Abstract
Several tools have proved useful in the study of invariant natural killer T (iNKT) cells, including CD1d-deficient mice, Jα281-deficient mice, synthetic lipid antigens and antigen-loaded CD1d tetramers. However, the generation and examination of long-term primary murine iNKT cell lines in vitro has been challenging. Here, we show the rapid generation of iNKT cell lines from splenic iNKT cells of Vα14 T-cell receptor (TCR) transgenic (Tg) mice. These purified iNKT cells were stimulated by bone marrow-derived dendritic cells (BMDCs) loaded with α-galactosylceramide (αGalCer) and cultured with interleukin (IL)-2 and IL-7. iNKT cells proliferated dramatically, and the cell number exhibited a 100-fold increase within 2 weeks and a 105-fold increase in 8 weeks after repeated stimulation with αGalCer. The iNKT cell lines consisted of iNKT cells expressing Vβ chains including Vβ8.1/8.2, Vβ14, Vβ10, Vβ6 and Vβ7, and responded to stimulation with αGalCer presented both by BMDCs and by plate-bound CD1d. In addition, the iNKT cell lines produced interferon (IFN)-γ when activated by lipopolysaccharide (LPS) or CpG oligodeoxynucleotide (ODN)-stimulated BMDCs. Further, we show that iNKT cell lines produced cytokines in response to microbial antigens. In summary, high-yield iNKT cell lines were generated very rapidly and robustly expanded, and these iNKT cells responded to both TCR and cytokine stimulation in vitro. Given the desire to study primary iNKT cells for many purposes, these iNKT cell lines should provide an important tool for the study of iNKT cell subsets, antigen and TCR specificity, activation, inactivation and effector functions.
Introduction
Invariant natural killer T cells (iNKT cells) are a subset of T cells that recognize lipid antigens presented by the CD1d molecule.1–3 iNKT cells express an invariant T-cell receptor α (TCR-α) chain (Vα14-Jα18 in mouse and Vα24-Jα18 in human) paired with TCR-β chains which are mostly restricted to Vβ8, Vβ7 and Vβ2 in mouse and Vβ11 in human. Even resting iNKT cells show a recently activated or memory phenotype and respond very rapidly upon stimulation. The evidence that iNKT cells from germ-free mice and human cord blood express activated/memory markers suggests that iNKT cells are already partially activated by autoantigens in vivo. When iNKT cells are activated by TCR stimulation with anti-CD3 antibodies, the pharmacological agent α-galactosylceramide (αGalCer) or microbial lipid antigens, they produce large amounts of cytokines including interferon (IFN)-γ and interleukin (IL)-4 and up-regulate CD40 ligand (CD40L).1–4 iNKT cell activation has a large impact on both innate and adaptive lymphocytes. For instance, iNKT cells can regulate major histocompatibility complex (MHC)-restricted T-cell polarization, dendritic cell (DC) maturation, and B-cell production of antibody.1–3 iNKT cell activation induces neutrophil recruitment, NK cell cytotoxicity and IFN-γ production.
iNKT cells can be activated by distinct lipid antigens from microbes, including α-galactosyldiacylglycerol from Borrelia species and α-glucuronosylceramide from Sphingomonas species.5–8 In addition, iNKT cells are also activated by cytokines produced by antigen-presenting cells (APCs) following stimulation by Toll-like receptor (TLR) agonists, such as lipopolysaccharide (LPS) and CpG olygodeoxynucleotide (ODN).9–12 IL-12 secreted by LPS-stimulated APCs induced iNKT cell IFN-γ production by enhancing NKT cell autoreactivity. The combination of IL-12 and IL-18 was shown to be sufficient for iNKT cell activation without APCs in an in vitro system.10 More recently, DCs stimulated with the TLR9 agonist CpG ODN were demonstrated to activate iNKT cells by producing type I interferons and to generate more stimulatory self-lipid antigens in the APC.11
iNKT cells are known to be involved in various types of immune responses, including infection, autoimmunity and tumour rejection. For instance, the activation of iNKT cells has been reported in mice infected with Cryptococcus neoformans, Leishmania major, Mycobacterium bovis bacillus Calmette–Guérin, Salmonella, Sphingomonas, and Ehrlichia.5,9,13–17 iNKT cells are critical for the control of infection with Streptococcus pneumoniae, Borrelia burgdorferi and Tripanosoma cruzi as well as methylcholanthrene-induced tumours.18–23 iNKT cells may also contribute to pathology in animal models of airway hyperreactivity, inflammatory arthritis, oxazalone-induced colitis, and atherosclerosis.24–29 In spite of many studies demonstrating iNKT cell involvement in immune responses, little is known about microbial iNKT cell antigens except for recently discovered lipid antigens such as α-glucuronosylceramide from Sphingomonas and α-galactosyldiacylglycerol from B. burgdorferi.5–8
Several tools have proved useful in the study of iNKT cells, including CD1d-deficient mice, Jα281-deficient mice, and antigen-loaded CD1d tetramers. However, the generation of primary mouse iNKT cell lines and clones has proved difficult, limiting in vitro studies to freshly isolated cells or to the use of iNKT cell hybridoma tumour cell lines. Reliable long-term primary iNKT cell lines would provide reagents essential to the study of the activation, inactivation and effector functions of iNKT cells. Here, we developed a method of rapid generation of iNKT cell lines from splenic iNKT cells of Vα14 TCR transgenic (Tg) mice. These cell lines consisted of iNKT cells expressing representative TCR-Vβ, including Vβ8, Vβ14, Vβ10, Vβ6 and Vβ7. The iNKT cell lines had the capacity to produce various kinds of cytokines upon stimulation with αGalCer presented by bone marrow-derived dendritic cells (BMDCs) or by plate-bound CD1d in an antigen-presenting cell-free system. In addition, we demonstrate that they could be activated either by lipid antigens or by cytokine-driven mechanisms.
Materials and methods
Mice
Vα14-Jα281 transgenic (Tg) mice were provided by Dr Albert Bendelac.30 CD1d−/− mice were provided by Dr Mark Exley.31 C57BL6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal studies were approved by the Dana-Faber Cancer Institute Office for the Protection of Research Subjects. The animals were housed in a specific pathogen-free animal facility. Bone marrow of MyD88−/− C57BL6 mice was provided by Dr Koichi Kobayashi (Dana-Farber Cancer Institute, Boston, MA).
Antigen-presenting cells
BMDCs were grown from bone marrow progenitors for 6 days in the presence of granulocyte–monocyte colony-stimulating factor (GM-CSF; 10 ng/ml) and IL-4 (1 ng/ml) (R&D Systems, Minneapolis, MN) in complete RPMI medium (RPMI supplemented with l-glutamine and penicillin/streptomycin; Life Technologies, Carlsbad, CA) containing 10% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT). αGalCer (100 ng/ml) was added to the media on day 6, and αGalCer-pulsed BMDCs were harvested on day 7.
Isolation of iNKT cells from Vα14Tg mouse spleens
A single-cell suspension of splenocytes from Vα14 Tg mice was obtained by pressing spleens through a 70-μm cell strainer. Cells were treated with erythrocyte lysis buffer. T cells were selected negatively using the Pan T Isolation Kit following the manufacturer’s protocol (Miltenyi Biotec, Auburn, CA). T cells were labelled with APC-conjugated CD1d tetramer loaded with PBS-57 lipid antigen (National Institutes of Health Tetramer Core Facility, Atlanta, GA) and iNKT cells were sorted using anti-APC beads (Miltenyi Biotec). The purity of iNKT cells was higher than 95%.
In vitro expansion of iNKT cells
2 × 106 iNKT cells were cultured with 2 × 105 irradiated BMDCs pulsed with αGalCer per well in 24-well plates in complete RPMI medium containing 10% FBS. Three to four days later, IL-2 (10 U/ml) (R&D Systems) and IL-7 (10 ng/ml) (PeproTech, Rocky Hill, NJ) were added to the media. iNKT cells were stimulated with irradiated αGalCer-pulsed BMDCs every 2–3 weeks.
Flow cytometry
Cells were incubated with anti-CD16/32 to avoid non-specific staining, and then stained with CD1d/PBS-57 tetramer conjugated with allophycocyanin (National Institutes of Health Tetramer Core Facility) and the following antibodies: anti-CD3, anti-CD4, anti-CD8, anti-CD19, anti-CD25, anti-CD28, anti-CD69, anti-CD44, anti-CD132, anti-CD212, anti-DX5, anti-NK1.1, anti-Vβ2, anti-Vβ6, anti-Vβ7, anti-Vβ8, anti-Vβ8.1/8.2, anti-Vβ10, anti-Vβ12, anti-Vβ14 (BD Biosciences, San Jose, CA), anti-IL-18Rα/IL-1RR5 (R&D Systems), anti-CD25, anti-CD122 and anti-CD127 (eBioscience, San Diego, CA). Samples were analysed with FACS Canto (Becton Dickinson, Franklin Lakes, NJ) and flowjo software was used to analyse the data (Tree Star Inc., Ashland, OR). PBS-57 is an analogue of αGalCer, and CD1d/PBS-57 tetramers have been shown to stain NKT cells comparably to CD1d/αGalCer tetramers. NKT cells were identified as CD3positive CD1d/PBS-57 tetramerpositive cells.
iNKT cell stimulation with BMDCs
iNKT cells (1 × 105) were cultured with BMDCs (1 × 105) or MyD88−/− BMDCs (1 × 105) in 96-well flat- or U-bottomed plates (Costar, Lowell, MA) in complete RPMI medium. Antigens or LPS (Sigma-Aldrich, St Louis, MO) or CpG ODN 1826 (Invitrogen, Carlsbad, CA) were added and cultured for 16–24 hr. The levels of IFN-γ, IL-2, IL-4, IL-13 and IL-17 in the culture supernatants were measured by standard sandwich enzyme-linked immunosorbent assay (ELISA) using purified and biotinylated monoclonal antibody (mAb) pairs and standard from BD Biosciences (IFN-γ, IL-2 and IL-4) and R&D Systems (IL-13 and IL-17).
mCD1d fusion protein iNKT cell stimulation assay
Mouse CD1d-Fc β2-microglobuin fusion protein was prepared as described previously.32 Ninety-six-well flat plates (Costar) were incubated with fusion protein (0·4 μg/well) in phosphate-buffered saline (PBS) for 2 hr and αGalCer was added at a molar ratio of 40 : 1 of antigen to fusion protein. After overnight incubation at 37°, plates were washed twice with PBS and once with culture medium. Cells were added to each well at a density of 5 × 105 cells per well and incubated at 37° for 16–24 hr.
Results
Rapid generation of murine iNKT cell lines
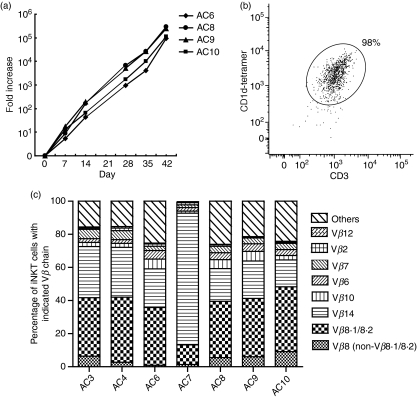
Upon TCR stimulation, iNKT cells become activated very rapidly in vivo, produce cytokines and proliferate. Within a few hours after TCR stimulation with αGalCer, activated iNKT cells down-modulate their TCR and become undetectable by αCD3mAb or CD1d tetramer.33–35 However, iNKT cells then proliferate and undergo significant expansion for 2–3 days and re-express TCR on the cell surface. After the expansion phase, most iNKT cells undergo cell death, and remaining NKT cells are anergic to re-stimulation with αGalCer for several weeks.15,36–38 This may be one of the reasons why iNKT cells are difficult to maintain in culture. Therefore, we set out to generate murine iNKT cell lines from purified iNKT cells instead of whole splenocytes. iNKT cells were sorted from spleen cells of Vα14 TCR Tg mice to obtain larger numbers of cells. 2 × 106 iNKT cells were co-cultured with 2 × 105 irradiated BMDCs loaded with αGalCer (100 ng/ml). Three to five days later, when iNKT cells started to actively proliferate, murine IL-2 (mIL-2) (10 IU/ml) and mIL-7 (10 ng/ml) were added to the culture medium. iNKT cells were re-stimulated with αGalCer-loaded BMDCs every 2–3 weeks, and cell numbers were counted at the time-points indicated in Fig. 1(a). iNKT cells expanded dramatically, and 42 days after the primary stimulation, cell number showed a 1·9 × 105-fold increase (Fig. 1a). To evaluate the purity of expanded cells, we stained cells for αCD3 and CD1d/PBS-57 tetramer (hereafter referred to as CD1d tetramer). iNKT cells down-modulate their TCR on the cell surface after αGalCer stimulation, and the proportion of CD3positive CD1d tetramerpositive cells was rather low 5 days after stimulation (82%). However, at day 14 post αGalCer-BMDC stimulation, 94% of cells were positive for CD3 and CD1d tetramer. We further studied cells maintained for 8 weeks in culture, and found that 98% of cells were positive for CD3 and CD1d tetramer (Fig. 1b), indicating that high-yield iNKT cell lines were generated.
Figure 1.
Rapid generation of murine invariant natural killer T (iNKT) cell lines in vitro. iNKT cells were purified from spleens of Vα14 transgenic (Tg) mice. 2 × 106 iNKT cells were co-cultured with 2 × 105 bone marrow dendritic cells (BMDCs) loaded with α-galactosylceramide (αGalCer) (100 ng/ml). Three to five days later, m (murine) IL-2 (10 U/ml) and mIL-7 (10 ng/ml) were added to the culture medium, and cells were stimulated with αGalCer-BMDCs every 2–3 weeks. (a) Fold increase in cell numbers of four independent cell lines (AC6, AC8, AC9 and AC10) at the indicated time-points. (b) Anti-CD3e-fluorescein isothiocyanate (FITC) and CD1d tetramer-allophycocyanin staining of an iNKT cell line at 8 weeks post in vitro culture. Results are representative of two separate experiments. (c) Vβ usage of iNKT cell lines. iNKT cell lines (AC3, AC4, AC6, AC7, AC8, AC9 and AC10) were stained with a panel of anti-Vβ monoclonal antibodies (mAbs). Frequencies of iNKT cells with indicated Vβ chains are expressed as percentages in the 100% stacked column chart. Each column represents the percentage of iNKT cells with the indicated Vβ chains contributing to the total iNKT cells.
Vβ usage of iNKT cell lines
The Vβ usage of murine iNKT cells is known to be predominantly restricted to Vβ8.1/8.2, Vβ7 and Vβ2. It has been reported that about 1–2% of iNKT cells express Vβ6, Vβ10 and Vβ14 in C57BL6 mice.39,40 To investigate whether iNKT cell lines consist of cells with various TCR-Vβ or iNKT cells with a specific TCR-Vβ expanded, we stained iNKT cell lines with mAbs against several TCR-Vβ chains. We found that all iNKT cell lines consist of various Vβ but are biased towards Vβ8, Vβ14, Vβ10, Vβ6 and Vβ7 (Fig. 1c and Table1). The percentages of Vβ14+, Vβ6+, Vβ7+ and Vβ2+ cells among iNKT cells in the spleens of Vα14Tg mice were about 1–2%. The percentages of Vβ6+, Vβ7+ and Vβ2+ cells of iNKT cell lines showed a 2- to 5-fold increase compared with those of Vα14Tg spleen iNKT cells (Fig. 1c and Table 1), and the average frequency of Vβ14+ cells in iNKT cell lines showed a 40-fold increase following culture (Table 1).
Table 1.
Vβ usage of spleen Invariant natural killer T (iNKT) cells and iNKT cell lines
| Vβ8.1/8.2 | Vβ14 | Vβ10 | Vβ6 | Vβ7 | Vβ2 | Vβ12 | |
|---|---|---|---|---|---|---|---|
| Spleen iNKT cell | 34·3 ± 3·7 | 0·8 ± 0·9 | 2·0 ± 0·8 | 0·8 ± 0·4 | 1·9 ± 0·3 | 0·2 ± 0·0 | 1·1 ± 0·5 |
| iNKT cell line | 32·8 ± 9·5 | 32·6 ± 23·8* | 3·7 ± 1·9 | 3·4 ± 1·3* | 3·8 ± 1·4* | 1·0 ± 0·4* | 0·6 ± 0·2 |
Splenocytes of Vα14Tg mice were stained with anti-CD19 monoclonal antibody (mAb), anti-CD3e mAb, CD1d-tetramer and each anti-Vβ mAb. iNKT cells were identified as CD19negative CD3positive CD1d-tetramer positive cells. iNKT cell lines were stained with a panel of anti-Vβ mAbs. The frequencies of cells expressing the indicated Vβ chains among iNKT cells are expressed as an average percentage ± standard deviation. n = 3–7 per group.
< 0·05, Welche’s t-test.
iNKT cell lines produce cytokines following αGalCer stimulation
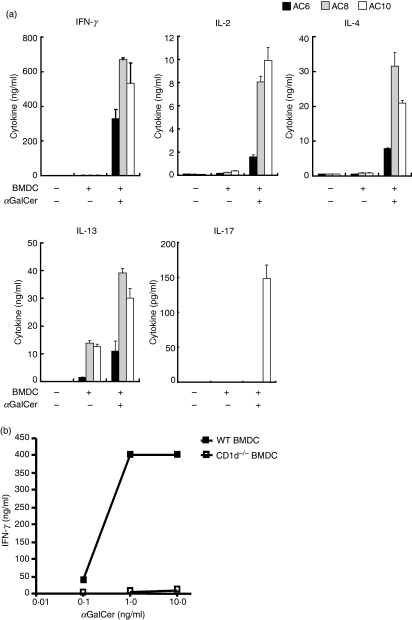
One of the unique characteristics of iNKT cells is their capacity to secrete various cytokines rapidly upon TCR engagement. We asked whether these iNKT cell lines are able to produce cytokines upon stimulation with αGalCer. iNKT cell lines were rested at least 2 weeks after stimulation with αGalCer-loaded BMDCs, and cells were washed and re-suspended with complete RPMI medium without cytokines. iNKT cells and BMDCs were incubated with or without αGalCer for 16–24 hr at 37°, and the culture supernatants were used for ELISA to evaluate cytokine production by iNKT cell lines. iNKT cell lines produced large amounts of cytokines, including IFN-γ, IL-2, IL-4 and IL-13, upon αGalCer stimulation (Fig. 2a). IFN-γ production by iNKT cell lines was CD1d dependent as αGalCer-stimulated CD1d−/− BMDCs failed to secrete any cytokines (Fig. 2b). Some NKT cell lines secreted IL-13 upon co-culture with BMDCs without αGalCer stimulation (Fig. 2a). However, they did not produce IL-13 when incubated with CD1d−/− BMDCs (data not shown). This finding is consistent with previous reports that human NKT cells produce IL-13 and GM-CSF by responding to autoantigens.41
Figure 2.
Invariant natural killer T (iNKT) cell lines produce cytokines after α-galactosylceramide (αGalCer) stimulation. iNKT cell lines (AC6, AC8 and AC10) stimulated with αGalCer more than 2 weeks earlier were washed and re-suspended with complete RPMI medium without cytokines. (a) 1 × 105 iNKT cells were incubated with 1 × 105 bone marrow dendritic cells (BMDCs) and αGalCer (100 ng/ml). Supernatants were collected and cytokine production was measured by enzyme-linked immunosorbent assay (ELISA). (b) 1 × 105 iNKT cells were incubated with 1 × 105 wild-type (WT) BMDCs or CD1d−/− BMDCs and αGalCer. The interferon (IFN)-γ content in culture supernatants was determined by ELISA. Results are representative of two separate experiments.
IL-17 is a proinflammatory cytokine that is known to be largely secreted by a newly identified T-cell subset, Th17 cells. Recently, several groups reported that iNKT cells also produce IL-17 upon stimulation.42–44 Therefore, we investigated whether iNKT cell lines secrete IL-17. We found that three out of six iNKT cell lines produced IL-17 in response to αGalCer stimulation (Fig. 2a, data not shown).
iNKT cell lines become activated by αGalCer and secrete cytokines in an APC-free system
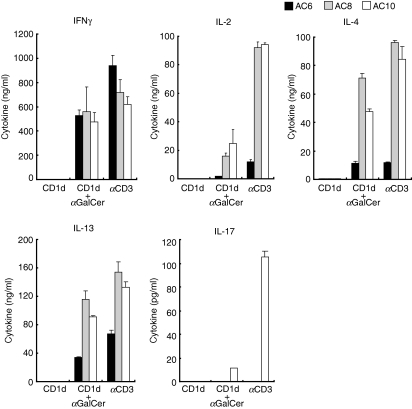
It is well recognized that iNKT cells are autoreactive and produce cytokines responding to APCs without exogenous antigens. In addition, cytokines produced by APCs stimulated with TLR agonists have also been shown to activate iNKT cells in the absence of foreign antigens. Therefore, we utilized an antigen presentation APC-free system recombinant CD1d to confirm the antigen specificity of the iNKT cell response. We asked whether iNKT cells can be fully activated by CD1d loaded with αGalCer without APCs. iNKT cell lines were incubated with plate-bound, recombinant, murine CD1d fusion protein loaded or unloaded with αGalCer. The culture supernatants were collected after 16–24 hr of incubation for analysis of the cytokine concentration. iNKT cell lines did not produce any cytokines when incubated with unloaded CD1d fusion protein, whereas iNKT cells secreted cytokines including IFN-γ, IL-2, IL-4 and IL-13 upon stimulation with CD1d fusion protein loaded with αGalCer (Fig. 3).
Figure 3.
Invariant natural killer T (iNKT) cell lines become activated and secrete cytokines in response to α-galactosylceramide (αGalCer) in an APC-free system. iNKT cell lines (AC6, AC8 and AC10) stimulated with αGalCer more than 2 weeks earlier were washed and re-suspended with complete RPMI medium without cytokines. 1 × 105 iNKT cells were incubated in the presence of plate-bound, recombinant CD1d-Fc fusion protein loaded with αGalCer or mock loaded with dimethyl sulphoxide (DMSO). Supernatants were collected and cytokine production was measured by enzyme-linked immunosorbent assay (ELISA). Results are representative of two separate experiments.
Expression of surface molecules on iNKT cell lines
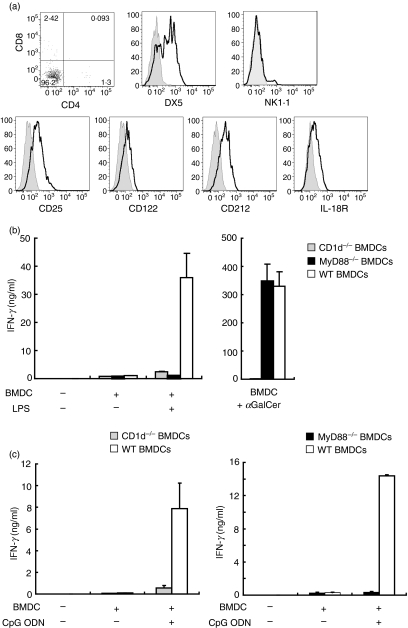
Many iNKT cells express natural killer markers, including NK1.1 and DX5.45–47 We found that, although 70% of iNKT cell lines were positive for DX5, NK1.1 was negative on all iNKT cell lines (Fig. 4a). αGalCer-activated iNKT cells down-modulate NK1.1 from the cell surface and this NK1.1 down-modulation lasts for several weeks. iNKT cell lines were stimulated with αGalCer-loaded BMDCs every 2–3 weeks; accordingly it was very likely that the majority of the cultivated iNKT cells were negative for NK1.1 as a result of TCR stimulation.
Figure 4.
Invariant natural killer T (iNKT) cell lines express cytokine receptors and become activated via a cytokine-driven mechanism. (a) Expression of surface molecules on iNKT cells. iNKT cell lines were stained with anti-CD3e-fluorescein isothiocyanate (FITC), CD1d-tetramer-allophycocyanin, and a panel of surface molecules. Expression of CD4, CD8, DX5, NK1.1, CD25, CD122, CD212 and interleukin (IL)-18R was determined on CD3-positive CD1d-tetramer-positive NKT cells. The open histograms indicate staining with each monoclonal antibody (mAb) and the grey histograms represent the isotype controls. Results are representative of two separate experiments. (b) iNKT cell line activation by lipopolysaccharide (LPS)-stimulated bone marrow dendritic cells (BMDCs) is CD1d and MyD88 dependent. 1 × 105 NKT cells were incubated with LPS (10 μg/ml) or α-galactosylceramide (αGalCer; 10 ng/ml) in the presence of 1 × 105 wild-type (WT) BMDCs, CD1d−/− BMDCs, or MyD88−/− BMDCs. Supernatants were collected and interferon (IFN)-γ production was determined by enzyme-linked immunosorbent assay (ELISA). (c) CD1d- and MyD88-dependent iNKT cell line activation by CpG oligodeoxynucleotide (ODN). 1 × 105 NKT cells were incubated with CpG ODN (125 ng/ml) in the presence of 1 × 105 WT BMDCs, CD1d−/− BMDCs or MyD88−/− BMDCs. IFN-γ production in supernatants was measured by ELISA. Results are representative of two separate experiments.
Most murine iNKT cells are CD4 positive and the remaining iNKT cells are double-negative (DN) in the periphery. The freshly isolated iNKT cells from Vα14Tg spleens were CD4+ (30%) or DN (data not shown). However, the proportions of CD4+ iNKT cells decreased at day 5 post αGalCer-BMDC stimulation (from 31·9 ± 0·61% to 5·8 ± 3·34%). The level of CD4+ iNKT cells at day 14 remained the same as the level at day 5, and most of the iNKT cells were DN by day 14 (Fig. 4a). It has been shown that, among iNKT cells, CD4negative NK1.1negative cells produce IL-17.42–44 However, the proportion of CD4negative iNKT cells did not correlate with the IL-17-producing ability of the iNKT cell lines examined here (data not shown).
Next, we sought to determine the surface expression of receptors of cytokines such as IL-2, IL-12 and IL-18. iNKT cell lines were positive for the IL-2 receptor α and β chains, indicating they are in an active state (Fig. 4a). Further iNKT cell lines also expressed IL-12 and IL-18 receptors, suggesting their potential ability to respond to these cytokines (Fig. 4a).
iNKT cell lines can also be activated by a cytokine-driven mechanism
Cytokines produced by APCs stimulated with TLR agonists have been shown to activate NKT cells without exogenous antigens.5,9–11 Therefore, we asked whether in vitro cultured iNKT cell lines can produce cytokines when activated by DCs stimulated by a TLR agonist in the absence of exogenously added antigens. iNKT cells and BMDCs were cultured with or without LPS and IFN-γ production in the culture supernatants was assessed by ELISA. iNKT cell lines cultured with wild-type (WT) BMDCs produced IFN-γ upon αGalCer or LPS stimulation (Fig. 4b). Although iNKT cell lines produced IFN-γ upon stimulation with αGalCer presented by MyD88−/− BMDCs, MyD88−/− BMDCs failed to activate iNKT cell lines following LPS stimulation (Fig. 4b). Further, iNKT cell lines were activated and produced IFN-γ when cultured with BMDCs stimulated when cultured with CpG ODN, whereas iNKT cell lines did not produce IFN-γ by CpG ODN-stimulated MyD88−/− BMDCs (Fig. 4c). These results indicate that iNKT cell lines can be activated by TLR agonist-stimulated BMDCs in a MyD88-dependent manner.
iNKT cell lines respond to a microbial antigens and produce cytokines
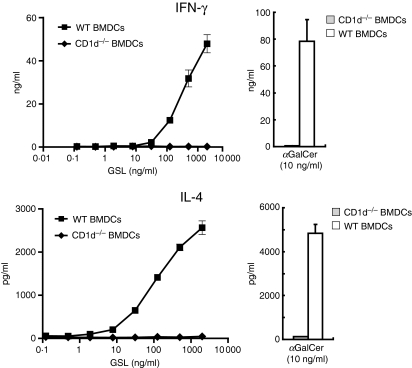
iNKT cells have been shown to be activated by glycolipid antigens from Sphingomonas bacterial species and B. burgdorferi. To assess whether the iNKT cell lines also recognize bacterial antigens and produce cytokines in vitro, iNKT cell lines were cultured with BMDCs and the glycolipid antigen glycosphingolipid (GSL)-1, and cytokine production in the culture supernatants was analysed by ELISA. iNKT cells did not respond to CD1d−/− BMDCs containing GSL-1 but responded to BMDCs cultured with GSL-1, and produced IFN-γ and IL-4 in an antigen dose-dependent manner (Fig. 5).
Figure 5.
Invariant natural killer T (iNKT) cell lines respond to a microbial antigen and produce cytokines. 1 × 105 NKT cells were incubated with the indicated concentrations of glycosphingolipid (GSL)-1 or α-galactosylceramide (αGalCer; 10 ng/ml) in the presence of 1 × 105 wild-type (WT) BMDCs or CD1d−/− BMDCs. Supernatants were collected to determine interferon (IFN)-γ and interleukin (IL)-4 production by enzyme-linked immunosorbent assay (ELISA). Results are representative of two separate experiments.
Together, these studies show that in vitro primary iNKT cell lines display characteristics of activation, specificity and cytokine production that are consistent with those expected for in vivo activated iNKT cells.
Discussion
It is possible to expand murine iNKT cells in vitro for a short time, but generation of durable iNKT cell lines is known to be difficult, as evidenced in the literature, as only two papers have reported that murine iNKT cells cultured for several weeks responded to αGalCer re-stimulation.48,49 In this report, we have demonstrated that high-yield murine iNKT cell lines can be generated within a few weeks by stimulating iNKT cells of Vα14Tg mice. iNKT cell lines were functional and responded to αGalCer and GSL-1 presented by BMDCs. We also showed that iNKT cell lines are able to produce cytokines when stimulated with αGalCer presented with recombinant, immobilized CD1d in an APC-free system. In addition, iNKT cell lines were activated by LPS or CpG ODN-stimulated BMDCs. Together, these results suggest that these iNKT cell lines will be a powerful tool for antigen identification and functional studies of iNKT cells.
Vβ usage of murine iNKT cells is known to be restricted to selected Vβ chains. It has been demonstrated that, without foreign antigens, APCs weakly stimulated iNKT cells that expressed Vβ2, Vβ7 and Vβ8.1/8.2. However, αGalCer-pulsed APCs activated iNKT cells with a broader set of Vβ, including Vβ2, Vβ6, Vβ7, Vβ8, Vβ9, Vβ10 and Vβ14.40 Consistent with this finding, all iNKT cell lines generated expressed multiple Vβ chains which were biased to Vβ8, Vβ14, Vβ10, Vβ6 and Vβ7. Molling et al.49 generated iNKT cell lines expressing Vβ7 or Vβ8.2 from spleen T cells of wild-type C57BL6 mice. In their protocol, T cells were stimulated with an αGalCer-loaded DC line, which expresses a low level of CD1d on the cell surface, and IL-7 was added to the culture media 1 week later. These specific conditions might be responsible for the generation of iNKT cell lines with dominant use of a single Vβ chain. We noted that the proportion of Vβ14+ iNKT cells in iNKT cell lines was higher than that in freshly isolated iNKT cells. We found that more Vβ14+ iNKT cells expressed IL-2 receptors than other Vβ14− iNKT cells at 2 weeks post stimulation with αGalCer-pulsed BMDCs (CD25+ CD122+ cells, Vβ14+ iNKT cells: 17·65 ± 1·7%; Vβ14− iNKT cells: 9·53 ± 3·2%). This suggests that Vβ14+ NKT cells remain in an active state and sensitive to IL-2 after αGalCer stimulation, which probably accounts for their expansion in vitro. This relative abundance of Vβ14 iNKT cells may provide a good opportunity to study their distinct nature.
Although iNKT cells are reactive to the same antigen, αGalCer, they nevertheless display clonal diversity based on their Vβ usage. Previously, we have shown that some but not all αGalCer-reactive iNKT cell hybridomas also respond to certain phospholipid antigens.32 It has been reported that CD1d tetramers loaded with synthetic Sphingomonas glycosphingolipid stained 25–50% of αGalCer-CD1d tetramerpositive cells, and there was a higher percentage of Vβ8.2-containing TCRs than Vβ7 TCRs among these glycosphingolipid-CD1d tetramerpositive cells.5,6 Vβ8.2 has been shown to have higher binding avidity to CD1d:αGalCer than Vβ7.50 As iNKT cell lines contain several Vβ chains which are preferentially used by iNKT cells, they will be useful for characterization of iNKT cells with each Vβ chain.
We found that the proportions of CD4+ iNKT cells decreased after culture and most cells were DN. Human CD4+ iNKT cells were reported to express more IL-7 receptor and respond to IL-7, and CD4− iNKT cells proliferate to IL-15 stimulation by expressing more IL-15 receptor.51 Murine CD4+ and CD4− iNKT cells may also have different cytokine requirements. It was also suggested that murine iNKT cells may lose surface expression of CD4 during culture, as, although only murine CD4+ NK1.1+ Ly49− thymocytes proliferated upon αGalCer stimulation, 30% of the cells were CD4+ when αGalCer-responsive cell lines were generated from thymocytes in the presence of IL-15.48 Therefore, in our culture condition, CD4− iNKT cells may preferentially respond to IL-2 and IL-7 or iNKT cells may down-modulate CD4 during culture.
In summary, we developed a method to generate high-yield murine iNKT cells as long-termin vitro cultivated cell lines. These iNKT cell lines consist of cells with various Vβ chains. They are activated by stimulation both with antigen and with cytokines produced by TLR-stimulated APCs. These iNKT cell lines should prove useful in the identification of self and foreign antigens for iNKT cells as well as in studies of iNKT cell activation, inactivation and effector functions.
Acknowledgments
The authors would like to thank the National Institute of Health (NIH) Tetramer Core Facility for the mouse CD1d tetramer and GSL-1, Albert Bendelac for Vα14-Jα281 transgenic mice, Mark Exley for CD1d−/− mice, and Koichi Kobayashi for providing bone marrow from MyD88−/− C57BL6 mice. This work was supported by the Arthritis Foundation (AC), the NIH (grant RO1AI063428-04 to MBB), and the Sandler Program for Asthma Research (grant 06-0058 to MBB).
Glossary
Abbreviations:
- BMDC
bone marrow-derived dendritic cell
- LPS
lipopolysaccharide
- TLR
Toll-like receptor
- αGalCer
α-galactosylceramide
Disclosures
The authors have no financial conflict of interest.
References
- 1.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–90. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg M. Toward an understanding of NKT cell biology. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–95. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 6.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 7.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycoshingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 8.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–86. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 9.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–7. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 10.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–13. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 11.Paget C, Mallevaey T, Speak AO, et al. Activation of invariant NKT cells by Toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Salio M, Speak AO, Shepherd D, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci USA. 2007;104:20490–5. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawakami K, Kinjo Y, Uezu K, et al. Monocyte chemoatrractant protein-1-dependent increase of Vα14 NKT cells in lungs and their role in Th1 response and host defense in cryptococcul infection. J Immunol. 2001;167:6525–32. doi: 10.4049/jimmunol.167.11.6525. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa H, Hisaeda H, Taniguchi M, et al. CD4+ Vα 14 NKT cells play a crucial role in an early stage of protective immunity against infection with Leishmania major. Int Immunol. 2000;12:1267–74. doi: 10.1093/intimm/12.9.1267. [DOI] [PubMed] [Google Scholar]
- 15.Chiba A, Dascher CC, Besra GS, Brenner MB. Rapid NKT cell responses are self-terminating during the course of microbial infection. J Immunol. 2008;181:2292–302. doi: 10.4049/jimmunol.181.4.2292. [DOI] [PubMed] [Google Scholar]
- 16.Dieli F, Taniguchi M, Kronenberg M, et al. Anti-inflammatory role for Vα14 NK T cells in Mycobacterium bovis Bacillus Calmette–Guérin-infected mice. J Immunol. 2003;171:1961–8. doi: 10.4049/jimmunol.171.4.1961. [DOI] [PubMed] [Google Scholar]
- 17.Berntman E, Rolf J, Johansson C, Anderson P, Cardell SL. The role of CD1d-restricted NK T lymphocytes in the immune response to oral infection with Salmonella typhimurium. Eur J Immuonol. 2005;35:2100–9. doi: 10.1002/eji.200425846. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami K, Yamamoto N, Kinjo Y, et al. Critical role of Vα14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol. 2003;33:3322–30. doi: 10.1002/eji.200324254. [DOI] [PubMed] [Google Scholar]
- 19.Nakamatsu M, Yamamoto N, Hatta M, et al. Role of interferon-γ in Vα14+ natural killer T cell-mediated host defense against Streptococcus pneumoniae infection in murine lungs. Microbes Infect. 2007;9:364–74. doi: 10.1016/j.micinf.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Kumar H, Belperron A, Barthold SW, Bockenstedt LK. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J Immunol. 2000;165:4797–801. doi: 10.4049/jimmunol.165.9.4797. [DOI] [PubMed] [Google Scholar]
- 21.Miyahira Y, Katae M, Takeda K, et al. Activation of natural killer T cells by α-galactosylceramide impairs DNA vaccine-induced protective immunity against Trypanosoma crusi. Infect Immun. 2003;71:1234–41. doi: 10.1128/IAI.71.3.1234-1241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duthie MS, Kahn M, White M, Kapur RP, Kahn SJ. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect Immun. 2005;73:181–92. doi: 10.1128/IAI.73.1.181-192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smyth MJ, Thia KYT, Street SEA, et al. Different tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–8. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–8. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 25.Miyake S, Yamamura T. NKT cells and autoimmune diseases: unraveling the complexity. Curr Top Miocrobiol Immunol. 2007;314:251–67. doi: 10.1007/978-3-540-69511-0_10. [DOI] [PubMed] [Google Scholar]
- 26.Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, Berne GP. CD1d-dependent activation of NKT cells aggravated atherosclerosis. J Exp Med. 2004;199:417–22. doi: 10.1084/jem.20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakai Y, Iwabuchi K, Fujii S, et al. Natural killer T cells accelerate atherogenesis in mice. Blood. 2004;104:2051–9. doi: 10.1182/blood-2003-10-3485. [DOI] [PubMed] [Google Scholar]
- 28.Major AS, Wilson MT, McCaleb JL, et al. Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:2351–7. doi: 10.1161/01.ATV.0000147112.84168.87. [DOI] [PubMed] [Google Scholar]
- 29.Aslanian AM, Chapman HA, Charo IF. Transient role for CD1d-restricted natural killer T cells in the formation of arherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2005;25:628–32. doi: 10.1161/01.ATV.0000153046.59370.13. [DOI] [PubMed] [Google Scholar]
- 30.Bendelac A, Hunziker RD, Lantz O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J Exp Med. 1996;184:1285–93. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Exley MA, Bigley NJ, Cheng O, et al. Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology. 2003;110:519–26. doi: 10.1111/j.1365-2567.2003.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gumperz JE, Roy C, Makowska A, et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–21. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 33.Crowe NY, Uldrich AP, Kyparissoudis K, et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003;171:4020–7. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 34.Wilson MT, Johanson C, Olivares-Villagómez D, Singh AK, Stanic AK, Wang CR, Joyce S, Van Kaer L. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100:10913–8. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harada M, Seino K, Wakao H, et al. Down-regulation of the invariant Vα14 antigen receptor in NKT cells upon activation. Int Immunol. 2004;16:241–7. doi: 10.1093/intimm/dxh023. [DOI] [PubMed] [Google Scholar]
- 36.Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–83. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uldrich AP, Crowe NY, Kyparissoudis K, et al. NKT cell stimulation with glicolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S, Lalani S, Parekh VV, Vincent TL, Wu L, Van Kaer L. Impact of bacteria on the phenotype, function and therapeutic activities of invariant NKT cells in mice. J Clin Invest. 2008;118:2301–15. doi: 10.1172/JCI33071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda JL, Gapin L, Fazilleau N, Warren K, Naidenko OV, Kronenberg M. Natural killer T cells reactive to a single glycolipid exhibit a highly diverse T cell receptor β repertoire and small clone size. Proc Natl Acad Sci USA. 2001;98:12636–41. doi: 10.1073/pnas.221445298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei D, Curran SA, Savage PB, Teyton L, Bendelac A. Mechanisms imposing the Vβ bias of Vα14 natural killer T cells and consequences for microbial glycolipid recognition. J Exp Med. 2006;203:1197–207. doi: 10.1084/jem.20060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hegde S, Chen X, Keaton JM, Reddington F, Besra GS, Gumperz JE. NKT cells direct monocytes into a DC differentiation pathway. J Leukoc Biol. 2007;81:1224–35. doi: 10.1189/jlb.1206718. [DOI] [PubMed] [Google Scholar]
- 42.Michel ML, Keller AC, Paget C, et al. Identification of an IL-17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rachitskaya AV, Hansen AH, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–71. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coquet JM, Chakravarti S, Kyparissoudis K, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4−NK1.1− NKT cell population. Proc Natl Acad Sci U S A. 2008;105:11287–92. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gadue P, Stein PL. NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J Immunol. 2002;169:2397–406. doi: 10.4049/jimmunol.169.5.2397. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Ueno A, Bao M, Wang Z, Im JS, Porcelli S, Yoon JW. Control of NKT cell differentiation by tissue-specific microenvironments. J Immunol. 2003;171:5913–20. doi: 10.4049/jimmunol.171.11.5913. [DOI] [PubMed] [Google Scholar]
- 47.Pellicci DG, Hammond KJL, Coquet J, et al. DX5/CD49b-positive T cells are not synonymous with CD1d-dependent NKT cells. J Immunol. 2005;175:4416–25. doi: 10.4049/jimmunol.175.7.4416. [DOI] [PubMed] [Google Scholar]
- 48.Maeda M, Lohwasser S, Yamamura T, Takei F. Regulation of NKT cells by Ly49: analysis of primary NKT cells and generation of NKT cell line. J Immunol. 2001;167:4180–6. doi: 10.4049/jimmunol.167.8.4180. [DOI] [PubMed] [Google Scholar]
- 49.Molling JW, Moreno M, van der Vliet HJ, von Blomberg BM, van den Eertwegh AJ, Scheper RJ, Bontkes HJ. Generation and sustained expansion of mouse spleen invariant NKT cell lines with preserved cytokine releasing capacity. J Immunol Methods. 2007;322:70–81. doi: 10.1016/j.jim.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Shümann J, Voyle RB, Wei BY, MacDonald HR. Influence of the TCR Vβ domain on the avidity of CD1d:α-galactosylceramide binding by invariant Va14 NKT cells. J Immunol. 2003;170:5815–9. doi: 10.4049/jimmunol.170.12.5815. [DOI] [PubMed] [Google Scholar]
- 51.Baev DV, Peng X, Song L, et al. Distinct homeostatic requirements of CD4+ and CD4− subsets of Vα24-invariant natural killer T cells in humans. Blood. 2004;104:4150–6. doi: 10.1182/blood-2004-04-1629. [DOI] [PubMed] [Google Scholar]