SUMMARY
A common property of long-term memory (LTM) induction is the requirement for repeated training sessions spaced over time. The phenomena of better memory being formed with resting intervals between training sessions is called the spacing effect, for which the underlying molecular and neural bases are largely unknown. Our study reveals that the duration of resting intervals required for inducing LTM can be regulated by activity levels of the protein tyrosine phosphatase corkscrew (CSW) in Drosophila. Our studies demonstrate that wild-type CSW overexpression dramatically shortens the inter-trial interval required for LTM induction. In contrast, overexpression of gain-of-function CSWs prolong the resting interval for induction of LTM formation. Further biochemical analysis reveals that LTM-inducing training regimens generate repetitive waves of CSW-dependent mitogen-activated protein kinase activation. The length of that kinase activity wave appears to define the duration of the resting interval. Thus, we establish that the spacing effect has a molecular basis and CSW/SHP2 is key in its regulation.
INTRODUCTION
Memory that is induced by repeated training improves when presentations are spaced over time compared to equal numbers of training exposures without spacing. This augmentation in memory formation is called the spacing effect and is a common phenomenon in the animal kingdom (Ebbinghaus, 1885; Carew et al., 1972; Tully et al., 1994; Gerber et al., 1998; Beck et al., 2000; Sutton et al., 2002; Cepeda et al., 2006; Philips et al., 2007). The spacing effect has been widely studied in both basic and applied research owing to its relevance for psychology, education, therapy and advertising (Bjork and Allen, 1970; Appleton-Knapp et al., 2005; Cepeda et al., 2006). Several psychological models have been proposed to explain the spacing effect. These models can be divided in two groups: those that attribute the benefit of the spaced learning to more efficient memory information encoding (e.g. Estes, 1955; Izawa, 1967; Hintzman, 1974; Martin, 1968) and those proposing superior memory consolidation (e.g. Fishman et al., 1968; Landauer, 1969; Wickelgren, 1970; for a meta-analysis and discussion, see Cepeda et al., 2006). In animal models, it is clear that the spacing effect requires protein synthesis (Quinn and Dudai, 1976) but the specific molecular mechanisms underlying this phenomenon are essentially unknown.
In the course of studying Drosophila models of Noonan syndrome (NS), we discovered that disease-associated mutations hampered memory formation and altered the spacing effect. NS is an autosomal dominant genetic disorder characterized by facial dysmorphia and other developmental abnormalities, including learning difficulties and mental retardation (Noonan, 1968; Tartaglia and Gelb, 2005). Mutations causing NS have been identified in five genes, PTPN11, SOS1, RAF1, BRAF and KRAS, all of which encode proteins that are components of the Ras/mitogen-activated protein kinase (MAPK) signaling pathway (Gelb and Tartaglia, 2006; Aoki et al., 2008). Gain-of-function (GOF) germline PTPN11 mutations cause about 50% of NS cases (Tartaglia et al., 2001) and somatic mutations with generally stronger GOF effects cause certain childhood leukemias (Tartaglia et al., 2003). The PTPN11 gene, and its orthologue in fruit flies corkscrew (csw), encodes the evolutionarily conserved protein tyrosine phosphatase SHP2 (Freeman et al., 1992; Neel et al., 2003), which is recruited to many receptor tyrosine kinases upon activation and is generally a positive regulator of Ras/MAPK signaling (Perkins et al., 1996; Bennett et al., 1994; Tang et al., 1995; Maroun et al., 2000; Oishi et al., 2006).
The spacing effect has been well demonstrated in Drosophila (Tully et al., 1994; Beck et al., 2000; Ho et al., 2007). With the extensively characterized odor and electric shock association task (Tully and Quinn, 1985; Tully et al., 1994), a single learning session elicits short-term memory (STM), mid-term memory (MTM), and a partial anesthesia-resistant memory (ARM) that lasts less than 24 hours, whereas ten consecutive learning sessions without rest in between (called massed training) produces STM, MTM and an ARM that lasts for four days. Long-term memory (LTM), a protein synthesis-dependent memory that does not decay after seven days, can be induced with ten learning sessions if rest intervals are provided between the training trials. This constitutes spaced training (For a detailed description, see Tully et al., 1994; Isabel et al., 2004; reviewed in Margulies et al., 2005).
Here, through the analysis of GOF csw mutations in Drosophila, we identified the first genetic factor controlling the spacing effect. We found that csw GOF mutations impair LTM formation by prolonging the length of the resting intervals between repetitive training required for induction of LTM. This prolonged resting interval correlated with altered training-induced MAPK activity within each resting interval.
RESULTS
We initiated our study by examining GOF csw mutations corresponding to amino acid substitutions that had been detected in Noonan syndrome (A72S, I282V and N308D), leukemia (D61Y and E76K), or individuals with both (T73I) (Figure 1A). These amino acid positions are conserved between SHP2 and CSW, and mutations at positions 72, 76 and 308 have been shown to increase MAPK activation and engender developmental phenotypes in Drosophila (Oishi et al., 2006). Since ubiquitous expression of the strongest csw GOF alleles is lethal (Oishi et al., 2006), we used GAL4 drivers specifying transgene expression in relevant portions of the central nervous system (Brand and Perrimon, 1993). Memory was assessed using the well-established odor and electric shock association learning procedures (Tully and Quinn, 1985).
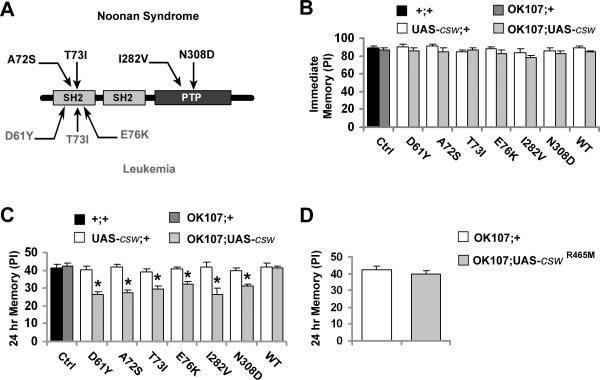
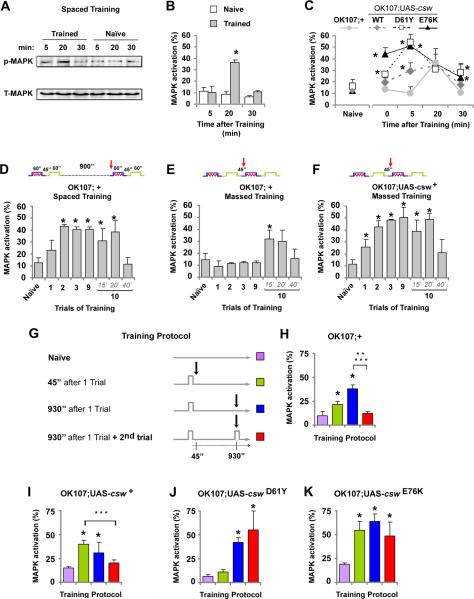
Figure 1. Expression of Gain-of-Function (GOF) csw Mutants in Mushroom Body (MB) Neurons Impairs 24-Hour Memory.
(A) Schematic representation of the SHP2 protein mapping point mutations detected in Noonan syndrome (top) and in leukemia (bottom) studied here. (B-C) Memory performance was determined in fly lines only carrying the transgene (UAS-csw;+) or expressing csw transgenes (OK107;UAS-csw). Transgene expression of csw mutant (from D61Y to N308D) and csw wild-type (WT) was targeted to the mushroom body using OK107-GAL4 (OK107;UAS-csw). (B) Immediate memory after a single trial was unaffected by the expression of the csw transgenes. (C) 24-hr memory after spaced training was reduced in all fly lines expressing mutants alleles (OK107;UAS-csw) compared with control (+/+ or OK107;+) flies, but not in lines only carrying those csw transgenes (UAS-csw;+). (D) 24-hr memory after spaced training in fly lines expressing a loss-of-function csw mutant (R465M) in the mushroom body neurons. Bars, mean ± SEM (n=8). Asterisks indicate p<0.05.
Expression of Clinically Relevant CSW Mutants in Mushroom Body Neurons Impairs 24-Hour Memory
We first assessed immediate memory, in which memory was measured immediately after one-cycle training (see Methods), and longer-lasting memory, for which memory scores were obtained 24 hr after spaced training that consisted of ten repetitive training trials with 15-min rest intervals between trials. Immediate memory mainly includes STM and MTM while 24-hr memory comprises LTM and ARM (Tully et al., 1994; Isabel et al., 2004).
Since the mushroom body (MB), which consists of a cluster of about 2,500 neurons in each hemisphere, has been shown to play a central role in the formation of immediate and 24-hr memory in Drosophila (De Belle and Heisenberg, 1994; Pascual and Preat, 2001; Yu et al., 2006; for reviews see Margulies et al., 2005), we targeted the expression of the csw transgenes to this region using a well-characterized GAL4 driver, OK107-GAL4 (Lee et al., 1999; also see Figure S1). Using that driver, all transgenic csw lines expressed CSW at comparable levels in adult heads (Figure S2). Our behavioral assays showed that immediate memory was not affected by overexpression of wild-type or mutant csw alleles (Figure 1B). In contrast, 24-hr memory was significantly reduced in transgenic fruit flies overexpressing any of the GOF csw mutant alleles (i.e. D61Y, A72S, T73I, E76K, I282V and N308D) but not in those overexpressing wild-type csw (Figure 1C and Table S1). This specific GOF-mutant effect on 24-hr memory was confirmed using another mushroom body driver, 247-GAL4 (Figure S3). As an additional control, we used the loss-of-function (LOF) mutation cswR465M, a point mutation that obliterates CSW's phosphatase activity (Flint et al., 1997; Kontaridis et al., 2004). Overexpression of this mutant in the MB neurons did not elicit any behavioral effects (Figures 1D and S4). Thus, overexpressing wild-type or LOF CSW in mushroom body neurons caused no effect on 24-hr memory. In contrast, overexpression of various GOF CSW mutants with increased or prolonged phosphatase activity led to a specific reduction in 24-hr memory. Thus, we focused our subsequent studies on 24-hr memory and used two alleles, D61Y and E76K, with the strongest biochemical GOF.
Involvement of CSW in Memory Formation
The data presented above were obtained with transgenic fruit flies in which the csw transgenes were expressed throughout development and in adulthood. To separate effects on neuronal development from those on the mature CNS, we analyzed the memories of adult flies in which the transgene expression was induced acutely using a heat shock-inducible GAL4 driver. After a single 1-hr heat shock, 24-hr memory formation was diminished in flies expressing the mutant allele D61Y but not in those expressing E76K or wild-type csw (Figures 2A and S5A-C). For the csw GOF mutant E76K, for which this protocol did not show detrimental effects on 24-hr memory, a stronger induction procedure did produce memory defects (Figure S5D). Taken together, these data showed that GOF CSW's adverse impact on 24-hr memory reflects an acute role in memory formation and does not depend on developmental abnormalities.
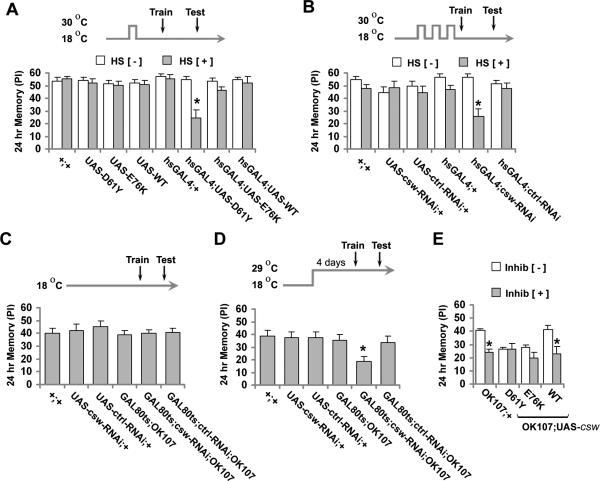
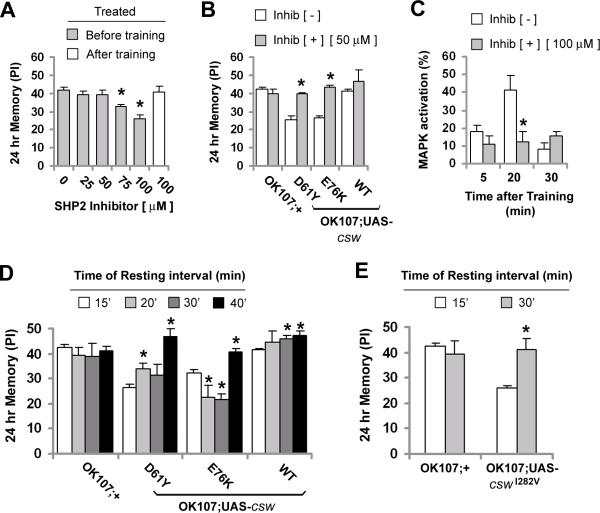
Figure 2. Acute Genetic or Pharmacological Interference of CSW Function Impairs 24-Hour Memory in Adult Fruit Flies.
(A-D) Heat shock or temperature protocol to induce transgene expression in adult flies (top) and effect on 24-hr memory after spaced training (bottom). HS+ represents heat shock and HS− represents no heat shock. (A) Effect of acute expression of mutant and wild-type csw induced with a heat shock-GAL4 driver and a single heat shock. The memory performance in hsGAL4;UAS-D61Y after heat shock (HS+) was reduced compared with the same line without heat shock (HS−) or control (+/+, UAS-D61Y, UAS-WT, hsGAL4;UAS-WT) groups in both conditions (HS+ or HS−). (B) Effect of acute expression of csw-RNAi (hsGAL4;csw-RNAi) induced with three heat shocks (HS+). Memory was reduced in hsGAL4;csw-RNAi after heat shock (HS+) compared with the same line without heat shock (HS−) or control (+/+, UAS-csw-RNAi, UAS-ctrl-RNAi or hsGAL4;+) groups in both conditions (HS+ or HS−). (C and D) Effect on memory performance of inducible RNAi expression targeted to mushroom bodies using the GAL80ts repressor of GAL4-mediated UAS-csw-RNAi expression, (C) at permissive temperature and (D) at restrictive temperature. Memory was reduced in the GAL80ts;csw-RNAi;OK107 group at restrictive temperature compared with control (+/+, UAS-csw-RNAi, GAL80ts;UAS-ctrl-RANi;OK107), but not at permissive temperature. (E) Effect of the SHP2 inhibitor, NSC-87877 (Inhib [+]) or vehicle (Inhib [−]) on 24-hr memory in flies expressing mutant or wild-type csw in mushroom body neurons. Memory was reduced in the control group (OK107;+) and in flies overexpressing the wild-type csw (WT) by the inhibitor (inhib [+]) compared with both groups fed with vehicle (Inhib [−]), but not in csw gof mutants. Bars, mean ± SEM (A, B and E n=8, and C and D n=5). Asterisks indicate p<0.05.
Although these observations indicated that GOF mutant CSW impairs 24-hr memory formation, it remained to be determined whether endogenous CSW played a role in memory formation. To examine that, we studied the effects of genetic reduction and pharmacological inhibition of CSW activity. First, we tested 24-hr memory in female flies heterozygous for hypomorphic, cswlf and csw6, or amorphic, cswLE120, alleles (Perkins et al., 1996). These heterozygous mutant flies did not show 24-hr memory defects (Figure S6). Second, since memory testing in homozygous was not possible due embryonic lethality, we attempted to achieve a greater reduction of CSW activity through the inducible expression of RNA interference (RNAi). Acute silencing of csw following a heat shock protocol produced a significant reduction in 24-hr memory compared with controls (Figures 2B and S7). Third, we confirmed this observation by expressing the RNAi in MB in adult flies by using the spatially and temporally defined TARGET system (McGuire et al., 2003), combining UAS-csw-RNAi with tubulin-GAL80ts;OK107-GAL4. The fly line GAL80ts;csw-RNAi;OK107-GAL4, as well as control groups, kept at permissive temperature (18°C), induced 24-hr memory normally (Figure 2C). In contrast, when the same fly lines were transferred to a restrictive temperature (29°C) for four days, csw silencing through expression of its RNAi reduced 24-hr memory whereas memory induction was unchanged in the control groups (Figures 2D and S8). Of note, the effects of the heat shock protocols or restrictive temperature specifically affected memory induction as sensorimotor abilities were unchanged (Table S2 and S3).
To further support our observation that endogenous CSW is critically involved in 24-hr memory formation, we inhibited CSW's phosphatase activity pharmacologically (Figure 2E). The selective SHP2 inhibitor, NSC-87877, binds to the catalytic cleft of the PTP domain, obliterating phosphatase activity (Chen et al., 2006). After feeding adult Drosophila NSC-87877 for several hours before and after training (see Experimental Procedures), 24-hr memory in control and wild-type csw transgenic fruit flies was reduced significantly (Figure 2E). Of note, treatment of the GOF mutant csw transgenic flies with NSC-87877 did not further impair their 24-hr memory formation. Taken together, the RNAi and phosphatase inhibitor data documented that CSW normally plays an important role in 24-hr memory formation. The observation that NSC-87877 did not further impair 24-hr memory in the GOF mutant csw transgenic flies suggested that they already lacked the memory component for which CSW normally plays its role. We attributed the lack of rescue by NSC-87877 in the GOF mutant csw transgenic flies to the high dose we used, a possibility we explored later in this study (see below).
Long-Term Memory is Specifically Affected
Next, we wanted to determine which of the components of 24-hr memory was affected in the GOF mutant csw transgenic flies, ARM or LTM. Two procedures were used to separate these two forms of memory. First, fruit flies were fed a protein synthesis inhibitor, cycloheximide, which blocks protein synthesis-dependent LTM formation while leaving the protein synthesis-independent ARM intact. As shown in Figure 3A, spaced training elicited significantly lower 24-hr memory scores in the control group and flies overexpressing normal CSW treated with cycloheximide as compared to those untreated. In contrast, cycloheximide treatment did not affect 24-hr memory scores significantly in transgenic fruit flies overexpressing GOF mutant CSW. These results suggested that LTM was reduced in most GOF mutants while ARM remained unaffected.
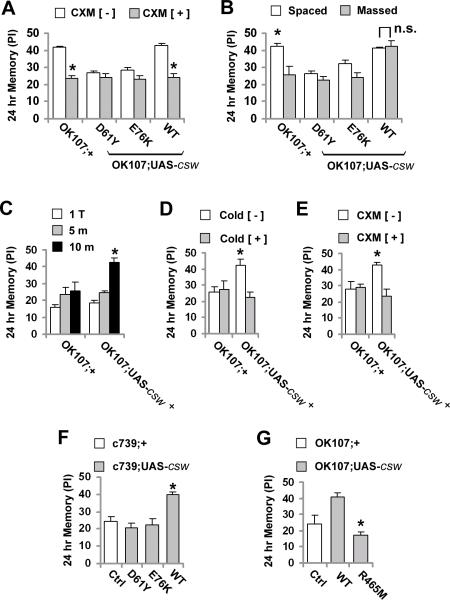
Figure 3. Altered LTM Formation via Genetic Manipulation of CSW.
(A) Effect of the protein synthesis inhibitor, cycloheximide (CXM+), or vehicle (CXM−) on 24-hr memory after spaced training in flies expressing mutant or wild-type csw in the mushroom body neurons. Memory was reduced in the control group (OK107;+) and in flies overexpressing the wild-type csw (WT) by the inhibitor (CXM +) compared with both groups fed with vehicle (CXM −), but not in csw gof mutants. (B) 24-hr memory after spaced or massed training in control (OK107;+) and transgenic flies expressing mutant and wild-type csw in the mushroom body neurons. Spaced training promoted a higher performance in control (OK107;+) flies compared with the massed training in the same line or the mutant lines (D61Y and E76K). Note that massed training in flies overexpressing wild-type csw produced a similar memory score as spaced training. (C) 24-hr memory after one trial (1 T), or 5 and 10 trials of massed training (5 m and 10 m, respectively) in control (OK107;+) and flies overexpressing wild-type csw in mushroom body neurons (OK107;UAS-csw+), which showed memory enhancement after 10 m compared with all the control bars (OK107;+) or the same lines trained with 1 or 5 trials. The effect of the cold shock (D) or cycloheximide (CXM) (E) on 24-hr memory after 10 trials of massed training in flies overexpressing wild-type csw in mushroom body neurons. Memory enhancement was detected in OK107;UAS-csw+ in the absence of cold shock or CXM compared with the same line untreated or with the control (OK107;+) line with or without treatment. (F) 24-hr memory after 10 trials of massed training in flies expressing wild-type or mutant csw in alpha/beta neurons of the mushroom bodies using the c739-GAL4 driver (c739;UAS-csw) compared with control (c739;+). Memory enhancement was detected in flies expressing wild-type csw compared with the control group or mutant lines. (G) 24-hr memory after 10 trials of massed training compared between flies overexpressing wild-type and phosphatase-dead mutant (R465M) csw in the mushroom body neurons. * indicates WT vs. R465M, Student's t test. Bars, mean ± SEM (N=6). Asterisks indicate p<0.05.
To test this further, we used massed training, which produces ARM but not LTM (Tully et al., 1994). The 24-hr memory scores elicited in this way were similar among the control group (OK107;+) and the transgenic fruit flies overexpressing GOF mutant CSW (Figure 3B), confirming that ARM remains normal in these transgenic mutants. Surprisingly, we observed that transgenic fruit flies overexpressing wild-type CSW, which showed normal 24-hr memory after spaced training, had enhanced 24-hr memory with massed training (Figure 3B). In fact, massed training was as effective in producing 24-hr memory as spaced training in these flies.
One possible explanation for the response of the wild-type csw transgenic flies to massed training was LTM formation requiring less repetitions of the conditioning (Ge et al., 2004). In testing this possibility, we found that one trial or 5-trial massed training did not induce any 24-hr memory enhancement in flies overexpressing wild-type CSW. However, 24-hr memory was enhanced in this fly line when 10 trials of massed training was used (Figure 3C). Additionally, every other trial of the massed protocol, which spread the five trials over the time used for 10-trial massed training, did not enhance 24-hr memory (Figure S9). Thus, the memory enhancement in the wild-type csw transgenic flies could not be attributed to a facilitated memory formation requiring fewer conditioning trials.
Next, we sought to determine if the enhanced 24-hr memory in response to massed training in the wild-type csw transgenic flies was LTM or ARM. First, we examined 4-hr memory elicited by one-trial training, which comprises only ARM (Tully et al., 1994; DeZazzo and Tully, 1995), and observed that it was normal in these transgenic flies (Figure S10). Second, we exposed these flies to a cold-shock treatment (2 min in ice water), to which ARM is resistant (Tully et al., 1994; DeZazzo and Tully, 1995), 2 hrs after training and found that it abolished the massed-training elicited memory enhancement (Figure 3D). Third, we found that the enhanced memory was disrupted with cycloheximide treatment (Figure 3E). This documented that this memory was protein-synthesis dependent, which is a major feature of LTM (Tully et al., 1994).
The MB in Drosophila contains three classes of neurons, named α/β, α′/β′ and γ. The α/β neurons have been shown to be critical for LTM formation (Pascual and Preat, 2001; Yu et al., 2006). To determine where the LTM induced by massed training in the wild-type csw transgenic flies was occurring, we used the GAL4 driver, c739, which limits expression mainly to the α/β MB neurons. We showed that the massed training-elicited 24-hr memory enhancement was retained with this GAL4 driver (Figure 3F and Table S4), documenting that this LTM was forming in the normal anatomic sites and not ectopically.
SHP2 (and, presumably CSW) has roles that are phosphatase-dependent and others, such as docking, that are phosphatase-independent. To test whether the massed training-induced LTM required CSW's phosphatase activity, we used the phosphatase-dead mutation, cswR465M (see earlier and refs. Flint et al., 1997; Kontaridis et al., 2004). Massed training failed to enhance 24-hr memory in transgenic fruit flies overexpressing this LOF mutant (Figure 3G), documenting that the phenomenon is phosphatase dependent.
Taken together, these observations showed that LTM was disrupted by overexpression of the GOF mutant csw transgenes, while overexpression of the wild-type csw transgene promoted LTM formation in response to massed training.
CSW Regulates Durations of Resting Intervals Needed to Elicit LTM
The discovery of novel LTM formation elicited via massed training in the wild-type csw transgenic flies led us to explore possible explanations for this phenomenon. A clue came from a close look at the massed training paradigm. In contrast to spaced training, the purpose of massed training is to leave minimal time between training trials. In reality, there is a separation of 150 sec between conditioning stimulations in the standard massed training protocol (Figure 4A). During these 150 seconds, 45 seconds are needed to purge the residual conditioned odor, 60 seconds are required for exposure to the unconditioned odor, and the final 45 seconds are used to purge the unconditioned odor. In order to establish a massed training protocol that would permit us to shorten the separation between conditioning stimuli, we first examined the effect of omitting the unconditioned odor on 24-hr memory. Thus, our modified massed training consisted of 10 repetitive training trials with 150 seconds between phases of conditioning, as the standard massed training, but without exposure to the unconditioned odor (named “Massed (150 sec)” in Figure 4A). Using this modified protocol, we found that 24-hr memory was not affected; the control group showed normal ARM and the memory enhancement in the wild-type csw transgenic flies persisted (Figure 4B).
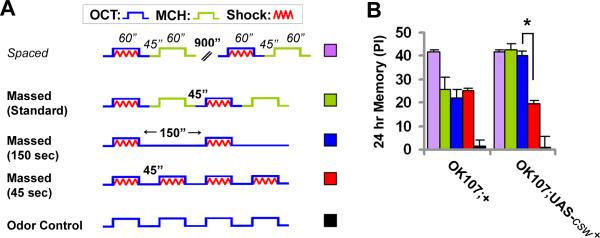
Figure 4. Overexpression of the Normal csw+ Transgene In MB Neurons Shortens Resting Intervals in LTM Induction.
(A) Schematic representation of the training protocols used in Panel B. These show the two initial trials of the protocols, which actually were composed of 10 trials. From top to bottom, first, symbols representing the conditioned stimuli (odors in blue or green) and the unconditioned stimuli (electric shock in red); and below training protocols. Spaced: training trials with a resting interval of 900 sec. Massed (standard): trials with a 45 sec of resting between trials. Massed (150 sec): trials separated by 150 sec of interval between electric shocks as in the standard massed protocol, but without control odor. Massed (45 sec): trials with the intervals between electric shocks reduced to 45 seconds. Odor control: control stimulation with odor. (B) 24-hr memory performance after 10 trials of training (schematized in Panel A) in flies overexpressing wild-type csw in the mushroom body neurons. * indicates memory reduction by shortening the inter-trial interval. Note: In these schematic representations, only the odor “OCT” is shown to be associated with the electric shock (US), whereas each performance index (PI) is the average of two experiments in parallel where the odor “OCT” and “MCH” were paired with electric shock. Bars, mean ± SEM (N=8). Asterisks indicate p<0.05.
Omitting the unconditioned odor allowed us to shorten the intervals between conditioning stimuli to 45 seconds. With this shorter resting interval, the enhancement of 24-hr memory in the wild-type csw transgenic flies disappeared, leaving a normal ARM-like 24-hr memory score that was similar to that observed with the control flies (Figure 4B). To exclude the possibility of biased behavioral effects through such intense exposure to the conditioned odor, we showed that odor exposure without pairing with electric shocks (or electric shock without paring with odor, not shown) did not elicit a detectable bias in behavior (Figure 4B). The data presented here together with those presented earlier demonstrate that modified massed training with 150-sec resting intervals was capable of producing the memory enhancement while only ARM-like was elicited when 45-sec resting intervals were employed with fruit flies overexpressing the wild-type csw transgene. In other words, the resting interval required to induce LTM was shortened from 15 min to 2.5 min, the first observation that the resting interval can be manipulated.
Correlation of Training-Induced CSW-Dependent MAPK Activity
To gain insights into how CSW activity might regulate the spacing effect, we assayed Drosophila head MAP kinase (MAPK) activation at different times after training because CSW is a positive regulator of signaling from a variety of growth factor receptors flowing through the Ras/MAP kinase cascade (Perkins et al., 1996; Gelb and Tartaglia, 2006; Aoki et al., 2008). In addition, MAPK activity has been implicated in memory formation in invertebrates and vertebrates (Kandel, 2001; Kelleher et al., 2004; Cammarota et al., 2007; Mayford, 2007). The activation of this cascade was assessed by western blot using an antibody specific for the diphosphorylated (activated) form of the MAP kinase, p-MAPK.
We first attempted to detect training-dependent activation of MAPK in control fruit flies. Western blots of homogenized heads at various times after training revealed a transient activation shortly after completing the training procedure. There was no significant difference between the trained and naïve groups at 5 min after spaced training (Figures 5A and B). An increase in MAPK activity was detected at 20 min after spaced training and that the increase decayed away by 30 min (Figure 5B). No training-dependent activation of MAPK was detected by unpaired or backward training (Figure S11), suggesting that the observed MAPK activation is specific to associative conditioning.
Figure 5. Training Induced CSW-Dependent Transient MAPK Activity.
Training-induced MAPK activation in heads of control and transgenic fruit flies overexpressing csw in the mushroom bodies. (A) Representative western blot showing activated MAPK (p-MAPK) and total MAPK (T-MAPK) after spaced training. (B) MAPK activation in non-transgenic flies after training (Trained) or just manipulated (Naive). * Indicates trained vs. naïve at 20 min. (C) MAPK activation in naive flies (left side) and at different times after spaced training (right side) in mutant csw transgenic (D61Y and E76K), wild-type csw transgenic (WT) or control (OK107;+) lines. * indicates differences between lines overexpressing csw compared with the control (OK107;+) at the same time point. For D, E and F, training paradigms are represented with a red arrow indicating the time of sampling (top) and MAPK activation (bottom) in naïve flies (left side) and at the end of the inter-trial interval after 1, 2, 3, 9 and at three time points after the 10th training trial. MAPK activation in the control line (OK107;+) during spaced training (inter-trial interval, 900 sec) (D), and during massed training (inter-trial interval, 45 sec) (E). (F) MAPK activation in fruit flies overexpressing wild-type csw (OK107;UAS-csw+) during massed training (inter-trial interval, 45 sec). For D, E and F, * indicates an increase in MAPK activation compared with the basal state (naïve). Data points, mean + SEM (n=3 in B) and (n= 4 in C, D, E and F). (G-K) Effect of a trial of training on training-induced MAPK activation produced by a previous trial of training. (G) Protocols of training represented by a line with a first trial of training and a second trial (2nd trial). Black arrows indicate the time at which the flies were harvested (45 sec or 930 sec) and processed for western blot. (H-K) MAPK activation in naïve (violet), at 45 sec (green) or 930’ sec (blue) after one trial; and at 930 sec after one trial plus a test trial of 30 sec (red) in the control group (OK107;+) (H); in fruit flies overexpressing wild-type CSW (OK107;UAS-csw+) (I); and flies overexpressing GOF mutants CSW D61Y (OK107;UAS-cswD61Y) (J) or E76K (OK107;UAS-cswE76K) (K). * indicates increased levels of MAPK compared with the basal state (naïve) and ** a statistical reduction of p-MAPK at 930 sec after the test trial compared with the same time point without a test trial (blue vs. red bar). Of note, in H and I, the level of MAPK activation after the test trial (red bar) is significantly smaller than the levels at the time of the peak of MAPK activation (blue and green bars, respectively; indicated by ***) and is not different from the naïve state. Data points, mean ± SEM (n=3). Asterisks indicate p<0.05.
We then compared MAPK activities among different genotypes, including transgenic fruit flies with targeted MB overexpression of wild-type csw and the csw mutant alleles, D61Y and E76K. These two mutants presented a higher activation immediately after training, however, all three transgenic lines showed significantly faster activation in response to training, i.e. strong MAPK activation was observed at 5 min after training (Figure 5C) while during the decay phase, MAPK activation associated with wild-type csw overexpression appeared to be normal but was slower with the D61Y and E76K mutants. Although intriguing, these data did not provide a clear idea about how the resting interval requirement is affected.
Next, we wanted to determine whether spaced and massed training activated MAPK differently in control flies. To do that, we harvested flies after 1, 2, 3, and 9 trials as well as at three time points up to 40 min after the 10th trial and then assessed the MAPK activation status of their heads. As shown in Figures 5D and E for spaced and massed training, respectively, MAPK activation and its decay after the 10th trial were comparable. Of note, there was a dramatic difference in the activation of MAPK observed after trials earlier in those protocols. MAPK activation was apparent after essentially every trial with spaced training, but that was never observed during massed training.
To determine whether the pattern of MAPK activation might explain the enhanced LTM formation that we observed in the transgenic flies overexpressing wild-type CSW, we assessed the status of MAPK activation of those flies during massed training (Figure 5F). Indeed, we observed that wild-type csw transgenic flies activated MAPK in manner comparable to that observed with control flies undergoing spaced training. This result is consistent with faster training-induced MAPK activation in fruit flies overexpressing wild-type CSW (Figure 5C, at 5 min) and suggested that activation of MAPK during resting intervals is critical for LTM formation.
GOF CSW disrupted Training-Induced MAPK Activity decay
We wanted to understand the mechanism explaining why spaced training resulted in inter-trial MAPK activation while massed training did not in control flies. The most facile explanation was that the 150-sec between phases of conditioning during massed training was insufficient to allow MAPK to be activated. That notion seemed inconsistent with the fact that the entire massed training protocol is in excess of 15 min but no MAPK activation was observed after the ninth trial (Figure 5E). Therefore, we established different experiments for which the status of MAPK activation was tested 45 sec after the first trial, 930 sec after the first trial, and in the midst of a second trial (930 sec after the first trial with a second trial inserted 30 sec prior to harvest; Figure 5G). When control flies were used, we observed modest MAPK activation at 45 sec and stronger activation at 930 sec after the first trial (Figure 5H). Of note, MAPK returned back to the basal level when a second trial was interposed prior to harvest (Figure 5H, red bar). Next, we tested transgenic flies overexpressing CSW using this paradigm. We observed that, as with the control flies, the second trial also reset MAPK compared with the time of highest activation (Figure 5I). Interestingly, that time of highest MAPK activation in flies overexpressing wild-type csw transgene occurred at 45 sec, consistent with our previous observation that these flies formed LTM when exposed to massed training because of faster MAPK activation.
Finally, we wanted to understand why the transgenic flies overexpressing GOF mutant CSW had diminished LTM formation in response to spaced training. This seemed enigmatic since we had shown that these mutants engendered rapid MAPK activation, even more than observed with the wild-type transgenic flies. To gain insights into that, we subjected the transgenic GOF csw fly strains to the protocol testing the effects of the second trial on MAPK activation (Figures 5J and 5K). While MAPK activation 45 sec after the first trial was comparable to that observed with the wild-type csw transgenic flies with one mutant, the striking finding was the MAPK activation remained quite elevated after interposition of the second trial for both GOF mutants.
To summarize these results, we observed that normal levels of CSW result in maximal MAPK activation 930 sec after a learning trial and that the subsequent trial resets MAPK to its basal state. Overexpression of wild-type CSW results in a more rapid MAPK activation but the reset mechanism appears to be largely conserved. In contrast, overexpression of GOF CSW may result in rapid MAPK activation but a slower decay in that activation appears to facilitate an overriding of the MAPK reset mechanism normally associated with the subsequent trial. Taken together, these findings provide a cogent explanation for why wild-type csw transgenic flies induce LTM with massed training. Moreover, the LTM deficit in the GOF csw transgenic flies implies that the switching on and off of MAPK activation plays a critical role in the formation of LTM.
Rescue of LTM Defects Induced by Gain-of-Function of csw Mutations
The data presented above suggested that csw GOF mutations cause LTM defects in Drosophila due to prolonged phosphatase activity, leading to the rest interval used during standard spaced training being inadequate to permit activated MAPK to be reset. Since the LTM defect occurs even when these CSW mutant proteins are expressed after development, we explored two strategies to rescue the memory deficit in adulthood. First, we attempted to reduce, but not eliminate, CSW activity in the GOF csw transgenic flies. Having shown that high-dose treatment with the phosphatase inhibitor NSC-87877 (100 μM) abolished LTM (see Figure 2E) in wild-type and csw transgenic flies, we tested whether more modest dosing could rescue the memory defect in the GOF csw transgenic flies. The dose-response curve to NSC-87877 was determined in control flies (Figure 6A). When these flies were fed for 8 h immediately before the standard spaced training, LTM formation was intact when doses below 75 □M were used. Moreover, drug feeding for 8 hr after training, even at a high concentration of 100 □M, did not affect memory (Figure 6A). On the basis of this dose response, we treated fly lines expressing GOF CSW mutants (D61Y and E76K) with NSC-87877 50 μM for 8 h immediately before the spaced training. We found that their 24-hr memory was completely rescued (Figure 6B). Then, we examined if this pharmacological treatment also modulates the training-induced MAPK activation. First, we studied the effect of a higher concentration of the SHP2 inhibitor (100 □M) or vehicle on the training-induced MAPK activation in control fruit flies treated for 8 h before training. The SHP2 inhibitor, but not the vehicle, precluded the training-induced activation of MAPK (Figure 6C). Next, we examined the effect of the lower concentration of the inhibitor (50 μM) on the MAPK activity after spaced training in flies overexpressing the E76K CSW mutant. At this lower concentration, the SHP2 inhibitor reduced slightly the overall training-induced MAPK activation compared with vehicle, but not at any specific time point after training (Figure S12). Thus, acute reduction of phosphatase activity is capable of allowing fruit flies overexpressing csw GOF mutations to form LTM normally and correlates with reduced training-dependent MAPK activation.
Figure 6. Rescue of the GOF Mutations-Induced LTM Phenotype.
(A) Dose response to the SHP2 inhibitor on 24-hr memory in non-transgenic flies (2022U). Flies were fed vehicle (0 μM) or varying doses of drug (25, 50, 75 and 100 μM) for eight hours before training, or for eight hours after training (100 μM). * indicates reduced memory performance compared with flies fed with vehicle (0 μM). (B) Effects of eight hours of SHP2 inhibitor 50 μM (inhib [+]) or vehicle (inhib [−]) before training on 24-hr memory in control (OK107;+) and flies overexpressing GOF or wild-type csw. * indicates memory rescue in mutants (D61Y and E76K) after drug feeding (inhib [+]) compared with the same line after vehicle (inhib [−]). (C) MAPK activation in non-transgenic flies treated with SHP2 inhibitor (100 μM) (Inhib [+]) or vehicle (Inhib [−]) after spaced training. * Indicates (Inhib [+]) vs. (Inhib [−]) at 20 min. Bars and data points, mean ± SEM (N=6 in A and B) and (n=3 in C). (D and E) 24-hr memory after 10 trials of spaced training with increasing resting intervals in controls (OK107;+) and flies overexpressing gain-of-function mutant (D61Y and E76K) or wild-type (WT) (D), or expressing a csw mutant with weaker gain-of function effect (I282V) (E). In D and E * indicates higher performance compared with the same fly line when the resting interval of 15 min was used. Bars, means ± SEM (N=8). Asterisks indicate p<0.05.
Our second strategy for LTM rescue was to increase the inter-trial rest interval during spaced training. For the control and wild-type csw transgenic flies, 24-hr memory was unaffected or slightly increased, respectively, as the resting interval was lengthened from the standard 15 min up to 40 min (Figure 6D). Remarkably, 24-hr memory for the GOF csw-mutant transgenic lines (D61Y and E76K) became normal as the resting interval was increased to 40 min (Figure 6D) and to 30 min (Figure 6E) with a third line expressing a csw mutant, I282V, with a milder biochemical GOF effect (Tartaglia et al., 2006). These results were consistent with the concept that MAPK activation must decay enough to permit a resetting with the subsequent trial during spaced training.
DISCUSSION
The work presented above began with the study of the effects of clinically relevant GOF csw mutations on learning and memory and led to the discovery that CSW plays a critical role in the regulation of the spacing effect for induction of LTM. We employed several measures to minimize biologic variation, including the use of an isogenic background for all genotypes examined, identical rearing and testing conditions, and batching the analysis for all data presented in the same figure. In addition, we used multiple mutant alleles to support any phenotypes observed. Finally, we used alternative approaches such as pharmacologic inhibition or RNAi when possible to bolster our initial observation.
CSW Signaling Pathways and Long-Term Memory Formation
Among the several functions of CSW, its phosphatase activity seems to be critical for LTM induction. Pharmacological phosphatase inhibition in wild-type fruit flies disrupted LTM (Figure 3E), overexpression of phosphatase-dead CSW had no effect on memory formation (Figure 1D), and NS- and leukemia-associated CSW mutants share the biochemical feature of having elevated phosphatase activity (Tartaglia et al., 2006).
The adverse effects of the GOF CSW on LTM formation are likely mediated through CSW-regulated Ras/MAPK activity. CSW is a key signaling relay in pathways in C. elegans, Drosophila, Xenopus and mammals (for review see, Neel et al., 2003). Our data indicated that GOF CSW deregulated the training-dependent MAPK activation/inactivation (Figures 5). Thus, the most parsimonious interpretation is that csw GOF mutations alter the time course of the activity of the MAPK pathway in such a way that a longer resting period between training sessions is required for promoting normal memory formation (Figure 6D).
Although the Ras/MAPK pathway is crucial for growth and differentiation, it was interesting to note that the defects in LTM formation associated with GOF CSW were not developmental (Figures 2 and 6). Thus, this study together with an increasing body of evidence suggest that the receptor tyrosine kinase-activated Ras/MAPK pathway might be a conserved mechanism from Drosophila to vertebrates and even humans in mediating memory formation (Kandel, 2001; Purcell and Carew, 2003; Kelleher et al., 2004; Cammarota et al., 2007; Mayford, 2007).
The Molecular Basis of the Spacing Effect
This study showed, for the first time, that genetic manipulation could modify the resting interval needed for the induction of LTM. In Drosophila, the spacing effect is well defined phenomenologically (Tully et al., 1994; Beck et al., 2000) and it is used as a behavioral strategy to induce protein synthesis-dependent LTM. It was previously established that LTM can be elicited with 10 repetitive training trials with an optimal spacing of 15 min and we showed that LTM is equally well formed as the rest interval is lengthened to 30-40 min (Figure 6). More strikingly, the minimum duration was shortened to 150 sec for transgenic fruit flies overexpressing wild-type csw (Figure 4), but was prolonged to 40 min in transgenic fruit flies with overexpression of GOF CSW mutants (Figure 6D). Of note, even though a 150-sec inter-trial was enough to induce LTM in fruit flies overexpressing wild-type csw, a longer interval did not produce more memory as only a small increase in performance was detected by using 30 or 40 min of spacing.
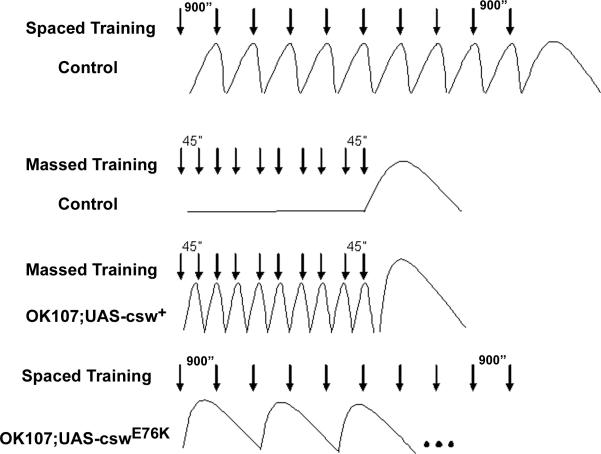
A biochemical correlate of this resting-interval dependence for LTM induction emerged from the analysis of MAPK activation patterns. For clarity, this idea is illustrated schematically in Figure 7. For wild-type flies subjected to spaced training, MAPK is activated during each 15-min rest interval and is reset to the basal level by the following training cycle. Thus, there is a wave of MAPK activity after each training trial, making for 10 peaks in all. In contrast, in massed training, there is only one peak of MAPK activity, which occurs 15-20 min after finishing the 10th training trial. For fruit flies overexpressing wild-type CSW, however, massed training does create 10 waves of MAPK activation due to the faster MAPK activation combined with a normal post-trial resetting mechanism. Although MAPK may also be activated faster in transgenic fruit flies overexpressing GOF CSW mutants, this activity is not reset by the subsequent training trial, apparently due to the slower kinetics for its decay. Therefore, the standard spaced training protocol with 15 min rest intervals engenders altered MAP activity peaks in these mutant CSW transgenic fruit flies, resulting in an LTM deficit. This is supported by the observation that lengthening the inter-trial interval to 40 min, which presumably provides more time for the decay of the MAPK activity, rescues LTM formation by restoring MAPK activation waves. Taken together, our finding suggests that CSW-dependent MAPK activation is involved in defining the duration of resting intervals necessary for LTM induction.
Figure 7. Schematic Representations of Training-Regulated MAPK Activity Correlated with Training Protocol and Genotype.
Arrows indicate individual training trails. The curves are schematic representation of MAPK activity (the vertical for amplitude of MAPK activity and horizontal for time). The duration for the resting intervals for each training paradigm is indicated.
EXPERIMENTAL PROCEDURES
Fly Stocks
Flies were raised at 22-24 °C on standard cornmeal medium. Drosophila stocks were purchased from the Bloomington Stock Center or Vienna RNAi Center, except when stated, and are described at FlyBase (www.flybase.org). The generation of the UAS-csw transgenic flies was described previously (Oishi et al., 2006). The UAS-csw transgenes were into the 2022U genetic background (Guo et al., 2000; Ho et al., 2007).
Conditioned Behavior Assay
Flies were trained and tested with a standard olfactory conditioning procedure (Tully and Quinn, 1985). Three to four-day-old flies were trained and tested in darkness in a group of ~100 individuals. Flies were exposed consecutively to odor #1 (CS+) (1 min) temporally paired with electric shock (US), and to odor # 2 (CS −) (1 min) without electric shock. This procedure constituted one training trial. The experiments had a balanced design; for one complete experiment, a second group of flies was trained with odor # 1 (as CS−) and odor # 2 (as CS+) and the two groups were used to estimate the behavioral performance index (PI). Fresh air was bubbled at 750 ml/min in mineral oil (Fisher) alone, or containing Octanol-3 (OCT) [1.5×10−3] or methylcyclohexanol (MCH) [1.0×10−3] (Fluka) as CS.
Performance Index
Conditioned odor avoidance responses were assessed for two minutes in a T-maze, where the CS+ and CS− were delivered simultaneously from the arms on currents of air. After testing, flies were trapped in their respective T-maze arms, counted and PI calculated.
Immediate and 24-hr Memory
For immediate memory, flies were tested immediately after one training trial. For 24-hr memory, flies received 10 training trials with 15 min intervals between each trial (spaced training) or without inter-trial intervals (massed training) as previously (Ho et al., 2007). Memory was tested 24 hrs after the end of the training protocol.
Drug Feeding Treatment
The cycloheximide (CXM) feeding regimen was performed as described previously (Tully et al., 1994). Briefly, groups of ~100 flies were fed with 35 mM of CXM (Calbiochem) in 5% glucose or vehicle only for 12-15 hrs before training and again during the 24-hr retention period.
Administration of the SHP2 inhibitor, NSC-87877 (Calbiochem), was performed according to the CXM protocol (Figure 2E), or only during an 8-hr period (Figure 6).
Heat Shock and Cold Shock Treatment
For expression of the csw transgenes, hsGAL4;UAS-csw flies were raised at 18°C, incubated at 30 °C in glass tubes in a thermostatic bath water for 1 hr and then remained at 18 °C until the end of the experiment. A 2.5-hr resting period was used between heat shock and training. For expression of csw-RNAi, hsGAL4;UAS-csw-RNAi, the conditions of fly culture and heat shock were the same, but three heat shocks separated by 8 hrs and a resting period of 8 hrs before training were used. For RNAi expression using the TARGET system, flies raised at 18°C, were incubated at 29°C for four days.
A 2-min cold-shock anesthesia was applied 2 hrs after training by submerging flies maintained in a glass tube in 0 °C ice water. After the cold-shock treatment, flies were transferred back to their food vials for the rest of the retention period.
MAPK Activity
MAPK activity was examined by western blot. Briefly, for each genotype and time point, 30 heads were homogenized; samples were run on 10% Tricine–glycine gels and transferred to nitrocellulose. Anti-phosphorylated p44/42 MAPK and anti-total p44/42 MAPK antibody was used (Cell Signaling Technology). Donkey anti-rabbit HRP-conjugated secondary antibody (1/2000) (Amersham) and ECL kit (Amersham) for detection of signal were used. Activation was quantified by ImageJ, as p-MAPK normalized to total MAPK.
Statistical Analysis
Multi-comparisons were performed by a one-way ANOVA followed by planned pair-wise comparisons. The alpha error = 0.05 was corrected for multiple comparisons using the Bonferroni method. The threshold for declaring significant differences was p<0.05.
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Rela, Y. Wang, J. Dubnau and the Zhong's lab for discussion on the experiments, S. Yichun for gal80ts;gal4 lines, L. Perkins for csw hypomorphic lines and antibody, and J. Beshel for comments on the manuscript. This work was supported by grants to Y.Z. from the National Institutes of Health (NIH) 5R01 DC05784-05A1 and Dept of Army W81XWH-05-1-0142, and to B.D.G. from NIH (HL71207). Conflict of Interest statement. One of the authors, Dr. Gelb, is an inventor on U.S. Patent 7,335,469 entitled “Methods for diagnosing Noonan syndrome.” He and his institution receive royalties for genetic testing of PTPN11 for Noonan syndrome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y. The RAS/MAPK syndromes: novel roles of the RAS pathway in human genetic disorders. Hum Mutat. 2008 doi: 10.1002/humu.20748. [DOI] [PubMed] [Google Scholar]
- Appleton-Knapp SL, Bjork RA, Wickens TD. Examining the spacing effect in advertising: encoding variability, retrieval processes, and their interactions. J. Consumer research. 2005;32:266–276. [Google Scholar]
- Beck CD, Schroeder B, Davis RL. Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. J Neurosci. 2000;20:2944–2953. doi: 10.1523/JNEUROSCI.20-08-02944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AM, Tang TL, Sugimoto S, Walsh CT, Neel BG. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc Natl Acad Sci U S A. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork RA, Allen TW. The spacing effect: consolidation or differential encoding? J. Verbal Learning and Verbal Behavior. 1970;9:567–572. [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Medina JH, Izquierdo I. ERK1/2 and CaMKII-mediated events in memory formation: Is 5HT regulation involved? Behav Brain Res. 2007 doi: 10.1016/j.bbr.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Pinsker HM, Kandel ER. Long-Term Habituation of a Defensive Withdrawal Reflex in Aplysia. Science. 1972;175:451–454. doi: 10.1126/science.175.4020.451. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D. Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychol Bull. 2006;132:354–380. doi: 10.1037/0033-2909.132.3.354. [DOI] [PubMed] [Google Scholar]
- Chen L, Sung SS, Yip ML, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J. Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol. 2006;70:562–570. doi: 10.1124/mol.106.025536. [DOI] [PubMed] [Google Scholar]
- De Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- DeZazzo J, Tully T. Dissection of memory formation: from behavioral pharmacology to molecular genetics. Trends Neurosci. 1995;18:212–218. doi: 10.1016/0166-2236(95)93905-d. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus HE. Memory: a contribution to experimental psychology. Dover; New York: 1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes WK. Statistical theory of distributional phenomena in learning. Psychol Rev. 1955;62:369–377. doi: 10.1037/h0046888. [DOI] [PubMed] [Google Scholar]
- Fishman EJ, Keller L, Atkinson RC. Massed versus distributed practice in computerized spelling drills. J Educ Psychol. 1968;59:290–296. doi: 10.1037/h0020055. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Tiganis T, Barford D, Tonks NK. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 1997;94:1680–1685. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman RM, Jr., Plutzky J, Neel BG. Identification of a human src homology 2-containing protein-tyrosine-phosphatase: a putative homolog of Drosophila corkscrew. Proc Natl Acad Sci U S A. 1992;89:11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Hannan F, Xie Z, Feng C, Tully T, Zhou H, Zhong Y. Notch signaling in Drosophila long-term memory formation. Proc Natl Acad Sci U S A. 2004;101:10172–10176. doi: 10.1073/pnas.0403497101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb BD, Tartaglia M. Noonan syndrome and related disorders: dysregulated RAS-mitogen activated protein kinase signal transduction. Hum Mol Genet. 2006;15:R220–226. doi: 10.1093/hmg/ddl197. Spec No 2. [DOI] [PubMed] [Google Scholar]
- Gerber B, Wustenberg D, Schutz A, Menzel R. Temporal determinants of olfactory long-term retention in honeybee classical conditioning: nonmonotonous effects of the training trial interval. Neurobiol Learn Mem. 1998;69:71–78. doi: 10.1006/nlme.1997.3801. [DOI] [PubMed] [Google Scholar]
- Guo HF, Tong J, Hannan F, Luo L, Zhong Y. A neurofibromatosis-1-regulated pathway is required for learning in Drosophila. Nature. 2000;403:895–898. doi: 10.1038/35002593. [DOI] [PubMed] [Google Scholar]
- Hintzman DL. Theoretical implications of the spacing effect. In: Solso RL, editor. Theories in cognitive psychology: The Loyola symposium, 77-97. Lawrence Erlbaum Associates; Potomac, MD: 1974. [Google Scholar]
- Ho IS, Hannan F, Guo HF, Hakker I, Zhong Y. Distinct functional domains of neurofibromatosis type 1 regulate immediate versus long-term memory formation. J Neurosci. 2007;27:6852–6857. doi: 10.1523/JNEUROSCI.0933-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabel G, Pascual A, Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
- Izawa C. Function of test trials in paired-associate learning. J Exp Psychol. 1967;75:194–209. doi: 10.1037/h0024971. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kontaridis MI, Eminaga S, Fornaro M, Zito CI, Sordella R, Settleman J, Bennett AM. SHP-2 positively regulates myogenesis by coupling to the Rho GTPase signaling pathway. Mol Cell Biol. 2004;24:5340–5352. doi: 10.1128/MCB.24.12.5340-5352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landauer TK. Reinforcement as consolidation. Psychol Rev. 1969;76:82–96. doi: 10.1037/h0026746. [DOI] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- Margulies C, Tully T, Dubnau J. Deconstructing memory in Drosophila. Curr Biol. 2005;15:R700–713. doi: 10.1016/j.cub.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun CR, Naujokas MA, Holgado-Madruga M, Wong AJ, Park M. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol. 2000;20:8513–8525. doi: 10.1128/mcb.20.22.8513-8525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E. Stimulus meaningfulness and paired-associate transfer: an encoding variability hypothesis. Psychol Rev. 1968;75:421–441. doi: 10.1037/h0026301. [DOI] [PubMed] [Google Scholar]
- Mayford M. Protein kinase signaling in synaptic plasticity and memory. Curr Opin Neurobiol. 2007;17:313–317. doi: 10.1016/j.conb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Noonan JA. Hypertelorism with Turner phenotype. A new syndrome with associated congenital heart disease. Am J Dis Child. 1968;116:373–380. doi: 10.1001/archpedi.1968.02100020377005. [DOI] [PubMed] [Google Scholar]
- Oishi K, Gaengel K, Krishnamoorthy S, Kamiya K, Kim IK, Ying H, Weber U, Perkins LA, Tartaglia M, Mlodzik M, et al. Transgenic Drosophila models of Noonan syndrome causing PTPN11 gain-of-function mutations. Hum Mol Genet. 2006;15:543–553. doi: 10.1093/hmg/ddi471. [DOI] [PubMed] [Google Scholar]
- Pascual A, Preat T. Localization of long-term memory within the Drosophila mushroom body. Science. 2001;294:1115–1117. doi: 10.1126/science.1064200. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Johnson MR, Melnick MB, Perrimon N. The nonreceptor protein tyrosine phosphatase corkscrew functions in multiple receptor tyrosine kinase pathways in Drosophila. Dev Biol. 1996;180:63–81. doi: 10.1006/dbio.1996.0285. [DOI] [PubMed] [Google Scholar]
- Purcell AL, Carew TJ. Tyrosine kinases, synaptic plasticity and memory: insights from vertebrates and invertebrates. Trends Neurosci. 2003;26:625–630. doi: 10.1016/j.tins.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Philips GT, Tzvetkova EI, Carew TJ. Transient mitogen-activated protein kinase activation is confined to a narrow temporal window required for the induction of two-trial long-term memory in Aplysia. J Neurosci. 2007;27:13701–13705. doi: 10.1523/JNEUROSCI.4262-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn WG, Dudai Y. Memory phases in Drosophila. Nature. 1976;262:576–577. doi: 10.1038/262576a0. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ide J, Masters SE, Carew TJ. Interaction between amount and pattern of training in the induction of intermediate- and long-term memory for sensitization in aplysia. Learn Mem. 2002;9:29–40. doi: 10.1101/lm.44802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TL, Freeman RM, Jr., O'Reilly AM, Neel BG, Sokol SY. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Gelb BD. Noonan syndrome and related disorders: genetics and pathogenesis. Annu Rev Genomics Hum Genet. 2005;6:45–68. doi: 10.1146/annurev.genom.6.080604.162305. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Martinelli S, Stella L, Bocchinfuso G, Flex E, Cordeddu V, Zampino G, Burgt I, Palleschi A, Petrucci TC, et al. Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. Am J Hum Genet. 2006;78:279–290. doi: 10.1086/499925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, van der Burgt I, Crosby AH, Ion A, Jeffery S, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Niemeyer CM, Fragale A, Song X, Buechner J, Jung A, Hahlen K, Hasle H, Licht JD, Gelb BD. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet. 2003;34:148–150. doi: 10.1038/ng1156. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Wickelgren WA. Multitrace strength theory. In: Norman DA, editor. Models of memory. Academic Press; New York: 1970. [Google Scholar]
- Yu D, Akalal DB, Davis RL. Drosophila alpha/beta mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52:845–855. doi: 10.1016/j.neuron.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.