Abstract
Introduction
The current study examined the effects of nicotine infusion into the dorsal hippocampus or anterior cingulate on fear conditioning and on ethanol-induced deficits in fear conditioning, and whether these effects involved receptor activation or inactivation.
Methods
Conditioning consisted of two white noise (30 seconds, 85 dB)–foot shock (2 seconds, 0.57 mA) pairings. Saline or ethanol was administered to C57BL/6 mice 15 minutes before training and saline or nicotine was administered 5 minutes before training or before training and testing. The ability of the high-affinity nicotinic acetylcholinergic receptor (nAChR) antagonist dihydro-beta-erythroidine (DHβE) to modulate the effects of ethanol and nicotine was also tested; saline or DHβE was administered 25 (injection) or 15 (infusion) minutes before training or before training and testing.
Results
Infusion of nicotine into the hippocampus enhanced contextual fear conditioning but had no effect on ethanol-induced learning deficits. Infusion of nicotine into the anterior cingulate ameliorated ethanol-induced deficits in contextual and cued fear conditioning but had no effect on learning in ethanol-naïve mice. DHβE blocked the effects of nicotine on ethanol-induced deficits; interestingly, DHβE alone and co-administration of sub-threshold doses of DHβE and nicotine also ameliorated ethanol-induced deficits but failed to enhance learning. Finally, DHβE failed to ameliorate ethanol-induced deficits in β2 nAChR subunit knockout mice.
Conclusions
These results suggest that nicotine acts in the hippocampus to enhance contextual learning, but acts in the cingulate to ameliorate ethanol-induced learning deficits through inactivation of high-affinity β2 subunit-containing nAChRs.
Keywords: Alcohol, Addiction, Learning and Memory, Hippocampus, Anterior Cingulate
Introduction
Despite the well-known consequences of alcohol and nicotine consumption, abuse and co-abuse of these drugs remains a major societal issue. A positive correlation exists between high-risk, heavy use of each drug (Dawson, 2000; John et al, 2003; Larsson and Engel, 2004). There is also a correlation between dependence on alcohol and dependence on nicotine; the National Institute of Health (2007) reports that nicotine-addicted smokers are four times more likely than non-smokers to also be addicted to alcohol, and that alcoholics are three times more likely than the rest of the population to smoke cigarettes.
While alcohol and nicotine have anxiolytic (Blanchard et al, 1993; Picciotto et al, 2002) and rewarding (White, 1996) effects, the co-abuse of these drugs may also result from the interaction of these drugs on cognitive function. For instance, ethanol disrupts contextual and cued fear conditioning (Escher and Mittleman, 2004; Gould, 2003; Gulick and Gould, 2007), while nicotine enhances contextual but not cued fear conditioning (Gould and Higgins, 2003a; Gould and Wehner, 1999) and ameliorates ethanol-induced deficits in both contextual and cued fear conditioning (Gould and Lommock, 2003b; Gulick and Gould, 2008). However, it is unclear whether the same mechanism involved in the enhancement of contextual fear conditioning by nicotine mediates nicotine amelioration of ethanol-induced deficits in fear conditioning.
Although nicotine infusion into the dorsal hippocampus enhances contextual fear conditioning (Davis et al, 2007), it is unknown if nicotine acts in this area to ameliorate ethanol-induced learning deficits. Nicotine modulates ethanol-induced deficits in both cued and contextual fear conditioning. Cued fear conditioning, however, is hippocampus-independent (Logue et al, 1997; Phillips and LeDoux, 1992); thus, nicotine may act in another brain area to ameliorate ethanol-induced deficits in contextual and cued fear conditioning. The anterior cingulate is critically involved in attentional processes (Allman et al, 2001), and mnemonic processes (Chiba et al, 1997; Faw, 2003; Malin et al, 2007; Malin and McGaugh, 2006; Tang et al, 2005). With roles in both attention and learning, the anterior cingulate represents an area where nicotine may act to alter ethanol-induced deficits in fear conditioning.
Just as the brain areas involved in the interactive effects of ethanol and nicotine on learning are unknown, so are the underlying receptor-level processes. Neuronal nicotinic acetylcholinergic receptors (nAChRs) are a broad family of pentameric ion channels comprised of either α (α2–10) or α and β (β2–4) subunits (Decker et al, 1995). β2–containing nAChRs are involved in both the nicotine enhancement of learning (Davis et al, 2007) and the interactive effects of nicotine and ethanol on learning (Wehner et al, 2004). Furthermore, α4β2* (* denotes potential unknown additional subunits) nAChRs are found in the dorsal hippocampus and anterior cingulate cortex (Alkondon and Albuquerque, 1993; Pichika et al, 2006). However, nicotine can both activate and desensitize nAChRs (for review, Picciotto et al, 2008); thus, nAChR activation and inactivation may differentially modulate the effects of nicotine on learning and on ethanol-induced deficits in learning.
In the current study, we compared the effects of nicotine infusion into the dorsal hippocampus and anterior cingulate on learning and on ethanol-induced deficits in learning. We also compared the effects of cingulate infusion of nicotine versus dihydro-beta-erythroidine (DHβE, a high-affinity nAChR antagonist) on ethanol-induced learning deficits to determine whether receptor activation or inactivation can ameliorate these deficits. Because DHβE antagonizes multiple high-affinity nAChRs (Harvey et al, 1996; Williams and Robinson, 1984), β2 nAChR subunit knockout (KO) mice were used to test if the effects of DHβE on ethanol-induced fear conditioning deficits involve β2–containing nAChRs.
Methods
Subjects
Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were tested at 8–12 weeks of age (20–30 g). Heterozygous β2 nAChR subunit knockout (KO) mice (original breeding pairs were backcrossed to C57BL/6 mice for at least seven generations and provided by Dr. Arthur Beaudet, Baylor College of Medicine) were bred to obtain male and female β2 nAChR subunit KO mice (ages 8–12 weeks), and wild-type littermates (WT; 8–12 weeks of age); genotype was verified by PCR. Numerous studies have used the β2 KO mice backcrossed onto a C57BL/6 background to investigate the effects of nicotine on behavior and brain function (Portugal et al, 2007; Raybuck and Gould, 2009; Salas et al, 2004; Wehner et al, 2004). Mice were housed in groups of 4 per cage prior to surgery and individually housed after surgery. Mice had ad libitum access to food and water. A 12-hr light–dark cycle (lights on at 0700) was maintained, with all testing done between 9:00 am and 5:00 pm. Procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Apparatus
Training and context testing took place in identical conditioning chambers housed in sound-attenuating boxes (MED Associates, St. Albans, VT). Each 17.78 × 19.05 × 38.10 cm chamber consisted of Plexiglas panels in the front, back, and ceiling and two stainless-steel walls on the sides. The metal grid floor of each chamber, through which the foot-shock unconditioned stimulus (US; 0.57 mA for 2 seconds) was delivered, was connected to a shock generator and scrambler. Speakers that delivered a white noise conditioned stimulus (CS; 85 dB for 30 seconds) were mounted on the right wall of each chamber. Background noise (69 dB) and air exchange were provided by ventilation fans mounted on the right wall of each sound-attenuating box. A computer running MED Associates (St. Albans, VT) software controlled stimuli presentation. Chambers were cleaned with 70% ethanol before each session.
Testing for freezing to the CS occurred in a separate room in altered context chambers housed in sound-attenuating boxes. Speakers that delivered the white noise CS were mounted on the left wall of each chamber. The 20.32 × 22.86 × 17.78 cm chambers were constructed of four Plexiglas walls, a Plexiglas ceiling, and an opaque white plastic floor. In addition to the differences in location, visual cues (e.g., the inside of the sound-attenuating boxes in the training context were white versus black in the altered context room), chamber dimensions, floor construction, and a vanilla extract olfactory cue (no such cue was present at training) further distinguished the altered context chambers from the original training chambers.
Fear Conditioning
At training, baseline freezing was recorded for the first 120 seconds (based on Gould et al, 2003a). At 120 and 270 seconds, the CS sounded for 30 seconds; the US occurred during the last 2 seconds of the CS; immediate freezing was measured during the inter-trial interval. The mice remained in the chamber for 30 seconds after the second CS-US presentation. Twenty-four hours later, freezing to the context was assessed for 5 minutes and one hour later, generalized freezing to the altered context test was measured for 3 minutes (pre-CS test) followed by a 3 minute test of freezing to the CS (CS test). Freezing, defined as the absence of visible movement with the exception of respiration, was assessed at 10-second intervals for a 1-second.
Surgery
Mice were anesthetized using isoflurane gas (5% induction, 2% maintenance) and placed in a stereotaxic apparatus from David Kopf Instruments (Tujunga, CA). Bilateral stainless steel guide cannulae (C232G, 22 gauge; Plastics One, Roanoke, VA) were stereotaxically inserted and fixed to the skull with dental cement. Dummy cannulae (C232DC; Plastics One) were inserted into the guide cannulae to prevent clogging. Coordinates determined from bregma using the mouse brain atlas (Paxinos and Franklin, 2001) were as follows: Hippocampus: −1.7 mm posterior; ±1.5 mm mediolateral; −2.3 mm ventral; Cingulate: +2.0 mm anterior; ±1.5 mm mediolateral (±2.5 mm mediolateral for lateral controls); −2.3 mm ventral (−4.0 mm ventral for below controls); it should be noted that in rats, functional differences in rostral (2.6 AP rats) and caudal (0.2 AP rats) anterior cingulate cortex (Johansen et al, 2001; Malin et al, 2007) exist. The mouse cingulate cortex extends from 2.34 AP to −0.22 AP, and then continues to −0.82 as the cingulate/retrosplenial complex. All of our cingulate infusions were restricted to a rostral range of 2.34 to 1.70 AP. Ketoprofen (2.0 mg/kg) was administered subcutaneously for post-operative pain. Animals were allowed at least 5 days to recover before behavioral procedures began.
Drugs and Infusion
Nicotine hydrogen tartrate salt (Sigma St. Louis, MO; 0.045 or 0.09 mg/kg intraperitoneal (i.p.) injection or 0.35 µg/0.50 µl/side infusion, reported as freebase weight, based on Davis and colleagues (2007) and Davis and Gould (2006)) and DHβE (Sigma St. Louis, MO; 3.0 or 6.0 mg/kg subcutaneous (s.c) injection or 18.0 µg/0.50 µl/side infusion based on Davis and colleagues (2007) and Davis and Gould (2006)) were dissolved in physiological saline; we were unable to test the effects of higher systemic doses of DHβE as doses of 8.0 mg/kg or higher induced motor depression. Injection volume for nicotine and DHβE was 0.01 ml/g body weight, and for ethanol (Fisher Scientific Pittsburgh, PA; 1.0 g/kg i.p. based on Gould, 2003) it was 20% vol/vol in saline. Controls received physiological saline. DHβE was infused (15 minutes) or injected (25 minutes) before training or before training and testing, ethanol was injected 15 minutes before training, and nicotine was infused or injected 5 minutes before training or before training and testing. Injection and infusion times were based on previous research (Davis and Gould, 2006; Davis et al, 2007; Gulick et al, 2008).
For direct infusions, mice were gently restrained and dummy cannulae were removed and replaced with 22 gauge infusion cannulae. Drugs were bilaterally infused at a rate of 0.50 µl/min. Infusion cannulae were attached to polyethylene tubing (PE50; Plastics One), which was attached to a 10 µl Hamilton (Reno, NV) syringe. Drug administration was controlled by a micro-infusion pump (KDS 100; KD Scientific, New Hope, PA). Injection cannulae were left in place for 1 minute after infusion to allow drug diffusion away from the cannula tip.
Histology
Brains were placed in a 10% formalin solution (Fisher Scientific, Pittsburgh, PA) for at least 24 hours before 60 µm thick coronal sections were sliced at −18°C. The sections were stained with cresyl violet and cannula placements were determined using a light microscope. Data from animals with incorrect placements were excluded from statistical analysis (<10%).
Experimental Design
We first tested the effects of nicotine infusion into the dorsal hippocampus or anterior cingulate at both training and testing on systemic ethanol-induced fear conditioning deficits. A second experiment examined whether nicotine administration at training only would be sufficient to ameliorate ethanol-induced cognitive deficits; the effects of systemic nicotine administration were tested first, followed by a test of the effects of direct infusion of nicotine into the cingulate cortex. To ensure that the effect of nicotine was specific to the anterior cingulate, we infused nicotine below and lateral to the anterior cingulate infusion site.
To determine if DHβE alters nicotine amelioration of ethanol-induced learning deficits, we systemically administered ethanol, nicotine, and DHβE before training. A follow-up experiment determined whether infusion of DHβE into the anterior cingulate cortex would alter the effects of systemic nicotine and ethanol on fear conditioning. Finally, we examined whether the effects of DHβE on ethanol-induced learning deficits involve β2-containing nAChRs by systemically administering the drugs to β2 KO and WT mice.
Shock sensitivity
We next determined whether the interactive effects of ethanol and nicotine on fear conditioning were mediated by changes in shock sensitivity. Animals were systemically administered either saline, 1.0 g/kg ethanol (10 minutes before testing), or 0.09 mg/kg nicotine (immediately before testing), and then exposed to a range of 2 second foot-shocks (0.10 mA to 0.60 mA) over a 15 minute testing period. There were three presentations at each shock intensity, with a 20 second inter-stimulus interval and a 90 second inter-trial interval. Each animal was administered all three drug treatments, one on each of three consecutive days, and testing sessions occurred 24 hours apart with counterbalancing of the order of drug administration. We scored vocalization (yes or no) and motion (0 = no response; 1= hop; 2 = jump; 3 = run; 4 = horizontal jump; 5= vertical jump) for each shock presentation (scoring based on Bardgett et al, 2003; Schrott and Crnic, 1994).
Statistical testing
Data were analyzed using a repeated-measures or two-way analysis of variance (ANOVA), or independent samples t-test in the DHβE- nicotine alone studies. Tukey’s post-hoc analysis was used to detect significant differences at p<0.05 (SPSS version 13; Chicago, IL).
Results
Nicotine infusion into the dorsal hippocampus
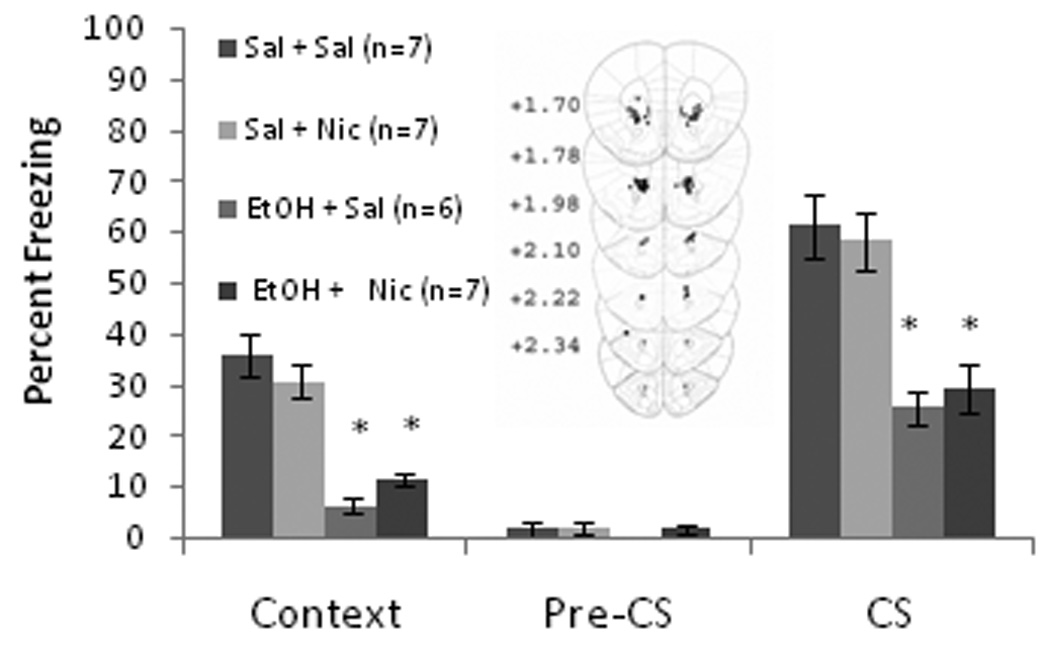
The ability of nicotine infusion into the dorsal hippocampus at training and testing, alone or concurrent with training day systemic ethanol administration, to alter contextual and cued fear conditioning was examined. The levels of baseline freezing, measured before the first CS presentation, and immediate freezing, measured after the first CS-US presentation, were similar across all groups. Significant effects of ethanol, F(1,27) = 99.17, p<0.001, and nicotine, F(1,27) = 6.59, p<0.05, on freezing to the context on testing day were found, but there was no interactive effect of ethanol and nicotine. There was also a significant effect of ethanol on freezing to the CS, F(1,27) = 45.67, p<0.001, but there was no effect of nicotine on freezing to the CS nor was there a significant ethanol by nicotine interaction. There were no significant differences in freezing during the pre-CS period (Figure 1).
Figure 1.
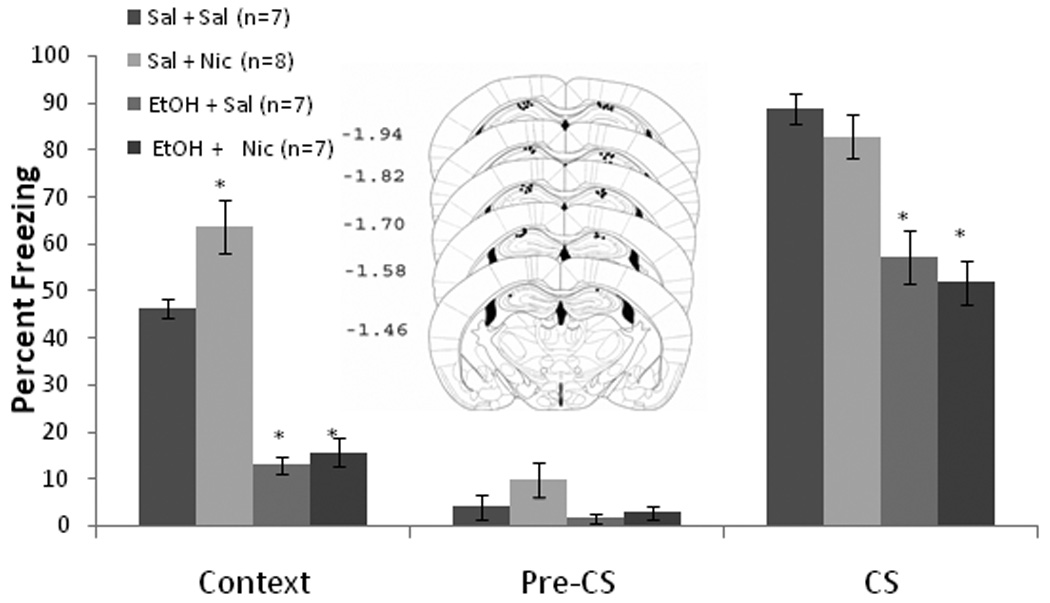
Bilateral nicotine infusion (0.35 µg/0.50 µl/side) into the dorsal hippocampus at training and testing enhanced contextual fear conditioning but failed to ameliorate systemic ethanol-induced (1.0 g/kg) impairments in contextual and cued fear conditioning (Mean ± SEM; * indicates significant difference from controls, p<0.05). Center figure represents drug infusion sites.
Post-hoc analysis revealed that during context testing, the ethanol-treated groups (n = 7 for each) froze significantly less than saline controls (n = 7) and less than the nicotine alone group (n = 8) (p<0.001). The nicotine alone group froze significantly more to the context than saline controls (p<0.05). For cued fear conditioning, both groups administered systemic ethanol froze significantly less than the saline or the nicotine alone group (p<0.001); no significant difference existed between the nicotine alone group and the saline controls. Thus, nicotine infusion into the dorsal hippocampus enhanced contextual fear conditioning but failed to ameliorate ethanol-induced learning deficits.
Nicotine infusion into the anterior cingulate
The ability of nicotine infusion into the anterior cingulate at training and testing, alone or concurrent with training day systemic ethanol administration, to alter contextual and cued fear conditioning was examined next. The levels of baseline and immediate freezing were similar across all groups. Significant effects of ethanol, F(1,35) = 41.61, p<0.001, and of nicotine, F(1,35) = 9.21, p<0.01, on freezing to the context on testing day were found, as well as an interaction of ethanol and nicotine F(1,35) = 18.57, p<0.001. There were also significant effects of ethanol, F(1,35) = 31.32, p<0.001, and of nicotine, F(1,35) = 9.50, p<0.01, on freezing to the CS, and an interaction of ethanol and nicotine F(1,35) = 8.58, p<0.01. There were no significant differences in freezing during the pre-CS period (Figure 2).
Figure 2.
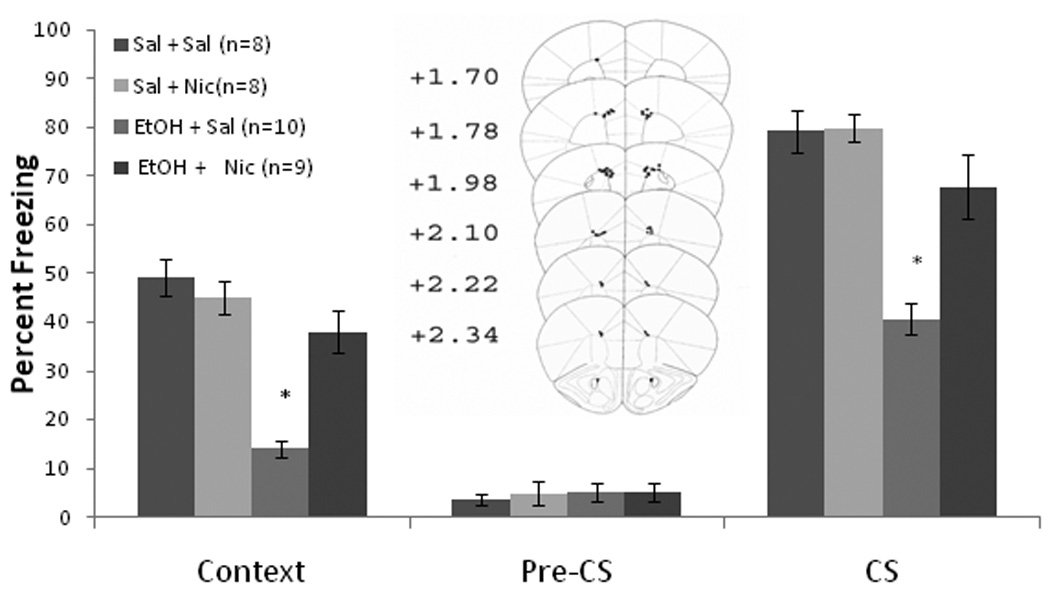
Bilateral nicotine infusion (0.35 µg/0.50 µl/side) into the anterior cingulate at training and testing ameliorated systemic ethanol-induced (1.0 g/kg) impairments in contextual and cued fear conditioning but failed to enhance contextual fear conditioning (Mean ± SEM; * indicates significant difference from controls, p<0.05). Center figure represents drug infusion sites.
Post-hoc analysis revealed that during context testing, the ethanol-alone group (n = 10) froze significantly less than saline controls (n = 8) and less than the nicotine infusion groups (p<0.001). The nicotine alone (n = 8) and nicotine and ethanol (n = 9) groups were similar to saline controls. The same pattern emerged for cued fear conditioning. Thus, nicotine infusion into the anterior cingulate did not enhance contextual fear conditioning, but ameliorated ethanol-induced deficits in contextual and cued fear conditioning.
Nicotine administration on training day only
In the previous studies, nicotine was administered at both training and testing. To test if training day administration of nicotine is sufficient to ameliorate ethanol-induced deficits, nicotine and ethanol were both administered systemically on training day only. Baseline and immediate freezing were similar across all groups. Significant effects of ethanol, F(1,36) = 215.01, p<0.001, and of nicotine, F(1,36) = 8.16, p<0.01, on freezing to the context on testing day were found, as well as an interaction of ethanol and nicotine F(1,36) = 4.39, p<0.05. There were also significant effects of ethanol, F(1,36) = 26.15, p<0.001, and of nicotine, F(1,36) = 4.39, p<0.05, on freezing to the CS, as well as an interaction of ethanol and nicotine F(1,36) = 5.54, p<0.05. There were no significant differences in freezing during the pre-CS period.
Post-hoc analysis revealed that for context testing, both ethanol-treated groups froze significantly less than saline controls and the nicotine alone group (p<0.001), but the group administered ethanol and nicotine also froze significantly more than the group administered ethanol alone (p<0.01). The group administered nicotine alone froze at levels similar to saline controls. For cued fear conditioning, the group administered ethanol alone froze significantly less than all other groups (p<0.01). Nicotine groups were not significantly different from saline controls. Thus, systemic nicotine administration on training day only did not enhance contextual fear conditioning, as has previously been shown (Gould et al, 2003a; Gould et al, 1999); but ameliorated ethanol-induced deficits in contextual and cued fear conditioning (Table 1).
Table 1.
Systemic nicotine on training day only (mean ± standard error of mean)
| Group | saline n=10 | ethanol n=9 | nicotine n=10 | EtOH & nic n=9 |
|---|---|---|---|---|
| Context | 46.7 ± 2.6 | 16.0 ± 1.3 | 45.3 ± 1.7 | 24.7 ± 1.3 |
| CS | 81.2 ± 2.6 | 56.1 ± 4.4 | 82.2 ± 2.9 | 71.7 ± 4.6 |
Based on the results with systemic nicotine, the effects of nicotine infusion into the anterior cingulate on training day only were assessed. Baseline and immediate freezing were similar across all groups. Significant effects of ethanol, F(1,28) = 35.20, p<0.001, and of nicotine, F(1,28) = 23.01, p<0.001, on freezing to the context on testing day were found, as well as an interaction of ethanol and nicotine F(1,28) = 33.30, p<0.001. There were also significant effects of ethanol, F(1,28) = 45.88, p<0.001, and of nicotine, F(1,28) = 10.03, p<0.01, on freezing to the CS, and an interaction of ethanol and nicotine F(1,28) = 31.04, p<0.001. There were no significant differences in freezing during the pre-CS period (Figure 3).
Figure 3.
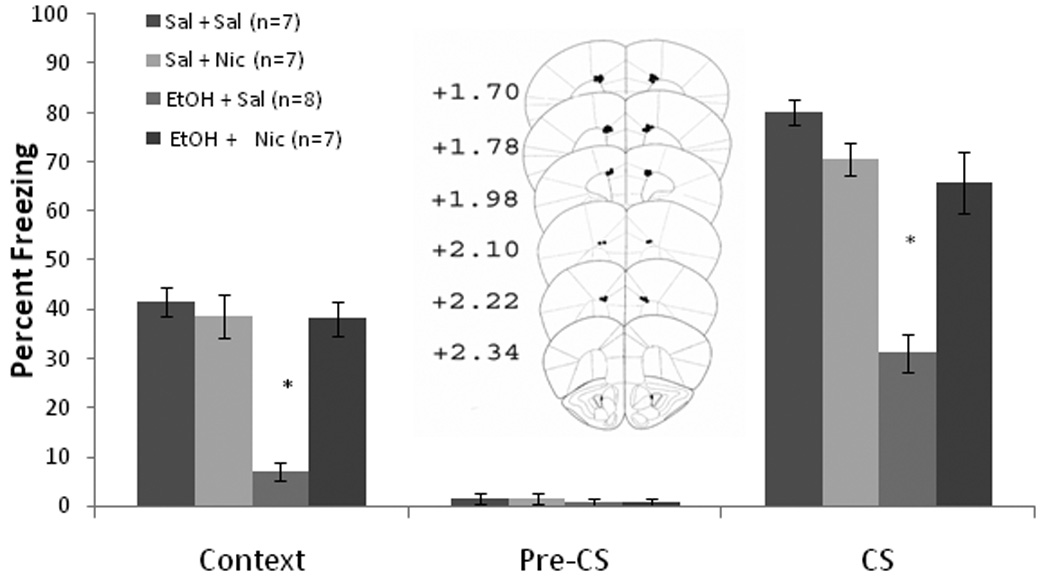
Bilateral nicotine infusion (0.35 µg/0.50 µl/side) into the anterior cingulate on training day only ameliorated systemic ethanol-induced (1.0 g/kg) impairments in contextual and cued fear conditioning but failed to enhance contextual fear conditioning (Mean ± SEM; * indicates significant difference from controls, p<0.05). Center figure represents drug infusion sites.
Post-hoc analysis revealed that during context testing, the ethanol-alone group (n = 8) froze significantly less than saline controls (n = 7) and less than the groups infused with nicotine (n = 7 for each) (p<0.001). The nicotine-infused groups froze at levels similar to saline controls. The same results were seen for cued fear conditioning. Thus, similar to systemic nicotine, nicotine infusion into the anterior cingulate on training day only did not enhance contextual fear conditioning but ameliorated ethanol-induced deficits in contextual and cued fear conditioning.
To ensure that the effects of nicotine were specific to the anterior cingulate, and not to diffusion into nearby areas, we also infused nicotine below (accessory optic tract) and lateral (motor cortex) to the anterior cingulate. This control was not necessary for the hippocampus study as it was previously conducted in our laboratory (Davis et al, 2007). For both sets of infusions, baseline and immediate freezing were similar across all groups. For the below infusions, there was a significant effect of systemic ethanol on freezing to the context, F(1,26) = 90.62, p<0.001, and to the CS, F(1,26) = 49.12, p<0.001, but no other significant effects. Post-hoc analysis revealed that during context and cued testing, both ethanol-treated groups (n = 7 for each) froze significantly less than saline controls (n = 7) and the nicotine alone group (n = 8) (p<0.001). For the lateral infusions, there was a significant effect of ethanol on freezing to the context, F(1,24) = 105.87, p<0.001, and to the CS, F(1,24) = 257.12, p<0.001, on testing day, but no other significant effects. Post-hoc analysis revealed that during context and cued testing, the ethanol-treated groups (n = 6 for EtOH-alone and n = 7 for Nic and EtOH) froze significantly less than saline controls (n = 7 for each) and the nicotine-alone group (n = 7) (p<0.001) (Figure 4). Thus, nicotine administration below or lateral to the anterior cingulate on training day only did not alter contextual and cued fear conditioning.
Figure 4.
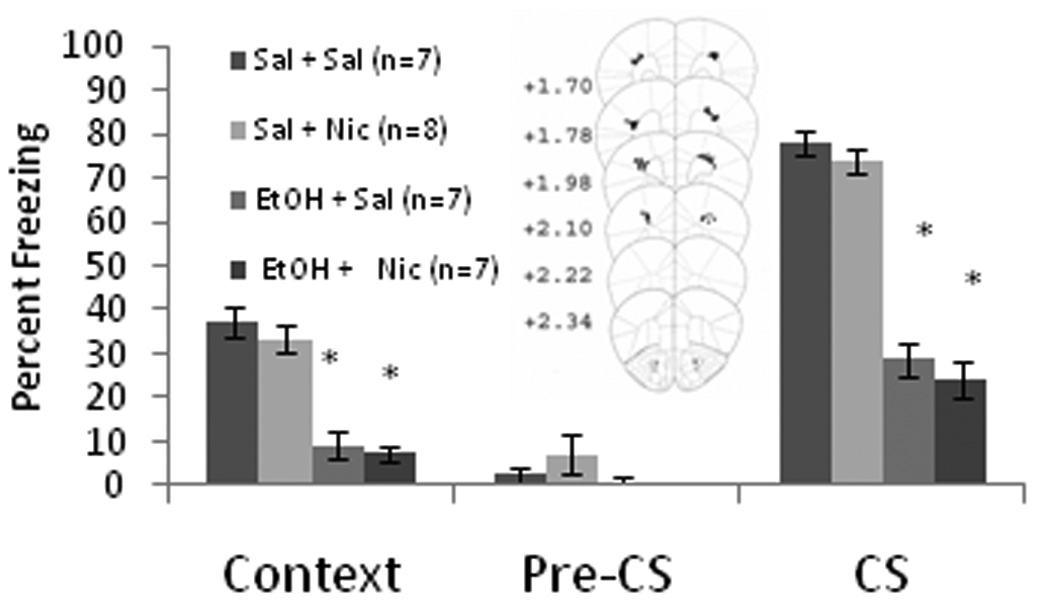
Bilateral nicotine infusion (0.35 µg/0.50 µl/side) lateral to (A) or below (B) the anterior cingulate had no effect on contextual and cued fear conditioning or on systemic ethanol-induced deficits (Mean ± SEM; * indicates significant difference from controls, p<0.05). Center figures represent drug infusion sites.
DHβE blockade of nicotine amelioration of ethanol-induced deficits
To determine whether the high-affinity nAChR antagonist DHβE would alter the interactive effects of nicotine and ethanol, we administered nicotine, ethanol, and DHβE systemically. Baseline and immediate freezing were similar across all groups. A significant effect of drug treatment on freezing to the context on testing day was found, F(3,24) = 28.35, p<0.001. There was also a significant effect of drug treatment on freezing to the CS, F(1,24) = 11.74, p<0.01. There were no significant differences in freezing during the pre-CS period. Post-hoc tests demonstrated that ethanol decreased freezing below saline control levels for both the contextual and cued tests (p<0.01), but the group treated with ethanol and nicotine froze significantly more than the ethanol-alone group (p<0.05). The group administered DHβE, ethanol, and nicotine froze at similar levels to the ethanol-alone group (Table 2).
Table 2.
Systemic DHβE, nicotine, and ethanol (mean ± standard error of mean)
| Group | saline n=6 | ethanol n=7 | EtOH & nic n=7 | DHβE, EtOH & nic n=7 |
|---|---|---|---|---|
| Context | 45.6 ± 1.5 | 11.0 ± 1.7 | 29.5 ± 1.2 | 8.6 ± 2.1 |
| CS | 89.8 ± 2.9 | 31.8 ± 4.4 | 76.2 ± 5.0 | 41.3 ± 4.7 |
To investigate whether the 0.09 mg/kg nicotine dose and the 6.0 mg/kg DHβE dose interact to alter learning in the absence of ethanol, we administered both drugs systemically before training. Baseline freezing and immediate freezing were similar across all groups. Co-administration nicotine and DHβE significantly disrupted freezing to the context, t(12) = 1.07, p<0.001, and freezing to the CS at testing, t(12) = 0.70, p<0.001 (Table 3). There was no effect on freezing during the pre-CS period.
Table 3.
Co-administration of systemic DHβE and nicotine (mean ± standard error of mean)
| Group | saline n=8 | DHβE & nicotine n=7 |
|---|---|---|
| Context | 32.1 ± 2.9 | 7.6 ± 1.9 |
| CS | 86.1 ± 3.0 | 33.3 ± 5.7 |
To examine whether the anterior cingulate is essential for the amelioration of ethanol-induced learning deficits by nicotine, we infused DHβE into the anterior cingulate and administered ethanol and nicotine systemically before training. Baseline and immediate freezing were similar across all groups. There was a significant testing day effect of drug treatment on freezing to the context, F(3,22) = 35.40, p<0.001, and on freezing to the CS, F(3,22) = 10.42, p<0.001. There were no significant differences in freezing during the pre-CS period (Figure 5).
Figure 5.
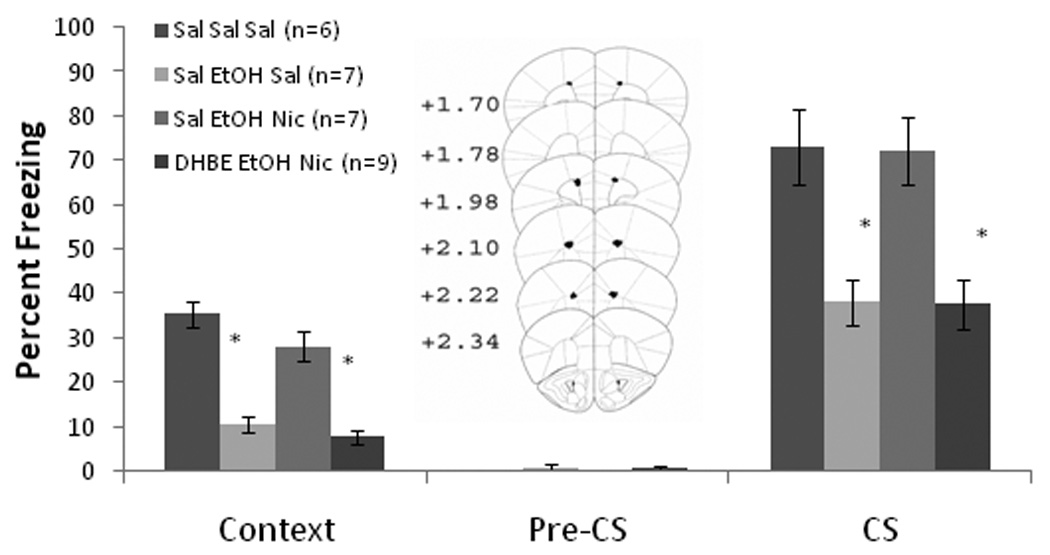
Bilateral DHβE infusion (18.0 µg/0.50 µl/side) into the anterior cingulate altered the effects of systemic nicotine (0.09 mg/kg) on systemic ethanol-induced (1.0 g/kg) impairments in contextual and cued fear conditioning (Mean ± SEM; * indicates significant difference from controls, p<0.05). Center figure represents drug infusion sites.
Post-hoc analysis revealed that the ethanol-alone (n = 7) and ethanol-nicotine-DHβE (n = 9) groups froze significantly less than saline controls (n = 6) and the ethanol-nicotine group (n = 7) to both the context (p<0.001) and the CS (p<0.01). The ethanol-nicotine group was not significantly different from saline controls in either test. Thus, DHβE infused into the anterior cingulate altered the effects of systemic nicotine on ethanol-induced learning deficits. This suggests that the effects of nicotine in the anterior cingulate are both necessary and sufficient to ameliorate ethanol-induced cognitive impairments.
DHβE amelioration of ethanol-induced deficits
To determine whether DHβE may alter learning or ethanol-induced learning deficits in the absence of nicotine, we systemically administered DHβE and ethanol at training. Baseline and immediate freezing were similar across all groups. There was a significant effect of ethanol, F(1,28) = 20.44, p<0.001, but not DHβE, on freezing to the context on testing day. There was a significant interaction of ethanol and DHβE on freezing to the context, F(1,28) = 19.17, p<0.001. There was also a significant effect of ethanol, F(1,28) = 32.81, p<0.001, and DHβE, F(1,28) = 7.81, p<0.05, on freezing to the CS on testing day, as well as a significant interaction of ethanol and DHβE on freezing to the CS, F(1,28) = 22.24, p<0.001. There were no significant differences in freezing during the pre-CS period.
Post-hoc tests revealed that the ethanol-alone group froze significantly less than saline controls to both the context and the CS (p<0.05). The group administered ethanol and DHβE froze significantly less than the control group to the context (p<0.05) but also froze significantly more to both the context and the CS than the group administered ethanol-alone (p<0.05) (Table 4). Thus, systemic DHβE partially ameliorated ethanol-induced deficits in fear conditioning.
Table 4.
Systemic DHβE and ethanol (mean ± standard error of mean)
| Group | saline n=6 | ethanol n=8 | DHβE n=7 | DHβE & EtOH n=8 |
|---|---|---|---|---|
| Context | 43.3 ± 3.0 | 13.8 ± 2.2 | 30.5 ± 5.1 | 30.0 ± 3.4 |
| CS | 82.4 ± 2.4 | 48.6 ± 2.7 | 79.2 ± 4.3 | 72.9 ± 3.8 |
We next examined whether DHβE infused into the anterior cingulate alters ethanol-induced learning deficits. Baseline and immediate freezing were similar across all groups. There was a significant effect of ethanol, F(1,25) = 33.25, p<0.001, but not DHβE, on freezing to the context on testing day, and a significant interaction of ethanol and DHβE, F(1,25) = 14.74, p<0.001. There was also a significant effect of ethanol, F(1,25) = 55.17, p<0.001, and DHβE, F(1,25) = 25.67, p<0.05, on freezing to the CS on testing day, and a significant interaction of ethanol and DHβE, F(1,25) = 15.17, p<0.001. No significant differences in freezing existed during the pre-CS period (Figure 6).
Figure 6.
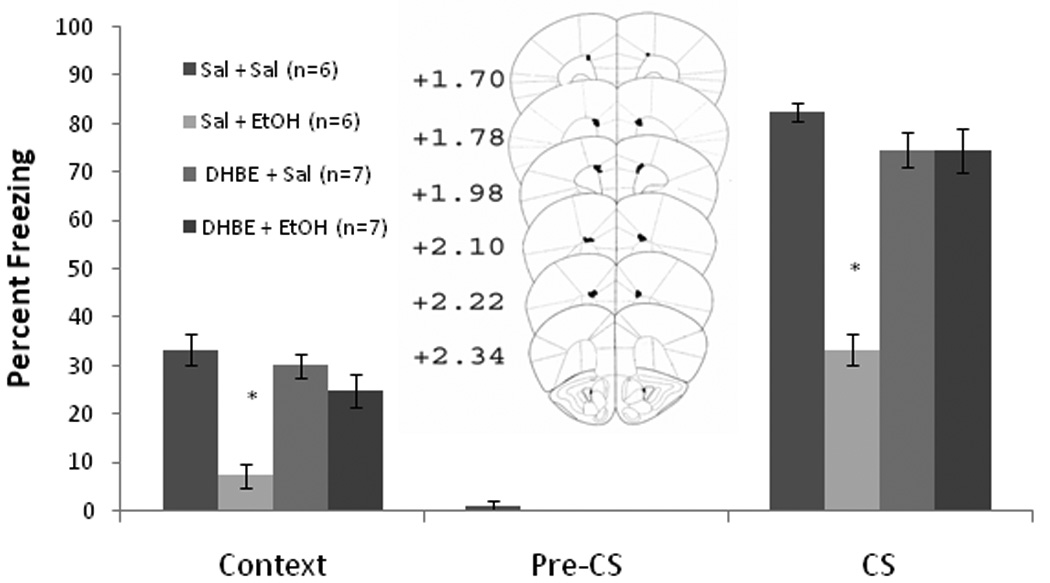
Bilateral DHβE infusion (18.0 µg/0.50 µl/side) into the anterior cingulate ameliorated systemic ethanol-induced (1.0 g/kg) impairments in contextual and cued fear conditioning but did not alter contextual or cued fear conditioning when administered alone (Mean ± SEM; * indicates significant difference from controls, p<0.05).
Post-hoc tests revealed that the group administered systemic ethanol alone (n = 6) froze significantly less to the context and to the CS than saline controls (n = 6) (p<0.05). The DHβE infusion groups (n = 7 each) were not significantly different from saline controls in freezing to the context or the CS. Thus, infusion of DHβE into the anterior cingulate cortex ameliorated ethanol-induced learning deficits but did not alter learning in the absence of ethanol.
Amelioration of ethanol-induced deficits by sub-threshold doses of nicotine and DHβE
Because both nicotine and DHβE ameliorated ethanol-induced deficits, we tested if co-administration of sub-threshold doses of each would act synergistically on ethanol-induced deficits. We first determined that a twofold dilution of our effective doses of nicotine (0.045 mg/kg) and DHβE (3.0 mg/kg) would not ameliorate ethanol-induced deficits. For nicotine, baseline and immediate freezing were similar across all groups. At context testing, there was only a significant effect of ethanol, F(1,27) = 196.68, p<0.001. There was a significant effect of ethanol, F(1,27) = 400.34, p<0.001, and nicotine, F(1,27) = 8.34, p<0.01, on freezing to the CS, but no interaction. There were no significant differences in freezing during the pre-CS period. Post-hoc tests revealed that both groups administered ethanol froze significantly less to both the context and the CS than saline controls (p<0.05), while the group administered nicotine alone was not significantly different from saline controls (Table 5). Thus, 0.045 mg/kg nicotine did not ameliorate ethanol-induced learning deficits.
Table 5.
Ethanol and sub-threshold doses of systemic nicotine or DHβE (mean ± standard error of mean)
| Group | saline n=7 | ethanol n=7 | nicotine n=7 | EtOH & nic n=7 |
|---|---|---|---|---|
| Context | 44.3 ± 2.5 | 14.3 ± 2.2 | 39.1 ± 2.4 | 12.4 ± 1.5 |
| CS | 81.7 ± 2.5 | 38.9 ± 3.2 | 89.5 ± 2.6 | 44.4 ± 1.3 |
| Group | saline n=8 | ethanol n=8 | DHβE n=8 | EtOH & DHβE n=9 |
| CS | 45.0 ± 2.8 | 16.3 ± 2.3 | 29.1 ± 2.6 | 13.7 ± 1.6 |
| Group | 85.4 ± 1.6 | 54.9 ± 4.8 | 65.6 ± 7.0 | 47.5 ± 3.4 |
For the 3.0 mg/kg DHβE dose, baseline and immediate freezing were similar across all groups. There was a significant effect of ethanol, F(1,29) = 95.69, p<0.001, and DHβE, F(1,29) = 22.34, p<0.01, on freezing to the context on testing day, and a significant interaction, F(1,29) = 12.60, p<0.05. There was a significant effect of ethanol, F(1,29) = 30.67, p<0.01, and DHβE, F(1,29) = 10.73, p<0.01, on freezing to the CS, but no significant interaction. There were no significant differences in freezing during the pre-CS period. Post-hoc tests revealed that both ethanol groups froze significantly less to both the context and the CS than saline controls (p<0.05), as did the group administered DHβE alone (Table 5). Thus, 3.0 mg/kg DHβE did not ameliorate ethanol-induced learning deficits.
We next co-administered the sub-threshold, systemic doses of both drugs with ethanol at training. Baseline and immediate freezing were similar across all groups. On testing day, there was a significant effect of drug treatment on freezing to the context, F(2,19) = 30.34, p<0.001, and the CS, F(2,19) = 27.54, p<0.001. There were no significant differences in freezing during the pre-CS period (Figure 7). Post-hoc analysis revealed that only the ethanol-alone group (n = 8) froze significantly less than saline controls (n = 8) to the context and to the CS (p<0.001). The group administered DHβE, ethanol, and nicotine (n = 9) and the group administered DHβE and nicotine (n = 8) were not significantly different from saline controls. Thus, co-administration of sub-threshold doses of DHβE and nicotine ameliorated ethanol-induced learning deficits.
Figure 7.
Co-administration of sub-threshold systemic doses of DHβE (3.0 mg/kg) and nicotine (0.045 mg/kg) ameliorated systemic ethanol-induced (1.0 g/kg) impairments in contextual and cued fear conditioning but did not alter conditioning in the absence of ethanol (Mean ± SEM; * indicates significant difference from controls, p<0.05).
To determine whether co-administration of systemic sub-threshold doses of nicotine and DHβE could enhance learning in the absence of ethanol, mimicking the effect of nicotine on contextual fear conditioning, DHβE and nicotine were administered before training and testing. Baseline and immediate freezing were similar across all groups. There was no significant drug effect on freezing to the context on testing day, but a significant effect of drug treatment on freezing to the CS on testing day, t(14) = 3.04, p<0.05; the group administered nicotine and DHβE froze significantly less than the saline group to the CS (Table 6). Thus, co-administration of sub-threshold doses of DHβE and nicotine do not enhance learning, suggesting that the enhancement of contextual fear conditioning and the amelioration of ethanol-induced deficits in contextual and cued fear conditioning by nicotine occur via different processes.
Table 6.
Co-administration of systemic sub-threshold doses of nicotine and DHβE (mean ± standard error of mean)
| Group | saline n=8 | DHβE & nicotine n=8 |
|---|---|---|
| Context | 32.3 ± 3.2 | 27.1 ± 1.7 |
| CS | 90.3 ± 2.7 | 79.9 ± 2.5 |
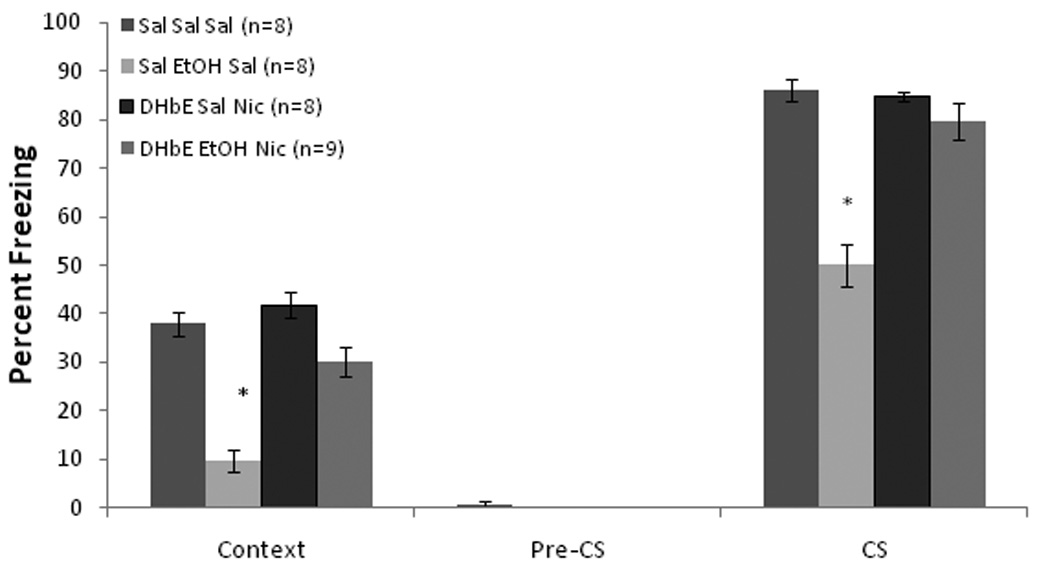
DHβE fails to ameliorate ethanol-induced deficits in β2 knockout mice
To determine whether β2–containing receptors are involved in the DHβE amelioration of ethanol-induced learning deficits, we co-administered systemic DHβE and ethanol to β2 KO and WT mice. Baseline and immediate freezing were similar across all groups. There was a significant effect of drug, F(2,34) = 49.17, p<0.001, and genotype, F(1,35) = 20.59, p<0.001, and a significant interaction on freezing to the context on testing day, F(2,34) = 17.98, p<0.001. For freezing to the CS on testing day, there was a significant effect of drug, F(2,34) = 45.41, p<0.001, and genotype, F(1,35) = 19.72, p<0.001, and a significant interaction, F(2,34) = 10.70, p<0.001. There were no significant differences in freezing during the pre-CS period (Figure 8).
Figure 8.
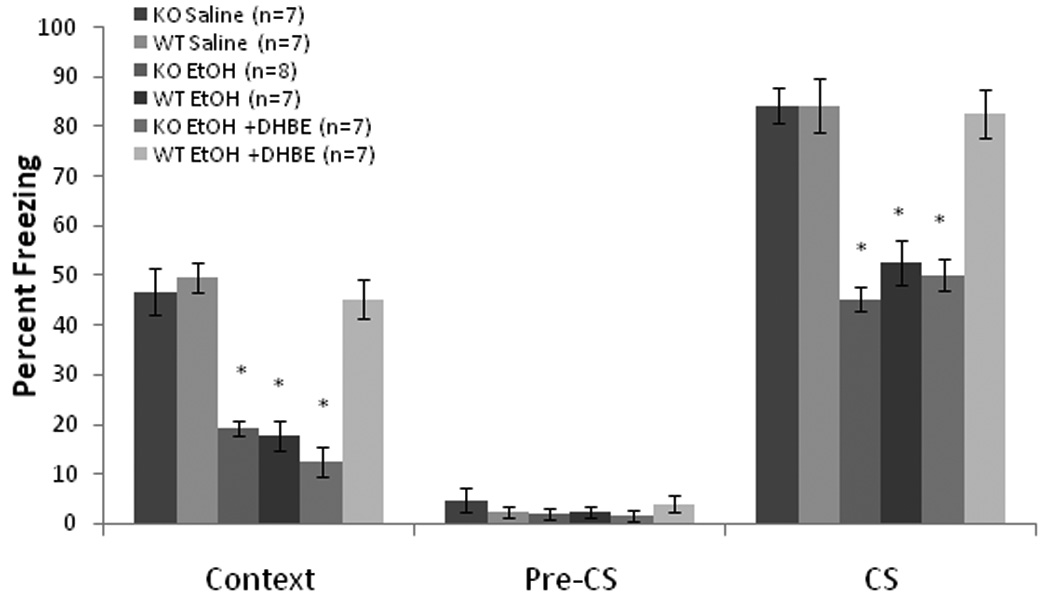
Systemic DHβE (6.0 mg/kg) ameliorated systemic ethanol-induced (1.0 g/kg) impairments in contextual and cued fear conditioning in wild-type mice, but failed to ameliorate these deficits in β2-knockout mice. There were no other differences between the genotypes in their responses to the drugs (Mean ± SEM; * indicates significant difference from controls, p<0.05).
Post-hoc tests revealed that the WT (n = 7) and KO (n = 7) saline controls froze at similar levels to both the context and the CS. The groups administered ethanol alone (WT, n = 7; KO, n = 8) froze significantly less than saline controls in both tests (p<0.05), but there were no differences between genotypes in the effect of ethanol. In the WT mice (n = 7), DHβE ameliorated the ethanol-induced deficit in both tests (p<0.05). No such effect was seen in the KO mice (n = 7). Thus, a β2–containing nAChR, such as the α4β2* receptor, is involved in the DHβE amelioration of ethanol-induced learning impairments.
Effects of ethanol and nicotine on shock sensitivity
Finally, we examined whether changes in sensitivity to the foot-shock may underlie the effects of ethanol or nicotine on fear conditioning. Repeated measures ANOVA revealed no significant effect of treatment order on motor responding or the number of vocalizations, so we collapsed across days. There were significant effects of shock intensity on motor responding, F(7,280) = 477.75, p<0.05, and vocalization, F(7,280) = 134.19, p<0.05, but no drug effects or interactions (n = 12 for all groups; Figure 9). Thus, neither ethanol nor nicotine significantly altered responding to the shock.
Figure 9.
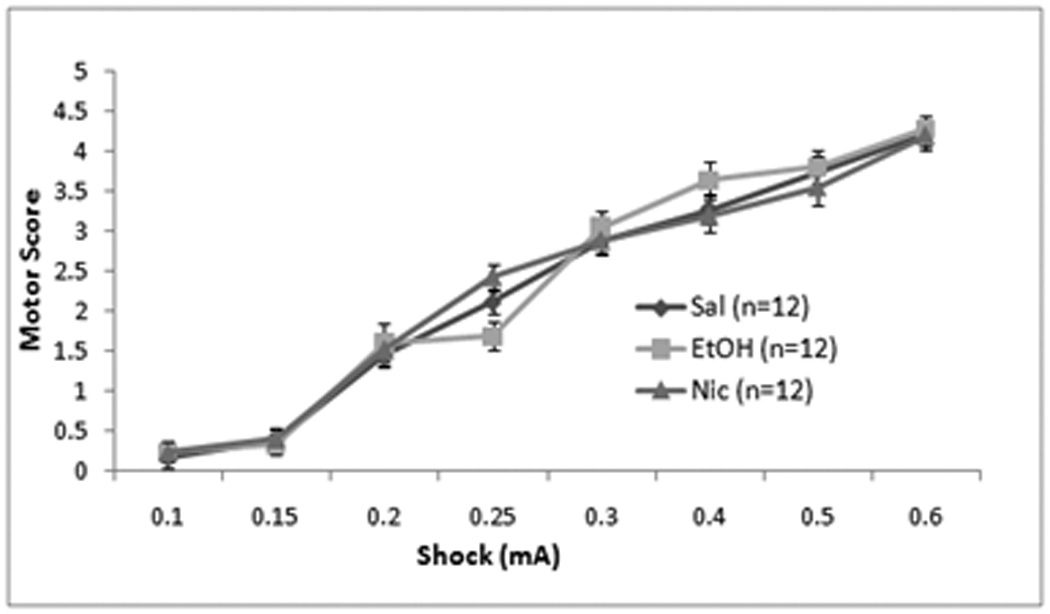
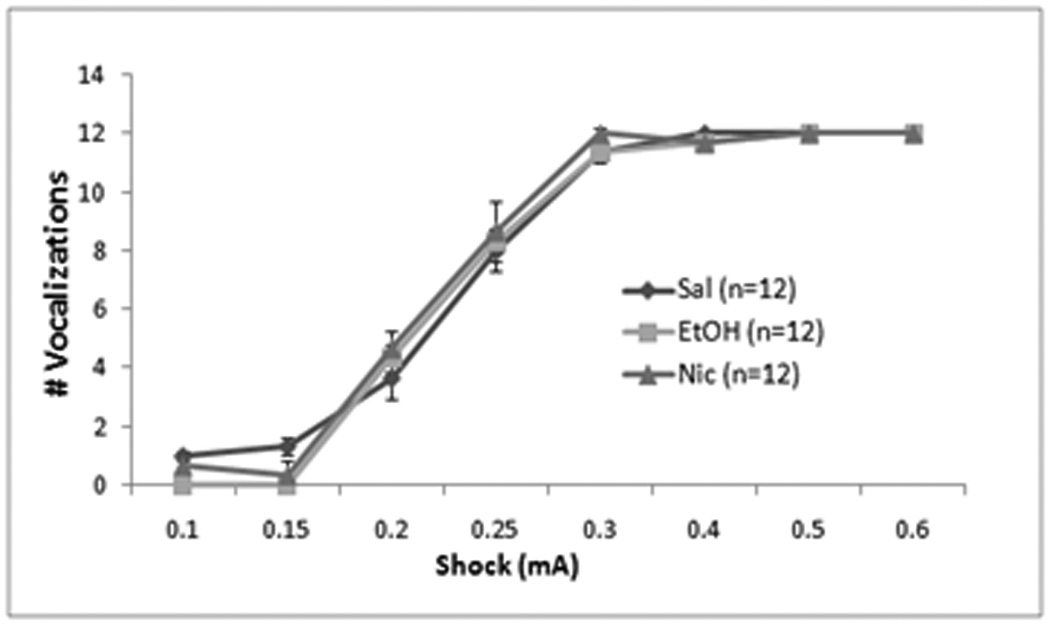
Effects of systemic saline, ethanol (1.0 g/kg), or nicotine (0.09 mg/kg) on shock sensitivity. There were no significant differences between groups in motor behavior (Mean ± SEM) (A) or in vocalizations (# vocalizations ± SEM) (B) at any shock intensity.
Discussion
Nicotine enhancement of learning and amelioration of ethanol-associated learning deficits are mediated by different neural processes. High-affinity nAChRs in the dorsal hippocampus are involved in the enhancement of contextual fear conditioning by nicotine, while high-affinity nAChRs in the anterior cingulate mediate the effects of nicotine on ethanol-induced deficits in contextual and cued fear conditioning. Moreover, nicotine may enhance learning by activating β2-containing hippocampal nAChRs but ameliorate ethanol-induced learning deficits by inactivating β2-containing anterior cingulate nAChRs.
The dorsal hippocampus processes contextual information during fear conditioning (Esclassan et al, 2008; Maren et al, 1997; Otto and Poon, 2006; Phillips and LeDoux, 1994). Previous research has demonstrated that nicotine infusion into the dorsal hippocampus enhances contextual but not cued fear conditioning (Davis et al, 2007), demonstrating that the dorsal hippocampus is sufficient for the effects of nicotine on contextual fear conditioning. If nicotine is given systemically and the high-affinity nAChR antagonist DHβE is infused into the dorsal hippocampus, no enhancement of contextual fear conditioning is seen (Davis et al, 2007), demonstrating that the dorsal hippocampus is necessary for the enhancement of contextual fear conditioning. However, if nicotine is infused into the dorsal hippocampus of ethanol-treated mice, there is no amelioration of the ethanol-induced deficits in contextual and cued fear conditioning.
In contrast, infusion of nicotine into the anterior cingulate cortex ameliorated ethanol-induced deficits in cued and contextual fear conditioning but had no effect in ethanol-naïve mice. Furthermore, infusion of DHβE into the anterior cingulate disrupted the effects of systemic nicotine on the ethanol-induced deficits. In addition, infusion of nicotine ventral or lateral to the anterior cingulate cortex had no effect on ethanol-induced deficits in fear conditioning, and neither nicotine nor ethanol changed sensitivity to the foot-shock. Together these studies suggest that the anterior cingulate cortex is necessary and sufficient for the effects of nicotine on ethanol-induced deficits in fear conditioning.
The anterior cingulate cortex is involved in attention and cognitive control (Cohen et al, 1999; Posner and Rothbart, 1998; Ridderinkhof et al, 2004). Ethanol-induced changes in these or other cingulate-mediated processes could alter fear conditioning. In support, numerous studies have demonstrated a link between the cingulate cortex and fear conditioning. In one study, lesions of the anterior cingulate cortex did not disrupt standard cued fear conditioning but did disrupt trace fear conditioning when tested 24 hours after training (Han et al, 2003). However, another study found that lesions of the anterior cingulate cortex disrupted cued but not contextual fear conditioning when testing occurred 24 hours after training (Bissiere et al, 2008). In addition, the same study found that disruption of anterior cingulate cortical function via infusion of the GABAa antagonist muscimol altered cued fear conditioning (the effects on contextual fear conditioning were not reported). Similarly, Tang et al. (2005) reported that infusion of the NMDA receptor antagonist APV into the anterior cingulate cortex disrupted cued fear conditioning only but infusion of muscimol disrupted both cued and contextual fear conditioning one and three days post training. Furthermore, infusion of the muscarinic cholinergic agonist oxotremorine into the anterior cingulate cortex post training enhanced foot-shock US-related learning (Malin et al, 2006). Finally, Frankland and colleagues (2004) demonstrated the involvement of this area in remote (36 day old) fear conditioning memories. These results suggest that in addition to being involved in remote fear conditioning memories, the anterior cingulate cortex modulates the early phase of fear conditioning such that disruption of anterior cingulate cortical function during this stage may be detrimental to fear conditioning. Therefore, ethanol may disrupt anterior cingulate function, resulting in deficits in fear conditioning, and nicotine administration may counter this effect of ethanol.
Equally important as identifying the brain areas involved in the interactive effects of nicotine and ethanol on learning is identifying the receptor-level changes that underlie these effects. The effects of nicotine at nAChRs are complex, as nicotine can both activate and desensitize nAChRs, and these effects vary across receptor subtypes (Picciotto et al, 2008). α4β2* nAChRs are high-affinity receptors that make up the majority of neuronal nAChRs (Whiting and Lindstrom, 1986a; Whiting and Lindstrom, 1986b). Previous work and the present study suggest that nicotine enhances contextual fear conditioning by activating or activating and then desensitizing β2-containing nAChRs in the dorsal hippocampus (Davis et al, 2006), as the effects of nicotine on contextual fear conditioning are different than the effects of nAChR antagonists. β2–containing nAChRs also mediate the amelioration of ethanol-induced learning deficits by nicotine (Wehner et al, 2004), but the current findings suggest that these effects may involve nAChR inactivation. Just as nicotine ameliorated ethanol-induced deficits in fear conditioning, infusion of the nAChR antagonist DHβE into the anterior cingulate ameliorated ethanol-induced learning deficits. The effects of DHβE on ethanol-induced deficits in fear conditioning involve β2-containing nAChRs as β2 KO mice did not show DHβE amelioration of ethanol-induced learning deficits. Furthermore, co-administration of sub-threshold doses of DHβE and nicotine ameliorated ethanol-induced learning deficits, suggesting that nicotine and DHβE may act synergistically on the ethanol-induced deficits. This interaction may have a dose-dependent inverted U-shaped function as co-administration of higher doses of nicotine and DHβE disrupted fear conditioning. The mechanism through which nicotine ameliorates ethanol-induced deficits in fear conditioning is still unknown but may involve the depression of nAChR activity as DHβE and nicotine had similar effects.
An important issue that needs further study is how a decrease of nAChR activity in the cingulate cortex could ameliorate ethanol-induced deficits in fear conditioning. Perhaps ethanol disrupts fear conditioning by increasing nAChR activity in the cingulate cortex. Whereas ethanol-induced up-regulation of nAChR activity in the cingulate cortex has not been directly observed, it has been shown that ethanol potentiates α4β2-like nAChR currents in rat frontal cortical cell cultures (Aistrup et al, 1999; Marszalec et al, 1999). If ethanol is decreasing learning by over-activating nAChRs, then a decrease in nAChR activity via nicotine desensitization of the receptors or via inhibition of the receptors by the nAChR antagonist DHβE could return the system to a level of activity optimal for learning. The finding that DHβE blocks the effects of ethanol on fear conditioning supports this theory; however, the finding that β2 KO mice show ethanol-induced deficits in fear conditioning (Wehner et al, 2004 and present results) suggests this may not be the case. It is possible that an unknown developmental compensatory change in the β2 KO mice alters the mechanism underlying ethanol effects on fear conditioning allowing ethanol to disrupt fear conditioning. Alternatively, ethanol and nicotine/DHβE may act on separate processes that have opposing actions on a common downstream target.
Interestingly, imaging studies in humans have shown that nicotine and ethanol have opposite effects on cingulate activity (Ghatan et al, 1998; Schreckenberger et al, 2004). In addition, a single nucleotide polymorphism in the gene CHRNA4 was associated with changes in attention-related activity in the anterior cingulate cortex (Winterer et al, 2007). This complements our finding that high-affinity nAChRs (which could include α4β2* nAChRs) in the cingulate cortex are involved in the effects of nicotine on ethanol-induced deficits in fear conditioning. However, the cellular location and the processes mediated by these nAChRs remain to be determined. Picciotto et al. (2008) put forward a model where nicotine desensitization of nAChRs on GABAergic interneurons in the ventral tegmental area underlies the behavioral effects of nicotine on reward and mood; thus, GABAergic interneurons in the cingulate cortex could similarly be involved in the effects of nicotine on ethanol-induced deficits in fear conditioning. This, however, remains to be tested as presynaptic nAChR regulation of the release of other neurotransmitters, such as dopamine, is also a viable mechanism (see Exley and Cragg, 2008 for a discussion of nAChR activation and inactivation mediating dopamine tone).
In summary, the effects of nicotine on learning in ethanol naïve and ethanol-treated mice are mediated by different brain regions and different nAChR processes. Nicotine enhances contextual fear conditioning through either activation or activation followed by desensitization of hippocampal β2-containing nAChR. In comparison, nicotine acts in the anterior cingulate to ameliorate ethanol-induced deficits in fear conditioning. This amelioration may depend on inactivation of β2–containing nAChRs, as nicotine and the nAChR antagonist DHβE have similar effects on the ethanol-induced deficits. Thus, inactivation of nAChRs in the cingulate cortex could ameliorate ethanol-induced deficits in fear conditioning by countering the effects of ethanol in the cingulate cortex or in areas efferent of the cingulate cortex.
Acknowledgements
This work was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant AA015515 (T.J.G.) and National Institute for Drug Abuse (NIDA) Grant DA01794901 (T.J.G.). All research complies with current US laws for the care and use of laboratory animals.
Footnotes
Disclosure/Conflict of Interest
Danielle Gulick has no conflict of interest to disclose for the research reported herein.
Thomas J. Gould has no conflict of interest to disclose for the research reported herein.
References
- Aistrup GL, Marszalec W, Narahashi T. Ethanol modulation of nicotinic acetylcholine receptor currents in cultured cortical neurons. Molecular pharmacology. 1999;55(1):39–49. doi: 10.1124/mol.55.1.39. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. The Journal of pharmacology and experimental therapeutics. 1993;265(3):1455–1473. [PubMed] [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Annals of the New York Academy of Sciences. 2001;935:107–117. [PubMed] [Google Scholar]
- Bardgett ME, Boeckman R, Krochmal D, Fernando H, Ahrens R, Csernansky JG. NMDA receptor blockade and hippocampal neuronal loss impair fear conditioning and position habit reversal in C57Bl/6 mice. Brain research bulletin. 2003;60(1–2):131–142. doi: 10.1016/s0361-9230(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Bissiere S, Plachta N, Hoyer D, McAllister KH, Olpe HR, Grace AA, et al. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biological psychiatry. 2008;63(9):821–831. doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Magee L, Veniegas R, Blanchard DC. Alcohol and anxiety: ethopharmacological approaches. Progress in neuro-psychopharmacology & biological psychiatry. 1993;17(2):171–182. doi: 10.1016/0278-5846(93)90041-p. [DOI] [PubMed] [Google Scholar]
- Chiba AA, Kesner RP, Gibson CJ. Memory for temporal order of new and familiar spatial location sequences: role of the medial prefrontal cortex. Learning & memory (Cold Spring Harbor, NY. 1997;4(4):311–317. doi: 10.1101/lm.4.4.311. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, Wilkinson H. Impairments of attention after cingulotomy. Neurology. 1999;53(4):819–824. doi: 10.1212/wnl.53.4.819. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology. 2006;184(3–4):345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27(40):10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug and alcohol dependence. 2000;59(3):235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Decker MW, Brioni JD, Bannon AW, Arneric SP. Diversity of neuronal nicotinic acetylcholine receptors: lessons from behavior and implications for CNS therapeutics. Life sciences. 1995;56(8):545–570. doi: 10.1016/0024-3205(94)00488-e. [DOI] [PubMed] [Google Scholar]
- Escher T, Mittleman G. Effects of ethanol and GABAB drugs on working memory in C57BL/6J and DBA/2J mice. Psychopharmacology. 2004;176(2):166–174. doi: 10.1007/s00213-004-1875-x. [DOI] [PubMed] [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G, Marchand AR. Differential contribution of dorsal and ventral hippocampus to trace and delay fear conditioning. Hippocampus. 2008 doi: 10.1002/hipo.20473. [DOI] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. British journal of pharmacology. 2008;153(Suppl 1):S283–S297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faw B. Pre-frontal executive committee for perception, working memory, attention, long-term memory, motor control, and thinking: a tutorial review. Conscious Cogn. 2003;12(1):83–139. doi: 10.1016/s1053-8100(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science (New York, NY. 2004;304(5672):881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, et al. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology. 1998;136(2):179–189. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Ethanol disrupts fear conditioning in C57BL/6 mice. Journal of psychopharmacology (Oxford, England) 2003;17(1):77–81. doi: 10.1177/0269881103017001702. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins SJ. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiology of learning and memory. 2003a;80(2):147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci. 2003b;117(6):1276–1282. doi: 10.1037/0735-7044.117.6.1276. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behavioural brain research. 1999;102(1–2):31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Acute ethanol has biphasic effects on short- and long-term memory in both foreground and background contextual fear conditioning in C57BL/6 mice. Alcoholism, clinical and experimental research. 2007;31(9):1528–1537. doi: 10.1111/j.1530-0277.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology. 2008;196(3):483–495. doi: 10.1007/s00213-007-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CJ, O'Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, et al. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(22):13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. Journal of neurochemistry. 1996;67(5):1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(14):8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U, Hapke U, Rumpf HJ. A new measure of the alcohol dependence syndrome: the severity scale of alcohol dependence. European addiction research. 2003;9(2):87–93. doi: 10.1159/000068806. [DOI] [PubMed] [Google Scholar]
- Larsson A, Engel JA. Neurochemical and behavioral studies on ethanol and nicotine interactions. Neuroscience and biobehavioral reviews. 2004;27(8):713–720. doi: 10.1016/j.neubiorev.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111(1):104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Malin EL, Ibrahim DY, Tu JW, McGaugh JL. Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: interaction with the basolateral amygdala. Neurobiology of learning and memory. 2007;87(2):295–302. doi: 10.1016/j.nlm.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(6):1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioural brain research. 1997;88(2):261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Marszalec W, Aistrup GL, Narahashi T. Ethanol-nicotine interactions at alpha-bungarotoxin-insensitive nicotinic acetylcholine receptors in rat cortical neurons. Alcoholism, clinical and experimental research. 1999;23(3):439–445. [PubMed] [Google Scholar]
- NIH. 2007 http://pubs.niaaa.nih.gov/publications/AA71/AA71.htm.
- Otto T, Poon P. Dorsal hippocampal contributions to unimodal contextual conditioning. J Neurosci. 2006;26(24):6603–6609. doi: 10.1523/JNEUROSCI.1056-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. San Diego: Academic; 2001. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learning & memory (Cold Spring Harbor, NY. 1994;1(1):34–44. [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Progress in neurobiology. 2008;84(4):329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13(9):1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Pichika R, Easwaramoorthy B, Collins D, Christian BT, Shi B, Narayanan TK, et al. Nicotinic alpha4beta2 receptor imaging agents: part II. Synthesis and biological evaluation of 2-[18F]fluoro-3-[2-((S)-3-pyrrolinyl)methoxy]pyridine (18F-nifene) in rodents and imaging by PET in nonhuman primate. Nuclear medicine and biology. 2006;33(3):295–304. doi: 10.1016/j.nucmedbio.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiology of learning and memory. 2007 doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philosophical transactions of the Royal Society of London. 1998;353(1377):1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Nicotine withdrawal-induced deficits in trace fear conditioning in C57BL/6 mice–a role for high-affinity beta2 subunit-containing nicotinic acetylcholine receptors. The European journal of neuroscience. 2009;29(2):377–387. doi: 10.1111/j.1460-9568.2008.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science (New York, NY. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24(45):10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreckenberger M, Amberg R, Scheurich A, Lochmann M, Tichy W, Klega A, et al. Acute alcohol effects on neuronal and attentional processing: striatal reward system and inhibitory sensory interactions under acute ethanol challenge. Neuropsychopharmacology. 2004;29(8):1527–1537. doi: 10.1038/sj.npp.1300453. [DOI] [PubMed] [Google Scholar]
- Schrott LM, Crnic LS. Sensitivity to foot shock in autoimmune NZB × NZW F1 hybrid mice. Physiology & behavior. 1994;56(5):849–853. doi: 10.1016/0031-9384(94)90314-x. [DOI] [PubMed] [Google Scholar]
- Tang J, Ko S, Ding HK, Qiu CS, Calejesan AA, Zhuo M. Pavlovian fear memory induced by activation in the anterior cingulate cortex. Molecular pain. 2005;1:6. doi: 10.1186/1744-8069-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, et al. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129(1):11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction (Abingdon, England) 1996;91(7):921–949. discussion 951–965. [PubMed] [Google Scholar]
- Whiting P, Lindstrom J. Pharmacological properties of immuno-isolated neuronal nicotinic receptors. J Neurosci. 1986a;6(10):3061–3069. doi: 10.1523/JNEUROSCI.06-10-03061.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, Lindstrom JM. Purification and characterization of a nicotinic acetylcholine receptor from chick brain. Biochemistry. 1986b;25(8):2082–2093. doi: 10.1021/bi00356a037. [DOI] [PubMed] [Google Scholar]
- Williams M, Robinson JL. Binding of the nicotinic cholinergic antagonist, dihydro-beta-erythroidine, to rat brain tissue. J Neurosci. 1984;4(12):2906–2911. doi: 10.1523/JNEUROSCI.04-12-02906.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Musso F, Konrad A, Vucurevic G, Stoeter P, Sander T, et al. Association of attentional network function with exon 5 variations of the CHRNA4 gene. Human molecular genetics. 2007;16(18):2165–2174. doi: 10.1093/hmg/ddm168. [DOI] [PubMed] [Google Scholar]


