Abstract
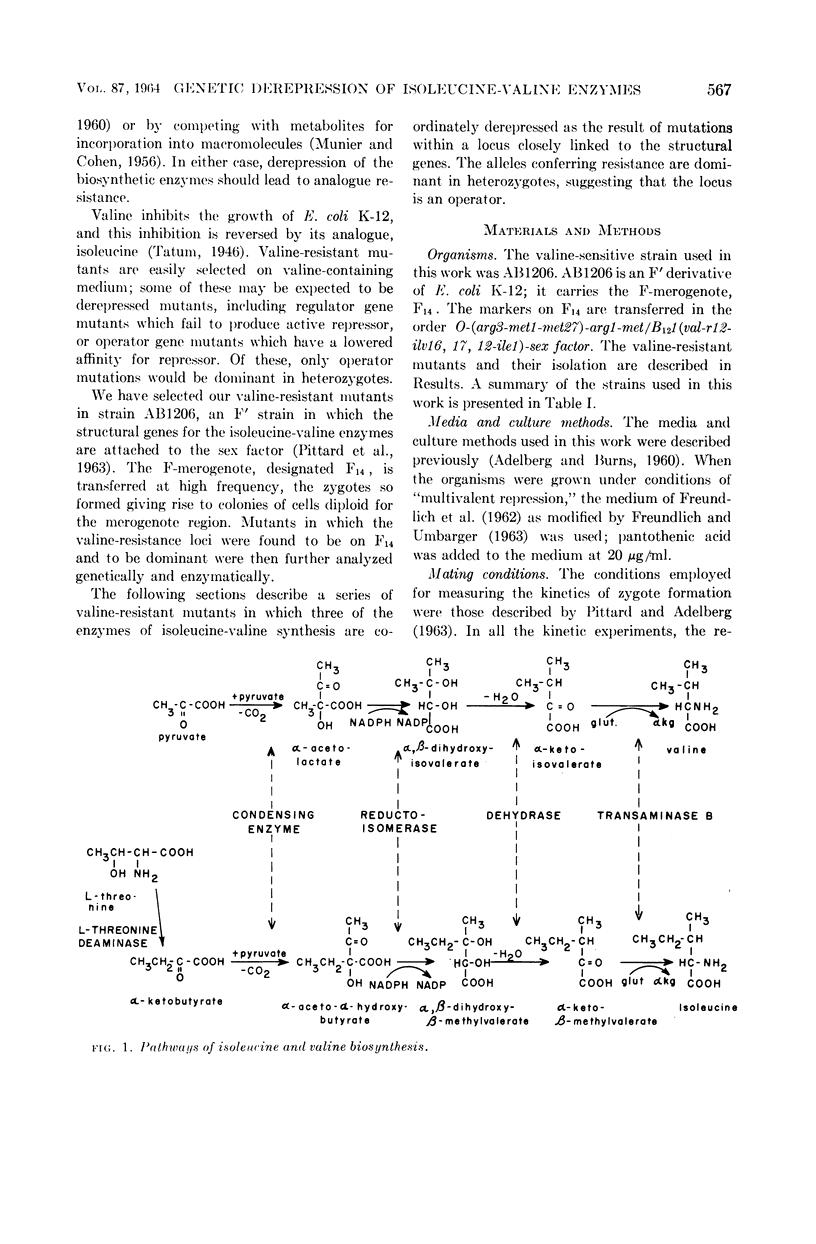
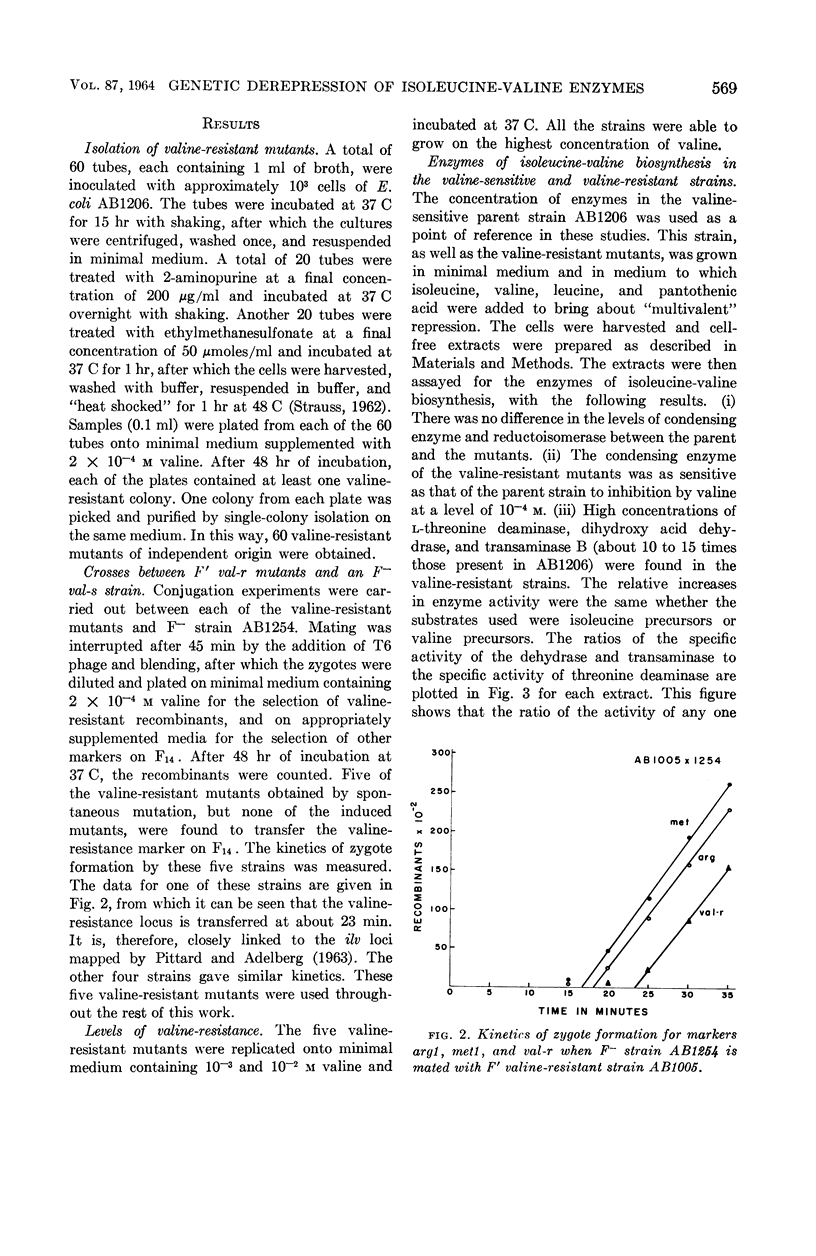
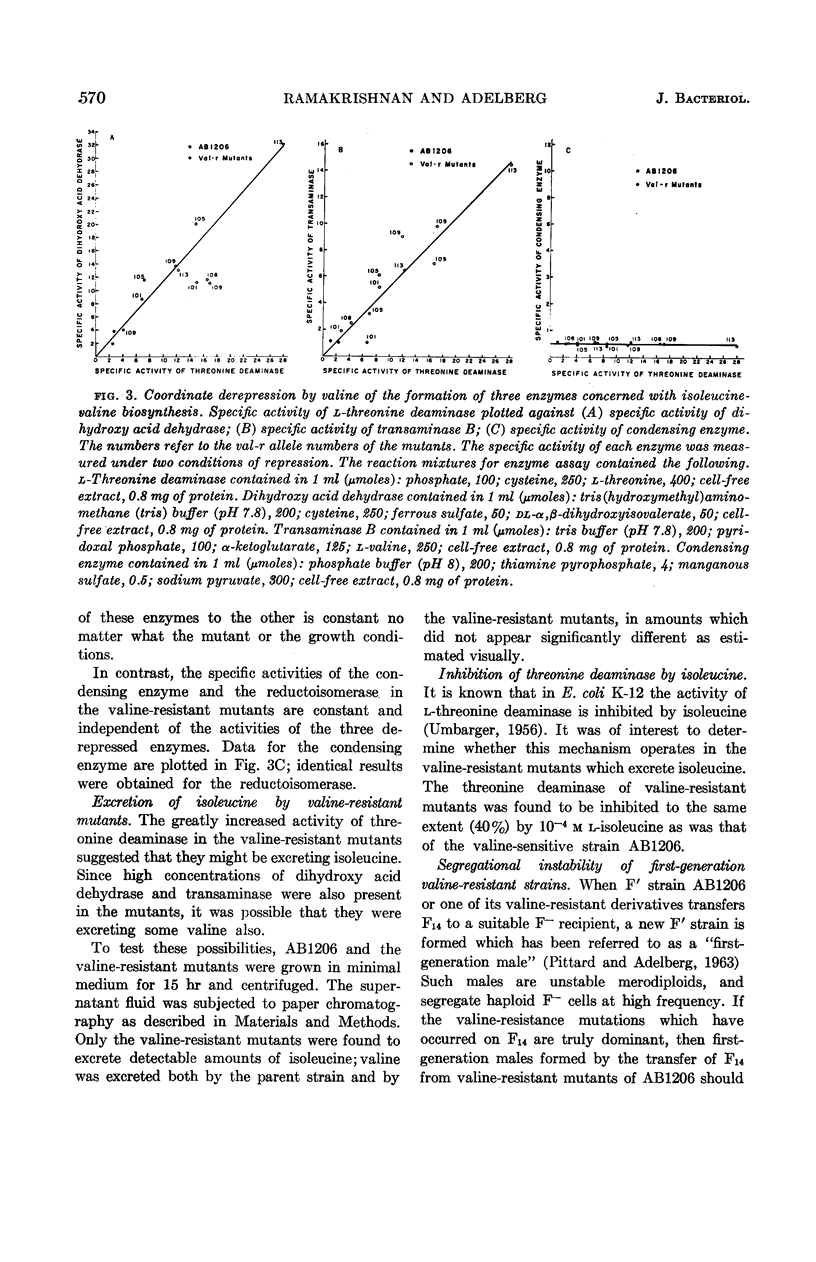
Ramakrishnan, T. (Yale University, New Haven, Conn.), and Edward A. Adelberg. Regulatory mechanisms in the biosynthesis of isoleucine and valine. I. Genetic derepression of enzyme formation. J. Bacteriol. 87:566–573. 1964.—A total of 60 mutants of Escherichia coli K-12 resistant to 10−2m valine were isolated from the valine-sensitive F′ strain AB1206. Conjugation experiments showed that in five of these mutants the valine-resistance locus is closely linked to the structural genes governing isoleucine-valine biosynthesis. In these five valine-resistant mutants, three enzymes of the isoleucine-valine pathway were found to be coordinately derepressed: l-threonine deaminase, dihydroxy acid dehydrase, and transaminase B. Two other enzymes of this pathway, the condensing enzyme and the reductoisomerase, were unaffected. The mutation from valine-sensitivity to valine-resistance appears to have altered an operator locus, because the derepressed state is dominant over the repressed state in diploids heterozygous for the valine-resistance locus. The valine-resistant mutants excrete isoleucine into the medium. The significance of these findings with respect to the valine-sensitivity of E. coli K-12 and the regulation of the biosynthesis of isoleucine and valine by this organism are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADELBERG E. A. Selection of bacterial mutants which excrete antagonists of antimetabolites. J Bacteriol. 1958 Sep;76(3):326–326. doi: 10.1128/jb.76.3.326-326.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARMSTRONG F. B., WAGNER R. P. Biosynthesis of valine and isoleucine. IV. alpha-Hydroxy-beta-keto acid reductoisomerase of Salmonella. J Biol Chem. 1961 Jul;236:2027–2032. [PubMed] [Google Scholar]
- Ames B. N., Garry B. COORDINATE REPRESSION OF THE SYNTHESIS OF FOUR HISTIDINE BIOSYNTHETIC ENZYMES BY HISTIDINE. Proc Natl Acad Sci U S A. 1959 Oct;45(10):1453–1461. doi: 10.1073/pnas.45.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANGEUX J. P. The feedback control mechanisms of biosynthetic L-threonine deaminase by L-isoleucine. Cold Spring Harb Symp Quant Biol. 1961;26:313–318. doi: 10.1101/sqb.1961.026.01.037. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., MUNIER R. Incorporation d'analogues structuraux d'aminoacides dans les protéines bactériennes. Biochim Biophys Acta. 1956 Sep;21(3):592–593. doi: 10.1016/0006-3002(56)90207-4. [DOI] [PubMed] [Google Scholar]
- COHEN G. N. Synthèse de protéines anormales chez Escherichia coli K 12 cultive en présence de L-valine. Ann Inst Pasteur (Paris) 1958 Jan;94(1):15–30. [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville E V, Demerec M. Threonine, Isoleucine, and Isoleucine-Valine Mutants of Salmonella Typhimurium. Genetics. 1960 Oct;45(10):1359–1374. doi: 10.1093/genetics/45.10.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. X. The enzymatic formation of acetohydroxybutyrate. J Biol Chem. 1961 Sep;236:2486–2491. [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOYED H. S. Induced phenotypic resistance to an antimetabolite. Science. 1960 May 13;131(3411):1449–1449. doi: 10.1126/science.131.3411.1449. [DOI] [PubMed] [Google Scholar]
- MYERS J. W. Dihydroxy acid dehydrase: an enzyme involved in the biosynthesis of isoleucine and valine. J Biol Chem. 1961 May;236:1414–1418. [PubMed] [Google Scholar]
- PITTARD J., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. II. INTERACTION BETWEEN F-MEROGENOTE AND CHROMOSOME DURING TRANSFER. J Bacteriol. 1963 Jun;85:1402–1408. doi: 10.1128/jb.85.6.1402-1408.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., LOUTIT J. S., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. I. DELAY IN INITIATION OF CHROMOSOME TRANSFER. J Bacteriol. 1963 Jun;85:1394–1401. doi: 10.1128/jb.85.6.1394-1401.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADHAKRISHANAN A. N., SNELL E. E. Biosynthesis of valine and isoleucine. 2. Formation of alpha-acetolactate and alpha-aceto-alpha-hydroxybutyrate in Neurospora crassa and Escherichia coli. J Biol Chem. 1960 Aug;235:2316–2321. [PubMed] [Google Scholar]
- RUDMAN D., MEISTER A. Transamination in Escherichia coli. J Biol Chem. 1953 Feb;200(2):591–604. [PubMed] [Google Scholar]
- STRAUSS B. S. Response of Escherichia coli auxotrophs to heat after treatment with mutagenic alkyl methanesulfonates. J Bacteriol. 1962 Feb;83:241–249. doi: 10.1128/jb.83.2.241-249.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. V. Antagonism between isoleucine and valine. J Bacteriol. 1955 Aug;70(2):241–248. doi: 10.1128/jb.70.2.241-248.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E. Evidence for a negative-feedback mechanism in the biosynthesis of isoleucine. Science. 1956 May 11;123(3202):848–848. doi: 10.1126/science.123.3202.848. [DOI] [PubMed] [Google Scholar]


