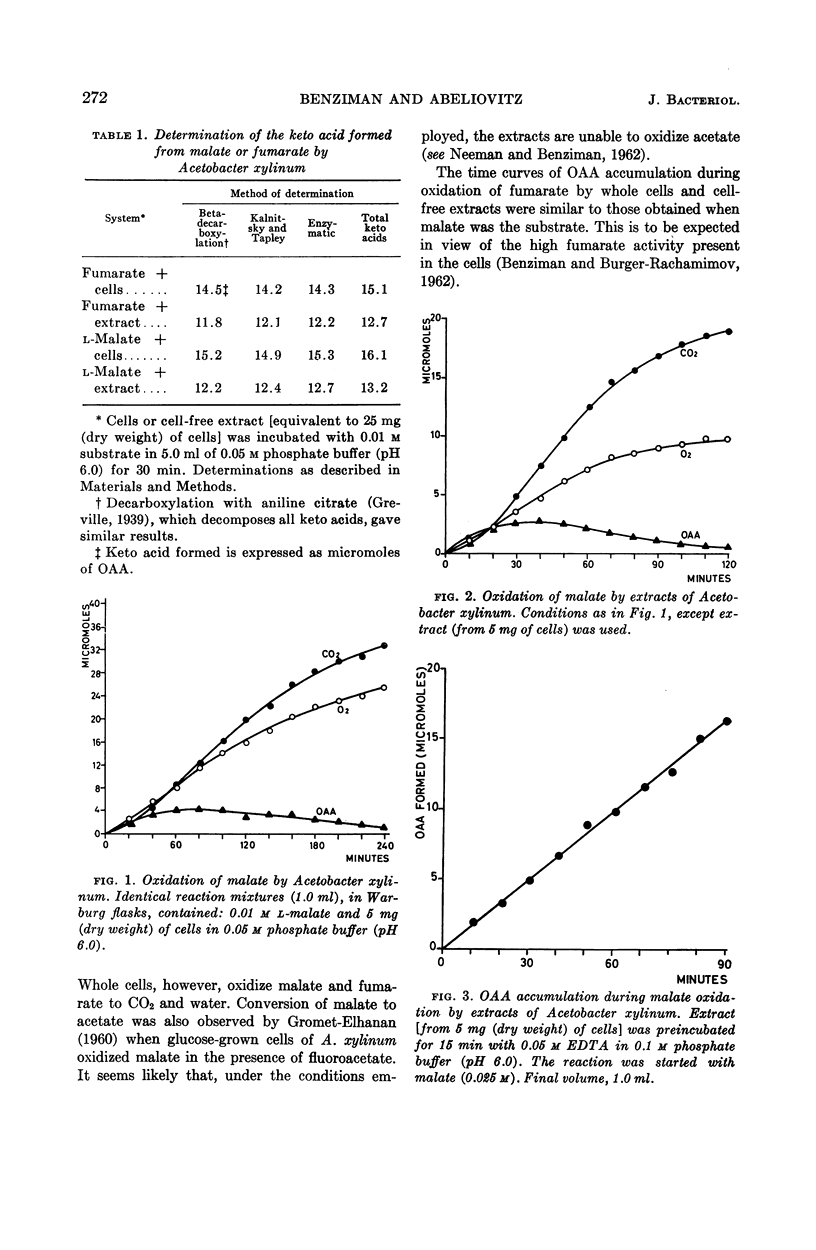
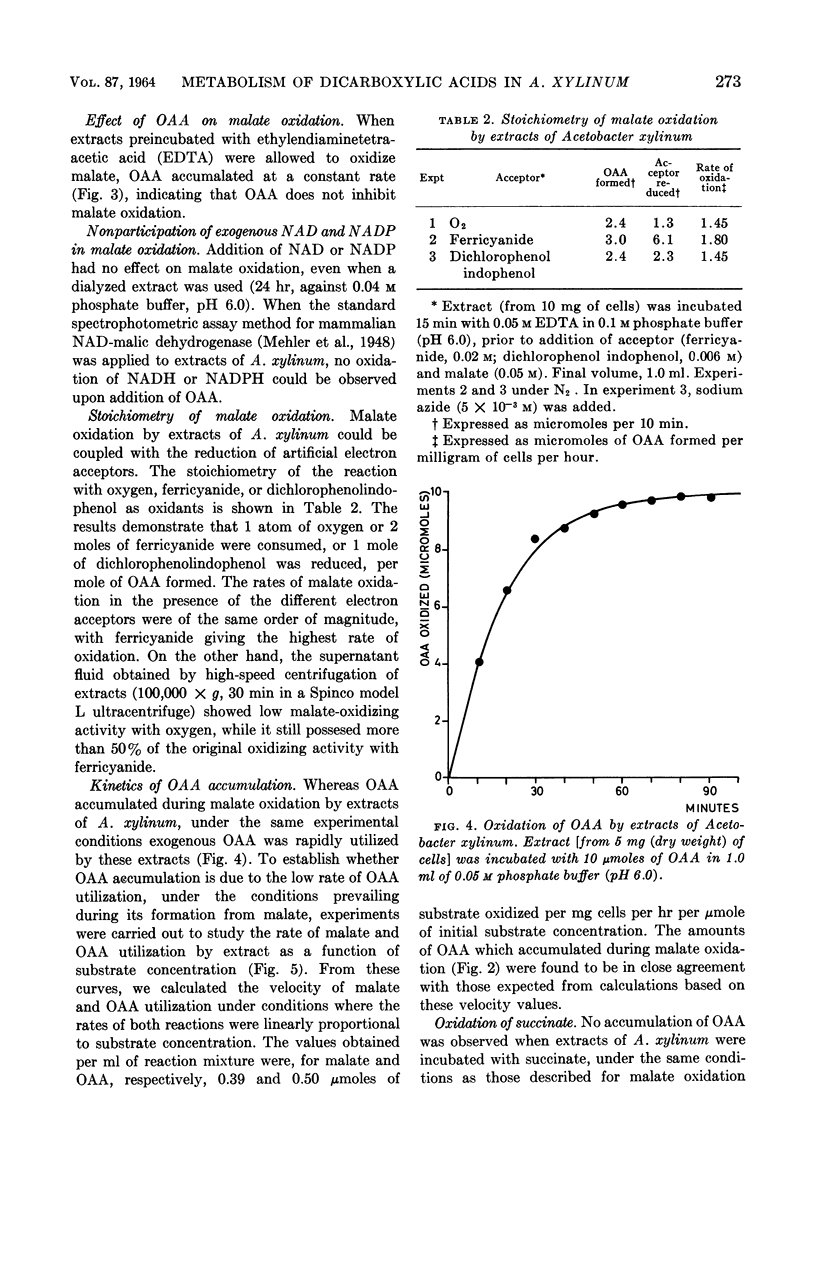
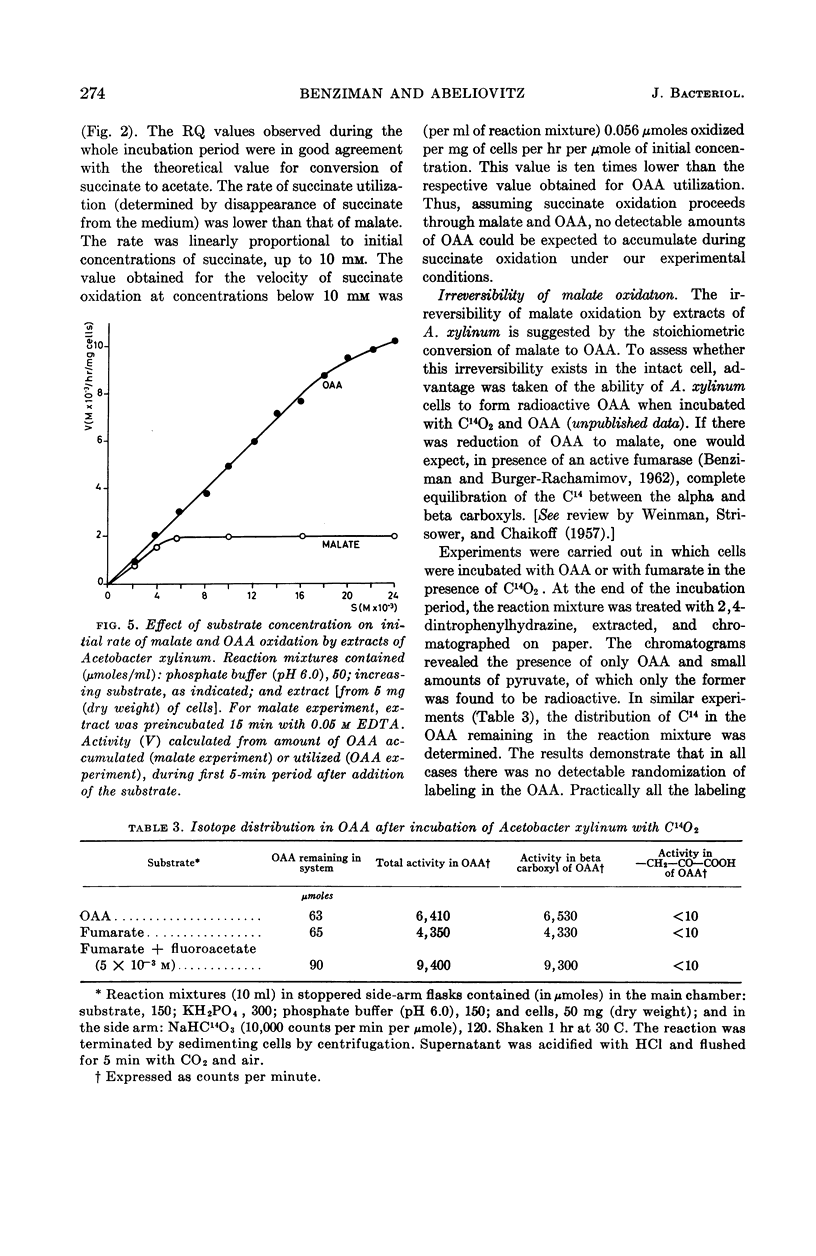
Benziman, Moshe (The Hebrew University of Jerusalem, Jerusalem, Israel), and A. Abeliovitz. Metabolism of dicarboxylic acids in Acetobacter xylinum. J. Bacteriol. 87:270–277. 1964.—During the oxidation of fumarate or l-malate by whole cells or extracts of Acetobacter xylinum grown on succinate, a keto acid accumulated in the medium in considerable amounts. This acid was identified as oxaloacetic acid (OAA). No accumulation of OAA was observed when succinate served as substrate. These phenomena could be explained by the kinetics of malate, succinate, and OAA oxidation. OAA did not inhibit malate oxidation, even when present at high concentrations. When cells were incubated with OAA or fumarate in the presence of C14O2, only the beta-carboxyl of residual OAA was found to be labeled. Evidence was obtained indicating that nicotinamide adenine dinucleotide (NAD) or nicotinamide adenine dinucleotide phosphate (NADP) are not directly involved in malate oxidation by cell-free extracts. The results suggest that malate oxidation in A. xylinum is irreversible, and is catalyzed by an enzyme which is not NAD- or NADP-linked.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENZIMAN M., BURGER-RACHAMIMOV H. Synthesis of cellulose from pyruvate by succinate-grown cells of Acetobacter xylinum. J Bacteriol. 1962 Oct;84:625–630. doi: 10.1128/jb.84.4.625-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN D. V. The enzymatic formation of oxalacetic acid by nonpyridine nucleotide malic dehydrogenase of Micrococcus lysodeikticus. J Biol Chem. 1958 Aug;233(2):299–304. [PubMed] [Google Scholar]
- COHN D. V. The oxidation of malic acid by Micrococcus lysodeikticus. J Biol Chem. 1956 Jul;221(1):413–423. [PubMed] [Google Scholar]
- EL HAWARY M. F. S., THOMPSON R. H. S. Separation and estimation of blood keto acids by paper chromatography. Biochem J. 1953 Feb;53(3):340–347. doi: 10.1042/bj0530340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. E., MII S., KOHOUT P. M. Studies on the terminal electron transport system. I. Succinic dehydrogenase. J Biol Chem. 1955 Dec;217(2):551–567. [PubMed] [Google Scholar]
- Greville G. D. The effect of calcium ion on tissue respiration; with a note on the estimation of oxaloacetic acid. Biochem J. 1939 May;33(5):718–722. doi: 10.1042/bj0330718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALNITSKY G., TAPLEY D. F. A sensitive method for estimation of oxaloacetate. Biochem J. 1958 Sep;70(1):28–34. doi: 10.1042/bj0700028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. Metabolism of acetoacetate in animal tissues. 1. Biochem J. 1945;39(5):408–419. [PMC free article] [PubMed] [Google Scholar]
- McMANUS I. R. A study of carbon dioxide fixation by Micrococcus lysodeikticus. J Biol Chem. 1951 Feb;188(2):729–740. [PubMed] [Google Scholar]
- RAVAL D. N., WOLFE R. G. Malic dehydrogenase. V. Kinetic studies of substrate inhibition by oxalacetate. Biochemistry. 1963 Mar-Apr;2:220–224. doi: 10.1021/bi00902a003. [DOI] [PubMed] [Google Scholar]
- RODGERS K. Estimation of succinic acid in biological materials. Biochem J. 1961 Aug;80:240–244. doi: 10.1042/bj0800240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLATER E. C., BORNER W. D., Jr The effect of fluoride on the succinic oxidase system. Biochem J. 1952 Oct;52(2):185–196. doi: 10.1042/bj0520185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick R. W., Wood H. G. THE ROLE OF TRANSCARBOXYLATION IN PROPIONIC ACID FERMENTATION. Proc Natl Acad Sci U S A. 1960 Jan;46(1):28–41. doi: 10.1073/pnas.46.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTTER M. F., KEECH D. B. Formation of oxaloacetate from pyruvate and carbon dioxide. J Biol Chem. 1960 May;235:PC17–PC18. [PubMed] [Google Scholar]
- UTTER M. F. The role of CO2 fixation in carbohydrate utilization and synthesis. Ann N Y Acad Sci. 1959 Feb 6;72(12):451–461. doi: 10.1111/j.1749-6632.1959.tb44173.x. [DOI] [PubMed] [Google Scholar]
- WEINMAN E. O., STRISOWER E. H., CHAIKOFF I. L. Conversion of fatty acids to carbohydrate; application of isotopes to this problem and role of the Krebs cycle as a synthetic pathway. Physiol Rev. 1957 Apr;37(2):252–272. doi: 10.1152/physrev.1957.37.2.252. [DOI] [PubMed] [Google Scholar]


