Abstract
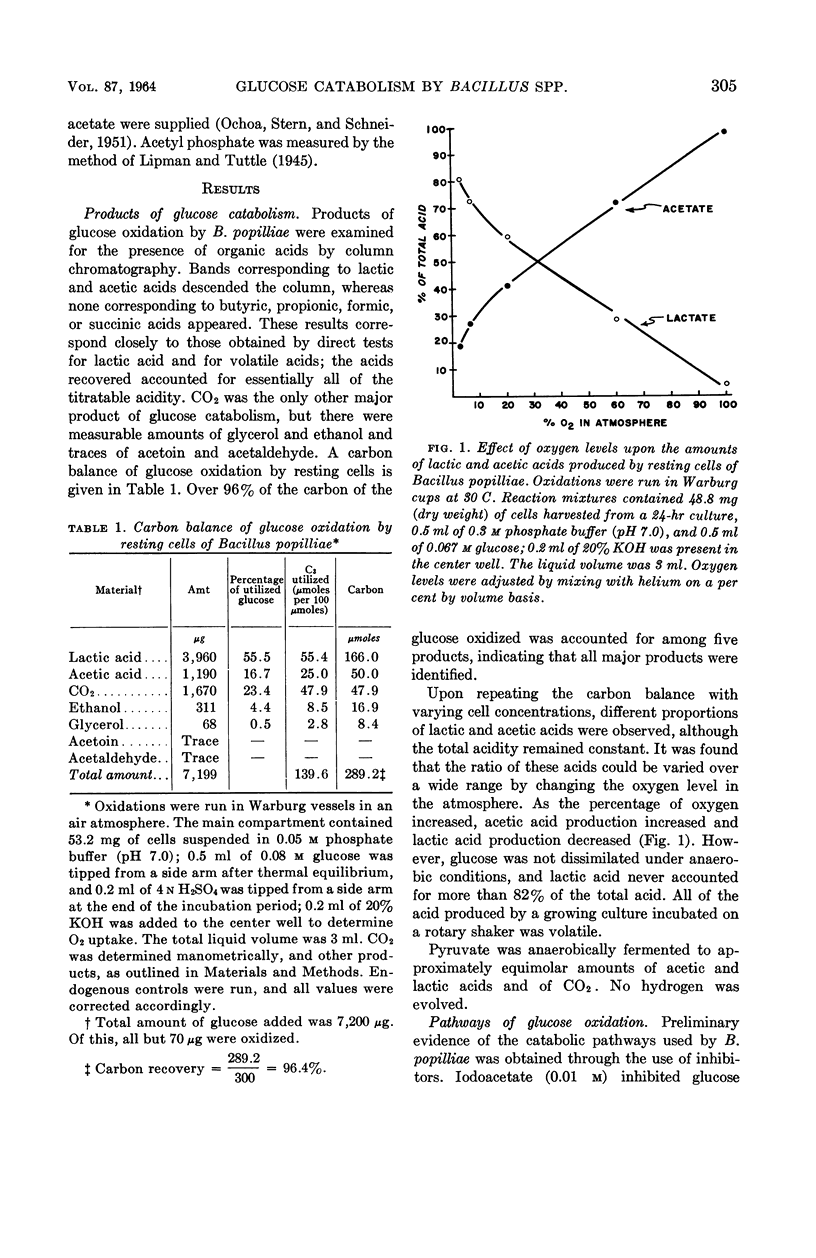
Pepper, Rollin E. (Michigan State University, East Lansing), and Ralph N. Costilow. Glucose catabolism by Bacillus popilliae and Bacillus lentimorbus. J. Bacteriol. 87:303–310. 1964.—Resting cells of Bacillus popilliae and B. lentimorbus catabolize glucose with the production of CO2, lactic acid, acetic acid, glycerol, ethanol, and trace amounts of acetoin and acetaldehyde. The first three products are the major ones, and their ratios may be varied by controlling the availability of oxygen. Practically no lactic acid is produced when oxygen is not limiting, whereas it may comprise up to 80% of the total acid when oxygen is greatly limited. However, no glucose is catabolized by resting cells in the absence of molecular oxygen. Isotope and inhibitor studies and assays for key enzymes of the established metabolic routes all indicate that these organisms utilize both the Embden-Meyerhof and hexosemonophosphate pathways for glucose dissimilation. With a concentrated resting-cell suspension, the extent of participation of the latter route was estimated to be as high as 40% in an atmosphere of pure oxygen, and as low as 2% in air. Acetate was oxidized by only one of the cultures of B. popilliae tested, which is apparently a mutant. Cells of this strain from stationary phase cultures oxidized acetate at pH 7.0 or higher, but not at pH 6.0; however, they oxidized succinate, fumarate, and malate more rapidly at pH 6.0 than at 7.0. The oxidation of tricarboxylic acid cycle intermediates, the presence of condensing enzyme in extracts of cells capable of oxidizing acetate, and the complete inhibition of acetate oxidation by arsenite and partial inhibition by malonate all indicate that terminal oxidation of acetate by this strain of B. popilliae is via the tricarboxylic acid cycle.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUTKY S. R. Preliminary observations on the growth requirements of Bacillus popilliae Dutky and Bacillus lentimorbus Dutky. J Bacteriol. 1947 Aug;54(2):267–267. [PMC free article] [PubMed] [Google Scholar]
- GARY N. D., BARD R. C. Effect of nutrition on the growth and metabolism of Bacillus subtilis. J Bacteriol. 1952 Oct;64(4):501–512. doi: 10.1128/jb.64.4.501-512.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEATH E. C., HURWITZ J., HORECKER B. L., GINSBURG A. Pentose fermentation by Lactobacillus plantarum. I. The cleavage of xylulose 5-phosphate by phosphoketolase. J Biol Chem. 1958 Apr;231(2):1009–1029. [PubMed] [Google Scholar]
- KOVACHEVICH R., WOOD W. A. Carbohydrate metabolism by Pseudomonas fluorescens. IV. Purification and properties of 2-keto-3-deoxy-6-phosphogluconate aldolase. J Biol Chem. 1955 Apr;213(2):757–767. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NAKATA H. M., HALVORSON H. O. Biochemical changes occurring during growth and sporulation of Bacillus cereus. J Bacteriol. 1960 Dec;80:801–810. doi: 10.1128/jb.80.6.801-810.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OCHOA S., STERN J. R., SCHNEIDER M. C. Enzymatic synthesis of citric acid. II. Crystalline condensing enzyme. J Biol Chem. 1951 Dec;193(2):691–702. [PubMed] [Google Scholar]
- STADTMAN E. R. The purification and properties of phosphotransacetylase. J Biol Chem. 1952 May;196(2):527–534. [PubMed] [Google Scholar]
- STEINKRAUS K. H. Studies on the milky disease organisms. II. Saprophytic growth of Bacillus popilliae. J Bacteriol. 1957 Nov;74(5):625–632. doi: 10.1128/jb.74.5.625-632.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


