Abstract
The typical symptoms of schizophrenia (SZ) are psychotic symptoms (hallucinations, delusions, disorders of thought or speech, grossly disorganized behavior) as well as cognitive impairments and negative symptoms. Not all patients respond to treatment and in those who do, only psychotic symptoms are usually improved. Imaging studies have shown that SZ subjects with high striatal dopamine release are far more responsive to antipsychotic drugs than those patients who have dopamine levels lower than or comparable to that of normal controls. In the present study we hypothesized that there was a link between psychosis and the number of dopaminergic synapses in the caudate nucleus in SZ. We examined dopaminergic synapses at the electron microscopic level in postmortem caudate from cases obtained from the Maryland Brain Collection. SZ were subdivided based on treatment response or resistance. The tissue was processed for the immunocytochemical localization of tyrosine hydroxylase (TH), the synthesizing enzyme for dopamine, and prepared for electron microscopy. The density of all TH labeled synapses was 43% greater in treatment responders than in controls and 62% greater in than in treatment resistant SZ. Axodendritic, but not axospinous, TH-labeled synapses showed this increase. TH-labeled axodendritic synapses in treatment responders were elevated in density (1.95±0.093/10μm3) compared to treatment resistant SZ (0.04±0.017/10μm3) and controls (0.11±0.044/10μm3). The results of the present study suggest that one anatomical underpinning of good treatment response may be a higher density of dopaminergic synapses and support a biological basis to treatment response and resistance. Moreover, these data have important implications for linking specific neuropathology with particular symptoms.
Keywords: basal ganglia, symptoms, psychosis, postmortem, treatment, ultrastructure
INTRODUCTION
Schizophrenia typically manifests itself in early adulthood with psychotic symptoms (hallucinations, delusions, disorders of thought or speech, grossly disorganized behaviour) as well as cognitive impairments and negative symptoms. Risk factors for schizophrenia suggest a developmental and genetic basis. Neuropathology and abnormalities in multiple neurotransmitter systems have been reported throughout the brain (Harrison, 1999; Powers, 1999). Not all patients respond to treatment and in those who do, only psychotic symptoms are usually improved (Conley and Kelly, 2001; Meltzer, 1997).
Disruption of dopamine transmission in the striatum is likely to be an important component of the pathophysiology of schizophrenia. The striatum is rich in dopamine receptors and all known effective antipsychotic medications are known to block dopamine D2 receptors (Creese, et al., 1976; Lahti et al., 2003; Seeman et al., 1975). Brain imaging studies show that the striatum of subjects with schizophrenia displays augmentation of presynaptic dopamine function, indicating an increase in dopamine synthesis capacity and/or an increase in presynaptic dopamine stores (Abi-Dargham et al., 1998, 2000; Breier et al., 1997; Dao-Castellana et al., 1997; Hietala et al., 1995, 1999; Laruelle et al., 1996, 1999). Specifically, there is an increase in the release of dopamine (Abi-Dargham et al., 1998; Laruelle et al., 1996, 1999) and in the density and occupancy of the D2 receptors (Abi-Dargham et al., 2000; Wong et al., 1986) in the striatum. In addition, caudate dopamine D2 receptor density is higher in the unaffected monozygotic co-twins of SZ compared to unaffected dizygotic co-twins and healthy control twins (Hirvonen et al., 2005). This suggests that dopamine transmission dysfunction confers a genetic risk for schizophrenia. Studies of patients with SZ have shown that those with high dopamine release are far more responsive to antipsychotic drugs than those patients who have dopamine levels lower than or comparable to that of healthy volunteers (Abi-Dargham et al., 2000).
Dopaminergic nigral inputs to the striatum have profound and complex effects on striatal neurons and function. Dopamine modulation depends on a number of factors such as the receptor subtype, the receptor location at pre or postsynaptic sites (Cepeda et al., 2001; Leveque et al., 2000; Onn et al., 2000; West and Grace, 2002), the concentration of ambient dopamine and the activity state of the spiny neuron (Cepeda and Levine, 1998). Considerable evidence indicates that endogenous dopamine modulates the membrane activity of the spiny neurons differentially via local dopamine D1 and D2 receptor activation (West and Grace, 2002). While activation of D1 receptor facilitates the depolarization and enhances the evoked activity of striatal neurons, activation of D2 receptor does the opposite. Dopaminergic axons form predominantly symmetric synapses (Bouyer et al., 1984; Descarries et al., 1996; Freund et al., 1984; Hattori et al., 1991; Pickel et al., 1981; Smith et al., 1994) yet have differential effects on striatal neurons (Gerfen et al., 1991). Dopaminergic and glutamatergic inputs converge on the same spines of medium spiny neurons (Bouyer et al., 1984; Smith et al., 1994) suggesting that a major function of dopaminergic inputs to the striatum is the regulation of glutamatergic pathways. The general pattern of dopamine terminals, as identified by tyrosine hydroxylase (TH) immunoreactivity, in human is similar to that of other species. For example, the synaptic arrangement of both a TH-labeled terminal forming a symmetric synapse and an unlabeled terminal forming an asymmetric synapse often occurs on the same spine (Kung et al., 1998).
Antipsychotic drugs act primarily to relieve positive symptoms (hallucinations, delusions) with little or no effect on negative (social withdrawal, avolition) and cognitive symptoms. The reported rate of treatment response can vary from 25 to 70% (Brenner, et al., 1990). Although numerous neuroimaging studies suggest a biological basis to treatment response/resistance, to our knowledge, no postmortem studies have addressed this issue. In the present study we sought to determine if there was a link between psychosis and the density of TH-labeled synapses in the caudate nucleus in SZ. Therefore we examined TH-labeled axon terminals and the synapses they formed at the electron microscopic level in postmortem caudate from SZs as a group and subdivided based on treatment response or resistance as defined by Kane (1988) and revised by Conley and Kelly (2000). Various aspects of this work have been presented in preliminary form (Roberts et al., 2001, 2005a, 2007).
Methods
Postmortem brain samples
Postmortem human brain tissue was obtained from the Maryland Brain Collection (MBC). The tissue was collected with family permission within 8 hours of death from subjects with schizophrenia (SZ) (n=13) and normal controls (NC) (n=6). The NCs had no history of central nervous system or neurological diseases and were matched to the SZ subjects for age, gender, postmortem interval and race when possible. Drug therapy, duration of illness and other medical details were obtained from hospital charts, autopsy reports and family interviews. The diagnosis of schizophrenia was made by two research psychiatrists according to the DSM-IV criteria using the Diagnostic Evaluation After Death (DEAD) (Salzman et. al., 1983) and the Scheduled Clinical Interview for the DSM III-R (SCID) (Spitzer et. al., 1992). The diagnoses of treatment response versus treatment resistance was made according to the following criteria (Conley, 2001; Conley and Kelly, 2000) which is a modification of the Kane criteria (1988): 1) Presence of a drug-refractory condition, which is defined as at least two prior drug treatment periods of 4 to 6-weeks duration at 400 to 600 mg/day of chlorpromazine (or equivalent) with no clinical improvement; 2) Persistence of illness, defined as at least a 5-year period with no period of good social or occupational functioning; and 3) Presence of persistent positive psychotic symptoms (e.g., hallucinations, delusions, suspiciousness, unusual thoughts) throughout the person's life. People are rated for presence of absence of these three items. If all are present, a diagnosis of treatment resistance is made. If item one is not present and one or no items are present from 2 and 3, a diagnosis of treatment responsive is made. These criteria identify subjects who did not respond to repeated trials of additional antipsychotic drugs but can respond to clozapine. Table I shows individual demographic and diagnostic information for each case. Table II shows the average demographic and diagnostic information for the cases comparing controls to SZs and controls to treatment responsive SZs and treatment resistant SZs. Treatment resistance/response was able to be diagnosed in 9 of the 13 subjects with SZ.
Table I.
Individual information is given for all of the cases. Abbreviations: SZ, subject with schizophrenia; PMI, postmortem interval; ≈, equivalent; na, not applicable; C, Caucasian; AA, African-American; M, male; F, female; un, unavailable information; MVA, motor vehicle accident; ASCVD, atherosclerotic cardio vascular disease; DVT, deep vein thrombosis; MI, myocardial infarction. Fluphenazine equivalents are shown; to convert to chlorpromazine equivalents, multiply value shown by 50 (Ezrin-Waters et al., 1981; Madras and Seeman, 1985).
| Case # | Group | Treatment Response | Age race gender | PMI hours | pH | Cause of death | Antipsychotic drug use | Fluphen-azine≈ | Toxicology report |
|---|---|---|---|---|---|---|---|---|---|
| 1 | control | na | 48AAM | 3 | un | MVA | na | na | None |
| 2 | control | na | 36CM | 4 | 6.70 | MVA | na | na | EtOH |
| 3 | control | na | 47CM | 6 | 7.23 | ASCVD | na | na | None |
| 4 | control | na | 32CF | 7 | 7.37 | cardiac arrhythmia | na | na | None |
| 5 | control | na | 43AAF | 8 | 7.18 | ASCVD | na | na | None |
| 6 | control | na | 21CM | 6 | un | MVA | na | na | EtOH |
| 7 | SZ | responsive | 37CM | 3 | 6.79 | polydipsia | Prolixin | un | Sertaline, Benztropine |
| 8 | SZ | responsive | 45CM | 6 | 7.17 | drug intoxication | Risperidone Olanzapine Fluoxetine | un | Fluoxetine, Nortriptaline |
| 9 | SZ | responsive | 45AAM | 8 | 7.17 | hanging | Olanzapine | un | Olanzapine |
| 10 | SZ | responsive | 53CM | 5 | 7.21 | ruptured MI | Perphenazine | 4mg | Carbamazapine |
| 11 | SZ | responsive | 48AAF | 6 | 7.26 | hanging | Haloperidol | 15mg | un |
| 12 | SZ | responsive | 60AAF | 6 | un | DVT | Loxapine | un | Loxapine |
| 13 | SZ | resistant | 43CF | 8 | 6.91 | Seizure, drug intoxication | Clozapine | un | Clozapine Carbamazapine Diphenylhydramine |
| 14 | SZ | resistant | 32CM | 7 | 7.28 | multiple injuries, jump | Clozapine | un | Clozapine |
| 15 | SZ | resistant | 52CF | 5 | 7.05 | aspirated food | Risperidone | 8mg | Diphenylhydramine |
| 16 | SZ | Off | 58AAF | 3 | 6.80 | ASCVD | Off | 0mg | na |
| 17 | SZ | un | 43AAM | 4 | na | seizure | Thioridizine | un | Thioridizine |
| 18 | SZ | un | 60AAM | 5 | na | amyloidosis | Olanzapine | 5mg | Olanzapine |
| 19 | SZ | un | 67AAM | 7 | 7.17 | ASCVD & drowning | Haloperidol or off | un | un |
Table II.
Summary table showing the mean ± SD for the subjects for various demographic and illness related details. Controls are compared to SZs on the top row for each measure. Controls are compared to treatment responsive SZS and treatment resistant SZS on the bottom row for each appropriate measure. Degrees of freedom (df, total), F and p values are shown for ANOVA comparisons between controls, treatment responsive SZS and treatment resistant SZS. Degrees of freedom (df), t values and p values are shown for t-tests when 2 groups were compared (controls versus SZ, or age of onset, illness duration and antipsychotic drugs for treatment responsive SZS versus treatment resistant SZS). The age of onset and duration of illness was only known for 4 of the treatment responsive SZS and 3 of the treatment resistant SZS. Abbreviations: t (typical); a (atypical); un, unknown; P, paranoid, U, undifferentiated.
| controls | SZ | Treatment responsive | Treatment resistant | df | t (F) | P value | |
|---|---|---|---|---|---|---|---|
| Number of cases | 6 | 13 | 6 | 3 | |||
| Age, years | 37.8±10.3 | 47.8±10.5 | 48.0±7.8 | 42.3±10. | 17 2 | 1.939 (1.904) | <0.069 <0.179 |
| Race (#AA, #C) | 2AA, 4C | 7AA, 6C | 3AA, 3C | 3C | 17 2 | -0.802 (0.719) | <0.434 <0.502 |
| Gender (#M, #F) | 4M, 2F | 8M, 5F | 2M, 4F | 2M, 1F | 17 2 | -0.204 (0.269) | <0.841 <0.767 |
| Postmortem interval, hours | 5.33±1.5 | 5.38±1.5 | 5.17±1.2 | 6.67±1.5 | 17 2 | 0.069 (1.458) | <0.946 <0.260 |
| pH | 7.12±0.29 | 7.05±0.18 | 7.05±0.19 | 7.08±0.19 | 11 2 | -0.563 (0.237) | <0.585 <0.793 |
| Age of onset, years | NA | 23.9±6.64 | 25.5±6.0 | 18.00±7.07 | 4 | -1.374 | <0.242 |
| Illness duration, years | NA | 25.0±9.1 | 21.8±11.5 | 29.5±0.70 | 4 | 0.898 | <0.420 |
| DSM-IV subtypes | NA | 4P,6U,3un | 2P, 2U, 2un | 3U | 5 | -0.378 | <0.721 |
| Type of APD a, atypical; t, typical | NA | 5t, 6a, 1un, 1off | 4t, 2a, | 3a | 5 | 3.162 | <0.025 |
Tissue processing
Coronal blocks from the head of the caudate were dissected from fresh human brain and immersed in a cold solution of 4% paraformaldehyde and 1% glutaraldehyde in 0.1M phosphate buffer (PB), pH=7.4 for at least one week at 4°C. The tissue was cut with a Vibratome (at a thickness of 40 μm) and 6-8 free floating sections (240μm apart) were processed from each case for the immunohistochemical localization of tyrosine hydroxylase (TH) as described previously (Kung et al., 1998). Briefly, the sections were incubated in normal horse serum, followed by mouse anti-TH (Boehringer Mannheim, Mannheim, Germany) at a dilution of 1:1,000 for 60 hours. Then the tissue was treated with reagents from the avidin-biotin peroxidase kit (ABC standard kit) using recommended dilutions and times. Then sections were incubated in diaminobenzidine (6 mg/10 ml PB) containing 0.03% hydrogen peroxide for 5 to 10 minutes to visualize the reaction product. Controls consisted of eliminating the primary antibody but otherwise processing the tissue in an identical fashion; control sections did not exhibit any specific staining.
Tissue samples were embedded using standard techniques. Briefly, the sections were rinsed in PB (3×10 minutes), immersed in 1% osmium tetroxide for 1 hour, dehydrated in ethyl alcohol, stained with uranyl acetate for 2 hours, further dehydrated in ethyl alcohol, embedded in resins on glass slides and heated at 60 °C for 72 hours. For each case at least 3 samples from different sections were randomly selected from dorsal or ventral regions of the caudate for electron microscopic analysis. The blocks were serially thin-sectioned on an ultramicrotome at a thickness of 90nm. The average length of each ribbon was six serial sections.
The pH of a sample of cerebellum was determined from several of the cases according to published techniques (Harrison et al., 1995; Johnson et al., 1996; Johnston et al., 1997). A sample piece of tissue (a 1.5 - 2.0 cm block) was dissected from the frozen brain with a Stryker autopsy bone saw. The tissue was homogenized for approximately one minute using an Omni PCR Tissue Homogenizing Kit; then the pH was measured. The pH value is often used as a marker of tissue integrity, especially in studies utilizing RNA (Harrison et al., 1995; Johnston et al., 1997; Preece and Cairns 2003; Tomita et al., 2004). Although a short postmortem interval is the primary measure of tissue integrity for electron microscopy, we have included pH information as well.
Data Collection and Analysis
Quantitative analysis was performed in the caudate in all six control and thirteen schizophrenic cases. In each sample, 6 photomicrographs (at a magnification of 10,000x) were taken that formed a montage. The montages were printed (final viewing magnification was approximately 25,000x), and a counting box (approximate are of 100μm2) was drawn in each. Sigma scan software was used to calculate the volume of each montage. The disector sterologic technique (Geinisman et al., 1996; Sterio, 1984) was utilized and described in more detail elsewhere (Perez-Costas et al., 2007; Roberts and Knickman 2002). All synapses appearing in the first montage in the series and all synapses that crossed the exclusion lines (top or right borders of the counting frame) in any of the series were excluded. Any synapses that appeared for the first time in subsequent montages, that met criteria, were numbered and followed in this three-dimensional reconstruction method. All synapses as well as TH-labeled synapses were quantified. TH-labeled synapses were then subcategorized as symmetric axospinous or symmetric axodendritic. Synapses were identified by the presence of parallel pre-and post-synaptic membranes with a discernable synaptic cleft and a postsynaptic density. Reaction product obscured the presence of synaptic vesicles, so this criterion was not used for the TH-labeled synapses. Over 100 synapses were counted for each case and data are reported as the mean ± standard deviation per 10μm3.
Statistics
Group means and standard deviations for demographic data were obtained for each group or subgroup (Table II). Unpaired t-tests were used to determine whether the density of TH-labeled synapses was different between the controls and the entire SZ group. To determine whether the density of TH-labeled synapses was different between the controls, treatment responsive SZs and treatment resistant SZs, an ANOVA followed by a posthoc t-test for multiple comparisons (least significant difference, LSD) was used. ANOVAs followed by the posthoc LSD t-test were used to determine if there were any group differences in age, PMI, race or gender between the three groups. Unpaired t-tests were used to compare parameters occurring between treatment responsive SZs and treatment resistant SZs- (but not applicable to controls) such as age of onset, duration of illness, or antipsychotic drug use. Since there was a significant difference in antipsychotic drug use between the treatment responsive SZs and the treatment resistant SZs, we performed a correlation analysis between fluphenazine equivalents and TH-labeled synapses. A Pearson bivariate correlation was used with a 2-tailed significance level.
Results
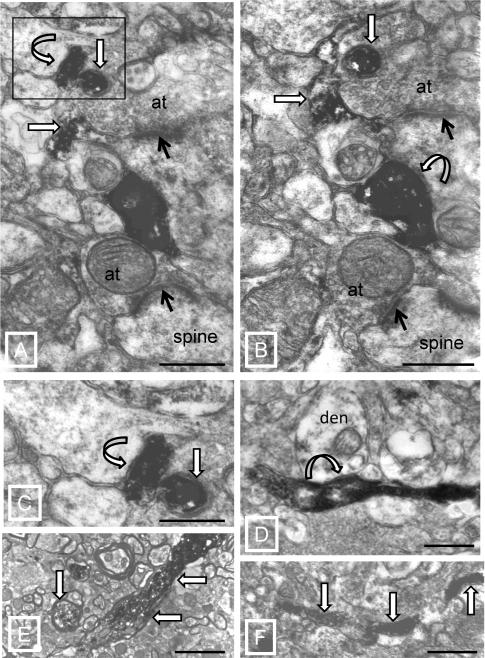
The features of TH-labeled structures were qualitatively similar between NCs and SZ subjects (Figure 1). TH-labeled axons were often in close proximity to large unlabeled terminals that formed asymmetric synapses (Figure 1A,B). Synapses were formed by TH-labeled axon terminals (Figure 1A-C) and boutons en passant (Figure 1D). TH-labeled axon terminals formed short symmetric synapses with spines and dendritic shafts (Figure 1A-D). While the majority of TH-labeled axons were small and unmyelinated (Figure 1D,F), some were myelinated (Figure 1E).
Figure 1.
Electron micrographs of TH immunocytochemistry. A,B) Serial sections showing several synaptic arrangements. TH-labeled axons (straight white arrows outlined in black) are adjacent to unlabeled axon terminals (at) that are forming asymmetric synapses (black arrows) with spines. TH-labeled terminals make symmetric synapses (curved white arrows outlined in black) in both micrographs. C) Boxed area in panel A is enlarged to show the symmetric synapse (curved white arrow outlined in black). D) TH-labeled axon makes a symmetric synapse (arrow) en passant with a dendrite (den). E) Examples of TH-labeled myelinated axons (arrows). F) Example of a small caliber unmyelinated axon (arrows). Scale bars = 0.5μm (A-E) and 1.0 μm (F).
In both control and SZ subjects, TH-labeled synapses accounted for 12% of total synapses (Table III). In both groups approximately 60% of the TH-labeled synapses were with spines, while 40% were on dendritic shafts. While the proportion of TH-labeled synapses to total was equivalent between groups, the SZ group had a 25% greater (albeit insignificant) density of TH-labeled synapses in comparison to that of the controls. The larger density of synapses was similar between synapses on spines and dendritic shafts.
Table III.
Summary table showing the means ± SD for all TH-labeled synapses, TH-labeled symmetric axospinous (SS) subtypes and TH-labeled symmetric axodendritic (SD) subtypes for the control group (NC) compared to the entire SZ cohort, without regard to treatment response/resistance. The % of total synapses refers to the % of each type of TH-labeled synapse to the total number of all synapses combined (the total of all synapses, labeled and unlabeled). Although the raw number of TH-labeled synapses in the SZ group is larger than that of the NC group, the overall percentages between NCs and SZs are equivalent.
| Groups | TH labeled | % of total | TH labeled SS | % of total | TH+SD | % of total |
|---|---|---|---|---|---|---|
| NC n=6 | 0.303±0.087 | 12.1% | 0.182±0.061 | 7.1% | 0.108±0.445 | 4.3% |
| SZ n=13 | 0.378±0.139 | 12.1% | 0.223±0.129 | 7.0% | 0.133±0.970 | 4.5% |
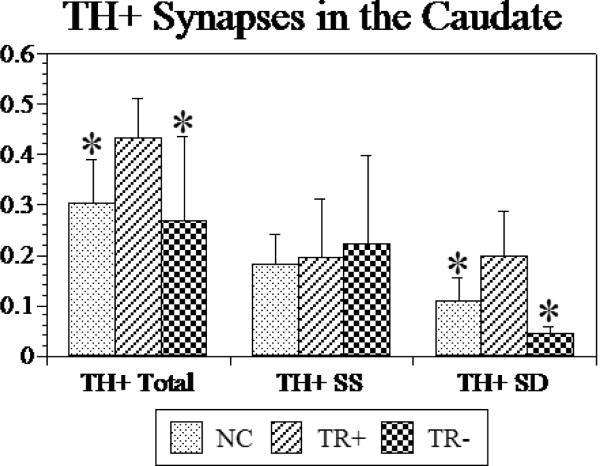
The total density of TH-labeled synapses was larger in treatment responsive SZs than either the controls or the treatment resistant SZs (Figure 2). This represented a 43% larger density in the treatment responsive SZs versus the controls, and a 51% larger density in the treatment responsive SZs versus the treatment resistant SZs. TH-labeled axodendritic synapses were higher in density in treatment responsive SZs compared to treatment resistant SZ and the controls (p<0.055). This represented an 80% higher density in the treatment responsive SZ versus controls and a 169% higher density in the treatment responsive SZ versus the treatment resistant SZs. The number of TH-labeled axospinous synapses was similar among all groups. Thus, the percentage of TH-labeled axospinous synapses in the treatment responsive SZs versus controls and the treatment responsive SZs versus treatment resistant SZs was 106% and 93%, respectively.
Figure 2.
Graph of the density (per 10μm3) of TH-labeled (TH+) terminals forming synapses are shown for controls (NC), treatment responsive (TR) and treatment resistant (TNR) subjects with schizophrenia. Total refers to all TH-labeled synapses regardless of subtype. SS, symmetric axospinous; SD, symmetric axodendritic. Error bars = standard deviation. ANOVA results: TH-labeled Total, p<0.057; TH-labeled SS, p<0.888; TH-labeled SD, p<0.017. Posthoc LSD t-tests show significant (*) results comparing TR to NCs or to TNR. There were no differences between the NCs and the TR-. *, p<0.05.
While both our subgroups were composed of cases on varied antipsychotic drugs, the proportion of cases on typical antipsychotics was significantly greater in the treatment responsive SZS than in the treatment resistant SZS group. Therefore we tested to see if there was a correlation between fluphenazine equivalents and any of the synaptic measures in which we found significant differences. We found no correlations.
Discussion
The results of the present study show that treatment responsive SZs have more dopaminergic synapses, as identified by TH-labeled terminals, than do treatment resistant SZs or controls. These changes were specific for the axodendritic subtype of TH-labeled synapses. These data have important implications for linking specific neuropathology with particular symptoms. The lack of replication data that is common in schizophrenia research may be due, in part, to the examination of cohorts of subjects with different antipsychotic drug histories (Roberts et al., 2005b), symptoms (Roberts et al., 2008) or, as suggested by the results of the present paper, treatment response. Our postmortem results are consistent with in vivo studies suggesting a biological basis to treatment response and resistance. The larger number of dopaminergic synapses in treatment responsive SZS may account for the higher levels of striatal dopamine in treatment responsive SZ patients shown in vivo imaging studies.
Potential confounds
The use of antipsychotic drugs to treat SZ imposes certain limitations on postmortem studies in SZ. For instance, any results found in a cohort of subjects may be caused or masked by the medications. Some medications cause a small (5-8%) enlargement of the striatum (Chakos et al., 1994; Gunduz et al., 2002; Gur et al., 1998; Shihabuddin et al., 1998). Our results, that there is a higher density of TH-labeled synapses in treatment responsive SZs as compared to controls, would probably be amplified rather than negated as discussed in more detail in our previous publications (Roberts et al., 2005b,c). Moreover, imaging studies show that the size of the caudate nucleus is the same in SZ subjects with good versus poor response (Buchsbaum et al. 2003), greatly reducing the size issue as a potential confound and substantiating our results. Another potential concern was that the proportion of cases on typical antipsychotics was significantly greater in the treatment responsive SZs than in the treatment resistant SZ group. We do not think this impacts on our results for several reasons. There were no correlations between fluphenazine equivalents and any of the synaptic measures in which we found significant differences. Moreover, the striatum of rats treated chronically with the typical antipsychotic drug, haloperidol, shows no increases in the number of TH-labeled axodendritic synapses (Roberts et al., 2002). In addition, chronic treatment with the atypical antipsychotic drug, olanzapine, produced no changes in density of any type of striatal synapse in rat (Roberts, 2001). Importantly, it has demonstrated that with the exception of clozapine, first- and second generation antipsychotic drugs alleviate positive symptoms to the same extent (Kane et al., 2008; Lieberman et al., 2005; McEvoy, 2006; McEvoy et al., 2006). Therefore, even though the SZ subgroups were composed of different numbers of subjects on typical versus atypical antipsychotic drugs the possibility that the difference in results between the groups is related to medication seems unlikely.
Another issue in postmortem SZ research is the reliability of the diagnosis. Fortunately it has been shown that the postmortem diagnosis of SZ using the diagnostic evaluation after death (DEAD) and the scheduled clinical interview for the DSM-IV (SCID), the instruments used in the present study, are highly accurate (Deep-Soboslay et al., 2005). The diagnosis of treatment response/resistance is novel in postmortem collections and does not correspond entirely to the categorization of good versus poor responders used in many of the imaging studies. It is usually well accepted that treatment response to APD is best defined along a gradient. One end is characterized by a very poor response also referred as treatment resistant. Postmortem brains were characterized using treatment resistant criteria to ensure correct classification in the absence of objective clinical ratings. Treatment resistant criteria included: lack of response to two drug treatment periods of adequate length, persistence of poor social and occupational functioning, and persistence of positive symptoms (Conley and Kelly, 2000, 2001, 2003; Kane et al., 1998). The diagnosis of treatment response/resistance in Maryland Brain Collection tissue was no more challenging or problematic than accurately subgrouping the SZ cohort in other ways such as the presence of the deficit syndrome (Kirkpatrick et al., 1999, 2003), different DSM-IV diagnostic subgroups (Roberts et al., 2008), or antipsychotic drug use (Kung et al., 1998; Roberts et al., 2005b). The results of the present study and of preliminary work (Roberts et al., 2005a; Somerville et al., 2003) show that there are neuroanatomical changes that differentiate treatment responsive SZs from treatment resistant SZs. However, differential response to treatment is not limited to schizophrenia. We would predict that if increased synaptic density of TH-labeled synapses is related to treatment response, we may find the same result in other psychotic disorders in which the subjects responded favorably to dopamine receptor blockade.
Structural, neurochemical and metabolic correlates of treatment response/resistance
Numerous neuroimaging studies have shown a relationship between pathophysiology and the degree of treatment response in SZ (Altamura et al., 2005; Arango et al., 2003; Beerpoot et al., 1996; Sheitman and Lieberman, 1998). MRI studies have shown that treatment resistant SZ subjects have greater cortical atrophy and larger cerebral ventricles than do treatment responsive SZs (Bilder et al. 1994; Staal et al., 2001; Stern et al., 1993). For example, decreases in volume in the posterior cingulate and retrosplenial cortices have been detected in poor compared to good-outcome patients (Mitelman et al., 2005). Lower pretreatment metabolic rate in the striatum is predictive of good treatment outcome, and good responders showed greater striatal response compared to poor responders (Buchsbaum et al., 1992).
Several imaging studies have demonstrated enhanced dopamine release in response to an amphetamine challenge in drug free SZ subjects (Abi-Dargham et al., 1998; Breier et al., 1997; Laruelle et al., 1996, 1999; Lindstrom et al.,1999) or neuroleptic naïve SZ subjects (Laruelle et al., 1999) compared to controls. Importantly, drug free patients who eventually responded to antipsychotic drugs had elevated dopamine release compared to those subjects who did respond to treatment (Abi-Dargham et al., 2000). The higher density of dopaminergic synapses in treatment responsive SZs may explain the results of in vivo studies that have measured dopamine content in live patients. However, more dopaminergic synapses may not relate to higher tonic dopamine levels. There could be several other explanations for these data, including but not limited to: differential affinity for dopamine receptors, different postsynaptic mechanisms, and/or different amounts of dopamine. Differential blockade of D2 receptors does not appear to be responsible since treatment resistant SZs have 95% D2 receptor occupancy (Coppens et al., 1991). The results of the present study suggest that one anatomical underpinning of good treatment response may a higher density of terminals synthesizing dopamine. There may also be more dopamine per terminal, but this measure was not performed in the present data set. An important issue still unresolved is whether treatment responsive SZ have more TH-labeled terminals before treatment of if they respond to treatment by making more terminals.
Significance from the synaptic perspective
As substantiated by ultrastructural studies in animals, the main striatal targets of dopaminergic inputs are the medium spiny projection neurons (Freund et al., 1984; Kubota et al., 1986a,b; Pickel et al., 1992). Inputs from the substantia nigra and glutamatergic afferents converge on the same spines of medium spiny neurons (Bouyer et al., 1984; Smith et al., 1994). This suggests that a major function of dopaminergic inputs to the striatum is the regulation of the glutamatergic pathways. It has long been known that cortical glutamatergic afferents and dopaminergic inputs converge on the same spines. Most thalamic inputs, except those from centromedian and parafascicular complex, also end on dendritic spines and therefore could also be modulated by dopaminergic afferents (Dube et al., 1988; Lapper and Bolam,1992; Meredith and Wouterlood, 1990; Raju et al., 2006; Sadikot et al., 1992b Sidibe and Smith, 1999; Smith et al., 2004). It is surprising therefore that the higher density of TH-labeled synapses in the treatment responsive SZs versus treatment resistant SZs was onto dendrites rather than spines. The targets of the axodendritic TH-labeled synapses are likely to be formed with interneurons as dopamine afferents form axodendricic synapses onto the various aspiny interneuron populations, including cholinergic (Chang, 1988; Dimova et al., 1993), GABAergic (Kubota et al., 1987), and those neurons containing neuropeptide Y/NADPH-diaphorase (Aoki and Pickel, 1988; Fujiyama and Masuko, 1996; Kubota et al., 1987; Vuillet et al., 1989). The targets of the TH-labeled synapses could also be the shafts of medium spiny neurons.
Taken together, our data suggest that difference in dopaminergic transmission in treatment responsive SZs versus treatment resistant SZs and controls may be present at sites not as tightly linked to glutamate inputs as the synaptic triad (glutamatergic and dopaminergic synapses on the same spine) described in most species studied, including human (Kung et al., 1998). Interestingly, dopaminergic transmission appears to be more complicated than simple synaptic interactions (Descarries et al., 1996). The relationship among striatal dopaminergic terminals, transporters and receptors suggests additional anatomical substrates for a control of glutamate release by dopamine, or the reciprocal. For instance, TH-labeled terminals are often adjacent to unlabeled axon terminals that form asymmetric axospinous synapses in human (present paper; Kung et al., 1998) and other species (Bouyer et al., 1984; Pickel et al., 1981; Roberts and Anderson, 1979). Most varicosities, at least in rat (Descarries et al., 1996) do not form synapses. The dopamine transporter is found at both synaptic and non-synaptic sites, consistent with the possibility of dopamine release from sites other than typical synapses (Descarries et al., 1996; Nirenberg et al., 1996). Moreover, the D2 receptor has been found in a small proportion of both TH-labeled and unlabeled striatal terminals, suggesting interactions between dopaminergic terminals and between dopaminergic and nondopaminergic terminals (Sesack et al., 1994).
In summary, the present results show an anatomical variation in dopaminergic synapses in the postmortem caudate nucleus that distinguishes treatment responsive SZs from treatment resistant SZs subjects. These results are consistent with that of imaging studies showing that SZ patients with high levels of striatal dopamine are treatment responsive. It is of course possible that there may be other factors that contribute to higher dopamine levels in treatment responsive SZs subjects in addition to a higher density of TH-labeled terminals and further studies are warranted.
ACKNOWLEDGMENTS
The authors wish to acknowledge the Maryland Brain Collection and Drs. David Fowler and Jack Titus (Office of the Chief Medical Examiner in Maryland) for their support and cooperation. We thank Tida Kumbalasiri and Michelle Force for their help with the EM analysis. We thank Rosie Ricks for help in preparation of the manuscript. This research was supported in part by MH60744 and MH66123 to RCR.
REFERENCES
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155(6):761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97(14):8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura AC, Bassetti R, Cattaneo E, Vismara S. Some biological correlates of drug resistance in schizophrenia: a multidimensional approach. World J Biol Psychiatry. 2005;6(Suppl 2):23–30. doi: 10.1080/15622970510030027. [DOI] [PubMed] [Google Scholar]
- Aoki C, Pickel VM. Neuropeptide Y-containing neurons in the rat striatum: ultrastructure and cellular relations with tyrosine hydroxylase- containing terminals and with astrocytes. Brain Res. 1988;459(2):205–225. doi: 10.1016/0006-8993(88)90637-3. [DOI] [PubMed] [Google Scholar]
- Arango C, Breier A, McMahon R, Carpenter WT, Jr., Buchanan RW. The relationship of clozapine and haloperidol treatment response to prefrontal, hippocampal, and caudate brain volumes. Am J Psychiatry. 2003;160(8):1421–1427. doi: 10.1176/appi.ajp.160.8.1421. [DOI] [PubMed] [Google Scholar]
- Beerpoot LJ, Lipska BK, Weinberger DR. Neurobiology of treatment-resistant schizophrenia: new insights and new models. Eur Neuropsychopharmacol. 1996;6(Suppl 2):S27–34. doi: 10.1016/0924-977x(96)00008-9. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Wu H, Chakos MH, Bogerts B, Pollack S, Aronowitz J, Ashtari M, Degreef G, Kane JM, Lieberman JA. Cerebral morphometry and clozapine treatment in schizophrenia. J Clin Psychiatry. 1994;55(Suppl B):53–56. [PubMed] [Google Scholar]
- Borrow MN, Harris TD, Shaughnessy KJ, Litt GJ. Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays. J Immunol Methods. 1989;124:279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- Bouyer JJ, Park DH, Joh TH, Pickel VM. Chemical and structural analysis of the relation between cortical inputs and tyrosine hydroxylase-containing terminals in rat neostriatum. Brain Res. 1984;302(2):267–275. doi: 10.1016/0006-8993(84)90239-7. [DOI] [PubMed] [Google Scholar]
- Brenner HD, Dencker SJ, Goldstein MJ, Hubbard JW, Keegan DL, Kruger G, Kulhanek F, Liberman RP, Malm U, Midha KK. Defining treatment refractoriness in schizophrenia. Schizophr Bull. 1990;16(4):551–561. doi: 10.1093/schbul/16.4.551. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997;94(6):2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Potkin SG, Siegel BV, Jr., Lohr J, Katz M, Gottschalk LA, Gulasekaram B, Marshall JF, Lottenberg S, Teng CY. Striatal metabolic rate and clinical response to neuroleptics in schizophrenia. Arch Gen Psychiatry. 1992;49(12):966–974. doi: 10.1001/archpsyc.1992.01820120054008. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Shihabuddin L, Brickman AM, Miozzo R, Prikryl R, Shaw R, Davis K. Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophr Res. 2003;64(1):53–62. doi: 10.1016/s0920-9964(02)00526-1. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez J, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA, Levine MS. Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol. 2001;85(2):659–670. doi: 10.1152/jn.2001.85.2.659. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20(1):1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151(10):1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Chang HT. Dopamine-acetylcholine interaction in the rat striatum: a dual-labeling immunocytochemical study. Brain Res Bull. 1988;21(2):295–304. doi: 10.1016/0361-9230(88)90244-4. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Berger TW. Interactions between dopamine and amino acid-induced excitation and inhibition in the striatum. Brain Res. 1986;375(1):198–203. doi: 10.1016/0006-8993(86)90976-5. [DOI] [PubMed] [Google Scholar]
- Conley R, Kelly DL. Pharmacologic treatment of schizophrenia. 1st edition Professional Communications; 2000. [Google Scholar]
- Conley R, Kelly DL. Pharmacologic treatment of schizophrenia. Professional Communications; Caddo, OK: 2003. [Google Scholar]
- Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50(11):898–911. doi: 10.1016/s0006-3223(01)01271-9. [DOI] [PubMed] [Google Scholar]
- Coppens HJ, Slooff CJ, Paans AM, Wiegman T, Vaalburg W, Korf J. High central D2-dopamine receptor occupancy as assessed with positron emission tomography in medicated but therapy-resistant schizophrenic patients. Biol Psychiatry. 1991;29(7):629–634. doi: 10.1016/0006-3223(91)90132-6. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192(4238):481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Dao-Castellana MH, Paillere-Martinot ML, Hantraye P, Attar-Levy D, Remy P, Crouzel C, Artiges E, Feline A, Syrota A, Martinot JL. Presynaptic dopaminergic function in the striatum of schizophrenic patients. Schizophr Res. 1997;23(2):167–174. doi: 10.1016/S0920-9964(96)00102-8. [DOI] [PubMed] [Google Scholar]
- Deep-Soboslay A, Akil M, Martin CE, Bigelow LB, Herman MM, Hyde TM, Kleinman JE. Reliability of psychiatric diagnosis in postmortem research. Biol Psychiatry. 2005;57(1):96–101. doi: 10.1016/j.biopsych.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Bosler O, Doucet G. Dual character, asynaptic and synaptic, of the dopamine innervation in adult rat neostriatum: a quantitative autoradiographic and immunocytochemical analysis. J Comp Neurol. 1996;375(2):167–186. doi: 10.1002/(SICI)1096-9861(19961111)375:2<167::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Dimova R, Vuillet J, Nieoullon A, Kerkerian-Le Goff L. Ultrastructural features of the choline acetyltransferase-containing neurons and relationships with nigral dopaminergic and cortical afferent pathways in the rat striatum. Neuroscience. 1993;53(4):1059–1071. doi: 10.1016/0306-4522(93)90489-3. [DOI] [PubMed] [Google Scholar]
- Dube L, Smith AD, Bolam JP. Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neostriatum. J Comp Neurol. 1988;267(4):455–471. doi: 10.1002/cne.902670402. [DOI] [PubMed] [Google Scholar]
- Ezrin-Waters C, Seeman MV, Seeman P. Tardive dyskinesia in schizophrenic outpatients: prevalence and significant variables. J Clin Psych. 1981;42(1):16–22. [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13(4):1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Masuko S. Association of dopaminergic terminals and neurons releasing nitric oxide in the rat striatum: an electron microscopic study using NADPH-diaphorase histochemistry and tyrosine hydroxylase immunohistochemistry. Brain Res Bull. 1996;40(2):121–127. doi: 10.1016/0361-9230(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Gundersen HJ, van der Zee E, West MJ. Unbiased stereological estimation of the total number of synapses in a brain region. J Neurocytol. 1996;25(12):805–819. doi: 10.1007/BF02284843. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, McGinty JF, Young WS., 3rd Dopamine differentially regulates dynorphin, substance P, and enkephalin expression in striatal neurons: in situ hybridization histochemical analysis. J Neurosci. 1991;11(4):1016–1031. doi: 10.1523/JNEUROSCI.11-04-01016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz H, Wu H, Ashtari M, Bogerts B, Crandall D, Robinson DG, Alvir J, Lieberman J, Kane J, Bilder R. Basal ganglia volumes in first-episode schizophrenia and healthy comparison subjects. Biol Psychiatry. 2002;51(10):801–808. doi: 10.1016/s0006-3223(01)01345-2. [DOI] [PubMed] [Google Scholar]
- Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155(12):1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Heath PR, Eastwood SL, Burnet PW, McDonald B, Pearson RC. The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett. 1995;200(3):151–154. doi: 10.1016/0304-3940(95)12102-a. [DOI] [PubMed] [Google Scholar]
- Hattori T, Takada M, Moriizumi T, Van der Kooy D. Single dopaminergic nigrostriatal neurons form two chemically distinct synaptic types: possible transmitter segregation within neurons. J Comp Neurol. 1991;309(3):391–401. doi: 10.1002/cne.903090308. [DOI] [PubMed] [Google Scholar]
- Hietala J, Syvalahti E, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, Solin O, Kuoppamaki M, Kirvela O, Ruotsalainen U, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346(8983):1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- Hietala J, Syvalahti E, Vilkman H, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, Solin O, Kuoppamaki M, Eronen E, Ruotsalainen U, Salokangas RK. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res. 1999;35(1):41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, van Erp TG, Huttunen J, Aalto S, Någren K, Huttunen M, Lönnqvist J, Kaprio J, Hietala J, Cannon TD. Increased caudate dopamine D2 receptor availability as a genetic marker for schizophrenia. Arch Gen Psych. 2005;62(4):371–8. doi: 10.1001/archpsyc.62.4.371. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Morgan DG, Finch CE. Extensive postmortem stability of RNA from rat and human brain. J Neurosci Res. 1986;16(1):267–280. doi: 10.1002/jnr.490160123. [DOI] [PubMed] [Google Scholar]
- Johnston NL, Cervenak J, Shore AD, Torrey EF, Yolken RH. Multivariate analysis of RNA levels from postmortem human brains as measured by three different methods of RT-PCR. Stanley Neuropathology Consortium. J Neurosci Methods. 1997;77(1):83–92. doi: 10.1016/s0165-0270(97)00115-5. [DOI] [PubMed] [Google Scholar]
- Kane JM, Honigfeld G, Singer J, Meltzer H. Clozapine in treatment-resistant schizophrenics. Psychopharmacol Bull. 1988;24(1):62–67. [PubMed] [Google Scholar]
- Kirkpatrick B, Conley RC, Kakoyannis A, Reep RL, Roberts RC. Interstitial cells of the white matter in the inferior parietal cortex in schizophrenia: An unbiased cell-counting study. Synapse. 1999;34(2):95–102. doi: 10.1002/(SICI)1098-2396(199911)34:2<95::AID-SYN2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias NC, Conley RR, Roberts RC. Interstitial cells of the white matter in the dorsolateral prefrontal cortex in deficit and nondeficit schizophrenia. J Nerv Ment Dis. 2003;191(9):563–567. doi: 10.1097/01.nmd.0000087181.61164.e1. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Inagaki S, Kito S. Innervation of substance P neurons by catecholaminergic terminals in the neostriatum. Brain Res. 1986a;375(1):163–167. doi: 10.1016/0006-8993(86)90969-8. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Inagaki S, Kito S, Shimada S, Okayama T, Hatanaka H, Pelletier G, Takagi H, Tohyama M. Neuropeptide Y-immunoreactive neurons receive synaptic inputs from dopaminergic axon terminals in the rat neostriatum. Brain Res. 1988;458(2):389–393. doi: 10.1016/0006-8993(88)90484-2. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Inagaki S, Kito S, Takagi H, Smith AD. Ultrastructural evidence of dopaminergic input to enkephalinergic neurons in rat neostriatum. Brain Res. 1986b;367(1-2):374–378. doi: 10.1016/0006-8993(86)91622-7. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Inagaki S, Kito S, Wu JY. Dopaminergic axons directly make synapses with GABAergic neurons in the rat neostriatum. Brain Res. 1987;406(1-2):147–156. doi: 10.1016/0006-8993(87)90779-7. [DOI] [PubMed] [Google Scholar]
- Kung L, Force M, Chute DJ, Roberts RC. Immunocytochemical localization of tyrosine hydroxylase in the human striatum: a postmortem ultrastructural study. J Comp Neurol. 1998;390(1):52–62. doi: 10.1002/(sici)1096-9861(19980105)390:1<52::aid-cne5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53(7):601–608. doi: 10.1016/s0006-3223(02)01602-5. [DOI] [PubMed] [Google Scholar]
- Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51(3):533–545. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46(1):56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93(17):9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveque JC, Macias W, Rajadhyaksha A, Carlson RR, Barczak A, Kang S, Li XM, Coyle JT, Huganir RL, Heckers S, Konradi C. Intracellular modulation of NMDA receptor function by antipsychotic drugs. J Neurosci. 2000;20(11):4011–4020. doi: 10.1523/JNEUROSCI.20-11-04011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lindstrom LH, Gefvert O, Hagberg G, Lundberg T, Bergstrom M, Hartvig P, Langstrom B. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(ß-11C) DOPA and PET. Biol Psychiatry. 1999;46(5):681–688. doi: 10.1016/s0006-3223(99)00109-2. [DOI] [PubMed] [Google Scholar]
- Madras BK, Seeman P. Drug potencies on partially purified brain D2 dopamine receptors. J Neurochem. 1985;44(3):856–61. doi: 10.1111/j.1471-4159.1985.tb12894.x. [DOI] [PubMed] [Google Scholar]
- McEvoy JP. An overview of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study. CNS Spectr. 2006;11(7 Suppl 7):4–8. doi: 10.1017/s1092852900026626. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, Swartz MS, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600–610. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Treatment-resistant schizophrenia--the role of clozapine. Curr Med Res Opin. 1997;14(1):1–20. doi: 10.1185/03007999709113338. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Wouterlood FG. Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: a light and electron microscopic study. J Comp Neurol. 1990;296(2):204–221. doi: 10.1002/cne.902960203. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophr Res. 2005;72(2-3):91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16(2):436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn SP, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci. 2000;23(10 Suppl):S48–56. doi: 10.1016/s1471-1931(00)00020-3. [DOI] [PubMed] [Google Scholar]
- Perez-Costas E, Melendez-Ferro M, Roberts RC. Microscopy techniques and the study of synapses. In: Mendez-Vilas A, Diaz J, editors. Modern Research and Educational Topics in Microscopy Badajoz. Spain Formatex; 2007. pp. 164–170. [Google Scholar]
- Pickel VM, Beckley SC, Joh TH, Reis DJ. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 1981;225(2):373–385. doi: 10.1016/0006-8993(81)90843-x. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Sesack SR. Cellular basis for interactions between catecholaminergic afferents and neurons containing Leu-enkephalin-like immunoreactivity in rat caudateputamen nuclei. J Neurosci Res. 1992;31(2):212–230. doi: 10.1002/jnr.490310203. [DOI] [PubMed] [Google Scholar]
- Powers RE. The neuropathology of schizophrenia. J Neuropathol Exp Neurol. 1999;58(7):679–690. doi: 10.1097/00005072-199907000-00001. [DOI] [PubMed] [Google Scholar]
- Preece P, Cairns NJ. Quantifying mRNA in postmortem human brain: influence of gender, age at death, postmortem interval, brain pH, agonal state and inter-lobe mRNA variance. Brain Res Mol Brain Res. 2003;118(1-2):60–71. doi: 10.1016/s0169-328x(03)00337-1. [DOI] [PubMed] [Google Scholar]
- Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y. Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. J Comp Neurol. 2006;499(2):231–243. doi: 10.1002/cne.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PJ, Anderson SD. Stimulatory effect of L-glutamate and related amino acids on [3H]dopamine release from rat striatum: an in vitro model for glutamate actions. J Neurochem. 1979;32(5):1539–1545. doi: 10.1111/j.1471-4159.1979.tb11096.x. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Knickman JK. The ultrastructural organization of the patch matrix compartments in the human striatum. J Comp Neurol. 2002;452(2):128–138. doi: 10.1002/cne.10351. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Kumbalasiri T, Force MM, Kung L. Ultrastructural organization of dopaminergic terminals in the striata of schizophrenic patients and animals treated chronically with haloperidol. Schizophrenia Res. 2001;49:56. [Google Scholar]
- Roberts RC, Roche JK, Conley RR. Perforated synapses in the patch matrix compartments in postmortem striatum in schizophrenia. Soc. Neurosci. Abstr. 2005a;31:912. [Google Scholar]
- Roberts RC, Roche JK, Conley R. Synaptic differences in the postmortem striatum of subjects with schizophrenia: a stereological ultrastructural analysis. Synapse. 2005b;56:185–197. doi: 10.1002/syn.20144. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, Conley RR. Synaptic differences in the patch matrix compartments of subjects with schizophrenia: a postmortem ultrastructural study of the striatum. Neurobiol Dis. 2005c;20(2):324–335. doi: 10.1016/j.nbd.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, Conley RR. Differential synaptic changes in the striatum of subjects with undifferentiated versus paranoid schizophrenia. Synapse. 2008;62(8):616–27. doi: 10.1002/syn.20534. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, Conley RR, Lahti AC. Synaptic organization in postmortem striatum in subjects with schizophrenia: treatment responders versus nonresponders. Schizophr Bull. 2007;33:271. [Google Scholar]
- Sadikot AF, Parent A, Smith Y, Bolam JP. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a light and electron microscopic study of the thalamostriatal projection in relation to striatal heterogeneity. J Comp Neurol. 1992;320(2):228–242. doi: 10.1002/cne.903200207. [DOI] [PubMed] [Google Scholar]
- Salzman S, Endicott J, Clayton P, Winokur G. Diagnostic evaluation after death (DEAD) National Institute of Mental Health, Neuroscience Research Branch; Rockville, MD: 1983. [Google Scholar]
- Seeman P, Chau-Wong M, Tedesco J, Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Nat Acad Sci. 1975;72(11):4376–80. doi: 10.1073/pnas.72.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14(1):88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheitman BB, Lieberman JA. The natural history and pathophysiology of treatment resistant schizophrenia. J Psychiatr Res. 1998;32(3-4):143–150. doi: 10.1016/s0022-3956(97)00052-6. [DOI] [PubMed] [Google Scholar]
- Shihabuddin L, Buchsbaum MS, Hazlett EA, Haznedar MM, Harvey PD, Newman A, Schnur DB, Spiegel-Cohen J, Wei T, Machac J, Knesaurek K, Vallabhajosula S, Biren MA, Ciaravolo TM, Luu-Hsia C. Dorsal striatal size, shape, and metabolic rate in never-medicated and previously medicated schizophrenics performing a verbal learning task. Arch Gen Psychiatry. 1998;55(3):235–243. doi: 10.1001/archpsyc.55.3.235. [DOI] [PubMed] [Google Scholar]
- Sidibe M, Smith Y. Thalamic inputs to striatal interneurons in monkeys: synaptic organization and co-localization of calcium binding proteins. Neuroscience. 1999;89(4):1189–1208. doi: 10.1016/s0306-4522(98)00367-4. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bennett BD, Bolam JP, Parent A, Sadikot AF. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. J Comp Neurol. 1994;344(1):1–19. doi: 10.1002/cne.903440102. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27(9):520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Somerville SM, Roche JK, Conley R, Roberts RC. Ultrastructural study of mitochondria in postmortem striatal patch and matrix compartments in schizophrenia. Soc Neurosci Abstr. 2003;29:316–313. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack HG, van Haren NE, Seifert N, Kahn RS. Structural brain abnormalities in chronic schizophrenia at the extremes of the outcome spectrum. Am J Psychiatry. 2001;158(7):1140–1142. doi: 10.1176/appi.ajp.158.7.1140. [DOI] [PubMed] [Google Scholar]
- Stern RG, Kahn RS, Harvey PD, Amin F, Apter SH, Hirschowitz J. Early response to haloperidol treatment in chronic schizophrenia. Schizophr Res. 1993;10(2):165–171. doi: 10.1016/0920-9964(93)90052-k. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Tomita H, Vawter MP, Walsh DM, Evans SJ, Choudary PV, Li J, Overman KM, Atz ME, Myers RM, Jones EG, Watson SJ, Akil H, Bunney WE., Jr. Effect of agonal and postmortem factors on gene expression profile: quality control in microarray analyses of postmortem human brain. Biol Psychiatry. 2004;55(4):346–352. doi: 10.1016/j.biopsych.2003.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillet J, Kerkerian L, Kachidian P, Bosler O, Nieoullon A. Ultrastructural correlates of functional relationships between nigral dopaminergic or cortical afferent fibers and neuropeptide Y-containing neurons in the rat striatum. Neurosci Lett. 1989;100(1-3):99–104. doi: 10.1016/0304-3940(89)90667-8. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci. 2002;22(1):294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Wagner HN, Jr., Tune LE, Dannals RF, Pearlson GD, Links JM, Tamminga CA, Broussolle EP, Ravert HT, Wilson AA. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science. 1986;234(4783):1558–1563. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]