Abstract
Prdx6 (peroxiredoxin 6), a bifunctional protein with both GSH peroxidase and PLA2 (phospholipase A2) [aiPLA2 (acidic calcium-independent PLA2)] activities, is responsible for the metabolism of lung surfactant phospholipids. We propose that the aiPLA2 activity of the enzyme is regulated through phosphorylation. Incubation of isolated rat alveolar type II cells (AECII) with PMA, a PKC (protein kinase C) agonist, had no effect on Prdx6 expression but led to ~75 % increase in aiPLA2 activity that was abolished by pretreatment of cells with the MAPK (mitogen-activated protein kinase) inhibitors, SB202190 or PD98059. Prdx6 phosphorylation after incubation of AECII with PMA was demonstrated by autoradiography after immunoprecipitation with either anti-phosphothreonine or -phosphoserine antibodies. In vitro, several active isoforms of ERK (extracellular-signal-regulated kinase) and p38 phosphorylated Prdx6, resulting in an 11-fold increase in aiPLA2 activity. The increased activity was calcium-independent and was abolished by the aiPLA2 inhibitors, surfactant protein A and hexadecyl-3-trifluorethylglycero-sn-2-phospho-methanol (MJ33). The peroxidase activity of Prdx6 was unaffected by phosphorylation. Mass spectroscopic analysis of in vitro phosphorylated Prdx6 showed a unique phosphorylation site at Thr-177 and mutation of this residue abolished protein phosphorylation and the increase in MAPK-mediated activity. These results show that the MAPKs can mediate phosphorylation of Prdx6 at Thr-177 with a consequent marked increase in its aiPLA2 activity.
Keywords: deacylation–reacylation, granular pneumocyte, lung surfactant, peroxidase, peroxiredoxin, phorbol ester
INTRODUCTION
Prdxs (peroxiredoxins) constitute an expanding family of non-seleno peroxidases that are ubiquitously found in archaea, bacteria and eukaryotes and include six mammalian members; they have been classified as either 1-Cys or 2-Cys Prdxs based on whether the protein contains one or two conserved cysteine residues that function in catalysis [1]. Prdx6, the unique 1-Cys member of the mammalian Prdx family [1,2], functions physiologically as an antioxidant enzyme related to its peroxidase activity [3–7]. In addition Prdx6 has PLA2 (phospholipase A2) activity that is maximal under acidic conditions and is calcium-independent, leading to the sobriquet aiPLA2 (acidic calcium-independent PLA2) [8,9]. 1-Hexadecyl-3-trifluroethyl-sn-glycero-2-phosphomethanol (MJ33) specifically inhibits the aiPLA2 activity of the protein [9,10]. The primary substrates for aiPLA2 are the PCs (phosphatidylcholines; l,2-diacyl-sn-glycero-3-phosphorylcholines), which are the predominant phospholipid components of the lung surfactant [11,12]. Thus it has been shown that Prdx6 has a major role in degradation of internalized DPPC (dipalmitoyl phosphatidylcholine; 1,2-dipalmitoyl-sn-glycero-3-phosphorylcholine) as well as in the remodelling of PC through the deacylation–reacylation pathway by lung granular pneumocytes (alveolar epithelial type II cells, AECII) [9,13–15].
Phorbol esters (e.g. phorbol myristic acid, PMA) are known surfactant secretagogues [16]. Studies with intact lungs have shown that secretion and re-uptake of surfactant by AECII are physiologically linked and, consistent with this observation, PMA increased the re-uptake of phospholipids from the alveolar space and increased the degradation and resynthesis of PC [17]. The increased degradation was reversed by the presence of the aiPLA2 inhibitor MJ33 [10,15]. Studies with isolated AECII (reported herein) showed that PMA treatment results in increased aiPLA2 activity. Considering the role of PMA as an activator of PKC (protein kinase C), we proposed that treatment of cells with this agonist results in Prdx6 activation through phosphorylation. However, subsequent evaluation with PKC isoforms in vitro failed to show phosphorylation of Prdx6. This led us to evaluate the effect of MAPKs (mitogen-activated protein kinases), which are now shown to phosphorylate Prdx6, resulting in increased aiPLA2 activity of the enzyme, and to be responsible for the effect of PMA on phospholipid metabolism by AECII.
EXPERIMENTAL
Animals and materials
Sprague–Dawley male rats weighing ~ 200 g were obtained from Charles River Breeding Laboratories (Kingston, NY, U.S.A.). All animal use was approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Isoforms of active MAPKs were purchased from Upstate Technology (Temecula, CA, U.S.A.). MAPK-specific inhibitors and human recombinant isoforms of active PKC were from Calbiochem (San Diego, CA, U.S.A.). H332PO4 was from ICN (MP Biomedicals, Irvine, CA, U.S.A.). [γ-32P]ATP was from PerkinElmer Life Science (Waltham, MA, U.S.A.). [3H]DPPC (1-palmitoyl-2-[3H]9,10-palmitoyl-sn-glycero-3-phosphorylcholine) was from American Radiolabeled Chemicals (St. Louis, MO, U.S.A.); all other lipids were from Avanti Polar Lipids (Alabaster, AL, U.S.A.). PLPCOOH (phospholipid hydroperoxide; 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphorylcholine hydroperoxide) was prepared by oxidation of the phospholipid with soyabean type V lipoxidase (Sigma, St. Louis, MO, U.S.A.) [18]. Disodium glycerol 2-phosphate, NaF, Na3VO4, protease inhibitor cocktail, PMA, sodium deoxycholate, Protein A/G beads and reagents for SDS/PAGE were from Sigma. Okadaic acid was purchased from LC Laboratories (Woburn, MA, U.S.A.). Rabbit anti-phosphoserine pAb (polyclonal antibody) and mouse anti-phosphothreonine mAb (monoclonal antibody) were purchased from Zymed Laboratories (South San Francisco, CA, U.S.A.). Antibodies specific for each of the non-phosphorylated and phosphorylated MAPKs [ERK (extracellular-signal-regulated kinase), p38 MAPK (p38) and JNK (c-Jun N-terminal kinase)] were obtained from Cell Signaling (Danvers, MA, U.S.A.); these antibodies were rabbit pAbs for ERK, JNK, p38 and phospho-p38, rabbit mAbs for phospho-ERK, and mouse mAbs for phospho-JNK. An anti-Prdx6 pAb was generated to a C-terminal region of Prdx6 and has been described previously [7]. For 2D (two-dimensional) gel analysis, IPG strips (immobilized pH gradient strips), IPG buffers, urea and CHAPS were from GE Healthcare (Princeton, NJ, U.S.A.). Modified trypsin was from Promega (Fitchburg, WI, U.S.A.). A Silverquest Silver Staining kit, a Novex Colloidal Blue Staining kit and a SYPRO Ruby protein gel stain were purchased from Invitrogen Life Technologies (Carlsbad, CA, U.S.A.).
Cell isolation and lysate preparation
AECII were isolated by proteolytic digestion of rat lungs followed by centrifugation and differential adherence as described previously [19]. Cells were studied after overnight incubation in minimal essential medium culture medium containing 10 % FCS (foetal calf serum). Cell lysates were prepared by addition of RIPA buffer (1 %, w/w, Nonidet P40, 1 %, w/v, sodium deoxycholate, 0.1 %, w/v, SDS, 0.15 MNaCl, 0.01 M sodium phosphate, pH 7.2, 2 mM EDTA and0.2 mM Na3VO4) followed by a brief sonication. Lysates were centrifuged at 16000 g for 15 min at 4°C, and the supernatant containing soluble protein was stored in aliquots at −80 °C until use.
Preparation of recombinant protein
Recombinant untagged rat full-length Prdx6 and human His-tagged (C-terminal) Prdx6 were prepared as previously described. The native rat and human proteins show 92 % amino acid identity [20]. Untagged proteins were purified by ion-exchange and size-exclusion chromatographies [8,21] and His-tagged proteins were purified on an Ni2+ column (His-Bind resin; Novagen). Mutants of threonine to alanine or glutamic-acid residues at position 177 were prepared for the human protein in the pET21b plasmid (Novagen) using the QuikChange II site-directed mutagenesis kit (Stratagene). The mutagenic oligonucleotides used were: 5′-CAGCAGAAAAAACCCTTGCCGCCCCAGTTGATTGGAA-GGATGGGG-3′ and its reverse complement for T177A and 5′-CAGCAGAAAAAAGGGTTGCCGAGCCAGTTGATTGGAA-GGATGGGG-3′ and its reverse complement for T177E. The resulting DNA was sequenced at University of Pennsylvania Cell Center to ensure fidelity. Tuner (DE3) cells containing the mutated plasmid were induced with 1 mM IPTG (isopropyl β-d-thiogalactoside) for several hours, harvested and lysed with Bugbuster (Novagen). Unlike the wild-type, either mutation caused the protein to accumulate in the pellet (inclusion bodies). For extraction, the pelleted protein was resuspended in Inclusion Body Solubilization Reagent (Pierce, Rockford, IL, U.S.A.) and then dialysed against 6 M urea using the protocol recommended by the manufacturer. In an alternative strategy designed to increase the soluble fraction of recombinant protein, we used the pPosKJ vector (a gift from Dr Kyung-Jin Kim, Pohang Accelerator Laboratory, Kyungbuk, Republic of Korea) in which the Prdx6 coding region with a His tag on the N-terminus was fused with an upstream bacterial Hb from Vitreoscilla [22]. The Thr-177 mutants were excised from the pET21b vector and recloned into the pPosKJ vector using the restriction enzymes NdeI and XhoI, transformed into Tuner (DE3) pLysS cells and induced and purified as described above.
Enzymatic activity
PLA2 activity was measured as described previously [23] using unilamellar liposomes containing DPPC/egg PC/phosphati-dylglycerol/cholesterol (5:2.5:1:1.5) with tracer [3H]DPPC. Enzyme was incubated with liposomal substrate at 37 °C for 1 h under acidic (40 mM sodium acetate, pH 4.0, and 5 mM EDTA) or alkaline (50 mM Tris/HCl, pH 7.4, and 1 mM EGTA) conditions in the presence of GSH (5 mM) [24,25]. aiPLA2 refers to assay specifically under acidic conditions in the absence of Ca2+. The reaction was stopped by the addition of chloroform/methanol (1:2) and lipids were extracted and separated by two-step TLC using hexane/diethyl ether/acetic acid. The radiolabeled non-esterified fatty acid (palmitate) spot was scraped and counted for d.p.m. using a Packard Tricarb 2900TR liquid-scintillation analyser (Packard, Downers Grove, IL, U.S.A.).
For studying PLA2 activity in intact cells, AECII were plated on to the top chamber of six-well 5 µm pore size Transwell® polycarbonate filters. After 24 h, cells were washed twice with serum-free MEM and incubated with [3H]DPPC-labelled liposomes for 2 h. Cells were washed three times with ice-cold PBS and harvested using trypsin–EDTA. Cell lysates were analysed for total cellular d.p.m. and the percentage of d.p.m. remaining in DPPC. The lipids were separated and analysed as described above for the in vitro assay. We have shown previously that liposomes of this composition are endocytosed by AECII, targeted to intracellular organelles for degradation, incorporated into surfactant phospholipids and re-secreted [9,19,26,27]. The processing of these liposomes is similar to that of natural surfactant by isolated AECII [15,28] as well as similar to liposome processing in intact lungs [10,29].
Peroxidase activity was measured by the decrease in NADPH absorbance using the coupled reaction based on oxidation of GSH to GSSG and its subsequent reduction by GSH reductase as described previously [8,18]. Activity was measured at pH 8 using as substrate 200 µM H2O2 or 100 µM PLPCOOH.
Immunoprecipitation and Immunoblotting
For immunoprecipitation, AECII lysates (~ 250 µg of total protein) were incubated with slow rotation overnight either with anti-phosphothreonine mAb (1:100) or with anti-phosphoserine pAb (1:100) followed by Protein A/G-conjugated agarose for an additional 45 min. As an internal control, 10% of the cell lysate protein (~ 25 µg) prior to the addition of antibodies was reserved for direct loading on to the gel and is labelled as the INPUT. The beads were washed four times with lysis buffer containing protease inhibitor cocktail and phosphatase inhibitors (NaF, 2-glycerophosphate, Na3VO4 and EDTA) and the supernatant was removed by centrifugation (1000 g, 3 min). The pellet was mixed with loading buffer and a denaturing agent, and the sample was heated to 95 °C for 5 min followed by SDS/PAGE with 12% Tris/glycine gels using the Xcell Surelock™ mini-cell system (Invitrogen Life Technologies) and then transferred on to an Immobilon-P membrane (Millipore, Bradford, MA, U.S.A.) using a Trans-blot® SD semi-dry transfer cell (Bio-Rad Laboratories). The membrane was blocked for 1 h with Odyssey blocking buffer containing 0.1% Tween 20, probed with the corresponding primary antibodies and then incubated with secondary IRDye™ antibodies (Rockland, Gilbertsville, PA, U.S.A.) following the manufacturer’s instructions. The membrane was analysed with an Odyssey® dual-colour IR excited imaging system (Licor, Lincoln, Nebraska).
Phosphorylation of Prdx6
To study the phosphorylation of Prdx6 in intact cells, AECII were serum-starved for 4 h and then incubated with 5 mCi of H332PO4 for 2 h in the presence of PMA and okadaic acid. After immunoprecipitation with either an anti-Prdx6 antibody or non-relevant IgG as a control, the samples were analysed by SDS/PAGE with silver staining and autoradiography. Additional cells were incubated for 2 h with or without MAPK inhibitors and then treated with PMA (200 nM) for another 1 h. The cell lysate was incubated with either anti-phosphothreonine mAb (1:100) or anti-phosphoserine pAb (1:100) followed by SDS/PAGE and immunoblot analysis.
For studying phosphorylation in vitro by MAPK isoforms, recombinant Prdx6 (150 ng/µl) was added to 20 µl of a buffer containing 50 mM Tris/HCl (pH 7.5), 20 µM EGTA, 10 mM MgCl2 and 2 mM [γ-32P]ATP (2 µCi/µmol) in the absence or presence of an active MAPK and incubated with slow shaking in a water bath at 30 °C for 90 min. Protein was analysed by SDS/PAGE that was stained with Coomassie Blue followed by exposure to an X-ray film for autoradiography. The target band on the Coomassie-Blue-stained gel was excised, added to complete liquid-scintillation counting solution (Liquiscint™), and analysed by liquid-scintillation counting. For in vitro phosphorylation by PKC isoforms, recombinant Prdx6 was added to 0.3 M Hepes buffer (pH 7.6) containing 0.06 M MgCl2, 3 mM [γ-32P]ATP, 10 mg/ml BSA, 25 mM EGTA, 50 mM dithiothreitol, 30 mM CaCl2 (omitted for PKCγ, ε and ζ), and carrier phosphati-dylserine/diacylglycerol (the latter was omitted for PKCζ) [30]. The products were separated with a DEAE cellulose/Domex AG-1X8 acetate column and analysed by scintillation counting.
Identification of phosphorylation site
For 2D gel analysis, samples (0.5 µg) of rat recombinant Prdx6 were diluted in a buffer containing 8 M urea, 2 M thiourea, 4% (w/v) CHAPS, 1% (w/v) dithiothreitol, 0.8% (v/v) IPG buffer (pH 4–7) and 0.8% (v/v) IPG buffer (pH 3–10) and separated on 11 cm pH 4–7 IPG strips in a Protean IEF (isoelectric focusing) cell apparatus (Sigma) using 10 h in-gel rehydration at 50 V followed by five phases of voltage steps from 250 to 8000 V with total focusing of 35 kV·h. After IEF, IPG strips were reduced and alkyated with iodoacetamide before separation on 11% Tris/Tricine SDS gels in a Criterion gel cassette (Sigma). After electrophoresis, gels were fixed in 40 % (v/v) ethanol and 10% (v/v) acetic acid and then stained with SYPRO Ruby fluorescent stain. Images were acquired on a ProExpress multiwavelength fluorescence scanner (PerkinElmer Life Science) using λex = 480 ± 30 nm and λem = 620 ± 30 nm with 100 µm resolution and 30 s exposure time. Spots were quantified using Image Master Platinum 5.0 (1.1.2) 2D gel analyser software (GE Healthcare). After scanning, the fluorescent gels were restained using the SilverQuest kit prior to spot excision. A second set of 2D gels containing higher protein loads (2.5 µg) was stained using the Novex Colloidal Blue Staining kit for peptide mapping.
Bands from 1D (one-dimensional) SDS gels and spots from 2D gels were excised, destained, and digested with 20 µl of 0.02 µg/µl modified trypsin in 40 mM ammonium bicarbonate at 37 °C overnight. Supernatants and a subsequent extraction of the gel slice were combined and analysed by LC (liquid chromatography) MS/MS (tandem MS) using a nanocapillary reverse-phase C18 column (75 µm × 15 cm) directly coupled to an LTQ or LTQ-FT quadrupole ion-trap mass spectrometer (ThermoFisher, Pittsburgh, PA, U.S.A.). Results were acquired in the data-dependent mode with fragmentation of the six most abundant ions per full scan and dynamic exclusion of previously fragmented ions. Resulting precursor masses and MS/MS spectra were searched against a non-redundant NCBI (National Center for Biotechnology Information) database or UniProt human–mouse database with concatenated reverse database using Proteomics Browser with a Sequest search engine [31].
Statistics
Results are expressed as means ± S.E.M. Significance was determined by ANOVA or a Student’s t test as appropriate by using SigmaStat (Jandel Scientific, San Jose, CA, U.S.A.), and the level of statistical significance was defined as P < 0.05.
RESULTS
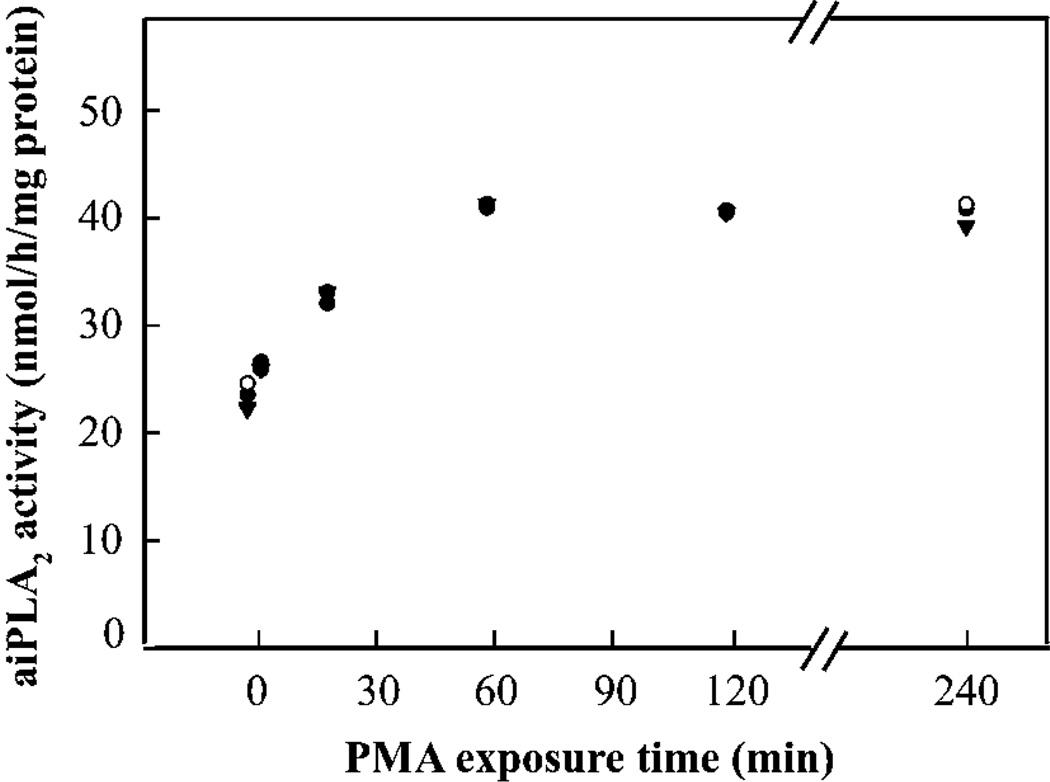
Incubation of isolated rat AECII with PMA resulted in a gradual increase in aiPLA2 activity in the cell lysate with a plateau value after 1 h of incubation at nearly double the baseline value (Figure 1). It should be noted that this activity was measured at pH 4 in Ca2+-free medium; we showed previously that this activity is decreased by 97 % in lung homogenate from Prdx6-null mice [13], indicating that the activity measured under these conditions is due to Prdx6. The effect of PMA on aiPLA2 activity was concentration-dependent reaching a plateau at 10−7–10−8 M (Table 1). H7 and calphostin C, inhibitors of PKC, abolished the increase in aiPLA2 activity induced by PMA, suggesting that PKC is involved in this process (Table 2). Neither 8-Br-cAMP (1 mM) nor 8-Br-cGMP (1 mM), specific agonists for PKA and PKG (protein kinase A and G) respectively, altered the aiPLA2 activity of AECII (results not shown).
Figure 1. Effect of PMA on aiPLA2 activity of rat AECII.
Adherent cells on 35 mm culture dishes were treated for the indicated time with 10−7 M PMA. Cells were then harvested and PLA2 activity of cell lysates was measured in Ca2+-free buffer at pH 4. Each symbol represents an independent experiment (n = 3).
Table 1. Effect of PMA concentration on aiPLA2 activity of AECII.
Cells were incubated with PMA for 1 h and the lysate was analysed for PLA2 activity. Results are the means ± S.E.M. for n = 3.
| PMA (M) | aiPLA2 activity (nmol/h per mg of protein) |
|---|---|
| 0 | 23.9 ± 1.1 |
| 10−9 | 31.1 ± 0.2* |
| 10−8 | 38.8 ± 1.2* |
| 10−7 | 40.6 ± 1.1 |
P < 0.05 compared with the value immediately above.
Table 2. Effect of PKC inhibitors on PMA-stimulated aiPLA2 activity of rat AECII.
Cells were incubated without (control) or with inhibitors of PKC activity for 1 h and then without (basal) or with PMA (1 × 10−8 M) for a subsequent 1 h. Cells were lysed for measurement of PLA2 activity. Values are means ± S.E.M. for three to five independent experiments.
| Activity (nmol/h per mg of protein) | Percentage of basal control | |||
|---|---|---|---|---|
| Basal | + PMA | Basal | + PMA | |
| Control | 22.3 ± 0.4 | 38.6 ± 0.4 | 100 | 173 |
| +H7, 60 µM | 21.6 ± 0.5 | 23.3 ± 1.1* | 97 | 104 |
| +Calphostine C (0.2 µM) |
23.7 ± 1.3 | 25.2 ±0.5* | 106 | 113 |
P < 0.05 compared with corresponding control.
PLA2 activity of intact AECII was evaluated by measuring the degradation of internalized [3H]DPPC during a 2 h incubation. Degradation was expressed as percentage of internalized liposomal PC substrate. [3H]DPPC degradation was increased by 1.5-fold when cells were stimulated with PMA (Table 3). Incubation with PMA for 24 h did not affect the level of Prdx6 protein expression (by Western blotting) in AECII (results not shown).
Table 3. Effect of MAPK inhibitors on degradation of internalized [3H]DPPC by intact rat AECII and on aiPLA2 activity of rat AECII lysate.
| [3H]DPPC degradation of intact cells* (d.p.m. in products × 100/total d.p.m.) |
aiPLA2 activity of lysate (nmol/h per mg of protein) | |||
|---|---|---|---|---|
| + PMA | Basal | + PMA | ||
| No additions | 74.6 ± 1.0† | 25.8 ± 0.44 | 45.2 ± 1.6† | |
| JNK II inhibitor (50 µM) | 71.7 ± 1.0 | 24.9 ± 0.5 | 45.7 ± 0.81† | |
| PD98059 (25 µM) | 25.1 ± 1.2†,‡ | 31.8±1.1 | 53.2 ± 0.30†,‡ | |
| SB202190(50 µM) | 53.2 ± 0.40‡ | 25.8 ± 0.73 | 33.2 ± 0.84†,‡ | |
| PD+SB | ND | 24.5 ± 0.47‡ | 24.5 ± 0.41‡ | |
| MJ33 (3 mol%) | 34.1±0.70‡ | ND | ND | |
Degradation was measured after 2 h incubation with substrate; degradation with no additions in the absence of PMA was 59.8. ND, not determined. Values are means ± S.E.M. for three independent experiments.
P < 0.05 compared with the corresponding basal.
P < 0.05 compared with no additions.
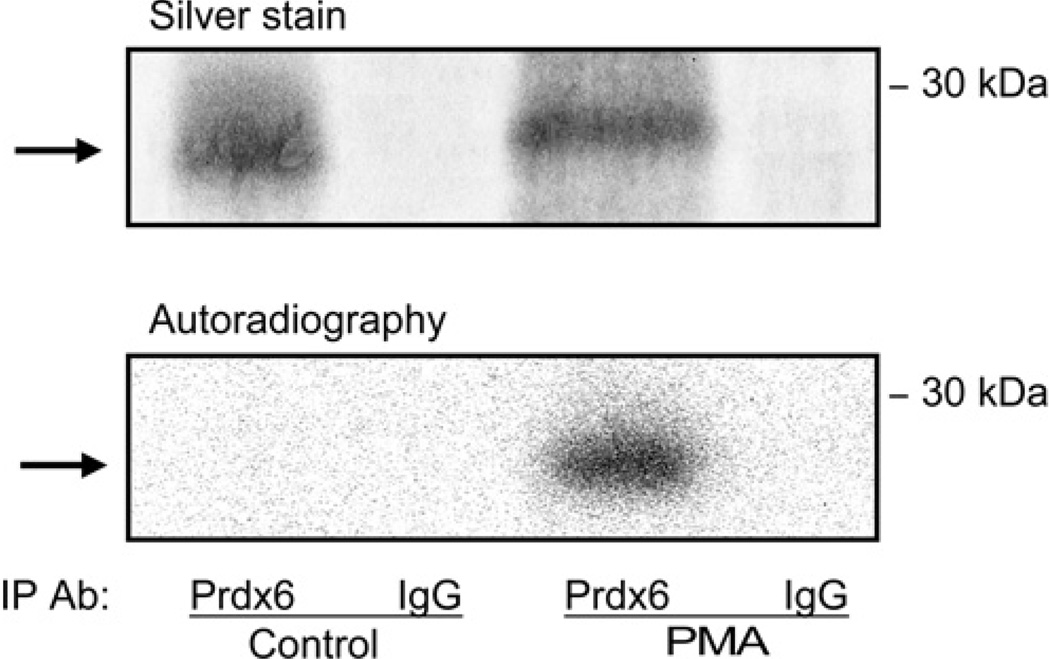
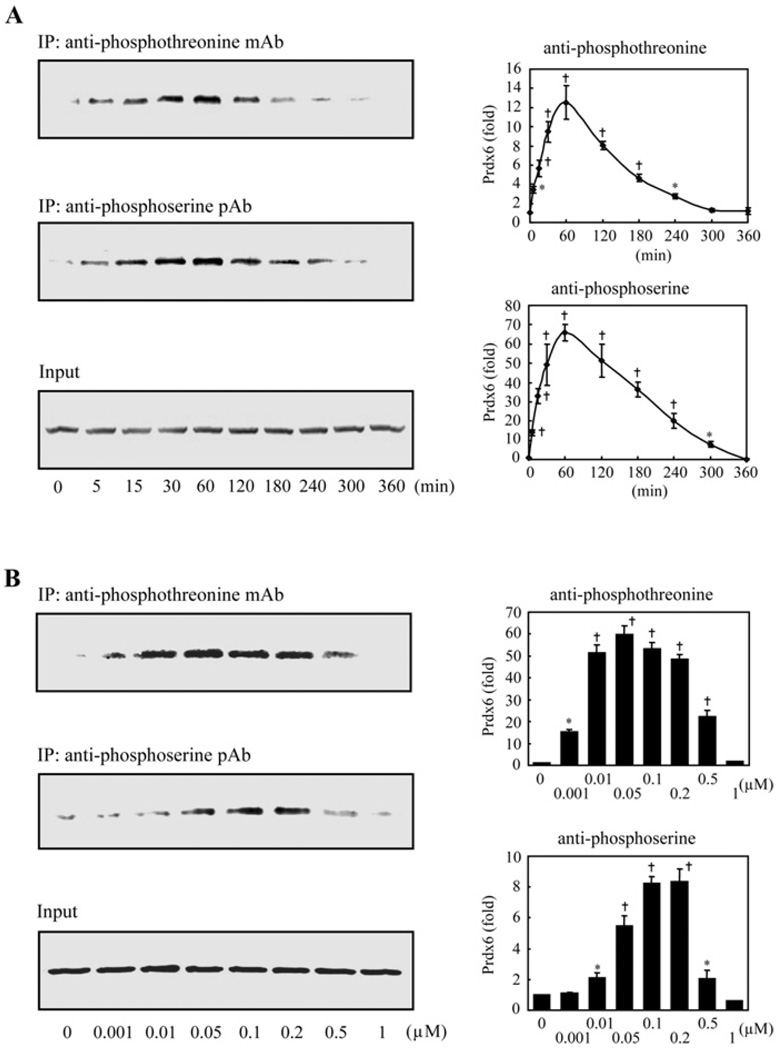
We evaluated whether PMA stimulation of rat AECII PLA2 activity is associated with Prdx6 phosphorylation. Cells were incubated for 2 h with H332PO4 in the presence of okadaic acid with or without PMA followed by immunoprecipitation with either an anti-Prdx6 antibody or non-specific IgG. The Prdx6 antibody immunoprecipitated a single protein of ~26 kDa corresponding to the molecular mass of Prdx6 (Figure 2). Autoradiography confirmed Prdx6 phosphorylation in AECII that were stimulated by PMA and not with the control incubation (no PMA) (Figure 2). Phosphorylation of Prdx6 in AECII exposed to PMA was also studied by immunoprecipitation with either anti-phosphothreonine or anti-phosphoserine antibodies followed by Western blotting using anti-Prdx6 pAb (Figure 3). Phosphorylation of Prdx6 in the cells was time- and concentration-dependent and reached maximal values, in the absence of a phosphatase inhibitor, at 30–60 min (Figure 3A) and 5–10 × 10−8 M PMA (Figure 3B).
Figure 2. Prdx6 phosphorylation in AECII induced by PMA.
Adherent AECII were incubated in serum-free MEM for 4 h and then incubated for an additional 2 h with 32P-labelled H3P04 (5 mCi) and okadaic acid (5 nM) in the absence (control) or presence of PMA (2 × 10−7 M). Immunoprecipitation (IP) was performed using either anti-Prdx6antibodyor a non-relevant antibody (anti-glutathione transferase IgG). The upper panel shows SDS/PAGE of the immunoprecipitated proteins visualized by silver staining and the lower panel shows autoradiography of the same gel. The position of the 30 kDa molecular mass marker is shown on the right. The arrow indicates the Prdx6 monomer.
Figure 3. Prdx6 phosphorylation in rat AECII as detected by immunoprecipitation with anti-phosphoprotein antibodies and immunoblotting with anti-Prdx6.
(A) Time course. AECII adherent on 35 mm culture dishes were treated with PMA (2 × 10−7 M) for various times. Cells were harvested in the presence of protease and phosphatase inhibitors followed by immunoprecipitation with either anti-phosphothreonine mAb (1:100) or anti-phosphoserine pAb (1:100). The immunoprecipitates were analysed by SDS/PAGE and immunoblotting using Prdx6 pAb (1:2500). The bottom panel indicates the input (10% of the original cell lysate) as a control. Values at each time point were normalized to the input and plotted as a percentage of the zero time value. (B) Effect of concentration. Rat AECII were treated with various concentrations of PMA for 1 h. Immunoprecipitation and immunoblot were then performed as described in (A). The plots indicate means ± S.E.M, for n = 4 (A) or n = 3 (B) separate experiments. P < 0.05 (*)or < 0.01 (†) versus zero time (A) or zero concentration (B).
Based on these results, we investigated whether Prdx6 can be phosphorylated in vitro directly by PKC. Incubation of recombinant human Prdx6 with [γ-32P]ATP and each (independently) of the nine known isoforms of active PKC showed autophosphorylation of PKC but phosphorylation of Prdx6 was not observed (n = 3 independent experiments, results not shown). This negative result led us to investigate a possible role for MAPKs in Prdx6 phosphorylation.
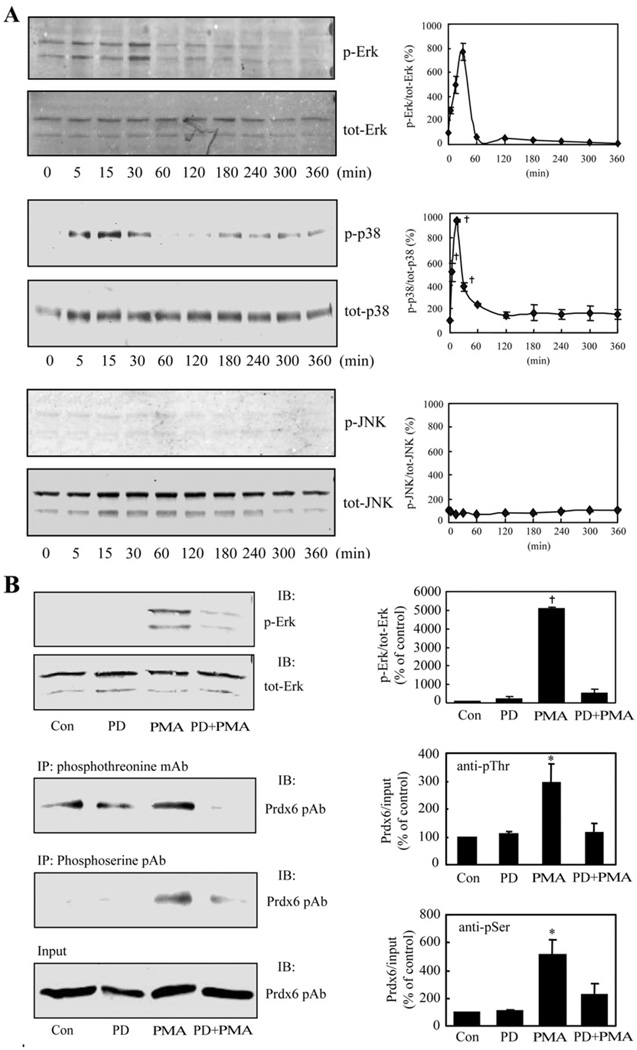
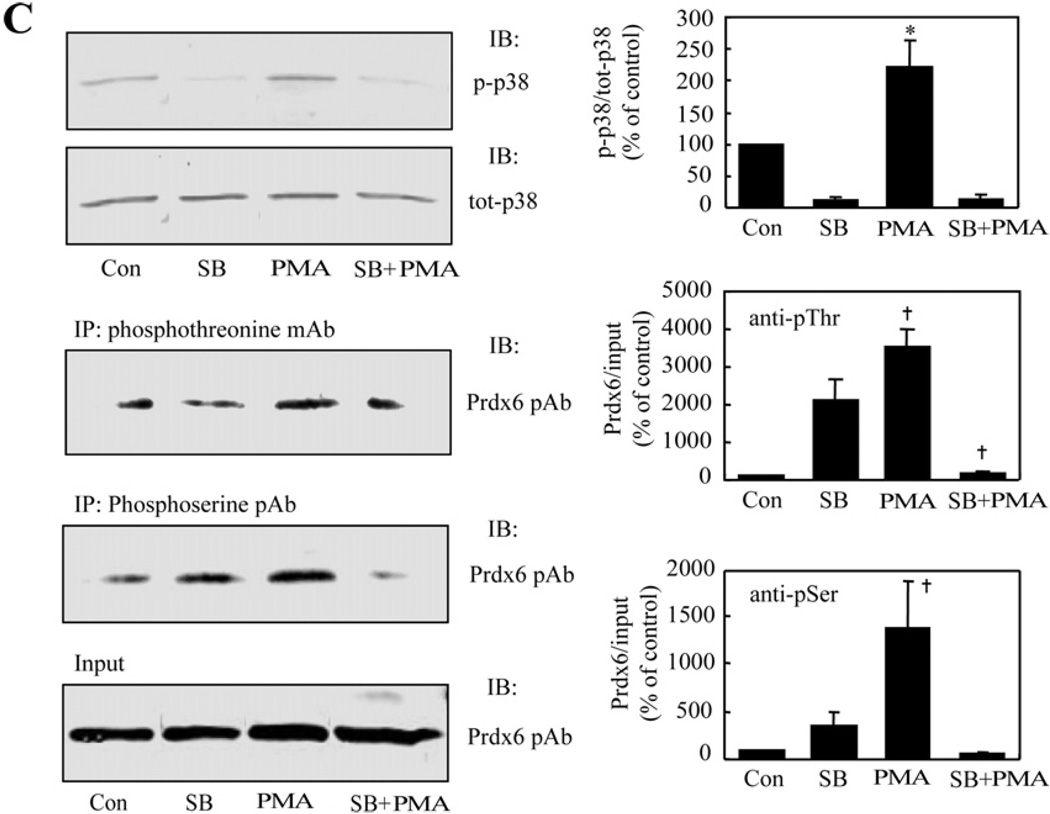
As a first step, we used immunoblots to evaluate the phosphorylation of isoforms of ERK, p38 and JNK in AECII treated with PMA. PMA induced phosphorylation of ERK and p38 within 5 min (the earliest time point that was studied) with a maximal level at 30 min followed by dephosphorylation (Figure 4A). There was no evidence for phosphorylation of JNK (Figure 4A). The cellular contents of total ERK, p38 and JNK proteins were unchanged (Figure 4A). Thus treatment of AECII with PMA results in activation of two of the MAPK enzymes (ERK and p38). Specific inhibitors were then used to examine whether MAPK phosphorylation induced by PMA results in Prdx6 phosphorylation. Treatment of cells with either PD98059 (25 µM), an ERK inhibitor, or SB202190 (50 µM), a p38 inhibitor, abolished the effect of PMA on phosphorylation of the respective kinase and also markedly diminished Prdx6 phosphorylation (Figures 4B and 4C). As expected, since JNK was not activated in AECII by PMA (Figure 4A), JNK II inhibitor (50 µM) had no effect on PMA-induced phosphorylation of Prdx6 (results not shown).
Figure 4. MAPK-mediated Prdx6 phosphorylation in PMA-stimulated rat AECII.
(A) Activation (phosphorylation) of MAPK proteins. Adherent AECII on 35 mm culture dishes were incubated with PMA (2 × 10−7 M) for the indicated times. Cells were collected in the presence of protease and phosphatase inhibitors, and lysates were analysed by Immunoblotting using anti-phospho-ERK mAb (1:1000)/anti-ERK pAb (1:1000), anti-phospho-p38 mAb (1:1000)/anti-p38 pAb (1:1000) and anti-phospho-JNK mAb (1:500)/anti-JNK pAb (1:500). Values indicate the density of the phosphorylated kinase signal as percentage of the density for the total kinase signal. Results are the means ± range for n = 2 for ERK, means ± S.E.M. for n = 3 for p38, and a single experiment for JNK. (B) Effect of ERK inhibitor on ERK and Prdx6 phosphorylation. Adherent AECII were treated for 2 h with or without the ERK inhibitor, PD98059 (PD, 25 µM), and then incubated for an additional 30 min in the absence or presence of PMA (2 × 10−7 M) and cells were harvested. Left panels: top, immunoblot (IB) using anti-phospho-ERK mAb (1:1000) or anti-ERK pAb (1:1000); middle, immunoprecipitation (IP) with anti-phosphothreonine mAb (anti-pThr, 1:100) or anti-phosphoserine pAb (anti-pSer, 1:100) followed by immunoblot using Prdx6 pAb (1:2500); bottom, input representing 10% of the original cell lysate. Right panels: values for PMA plus or minus PD98059 were normalized to the corresponding input and plotted as percentage of the control (Con, no PMA or PD98059) value. IB, antibody used for immunoblot; IP, antibody used for immunoprecipitation. (C) Effect of p38 inhibitor on p38 and Prdx6 phosphorylation. Cells were incubated with or without the p38 inhibitor, SB202190 (SB, 50 µM), for 2 h followed by incubation with/without PMA (2 × 10−7 M) for an additional 30 min. Cell lysate was analysed and presented as described in (B). Values are means ± S.E.M. for n = 4 (B) or n = 3 (C). P < 0.05 (*) or < 0.01 (†) in comparison with (A) zero time or (B, C) either the control (con) for PMA or inhibitor (PD98059 or SB202190) for inhibitor plus PMA. tot-, total; p-, phosphorylated.
Based on the evidence for phosphorylation of Prdx6 through MAPK, we investigated this pathway as an activator of PLA2 activity in AECII (Table 3). The effect of PMA on DPPC metabolism in intact AECII was blocked partially by either an ERK (PD98059) or p38 (SB202190) inhibitor. The PMA-induced increase in aiPLA2 activity as evaluated in AECII lysates was also abolished by the ERK and p38 inhibitors. Incubation of AECII with maximal inhibitory concentrations of both inhibitors resulted in greater inhibition of aiPLA2 activity than either inhibitor alone, suggesting that ERK and p38 may phosphorylate Prdx6 at least in part at different sites. The JNK inhibitor had no effect on PMA-induced PLA2 activity, either in the intact cells or cell lysates. These results with intact cells and cell lysates indicate that phosphorylation of Prdx6 activates its PLA2 activity.
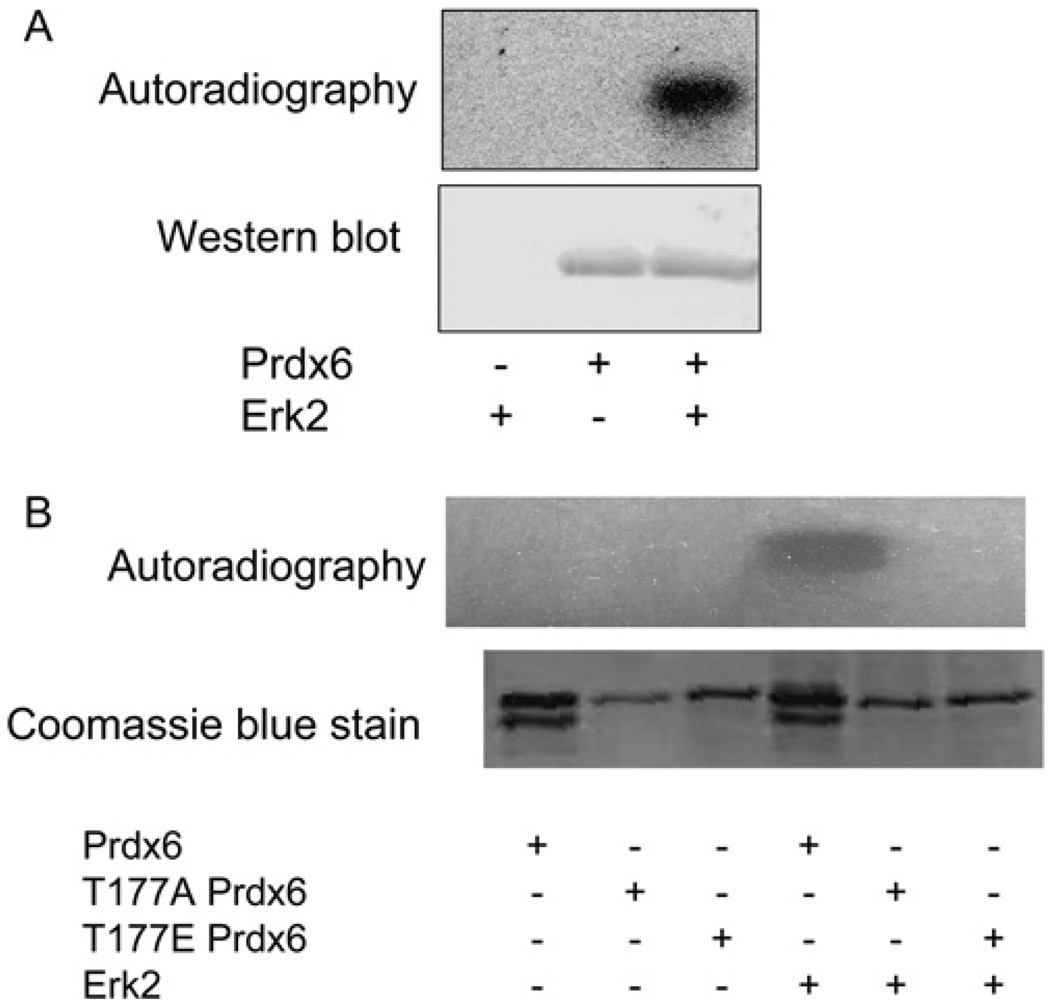
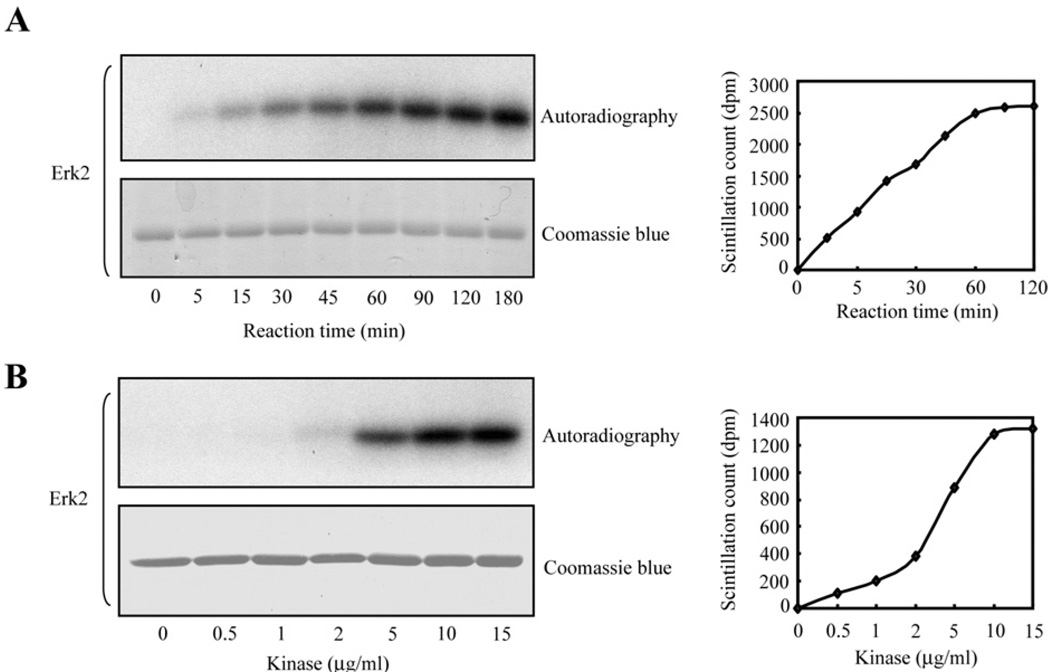
Phosphorylation by MAPK in vitro was studied by incubation of recombinant Prdx6 with commercially available active isoforms of ERK and p38 (ERK1, ERK2, p38α, p38β2, p38γ and p38δ). All isoforms led to Prdx6 phosphorylation as determined by autoradiography in association with Western blotting (shown in Figure 5A for ERK2). By autoradiography, ERK2, p38γ and p38δ appeared to give the greatest degree of phosphorylation in vitro and was confirmed by scintillation counting of the bands excised from the gel (results not shown). Phosphorylation of rat Prdx6 in vitro by ERK2 was observed as early as 5 min of incubation and reached a maximal level at 180 min (Figure 6A); phosphorylation of Prdx6 was seen at 5 µg/ml and was maximal at 15 µg/ml (Figure 6B). Similar kinetic results were seen for p38δ (results not shown). His-tagged human Prdx6 incubated with ERK2 also was phosphorylated similar to recombinant rat protein (Figure 5B).
Figure 5. Prdx6 phosphorylation in vitro.
Phosphorylation of (A) recombinant rat Prdx6 and (B) recombinant His-tagged human wild-type and T177A and T177E mutant proteins. Incubation was at 30°C for 60 min with and without Prdx6 (150 ng/µl) and with and without ERK2 (1 µg/ml) in a buffer containing 10 mM MgCI2 ± [γ-32P]ATP (2 mM). After SDS/PAGE, the gel was analysed by Western blotting with anti-Prdx6 antibody (A) or by Coomassie Blue staining (B) followed by autoradiography.
Figure 6. Prdx6 phosphorylation in vitro.
Recombinant rat Prdx6 (150 µg/ml) was mixed with active ERK2 in reaction buffer containing [γ-32P]ATP (2 mM final concentration) plus 10 mM MgCI2 and incubated with slow shaking at 30°C. Samples were analysed by SDS/PAGE, stained with Coomassie Blue and then exposed to a film for autoradiography. (A) Time dependence with 10 µg/ml of active kinase. (B) Concentration dependence with a 90 min incubation.
The effect of in vitro phosphorylation of recombinant rat Prdx6 on its PLA2 activity was studied. Basal activity of the protein at pH 4 (Table 4) is similar to our previously reports [24,25]. Phosphorylation of Prdx6 by ERK2 results in a marked increase in activity (approx. 11-fold) over the basal level (Table 4). The human wild-type protein showed a similar increase in PLA2 activity after MAPK-mediated phosphorylation (Table 5). The basal PLA2 activity at pH 7.4 is approximately one-half the activity at pH 4, while phosphorylated Prdx6 has similar activity at the two different pH values (Table 4). These activities were measured in the absence of Ca2+. In the presence of 10 mM Ca2+, PLA2 activity (nmol/min per mg of protein) of phosphorylated Prdx6 was 1186 ± 19 at pH 4 and 1203 ± 12 at pH 7.4 (n = 4). Thus PLA2 activity was unaltered by the presence of Ca2+. Ca2+-independent PLA2 activity of phosphorylated rat Prdx6 was abolished by the addition of either MJ33 or SP-A (surfactant protein A) (Table 4), known inhibitors of aiPLA2 activity [9,10,14,25].
Table 4. Effect of the phosphorylation of rat Prdx6 in vitro on PLA2 activity.
Recombinant rat Prdx6 was phosphorylated by incubation with ERK2 (10 µg/ml). Activity was measured in the absence of Ca2+. Values are means ± S.E.M. for three to five independent experiments.
| Basal (nmol/min per mg of protein) | Phosphorylated (nmol/min per mg of protein) |
|||
|---|---|---|---|---|
| pH 4.0 | pH 7.4 | pH4.0 | pH7.4 | |
| No additions | 103 ± 1 | 50 ± 1 | 1281±22* | 1208 ± 16* |
| +MJ33 (3 mol%) | 19 ± 1† | 18 ± 2† | 18 ± 1† | 17 ± 1† |
| +SP-A (50 mg/ml) | 36 ± 3† | 22 ± 1† | 24 ± 1*,† | 22 ± 1† |
P < 0.05 compared with corresponding basal.
P < 0.05 compared with the corresponding no additions.
Table 5. Effect of Thr-177 mutation on the aiPLA2 activity of human Prdx6.
Recombinant human Prdx6 (with C-terminal His tag) was incubated with 10 µg/ml ERK2 at pH 4.0 in the absence of Ca2+. Units are nmol/min per mg of protein. Values are means ± S.E.M. for n = 3.
P < 0.05 compared with the corresponding basal.
P < 0.05 compared with the corresponding wild-type.
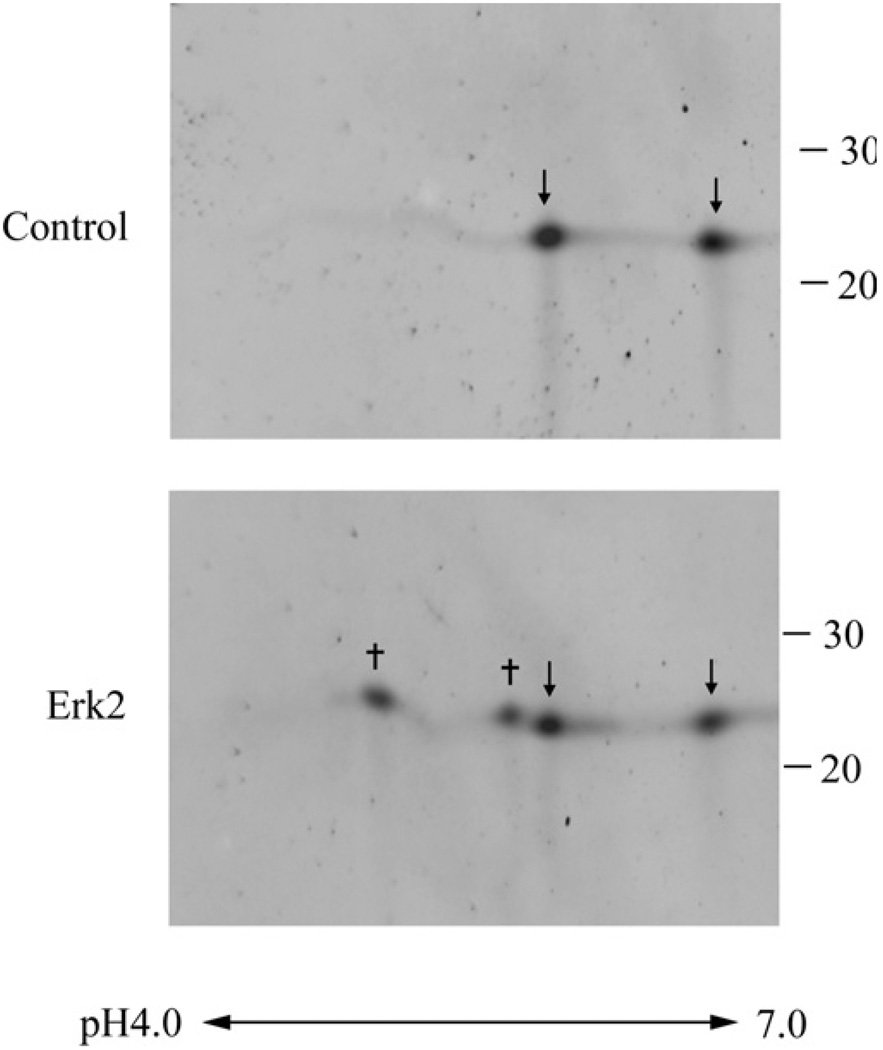
Rat Prdx6 phosphorylated in vitro was evaluated for the site of phosphorylation by 2D gel electrophoresis followed by analysis of tryptic digests of the protein spots by MS. By Immunoblotting, two clear spots were observed for non-phosphorylated Prdx6, compatible with the known two different oxidation states of this protein (Figure 7). Two new major spots, presumably representing phosphorylated protein, were observed for Prdx6 that had been treated with ERK2 (Figure 7). 2D gel analysis of human Prdx6 made to react with ERK2 or of rat Prdx6 incubated with p38δ was similar (results not shown). By mass spectroscopic analysis, ~50 % of total Prdx6 was phosphorylated in vitro by ERK2. After trypsin digest of the two new major Prdx6 spots, a unique peptide was detected, which on further analysis indicated phosphorylation of Thr-177. In some gels, an additional two more acidic but much fainter spots were observed but it was not possible to accurately identify these as phosphorylated protein by mass spectroscopy.
Figure 7. 2D gel mapping of the site of Prdx6 phosphorylation.
Upper panel: conditions were native Prdx6 (control); lower panel: Prdx after incubation for 90 min with 10 µg/ml ERK2. Arrows indicate non-phosphorylated Prdx6; † indicates major spots of phosphorylated Prdx6. The pH gradient is indicated below the Figure and the position of molecular mass standards in kDa is shown on the far right.
Mutation of Thr-177 in human His-tagged Prdx6 was studied for its effect on phosphorylation and activity. We evaluated T177A mutant and also T177E mutant protein since the negative charge imparted by the glutamic-acid residue has been noted to mimic phosphorylation in some proteins [32,33]. Neither of these mutant proteins showed phosphorylation when incubated with ERK2 and [γ-32p]ATP (Figure 5B). Basal PLA2 activity was present in the T177A mutant protein although moderately decreased compared with wild-type (Table 5). The T177E mutant showed basal activity that was approx. 2.4 times the activity of the T177A mutant. There was no significant change in activity of either mutant protein after incubation with ERK2 (Table 5) or p38δ (results not shown). These Thr-177 mutant proteins were generated with Tuner (DE3) cells, but essentially identical results for activity were seen using the pPosKJ vector in Tuner (DE3) pLysS cells (results not shown). These results indicate that phosphorylation of Thr-177 is necessary for the increased PLA2 activity after incubation of Prdx6 with MAPK.
Peroxidase activity (n = 3) of the wild-type human His-tagged Prdx6 was 5.3 ± 0.15 µmol/min per mg of protein with PLPCOOH substrate and was the same with H2O2, similar to our previously reported values [8,18]. Phosphorylation of rat or human Prdx6 had no effect on its peroxidase activities (results not shown). Activity of human T177A was 4.9 ± 0.32 and 4.9 ± 0.25 µmol/min per mg of protein respectively for the two substrates and was 4.8 ± 0.39 and 5.2 ± 0.20 µmol/min per mg of protein for T177E. None of these values was statistically different from wild-type protein. The intact peroxidase activity with PLPCOOH confirms that the Thr-177 mutant proteins are able to bind to the phospholipid substrate.
DISCUSSION
Prdx6 plays a key role in lung surfactant metabolism through its aiPLA2 activity within the secretory organelles and lysosomes of AECII [12,15]. aiPLA2 activity degrades surfactant phospholipids and also provides the lyso-PC substrate that is required for DPPC synthesis by the deacylation–reacylation pathway. The present study shows that stimulation of AECII with PMA markedly increases aiPLA2 activity of the cell lysate as well as DPPC degradation by the cells, which, as we have shown previously, indicates an increase in the aiPLA2 activity of Prdx6 [9,13–15,23]. The effect of PMA was seen within 5–30 min so that protein induction would be an unlikely mechanism, compatible with results from another laboratory [34], and the lack of change in Prdx6 protein levels in the present study. The effect of PMA was not due to the direct phosphorylation of Prdx6 by PKC as studied in vitro. Therefore we investigated whether PMA-mediated activation of MAPK was the mechanism for increased PLA2 activity. Indeed, PMA treatment of AECII resulted in phosphorylation of ERK and p38 as well as Prdx6 phosphorylation. The kinetics for phosphorylation (and dephosphorylation) of ERK and p38 in AECII were slightly different and the results with inhibitors suggest that activation of both kinases is required for maximal Prdx6 phosphorylation. The phosphorylation of Prdx6 (Figure 3A) persisted long after the MAPK activity had returned to baseline (Figure 4A), suggesting relative stability of the phosphorylated protein and a relatively low rate of dephosphorylation. Treatment of AECII with inhibitors of ERK or p38 blocked the PMA-mediated phosphorylation of the MAPK, the phosphorylation of Prdx6 and the increased aiPLA2 activity. Thus it appears that activation of MAPK pathways is responsible for the PMA effect on Prdx6 activity in AECII.
Supporting the results with intact cells, recombinant Prdx6 was phosphorylated in vitro by ERK and p38, but not by JNK. Phosphorylation in vitro resulted in a marked increase in PLA2 activity that was Ca2+-independent and was inhibited by MJ33 and by SP-A, similar to native lung enzyme. Both ERK1 and 2 and all four p38 isoforms could phosphorylate Prdx6 in vitro. In HeLa cells, PMA has been reported to activate only p38γ and δ, while SB202190 has been reported to inhibit only p38α and β [35,36]. However, another study indicated that phosphorylation by p38γ is inhibited by SB202190 suggesting that unknown factors may modify the response [37]. Thus the specific isoform of p38 that leads to the activation of PLA2 in the intact cell is unclear, although p38γ would be a leading candidate. Likewise, the ERK isoform responsible for Prdx6 phosphorylation in the cell is also not known.
MAPKs preferentially catalyse the phosphorylation of substrates containing the minimal consensus sequence Ser/Thr-Pro, which is recognized by the active site of the kinase [38–40]. The Prdx6 sequence [20] shows three conserved putative threonine phosphorylation sites at positions Thr-44, Thr-177 and Thr-221 [20] (see Supplementary Figure S1A at http://www.BiochemJ.org/bj/419/bj4190669add.htm). Both ERK2 and p38γ led to in vitro phosphorylation at Thr-177 of recombinant Prdx6 by mass spectroscopic analysis and increased PLA2 activity. These effects were not observed in Thr-177 mutant protein. The mutant proteins were otherwise intact since both peroxidase and basal PLA2 activities were similar to wild-type protein. Thus phosphorylation of Thr-177 appears to be responsible for the increased PLA2 activity of the phosphorylated protein. Of note, the crystal structure of the oxidized Prdx6 homodimer [41] shows that Thr-177 is not located near the proposed aiPLA2 catalytic triad (see Supplementary Figure S1B at http://www.BiochemJ.org/bj/419/bj4190669add.htm), so the mechanism for increased PLA2 activity of Prdx6 by Thr-177 phosphorylation is unresolved. The 2D gels suggest possible phosphorylation of another residue besides Thr-177 that could not be identified by mass spectroscopy in the present study because of its relatively low yield. Since Prdx6 was immunoprecipitated from PMA-treated AECII by an anti-phosphoserine antibody, a serine residue in Prdx6 may be the other phosphorylated moiety; however, putative serine residue(s) that fit the MAPK consensus phosphorylation sequence could not be identified by inspection of the Prdx6 amino acid sequence. Thus identification of possible serine phosphorylation sites and the responsible enzymes will require further study.
Scansite (http://scansite.mit.edu) [42] analysis of the Prdx6 amino acid sequence for possible MAPK recognition motifs revealed the presence of two docking sites (D-domains), D1 (R64NVKLIALSI73) and D2 (R106NRELAILL114) that are conserved in Prdx6 in mammalian and other vertebrate species (see Supplementary Figure 1C at http://www.BiochemJ.org/bj/419/bj4190669add.htm). Both of the D-domain peptides in Prdx6 fit the consensus motif sequence (R/K)-X-X-(R/K)-X(1–4)-ΦA-X-ΦB, where X is any amino acid and Φ is most likely leucine/isoleucine residue (L/I) or any other hydrophobic amino acid [38–40]. The Prdx6 D2-domain is one residue shorter than the D1-domain since it lacks a single amino acid within the (R/K)-X-X-(R/K) D-domain sequence (see Supplementary Figure S1C at http://www.BiochemJ.org/bj/419/bj4190669add.htm). The hydrophobic core (L/I)-X-(L/I) is well conserved in both D-domains of Prdx6, I69 ALSI78 and L110AILL114 in rat and human Prdx6 Dl- and D2-domains respectively. The Prdx6 D2-domain also has a cluster of hydrophobic amino acids outside the consensus D-domain sequence (G115MLDP119). A stretch of hydrophobic amino acids downstream from the D-domain has been reported in several MAPK substrates such as MAPK phosphatases and the ETS (E twenty-six) domain transcription factors, Sap-1 and Sap-2 [40,43]. Based on the crystal structures, the D1-domain of Prdx6 is partially and the D2-domain is fully accessible to the protein surface (see Supplementary Figure S1D at http://www.BiochemJ.org/bj/419/bj4190669add.htm). In addition to the presence of the D-domains, the Prdx6 protein sequence contains another MAPK recognition motif called the DEF (docking site of ERKFXF) domain [38–40] that fits the minimal consensus sequence FXF (see Supplementary Figure S1B at http://www.BiochemJ.org/bj/419/bj4190669add.htm). In contrast with the D-domains, the DEF domain is not as well conserved among Prdx6 homologues, suggesting that the D-domains of Prdx6 are more likely to represent the MAPK docking site.
The present results suggest that phosphorylation of Prdx6 may be important for its functional role as a PLA2. In the non-phosphorylated state, PLA2 activity is maximal at pH4, compatible with its function as a lysosomal enzyme. However, phosphorylated Prdx6 is active at both acidic and cytosolic pH. Thus phosphorylated Prdx6 could possibly hydrolyse membrane phospholipids that are accessible from the cytosol. This suggests a physiologically regulated system where membrane damage such as that associated with oxidative stress results in MAPK-mediated phosphorylation of Prdx6, thereby promoting its PLA2 activity in the cytosol. In this circumstance, Prdx6 could function either as a peroxidase to directly reduce peroxidized membrane phospholipids or as a PLA2 to ‘excise’ the oxidized sn-2 fatty acid.
Previous studies have provided evidence that phosphorylation is also of importance for other PLA2 enzymes. Phosphorylation of cytosolic PLA2 (cPLA2) enhances its PLA2 activity [44–46] and treatment with PKC (the α isoform in this case) could directly phosphorylate the protein in vitro [47]. cPLA2 can also be phosphorylated in vitro by a MAPK (ERK2), which enhanced its activity to a greater degree than that seen with PKC [45]. The sites of phosphorylation of cPLA2 by PKC and ERK2 were different for the two kinases [46,48].
The possible phosphorylation of Prdx6 has not been reported previously. However, phosphorylation of Prdx1 at Thr-90 by cyclin-dependent kinases is known [49]. Of note, Prdx1 and Prdx6 show only ~15 % identity of amino acid sequence and Thr-90 is conserved in Prdx1–4 but not in Prdx6 [50]. Furthermore, PLA2 activity of Prdx1 has not been reported and is unlikely on the basis of the lack of a lipase motif in its amino acid sequence [50]. Phosphorylation of Prdxl led to a marked decrease in its peroxidase activity [49], but peroxidase activity of Prdx6 in the present study was unaffected by MAPK-mediated phosphorylation.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Susan Turbitt for typing the manuscript. This work was presented in preliminary form at the May 2007 Annual Meeting of the American Thoracic Society held in San Francisco, CA, U.S.A.
FUNDING
This work was supported by grants from the National Institutes of Health [grant numbers HL19737 and HL79063].
Abbreviations used
- 2D
two-dimensional
- [3H]DPPC
1-palmitoyl-2-[3H]9,10-palmitoyl-sn-glycero-3-phosphorylcholine
- AECII
alveolar epithelial type II cells
- DEF domain
docking site of ERK FXF domain
- ERK
extracellular-signal-regulated kinase
- IEF
isoelectric focusing
- IPG
immobilized pH gradient
- JNK
c-Jun N-terminal kinase
- PC
phosphatidylcholine
- DPPC
dipalmitoyl phosphatidylcholine
- mAb
monoclonal antibody
- MAPK
mitogen-activated protein kinase
- MS/MS
tandem MS
- pAb
polyclonal antibody
- PKC
protein kinase C
- PLA2
phospholipase A2
- aiPLA2
acidic calcium-independent PLA2
- PLPCOOH
phospholipid hydroperoxide
- Prdx
peroxiredoxin
- Sp-A
surfactant protein-A
REFERENCES
- 1.Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 2.Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Pak JH, Manevich Y, Kim HS, Feinstein SI, Fisher AB. An antisense oligonucleotide to 1-cys peroxiredoxin causes lipid peroxidation and apoptosis in lung epithelial cells. J. Biol. Chem. 2002;277:49927–49934. doi: 10.1074/jbc.M204222200. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Feinstein SI, Manevich Y, Ho YS, Fisher AB. Lung injury and mortality with hyperoxia are increased in peroxiredoxin 6 gene-targeted mice. Free Radical Biol. Med. 2004;37:1736–1743. doi: 10.1016/j.freeradbiomed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Feinstein SI, Manevich Y, Ho YS, Fisher AB. Peroxiredoxin 6 gene-targeted mice show increased lung injury with paraquat-induced oxidative stress. Antioxid. Redox Signaling. 2006;8:229–237. doi: 10.1089/ars.2006.8.229. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Phelan SA, Manevich Y, Feinstein SI, Fisher AB. Transgenic mice overexpressing peroxiredoxin 6 show increased resistance to lung injury in hyperoxia. Am. J. Respir. Cell. Mol. Biol. 2006;34:481–486. doi: 10.1165/rcmb.2005-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11599–11604. doi: 10.1073/pnas.182384499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JW, Dodia C, Feinstein SI, Jain MK, Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J. Biol. Chem. 2000;275:28421–28427. doi: 10.1074/jbc.M005073200. [DOI] [PubMed] [Google Scholar]
- 9.Fisher AB, Dodia C. Role of phospholipase A2 enzymes in degradation of dipalmitoylphosphatidylcholine by granular pneumocytes. J. Lipid Res. 1996;37:1057–1064. [PubMed] [Google Scholar]
- 10.Fisher AB, Dodia C, Chander A, Jain M. A competitive inhibitor of phospholipase A2 decreases surfactant phosphatidylcholine degradation by the rat lung. Biochem. J. 1992;288:407–411. doi: 10.1042/bj2880407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim TS, Sundaresh CS, Feinstein SI, Dodia C, Skach WR, Jain MK, Nagase T, Seki N, Ishikawa K, Nomura N, Fisher AB. Identification of a human cDNA clone for lysosomal type Ca2+-independent phospholipase A2 and properties of the expressed protein. J. Biol. Chem. 1997;272:2542–2550. doi: 10.1074/jbc.272.4.2542. [DOI] [PubMed] [Google Scholar]
- 12.Akiba S, Dodia C, Chen X, Fisher AB. Characterization of acidic Ca(2+)-independent phospholipase A2 of bovine lung. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998;120:393–404. doi: 10.1016/s0305-0491(98)10046-9. [DOI] [PubMed] [Google Scholar]
- 13.Fisher AB, Dodia C, Feinstein SI, Ho YS. Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal-type phospholipase A2. J. Lipid Res. 2005;46:1248–1256. doi: 10.1194/jlr.M400499-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Fisher AB, Dodia C. Role of acidic Ca2+-independent phospholipase A2 in synthesis of lung dipalmitoyl phosphatidylcholine. Am. J. Physiol. 1997;272:L238–L243. doi: 10.1152/ajplung.1997.272.2.L238. [DOI] [PubMed] [Google Scholar]
- 15.Fisher AB, Dodia C. Lysosomal-type PLA2 and turnover of alveolar DPPC. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L748–L754. doi: 10.1152/ajplung.2001.280.4.L748. [DOI] [PubMed] [Google Scholar]
- 16.Chander A, Fisher AB. Regulation of lung surfactant secretion. Am. J. Physiol. 1990;258:L241–L253. doi: 10.1152/ajplung.1990.258.6.L241. [DOI] [PubMed] [Google Scholar]
- 17.Fisher AB, Dodia C, Chander A. Secretagogues for lung surfactant increase lung uptake of alveolar phospholipids. Am. J. Physiol. 1989;257:L248–L252. doi: 10.1152/ajplung.1989.257.4.L248. [DOI] [PubMed] [Google Scholar]
- 18.Fisher AB, Dodia C, Manevich Y, Chen JW, Feinstein SI. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J. Biol. Chem. 1999;274:21326–21334. doi: 10.1074/jbc.274.30.21326. [DOI] [PubMed] [Google Scholar]
- 19.Chinoy MR, Dodia C, Fisher AB. Increased surfactant internalization by rat type II cells cultured on microporous membranes. Am. J. Physiol. 1993;264:L300–L307. doi: 10.1152/ajplung.1993.264.3.L300. [DOI] [PubMed] [Google Scholar]
- 20.Kim TS, Dodia C, Chen X, Hennigan BB, Jain M, Feinstein SI, Fisher AB. Cloning and expression of rat lung acidic Ca(2+)-independent PLA2 and its organ distribution. Am. J. Physiol. 1998;274:L750–L761. doi: 10.1152/ajplung.1998.274.5.L750. [DOI] [PubMed] [Google Scholar]
- 21.Manevich Y, Feinstein SI, Fisher AB. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with Pi GST. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3780–3785. doi: 10.1073/pnas.0400181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon SY, Choi YJ, Kang TH, Lee KH, Cha SS, Kim GH, Lee HS, Kim KT, Kim KJ. Highly efficient protein expression and purification using bacterial hemoglobin fusion vector. Plasmid. 2005;53:274–282. doi: 10.1016/j.plasmid.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Fisher AB, Dodia C, Chander A. Inhibition of lung calcium-independent phospholipase A2 by surfactant protein A. Am. J. Physiol. 1994;267:L335–L341. doi: 10.1152/ajplung.1994.267.3.L335. [DOI] [PubMed] [Google Scholar]
- 24.Manevich Y, Reddy KS, Shuvaeva T, Feinstein SI, Fisher AB. Structure and phospholipase function of peroxiredoxin 6: identification of the catalytic triad and its role in phospholipid substrate binding. J. Lipid Res. 2007;48:2306–2318. doi: 10.1194/jlr.M700299-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Wu YZ, Manevich Y, Baldwin JL, Dodia C, Yu K, Feinstein SI, Fisher AB. Interaction of surfactant protein A with peroxiredoxin 6 regulates phospholipase A2 activity. J. Biol. Chem. 2006;281:7515–7525. doi: 10.1074/jbc.M504525200. [DOI] [PubMed] [Google Scholar]
- 26.Chander A, Reicherter J, Fisher AB. Degradation of dipalmitoyl phosphatidylcholine by isolated rat granular pneumocytes and reutilization for surfactant synthesis. J. Clin. Invest. 1987;79:1133–1138. doi: 10.1172/JCI112929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller WJ, Zen K, Fisher AB, Shuman H. Pathways for uptake of fluorescently labeled liposomes by alveolar type II cells in culture. Am. J. Physiol. 1995;269:L11–L19. doi: 10.1152/ajplung.1995.269.1.L11. [DOI] [PubMed] [Google Scholar]
- 28.Fisher AB, Chander A, Reicherter J. Uptake and degradation of natural surfactant by isolated rat granular pneumocytes. Am. J. Physiol. 1987;253:C792–C796. doi: 10.1152/ajpcell.1987.253.6.C792. [DOI] [PubMed] [Google Scholar]
- 29.Fisher A, Chander A. Intracellular processing of surfactant lipids in the lung. Ann Rev. Physiol. 1985;47:789–802. doi: 10.1146/annurev.ph.47.030185.004041. [DOI] [PubMed] [Google Scholar]
- 30.Xiao DM, Pak JH, Wang X, Sato T, Huang FL, Chen HC, Huang KP. Phosphorylation of HMG-I by protein kinase C attenuates its binding affinity to the promoter regions of protein kinase C gamma and neurogranin/RC3 genes. J. Neurochem. 2000;74:392–399. doi: 10.1046/j.1471-4159.2000.0740392.x. [DOI] [PubMed] [Google Scholar]
- 31.Yates JR, III, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal. Chem. 1995;67:1426–1436. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
- 32.Maciejewski PM, Peterson FC, Anderson PJ, Brooks CL. Mutation of serine 90 to glutamic acid mimics phosphorylation of bovine prolactin. J. Biol. Chem. 1995;270:27661–27665. doi: 10.1074/jbc.270.46.27661. [DOI] [PubMed] [Google Scholar]
- 33.Burkart EM, Arteaga GM, Sumandea MP, Prabhakar R, Wieczorek DF, Solaro RJ. Altered signaling surrounding the C-lobe of cardiac troponin C in myofilaments containing an alpha-tropomyosin mutation linked to familial hypertrophic cardiomyopathy. J. Mol. Cell. Cardiol. 2003;35:1285–1293. doi: 10.1016/s0022-2828(03)00240-2. [DOI] [PubMed] [Google Scholar]
- 34.Tolle A, Schlame M, Charlier N, Guthmann F, Rustow B. Vitamin E differentially regulates the expression of peroxiredoxin-1 and -6 in alveolar type II cells. Free Radical Biol. Med. 2005;38:1401–1408. doi: 10.1016/j.freeradbiomed.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S, McDonnell PC, Gum RJ, Hand AT, Lee JC, Young PR. Novel homologues of CSBP/p38 MAP kinase: activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem. Biophys. Res. Commun. 1997;235:533–538. doi: 10.1006/bbrc.1997.6849. [DOI] [PubMed] [Google Scholar]
- 36.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Jiang Y, Ulevitch RJ, Han J. The primary structure of p38 gamma: a new member of p38 group of MAP kinases. Biochem. Biophys. Res. Commun. 1996;228:334–340. doi: 10.1006/bbrc.1996.1662. [DOI] [PubMed] [Google Scholar]
- 38.Biondi RM, Nebreda AR. Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem. J. 2003;372:1–13. doi: 10.1042/BJ20021641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs D, Glossip D, Xing H, Muslin AJ, Kornfeld K. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 1999;13:163–175. [PMC free article] [PubMed] [Google Scholar]
- 40.Sharrocks AD, Yang SH, Galanis A. Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem. Sci. 2000;25:448–453. doi: 10.1016/s0968-0004(00)01627-3. [DOI] [PubMed] [Google Scholar]
- 41.Choi HJ, Kang SW, Yang CH, Rhee SG, Ryu SE. Crystallization and preliminary X-ray studies of h0RF6, a novel human antioxidant enzyme. Acta Crystallogr. D Biol. Crystallogr. 1998;54:436–437. doi: 10.1107/s0907444997011153. [DOI] [PubMed] [Google Scholar]
- 42.Obenauer JC, Yaffe MB. Computational prediction of protein–protein interactions. Methods Mol. Biol. 2004;261:445–468. doi: 10.1385/1-59259-762-9:445. [DOI] [PubMed] [Google Scholar]
- 43.Masuda K, Shima H, Katagiri C, Kikuchi K. Activation of ERK induces phosphorylation of MAPK phosphatase-7, a JNK specific phosphatase, at Ser-446. J. Biol. Chem. 2003;278:32448–32456. doi: 10.1074/jbc.M213254200. [DOI] [PubMed] [Google Scholar]
- 44.Xing M, Insel PA. Protein kinase C-dependent activation of cytosolic phospholipase A2 and mitogen-activated protein kinase by alpha 1-adrenergic receptors in Madin–Darby canine kidney cells. J. Clin. Invest. 1996;97:1302–1310. doi: 10.1172/JCI118546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 46.Nemenoff RA, Winitz S, Qian NX, Van Putten V, Johnson GL, Heasley LE. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule-associated protein 2 kinase and protein kinase C. J. Biol. Chem. 1993;268:1960–1964. [PubMed] [Google Scholar]
- 47.Li Q, Subbulakshmi V, Oldfield CM, Aamir R, Weyman CM, Wolfman A, Cathcart MK. PKCalpha regulates phosphorylation and enzymatic activity of cPLA2 in vitro and in activated human monocytes. Cell. Signalling. 2007;19:359–366. doi: 10.1016/j.cellsig.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Hefner Y, Borsch-Haubold AG, Murakami M, Wilde JI, Pasquet S, Schieltz D, Ghomashchi F, Yates JR, III, Armstrong CG, Paterson A, et al. Serine 727 phosphorylation and activation of cytosolic phospholipase A2 by MNK1-related protein kinases. J. Biol. Chem. 2000;275:37542–37551. doi: 10.1074/jbc.M003395200. [DOI] [PubMed] [Google Scholar]
- 49.Chang TS, Jeong W, Choi SY, Yu S, Kang SW, Rhee SG. Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation. J. Biol. Chem. 2002;277:25370–25376. doi: 10.1074/jbc.M110432200. [DOI] [PubMed] [Google Scholar]
- 50.Phelan SA. A0P2 (antioxidant protein 2): structure and function of a unique thiol-specific antioxidant. Antioxid. Redox Signaling. 1999;1:571–584. doi: 10.1089/ars.1999.1.4-571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.