Abstract
Although the administration of monocrotaline (MCT) into experimental animals is in widespread use today in investigations of pulmonary arterial hypertension (PAH), the underlying cellular and subcellular mechanisms that culminate in vascular remodeling are incompletely understood. Bovine pulmonary arterial endothelial cells (PAECs) in culture exposed to monocrotaline pyrrole (MCTP) develop “megalocytosis” 18–24 h later characterized by enlarged hyperploid cells with enlarged Golgi, mislocalization of endothelial nitric oxide synthase away from the plasma membrane, decreased cell-surface/caveolar nitric oxide (NO), and hypo-S-nitrosylation of caveolin-1, clathrin heavy chain, and N-ethylmaleimide-sensitive factor. We investigated whether MCTP did in fact affect functional intracellular trafficking. The NO scavenger (4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO) and the NO donor diethylamine NONOate were used for comparison. Both MCTP and c-PTIO produced distinctive four- to fivefold enlarged PAECs within 24–48 h with markedly enlarged/dispersed Golgi, as visualized by immunostaining for the Golgi tethers/matrix proteins giantin, GM130, and p115. Live-cell uptake of the Golgi marker C5 ceramide revealed a compact juxtanuclear Golgi in untreated PAECs, brightly labeled enlarged circumnuclear Golgi after MCTP, but minimally labeled Golgi elements after c-PTIO. These Golgi changes were reduced by NONOate. After an initial inhibition during the first day, both MCTP and c-PTIO markedly enhanced anterograde secretion of soluble cargo (exogenous vector-expressed recombinant horseradish peroxidase) over the next 4 days. Live-cell internalization assays using fluorescently tagged ligands showed that both MCTP and c-PTIO inhibited the retrograde uptake of acetylated low-density lipoprotein, transferrin, and cholera toxin B. Moreover, MCTP, and to a variable extent c-PTIO, reduced the cell-surface density of all receptors assayed (LDLR, TfnR, BMPR, Tie-2, and PECAM-1/CD31). In an important distinction, c-PTIO enhanced mitosis in PAECs but MCTP inhibited mitosis, even that due to c-PTIO, despite markedly exaggerated Golgi dispersal. Taken together, these data define a broad-spectrum Golgi and subcellular trafficking dysfunction syndrome in endothelial cells exposed to MCTP or NO scavenging.
Keywords: vascular remodeling, megalocytosis, anterograde and retrograde trafficking, β-actin
ingestion or administration of pyrrolizidene alkaloids leads to vascular occlusive disease including pulmonary arterial hypertension (PAH) in cattle, horses, pigs, dogs, and even primates (reviewed in Refs. 20, 48 and citations therein). Over the last four decades the experimental administration of the pyrrolizidine alkaloid monocrotaline into rats, pigs, and dogs has come into widespread use in experimental studies of pulmonary arterial hypertension PAH (20, 24, 30, 34–36, 46, 62). As one example, a single injection of monocrotaline (MCT) leads to increased pulmonary arterial pressure in 10–14 days in the juvenile male rat (24, 34–36). Despite the extensive experimental use of the MCT model over the years, there is an incomplete understanding of the underlying cellular and subcellular changes that culminate in pulmonary vascular remodeling.
The administered MCT is converted into the bioactive MCT-pyrrole (MCTP) in the liver (reviewed in Refs. 20, 48). Histologically, the pulmonary arterial lesions produced by MCTP include enlarged pulmonary arterial endothelial cells (PAECs) and hypertrophic medial smooth muscle cells (PASMCs) with increased DNA synthesis (27, 28, 34). The term “megalocytosis” was coined by Bull in 1955 to reflect this dramatic phenotypic change caused by pyrrolizidene alkaloids (6; also see Refs. 1, 16). Neointima development, thrombotic obliteration of arterial lumen, and development of plexiform lesions, reminiscent of human PAH, have also been reported with MCT when combined with partial pneumonectomy (44, 65 and citations therein). Additionally, perivascular/adventitial infiltrative cells and their cytokine contributions (such as IL-6) and transcription factors activated by such cytokines (STAT3 and NF-κB) have been posited as contributory factors in the development of disease in this model (2, 14, 15, 30, 41, 64).
Over the last two decades bovine PAECs and PASMCs in culture exposed to MCTP have been used as experimental models to investigate some of the cellular, subcellular, and biochemical changes elicited by MCTP (18, 25, 26, 30, 38–42, 48, 49, 53–55, 57, 61, 66). A single exposure of confluent bovine PAEC cultures to MCTP for as short a time as 2 min leads to apoptosis of a small subset of cells with the development of marked megalocytosis of cells in the surviving, but still confluent, cell sheet 18–24 h later (18, 38, 48, 49, 68, 73). As with the in vivo observations (27, 34–36), the cell culture studies showed dramatic increases in cell size, in Golgi stacks, and in the endoplasmic reticulum; increased DNA synthesis; and a unique premitotic cell cycle arrest despite the continued DNA synthesis resulting in hyperploidy of the affected cells (18, 25, 33, 36, 39–41, 48, 49, 55, 57, 61, 66; reviewed in Refs. 53, 54). Indeed, Roth and colleagues (18, 48, 49) posited as early as 1991 that these changes, observed in bovine PAEC culture, likely underlie the vascular remodeling seen in PAH in experimental animals. Nevertheless, how these changes are biochemically initiated by MCTP within 2 min, what subcellular mechanisms effectuate development of the overt megalocytosis phenotype over the next 18–24 h, and the extent of the functional phenotypic alterations remain, unclear.
Studies (30, 42, 55, 57) on the mechanism of action of MCTP from this laboratory in cultured bovine PAECs and in the MCT/rat model disclosed the loss of caveolin-1 (cav-1) and endothelial nitric oxide synthase (eNOS) from the plasma membrane of megalocytotic PAECs with the aberrant sequestration of eNOS in the Golgi and in cytoplasmic vesicles. There was marked enlargement of the Golgi with marked accumulation of diverse vesicle trafficking tethers and soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) and SNAP receptors (SNAREs) in the Golgi in both bovine PAECs in culture and in PAECs in the MCT/rat model (30, 42, 53–55, 57). Various cargo molecules such as cav-1, eNOS, and bone morphogenetic receptor type-2 (BMPR-2) were trapped, at least in part, in the Golgi (55). The mislocalization of eNOS was accompanied by reduced cell surface/caveolar production of NO as assayed by live-cell 4–5 diaminofluorescein diacetate (DAF-2DA) imaging in PAEC culture (42). Biochemically, this resulted in the hypo-S-nitrosylation of the trafficking mediator proteins cav-1, clathrin heavy chain, and N-ethylmaleimide-sensitive factor and of eNOS itself (38). This raised the question of whether, consequently, functional endocytic and caveolar trafficking would be inhibited in MCTP-exposed PAECs.
In the present study, we investigated whether the MCTP produced hypo-S-nitrosylation of trafficking mediator proteins in PAECs (38) translated into defects in functional intracellular trafficking and Golgi function. For comparison, we included the NO scavenger (4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO) and the NO donor diethylamine NONOate (NONOate). We observed that both MCTP and NO scavenging produced extensive changes in Golgi structure and function, subcellular mislocalization of vasorelevant proteins and receptors, and functional defects in intracellular trafficking. Moreover, NO scavenging dramatically enhanced mitosis and proliferation of PAECs. The new data define a novel broad-spectrum subcellular trafficking dysfunction syndrome in PAECs characterized by multiple simultaneous functional changes in the Golgi, intracellular trafficking, and cell surface receptors and increased mitosis.
Our companion article appearing in this issue (54a) tests the applicability of this Golgi dysfunction syndrome to cells in pulmonary arterial vasculopathies in human idiopathic PAH and in SHIV-nef-infected macaques.
MATERIALS AND METHODS
Cell culture.
Bovine PAECs were grown in T-75 flasks or 6-well or 35-mm plates in DMEM and 15% FBS as described by us previously (38–42, 55–57). MCTP was prepared by the method of Mattocks et al. (32) as described by us previously (38–42, 55, 57). MCTP in dimethylformamide (equivalent to 200 μM MCT with 25–30% conversion to the active pyrrole, see Ref. 60for details) was added directly to the culture medium with a rapid swirling motion. Typically MCTP was added once to cultures 1 day after seeding and growth medium refreshed on a daily basis thereafter. Megalocytosis began to develop within 24 h and was fully evident by 36–48 h. Carboxy-PTIO (c-PTIO; BioMol International, Plymouth Meeting, PA) was used at 100 μM and diethylamine NONOate sodium salt hydrate (NONOate; Sigma-Aldrich, St. Louis, MO) at 400 μM with daily refreshing of cultures with growth medium containing c-PTIO and NONOate. Phase-contrast microscopy was carried out using a Nikon Diaphot microscope equipped with a Nikon Coolpix digital camera.
Immunofluorescence microscopy.
Immunofluorescence assays were performed essentially as described previously (38–42, 55–57). Typically, PAECs in 6-well plates appropriately exposed to MCTP and/or c-PTIO and/or NONOate (see respective figure legends in results) were fixed using 4% cold paraformaldehyde for 1 h and permeabilized using 0.1% Triton X-100. Fixed cultures were then stained using various combinations of goat and rabbit pAbs and murine mAbs. Respective donkey AlexaFluor 488- or AlexaFluor 594-tagged secondary antibodies were used in this study (Molecular Probes, Eugene, OR). Images were collected using a Nikon Eclipse 50i epifluorescence microscopy system (objectives: low-magnification Nikon Plan 10×/NA 0.25 and high-magnification Nikon Plan 40×/NA 0.65) equipped with a red-green-blue charged-couple device (CCD) camera and RS Image 1.9.2 software (Roper Scientific, Tucson, AZ). In epifluoresence microscopy, nuclei were demarcated using DAPI (Sigma-Aldrich). Alternatively, images were collected using an MRC 1024 ES (Bio-Rad) confocal laser scanning microscopy system (objective: Olympus Plan 10×/NA 0.25) with a black-and-white CCD camera using the manufacturer's software and then rendered in pseudocolor. All data within each experiment were collected at identical imaging settings. Controls included omission of the primary antibody and peptide competition assays and the use of multiple different antibodies toward the same antigen (see our previous Refs. 38–42, 55–57). Because of the limitations of sensitivity of the existing confocal microscope available for this project, confocal imaging was limited to data collection in the green channel (such as using AlexaFluor 488), while the epifluoresence microscope was used for data collection in the green, red (such as with AlexaFluor 594), and blue (DAPI) channels.
Endogenous receptors on the surface of PAECs were displayed by carrying out “surface-accessible immunostaining” after paraformaldehyde fixation but before permeabilization with detergent using antibodies to extracellular domains of respective receptors. After quantitative imaging, the immunostaining was repeated with Triton X-100 (0.1%) permeabilization to display distribution of all of the respective antigens within the cell.
Live-cell quantitative horseradish peroxidase secretion assay.
Gene-transfer assays for horseradish peroxidase (HRP) secretion were carried out essentially as described by Connolly et al. (9) and by us earlier (55). Briefly, 1-day-old PAEC cultures in 6-well plates were transfected with the constitutive expression vectors for secreted HRP species (pSRα.ssHRP of Ref. 9; 2.5 μg/well in triplicate wells for each experimental variable) using the lipofectamine reagent (Polyfect, Qiagen, Valencia, CA) and the manufacturer's protocol. As a baseline control, PAECs were transfected with pcDNA3.1 vector alone. After 1 day, the culture medium was harvested (“0” day samples; 1 ml per well) and MCTP or c-PTIO was added. Thereafter, the medium was harvested and replenished (1 ml each time) every day for 5 days with the daily addition of fresh c-PTIO. The harvested medium samples were assayed quantitatively for HRP in triplicate (9, 55), the baseline values derived from pcDNA3.1-transfected cells were subtracted, and the HRP secretion data for days 1 to 5 were normalized to the HRP in the medium of the same well in the “0” day sample.
Live-cell imaging of caveolar NO using DAF-2DA fluorescence.
This was carried out using the membrane-permeant probe DAF-2DA as described previously (38, 42). Briefly, PAEC cultures in 6-well plates were washed with PBS and replenished with HBSS medium containing 0.1 mM l-arginine (Arg-HBSS). The cells were then loaded with DAF-2DA (10 μM) for 20–30 min at 37○C, and the fluorescence was imaged using the Nikon epifluorescence microscope and RS Image software as indicated in Immunofluorescence microscopy.
Live-cell endocytic/caveolar trafficking assays.
Ligand uptake and internalization assays were carried out using AlexaFluor 594-tagged transferrin, AlexaFluor 594-tagged cholera toxin subunit B (CTB; recombinant), and AlexaFluor 488-tagged acetyl low density lipoprotein (AcLDL) purchased from Invitrogen Molecular Probes and the manufacturer's respective protocols. Typically, PAEC cultures in 6-well or 35-mm plates were washed with warm PBS and replenished with 0.5 ml each of growth medium containing the respective tagged ligands (10 μM final concentration). The cultures were incubated at 37°C for 30 min, washed extensively with growth medium, and imaged over the next 5–10 min by epifluorescence microscopy and RS Image software indicated as in Immunofluorescence microscopy. The imaging data represent the sum of the residual bound ligand at the cell surface plus the internalized ligand (see Figs. 5 and 6 and Supplemental Fig. 3; supplemental data for this article are available online at the Am J Physiol Lung Cell Mol Physiol website). Alternative uptake and chase protocols included extending the labeling period to 60 or 90 min at 37°C before imaging or exposing cells to ligand for 30 min in the cold followed by a chase for 30 min at 37°C. Selected uptake experiments (as those using AlexaFluor 488-AcLDL visible in the green channel) were visualized using the epifluoresence as well as confocal microscopy systems.
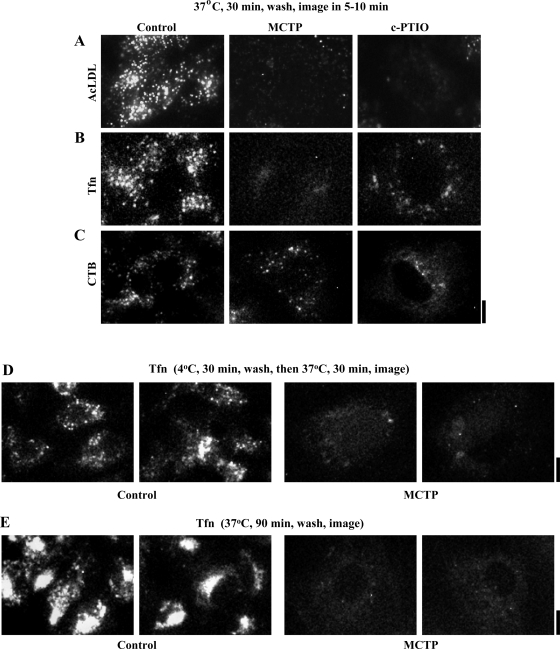
Fig. 5.
Epifluoresence imaging of functional retrograde uptake and internalization of acetylated low-density lipoprotein (AcLDL), transferrin (Tfn), and cholera toxin B (CTB) in PAECs exposed to MCTP and c-PTIO for 4 days. Respective fluorescent ligand uptake and internalization assays were carried out in PAEC cultures 4 days after commencement of MCTP or c-PTIO treatment using procedures summarized in Materials and Methods with variations as indicated in the figure. Multiple epifluoresence images (×40 objective, n = 4–6 per variable) were collected in each experiment. A–E: representative illustrations of ×4 zoomed-in sections of representative image frames from separate experiments; full ×40 objective image frames corresponding to these experiments are illustrated in Supplemental Fig. 3. Scale bars = 12.5 μm.
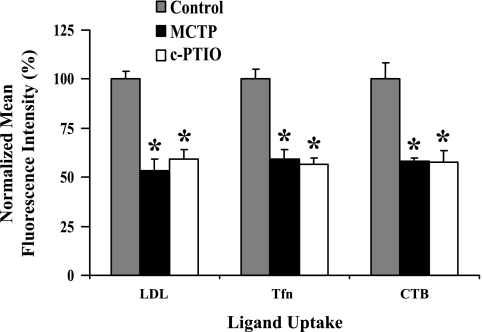
Fig. 6.
Quantitation of ligand uptake and internalization in MCTP- or c-PTIO-treated PAEC cultures. Epifluoresence imaging data such as from experiments shown in Fig. 5 and Supplemental Fig. 3 (4–20 images per variable) were quantitated in terms of fluorescence intensity per unit cell surface area, which was then normalized by taking mean values derived from control cultures to be 100 and are expressed as means ± SE over all images collected. *P = 0.001 in comparisons with the untreated control cells.
Live-cell labeling of cytoplasmic compartments (Golgi, lysosome, endoplasmic reticulum, and mitochondria).
This was carried out in 6-well plates or 35-mm culture dishes using BODIPY TR C5 ceramide complexed to BSA (5 μM), ER-Tracker Red glibenclamide BODIPY FL (1 μM), LysoTracker Red DND-99 (100 nM), and MitoTracker Blue (1 μM) purchased from Invitrogen Molecular Probes, and used essentially per manufacturer's protocols. C5 ceramide labeling was carried out in serum-free DMEM in two ways: 1) binding for 30 min on ice, washing, and then warming to 37°C for 30 min; or 2) binding for 30 min at 37°C and then washing followed by imaging. The two methods gave similar data. Double-label experiments with DAF-2DA (green) and C5 ceramide (red) were carried out in Arg-HBSS. Imaging of the respective subcellular compartments was carried out by epifluorescence microscopy using a red-green-blue camera as well as the confocal microscopy system with a black-and-white CCD camera.
Quantitative image analyses.
Representative images from respective experiments were deconvolved using NIH Image J software and respective utility plugins (available as free downloads from http://rsb.info.nih.gov/ij/) as described previously (38, 41, 42, 55, 56) for the purposes of illustration in the respective figures in Results. Quantitation was carried out using Image J software by pooling data from all of the raw unadjusted images collected per variable. Images collected using epifluorescence microscopy and the RS Image software were quantitated by drawing outlines of individual cells to derive the cell area, the integrated pixel intensity per cell, and the average image intensity per pixel within the cell outline (thus correcting for changes in cell size). The numerical correction for background was generated from within each raw image by outlining cell-free areas within the image, deriving the average pixel intensity within the outlined area, and then using this value to calculate the background over respective cell areas within the same image. For confocal images of confluent sheets of cells collected using Bio-Rad imaging software, the entire integrated pixel intensity per frame was used after subtracting for background integrated pixel intensity over the entire frame derived from blank culture plates. Such blank images gave the same instrumental background pixel intensity readings as PAEC cultures processed using the secondary antibody alone.
Antibody and inhibitor reagents.
Rabbit pAb to giantin was from Abcam (Cambridge, MA) and that to p115 was a gift from the late Dr. Dennis Shields (Albert Einstein College of Medicine; Ref. 38). Murine mAbs to GM130 (Golgi Matrix 130) were purchased from BD Biosciences (Eugene, OR). Rabbit pAbs to eNOS, cav-1, LDL receptor (LDLR), and transferrin receptor (TfnR) and goat pAbs to BMPR-2, Tie-2, platelet endothelial cell adhesion molecule (PECAM-1), and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Respective AlexaFluor 488-, AlexaFluor 594-, or AlexaFluor 647-tagged secondary donkey antibodies to rabbit, goat or mouse IgG were from Invitrogen Molecular Probes (Eugene, OR). The STAT3 inhibitor indirubin E804 (43) was purchased from Sigma-Aldrich.
Statistical analyses.
NCSS 2007 Statistical Analysis Software (Kaysville, UT) was used for comparisons between respective experimental groups and their matched controls using ANOVA tests (one-way and repeated measures). Post hoc comparisons were carried out using the Newman-Keuls Multiple comparison test.
RESULTS
PAEC megalocytosis and Golgi enlargement/dispersal after MCTP and NO scavenging.
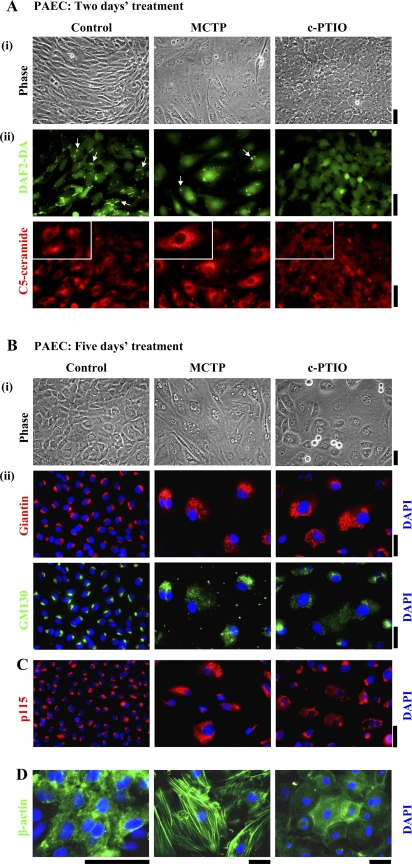
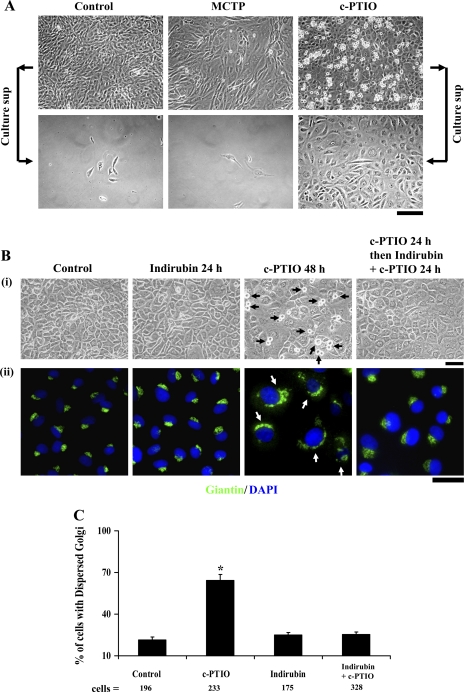
Given our previous data showing Golgi enlargement in MCTP-treated PAECs assayed after fixation and detergent permeabilization (55), we investigated whether the live-cell Golgi marker BODIPY TR C5 ceramide would be taken up by such cells and whether it would then be transported to and concentrated in the enlarged Golgi (C5 ceramide is taken up through endocytosis and is then localized to Golgi membranes via retrograde trafficking; Ref. 45). For comparison, PAEC cultures were exposed to the NO scavenger c-PTIO (100 μM). Figure 1A and Supplemental Figure 1 summarize the live-cell C5 ceramide uptake data as well as several controls. Phase-contrast microscopy showed that MCTP-treated PAECs were enlarged and angular, while c-PTIO-treated cells were enlarged and cuboidal (Fig. 1, A, i, B, ii, and D, and Supplemental Fig. 1A, top row). As described previously (42), DAF-2DA fluorescence confirmed the loss of cell surface/caveolar NO after MCTP and after c-PTIO (Fig. 1A, ii, top row, arrows). Strikingly, while BODIPY TR C5 ceramide localization revealed a compact juxtanuclear Golgi in untreated PAECs, C5 ceramide brightly labeled the enlarged circumnuclear Golgi after MCTP but minimally labeled Golgi elements after c-PTIO (Fig. 1A, ii, bottom row and Supplemental Fig. 1B, top row). Critically, 1) the changes in endothelial morphology and C5 ceramide uptake could be reduced by the NO donor diethylamine NONOate (Supplemental Fig. 1), and 2) the changes were selective in that the uptake and compartmental localization of LysoTracker, MitoTracker, or ER-Tracker was not affected by either MCTP or c-PTIO (data not shown). These data 1) confirm the selective enlargement of a functional Golgi in MCTP-treated PAECs and that the Golgi in such cells retains the ability to concentrate C5 ceramide, 2) show that NO scavenging inhibited uptake and transport of C5 ceramide to the Golgi, and 3) show that the effects of both MCTP and c-PTIO were, at least in part, due to a hypo-NO state in that NONOate reduced the changes.
Fig. 1.
Golgi dysfunction in megalocytotic pulmonary arterial endothelial cells (PAECs) exposed to monocrotaline pyrrole (MCTP) or nitric oxide (NO) scavenging. One-day-old confluent PAEC cultures in 6-well plates were exposed to MCTP once (equivalent to ∼50 μM of active pyrrole) followed by daily replenishment with normal growth medium or to (4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO; 100 μM) with daily replenishment with c-PTIO-containing medium for 2 (A), 5 (B and D), or 4 days (C). A: cultures were imaged under phase contrast (i) and switched to Arg-HBSS medium at 4°C (1 ml/well) containing BODIPY TR C5 ceramide (5 μM) for 30 min. Cultures were then washed and replenished with Arg-HBSS at 37°C for 30 min. Fifteen minutes into the latter incubation, 4–5 diaminofluorescein diacetate (DAF-2DA; 10 μM) was added and cells were imaged 15 min later using epifluorescence microscopy (DAF-2DA in the green channel and C5 ceramide in the red channel). A, ii: same fields in the two colors; scale bars = 50 μm. Insets: ×4 zoomed-in views from within the larger frame. Puncta of green DAF-2DA fluorescence seen in controls indicating surface/caveolar NO (white arrows; Ref. 43) are lost after MCTP and c-PTIO. B, C, and D: cultures were imaged under phase contrast, then fixed, permeabilized, and immunostained for the Golgi tethers/scaffolding proteins giantin and p115 (using rabbit pAbs) or GM130 (using a murine mAb), as well as for β-actin (goat pAb) and respective different secondary Abs. DAPI was used for nuclear DNA staining. B, ii: giantin and GM130 images are of same cells. In B, percentage of cells with Golgi enlargement/dispersal was as follows: controls 14.4 ± 3.6 (means ± SE over all images collected; n = 315 cells), MCTP 93.54 ± 2.4 (n = 41 cells; P < 0.001), and c-PTIO 97.6 ± 1.6 (n = 63 cells; P < 0.001). Rounded cells apparent as doublets in B, i (phase contrast) in the c-PTIO-treated culture are cells traversing through mitosis (see Figs. 8 and 9). Scale bars = 50 μm.
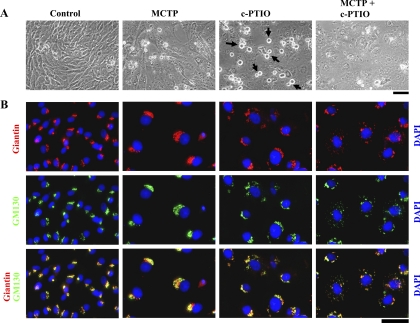
Despite the inhibition of C5 ceramide uptake after c-PTIO treatment, the Golgi elements in such cells were markedly increased in amount but extensively dispersed in the cytoplasm (Fig. 1, B and C, Fig. 2, and Supplemental Fig. 2). Figure 1B, i and Figure 2 show that 5 days after exposing PAECs to either MCTP or c-PTIO there was a marked increase in cell size. This was accompanied by marked enlargement and cytoplasmic dispersal of the Golgi as assayed by immunostaining for either of the Golgi tethers/scaffolding proteins giantin, GM130, or p115 (Fig. 1, B, ii and C, and Fig. 2). Consistent with prior observations that Golgi dispersal typically precedes entry of cells into mitosis (60), c-PTIO-treated cultures showed rounded doublets characteristic of mitotic cells (Fig. 1B, i; also see Figs. 8 and 9). The data in Supplemental Fig. 2, A and B, confirm that NONOate reduced the Golgi enlargement and dispersal due to either of MCTP or c-PTIO, again implicating NO in the structural integrity of the Golgi apparatus in endothelial cells. Nevertheless, the effects of MCTP and c-PTIO on PAECs were distinctive in that β-actin was present in prominent stress fibers within the cell in MCTP-induced megalocytosis but accumulated at the cell periphery in c-PTIO-induced megalocytosis (Fig. 1D).
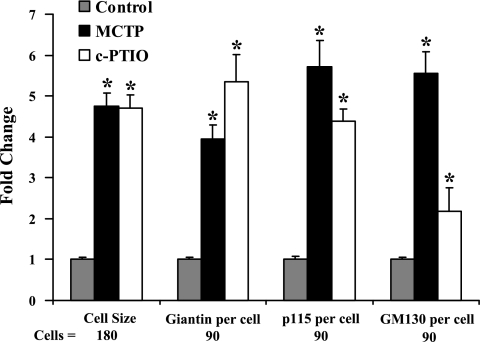
Fig. 2.
Quantitation of increase in cell size and cellular content of the Golgi tethers/scaffolding proteins giantin, p115, and GM130 in PAECs exposed to MCTP or c-PTIO. Immunofluorescence images derived from the experiments in Fig. 1, B and C, were quantitated as described in Materials and Methods to obtain the respective integrated intensities per cell. Data are expressed in terms of fold change compared with respective means derived from control cells. *P < 0.05, compared with respective untreated controls.
Fig. 8.
NO scavenging drives PAECs into mitosis and proliferation. A: PAEC cultures in 6-well plates were exposed to MCTP or c-PTIO for 5 days as indicated in Fig. 1 legend. After phase-contrast imaging, cultures were vigorously shaken horizontally and culture medium containing floating cells harvested. Cell harvests from two wells per variable were pooled, washed with fresh growth medium, and replated into fresh 6-well cultures. Replated cells were imaged one-day later by phase-contrast microscopy. Low-magnification fields are illustrated. Scale bar = 100 μm. B and C: STAT3 inhibitor indirubin E804 inhibits mitosis and Golgi dispersal due to NO scavenging. PAEC cultures in 6-well plates were exposed to c-PTIO for 48 h. In some cultures, indirubin E804 (5 μM) was included during the last 24 h. Phase-contrast images and giantin immunostaining show that indirubin inhibited entry of cells into mitosis (black arrowheads) and Golgi dispersal (white arrowheads). Quantitation of the fraction of cells with dispersed Golgi in all images collected from this experiment from respective cultures (mean % ± SE) is summarized in C. Scale bar = 50 μm in B. *P < 0.01.
Fig. 9.
MCTP inhibits c-PTIO-driven entry of cells into mitosis despite exaggerating the marked increase in Golgi dispersal. PAEC cultures were exposed to MCTP for 20 min and then washed. c-PTIO was then added (1 mM for 30 min; 100 μM thereafter). Cultures were imaged by phase contrast (A) 2 days later and then fixed and processed for immunostaining for giantin and GM130 and counterstained with DAPI (B). Black arrows in A point to rounded cells traversing mitosis. Scale bars = 50 μm.
Functional changes in anterograde trafficking after MCTP or NO scavenging.
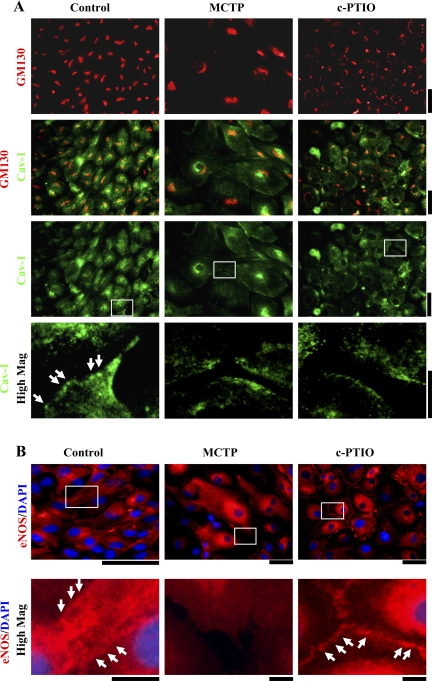
The Golgi apparatus is the key nodal point in regulating the anterograde trafficking from the cell interior to the plasma membrane (3, 9, 37, 59, 67). Thus soluble cargo destined for secretion traverses through the Golgi as does membrane-associated cargo such as cav-1, eNOS, and cell surface receptors. Figure 3A shows that both MCTP and c-PTIO reduced the localization of cav-1 to the plasma membrane with increased sequestration in a GM130-positive Golgi compartment. However, although eNOS was sequestered in a cell-centric compartment after both MCTP and c-PTIO, it was markedly lost from the plasma membrane after MCTP but continued to be retained in the intercellular plasma membrane contacts after c-PTIO (Fig. 3B). In comparison, both MCTP and c-PTIO reduced the localization of PECAM-1/CD31 from intercellular plasma membrane contacts with sequestration in an intracellular compartment (see data in Fig. 7B, ii). Thus MCTP and c-PTIO each produce specialized phenotypic changes in PAECs that can be both similar (loss of cav-1 and PECAM-1 from plasma membrane after both) and dissimilar (retained eNOS in intercellular plasma membrane contacts after c-PTIO).
Fig. 3.
Subcellular mislocalization of cav-1 and endothelial nitric oxide synthase (eNOS) in PAECs exposed to MCTP or c-PTIO. PAEC cultures in 6-well plates were exposed to MCTP or c-PTIO for 4 days as indicated in Fig. 1 legend. The fixed permeabilized cultures were then immunostained for GM130 (mAb), cav-1 (pAb), and eNOS (pAb) together with DAPI. A and B: each include high-magnification insets zooming (×4) into indicated areas. Cav-1 and eNOS were localized to the plasma membrane in controls (arrowheads). Cav-1 was lost from the plasma membrane after MCTP or c-PTIO and sequestered in a cell-centric compartment (A). eNOS was lost from the plasma membrane after MCTP but was increased at intercellular membrane contacts after c-PTIO (B, arrowheads). Scale bars = 50 and 25 μm, respectively, in low- and high-magnification frames in A and 50 and 12.5 μm, respectively, in low- and high-magnification frames in B.
Fig. 7.
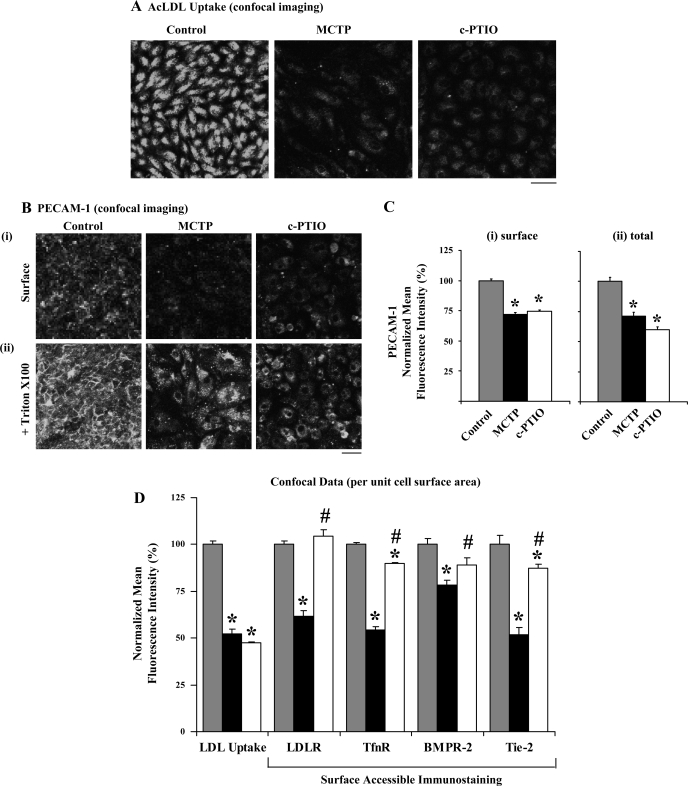
Confocal imaging assays of retrograde AcLDL uptake, of surface-accessible and total platelet endothelial cell adhesion molecule (PECAM-1)/CD31, and cell-surface accessible densities of LDLR, TfnR, BMPR-2 and Tie-2 in PAECs exposed to MCTP or c-PTIO for 4 days. A: PAEC cultures in 6-well plates were exposed to MCTP or c-PTIO as indicated in Fig. 1 legend and AcLDL uptake was assayed (30 min at 37°C) and imaged using confocal microscopy and Bio-Rad imaging software. Scale bar = 60 μm. Quantitation of this experiment is shown in D, left-most set. B: parallel cultures to those in A were fixed with 4% paraformaldehyde, immunostained for PECAM-1 without permeabilization, and imaged using confocal microscopy (i). Subsequently, these were treated with 0.1% Triton X-100 and PECAM-1 immunostaining and confocal microscopy was repeated (ii). Scale bar = 60 μm; the laser intensity used for imaging in ii was 30% of that in i. C: quantitation of surface-accessible and total PECAM-1 immunostaining per unit cell surface area in experiment in B. *P < 0.01 in comparisons with respective untreated control cells. D: PAECs in 6-well plates (at least in duplicate per variable) were exposed to MCTP or c-PTIO for 4 days and processed for LDL uptake (as in A) and surface-accessible immunostaining for LDLR, TfnR, bone morphogenetic receptor type-2 (BMPR-2), and Tie-2 after fixation but without permeabilization (as in B, i). Imaging was carried out using confocal microscopy (n = at least 4 images per variable). Quantitation (fluorescence intensity per unit cell surface area; mean ± SE) was carried out as indicated in C, i and normalized by taking respective mean values in control cells as 100. *P < 0.05 in comparisons with respective untreated control cells; #P < 0.05 in comparisons between the MCTP and c-PTIO groups.
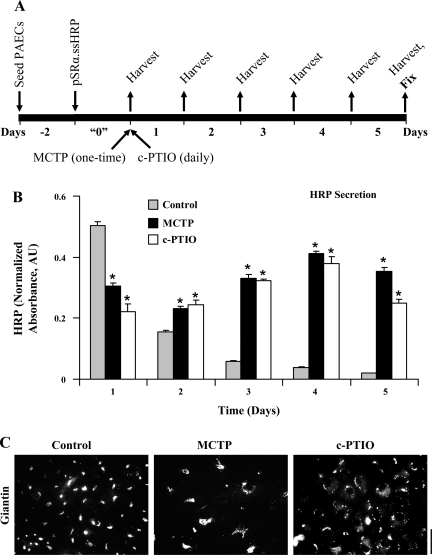
An extraordinary aspect of this specialized phenotype is revealed in the live-cell anterograde secretion assays in Fig. 4. Previously, we reported that PAECs expressing an engineered secretory form of recombinant HRP synthesized off a transiently transfected expression plasmid showed diminished secretion when exposed to MCTP for 1 day (Fig. 1 in Ref. 55). Yet, it is well known that such MCTP-treated cells survive and increase in size over the next several days and weeks (27, 28, 48, 49, 55, 57, 61, 66). Figure 4A illustrates the experimental protocol used to investigate the long-term secretion of HRP by PAECs derived from a single transient transfection of the cells with the constitutive expression plasmid. The data in Fig. 4B show that with both MCTP and c-PTIO, while there was an inhibition of HRP secretion during the first day compared with untreated controls (confirming our previous observation in Ref. 55), there was continued and sustained secretion of HRP by treated cells over the next 4 days even though this rapidly declined in untreated controls. This occurred even though the Golgi elements were markedly dispersed in the cytoplasm (Fig. 4C).
Fig. 4.
Prolonged and enhanced long-term anterograde secretion of soluble cargo [recombinant horseradish perioxidase (HRP)] by PAECs exposed to MCTP or c-PTIO. PAEC cultures (in triplicate per variable) were transfected with the ssHRP expression vector and exposed to MCTP or c-PTIO 1 day later, the culture medium (1 ml per well) was harvested for the next 5 days, and the cultures were then fixed for immunostaining (A). Each sample of culture medium was assayed in triplicate for peroxidase activity [expressed in arbitrary absorbance units (AU); Ref. 60]; values for background subtraction were generated from medium samples simultaneously harvested from PAEC cultures transfected with only the pCDNA3 vector. HRP activity data derived from each well during the 5-day experiment were normalized in terms of HRP activity obtained from that same well in the first 24-h period (time interval indicated as “0” in A); thus each well acted as its own transfection control. A: experimental protocol. B: summary of data obtained (means ± SE). C: confirmation of Golgi dispersal in cultures at the conclusion of this experiment (scale bar = 50 μm). *P < 0.01 in intraday comparison with respective untreated control at each time point; n = 9 at each time point for each variable (triplicate cultures per variable with each medium sample assayed in triplicate).
Functional changes in retrograde trafficking after MCTP or NO scavenging.
The observed hypo-S-nitrosylation of clathrin heavy chain and cav-1 after MCTP or c-PTIO (38) suggested that there might be global dysfunction of endocytic pathways. We used ligand uptake and internalization assays to monitor the functional integrity of clathrin (using AcLDL and Tfn)- or cav-1 (using CTB)-endocytic pathways in live PAECs exposed to MCTP or c-PTIO using epifluoresence imaging methods. Such ligand uptake assays are typically carried out by binding the respective fluorescently tagged ligand to cells in culture at 4°C for 30 min, washing excess ligand away, and then assaying for internalization by shifting the temperature up to 37°C. However, in our hands, PAECs in culture tended to retract when exposed to 4°C for 30 min. Therefore, in addition to the customary binding in the cold protocol (as in Fig. 5D and Supplemental Fig. 3D), in most experiments we incubated PAEC cultures to tagged ligands at either 37°C for 30 min (as in Fig. 5, A–C, and Supplemental Fig. 3, A–C) to assay for localization of ligand soon after internalization or for 37°C for 90 min (as in Fig. 5E and Supplemental Fig. 3E) to assay for the eventual destination of the tagged ligand. The data in Supplemental Fig. 3, A–C (compilation of low-magnification images), and in Fig. 5, A–C (compilation of high-magnification images), and the quantitation summarized in Fig. 6 show that PAECs exposed to either MCTP and c-PTIO had reduced ligand uptake and internalization on a per unit cell surface area basis with each of the ligands assayed using 37°C for the 30-min incubation protocol. The reduction in Tfn uptake and internalization in MCTP-treated cells was also observed when assayed by binding ligand at 4°C followed by a 30-min chase at 37°C (Fig. 5D and Supplemental Fig. 3D). Moreover, in MCTP-treated cells the endocytic transit of ligands to the Golgi was incomplete in that Tfn did not reach the Golgi even after a 90-min labeling period (Fig. 5E and Supplemental Fig. 3E). Additionally, imaging of fluorescently tagged AcLDL uptake using the alternative approach of confocal imaging (Fig. 7, A and D, left) also confirmed a reduction in uptake and internalization in MCTP- and c-PTIO-treated PAECs.
Mechanistically, impaired ligand uptake and internalization could result from either a reduction in the cell surface density of the respective receptor or impaired endocytic transit from the plasma membrane to the cell interior or both. This question was investigated by quantitative imaging of the cell-surface accessible fraction of the respective receptors after fixation with paraformaldehyde but without detergent permeabilization. The data in Fig. 7, B, i and C, i, confirm the effectiveness of this approach and show that MCTP and c-PTIO both reduced cell-surface accessible PECAM-1/CD31 immunostaining. Reimmunostaining after detergent permeabilization showed that while PECAM-1 was lost from intercellular membrane contact areas after MCTP and c-PTIO, this antigen was increasingly sequestered in cell-centric cytoplasmic vesicles but with a net loss (Fig. 7, B, ii and C, ii).
This surface-accessible immunostaining procedure was then used to assess the surface density of LDLR, TfnR, Tie-2, and BMPR-2. The data summarized in Fig. 7D show that MCTP reduced the surface density of LDLR, TfnR, Tie-2, and to a lesser extent that of BMPR-2. In comparison, c-PTIO had smaller effects, or not at all, on the surface densities of all of these receptors. Nevertheless, both MCTP and c-PTIO had equivalent inhibitory effects on functional LDL, Tfn, and CTB uptakes (Fig. 6). Thus while the reduced uptake in MCTP-treated cells might be accounted for by reduced surface receptors, that due to c-PTIO cannot. Thus NO scavenging likely has additional inhibitory effects on endocytic transit from the plasma membrane to the cell interior.
NO scavenging enhances mitosis in PAEC cultures.
Endothelial cell proliferation, i.e., an increase in endothelial cell number, is characteristic of vascular lesions and neointima development in PAH (46, 47, 62). The data in Fig. 8A show that NO scavenging led to increased entry of endothelial cells into mitosis and cell proliferation. The rounded cells in Fig. 8A, top row, after c-PTIO were not cells in apoptosis in that these cells were 1) annexin-V-FITC negative (38) and 2) could be mechanically shaken off one culture and replated into a fresh one (Fig. 8A, bottom row). Additional data using DAPI to stain chromosomes confirmed an increase in mitotic figures after c-PTIO (not shown). This enhanced mitosis due to c-PTIO occurred concomitant with marked Golgi enlargement and dispersal (Fig. 8, B and C). The megalocytosis, Golgi dispersal, and increased mitosis due to NO scavenging were all reversed by the STAT3 inhibitor indirubin E804 (Fig. 8, B and C; Ref. 43). In contrast, while MCTP-treated cells were enlarged and megalocytotic with enlarged nuclei, these cells did not enter mitosis (Fig. 8A).
Figure 9 shows the results of an experiment in which PAECs were exposed to both MCTP and NO scavenging. Each of the MCTP and c-PTIO alone produced enlarged cells as did the combination (Fig. 9A). However, while c-PTIO alone clearly enhanced entry of cells into mitosis, the inclusion of MCTP together with c-PTIO inhibited this traverse into mitosis (Fig. 9A). Nevertheless, although each of the MCTP and c-PTIO alone enlarged the Golgi, albeit with different fine structural changes (enlargement with intact Golgi ribbon after MCTP and extensive cytoplasmic dispersal after c-PTIO), the combination of the two caused a dramatic and exaggerated cytoplasmic dispersal and fragmentation of the Golgi elements (Fig. 9B). Thus 1) with respect to the Golgi, MCTP and c-PTIO must have distinct subcellular/biochemical targets, 2) MCTP has a distinct and unique effect on cell cycle traverse consisting of a premitosis block, and 3) NO scavenging stimulates mitosis.
DISCUSSION
The present data define a broad-spectrum subcellular dysfunction syndrome in PAECs exposed to either MCTP or NO scavenging characterized by multiple simultaneous functional changes in cell size, the Golgi apparatus, intracellular trafficking, surface receptors, and mitosis (Table 1). MCTP, an agent widely used to generate experimental models of PAH, was used as the index probe to uncover this subcellular dysfunction syndrome. Many of the effects of MCTP on trafficking were similar to those of NO scavenging. However, there were distinct differences in terms of the enlarged cell morphology including the disposition of β-actin fibers, the fine structure of Golgi enlargement/dispersal, and the entry into mitosis. Remarkably, the present data showing a 40–50% inhibition of the functional uptake of multiple different ligands (LDL, Tfn, and CTB) by bovine PAECs exposed to MCTP in cell culture recapitulate the observations of Gillis et al. (12) who reported in 1978 that pulmonary vessels in MCT-treated rats showed a 50–60% reduction in uptake of the ligands 14C-norepinephrine and 14C-5-hydroxy-tryptamine in an ex vivo perfused lung assay. Taken together the data show that it is no longer possible to view the cellular changes underlying vascular remodeling in MCT-induced PAH, and in PAH in general, in terms of one or other discrete change alone at the level of the single endothelial cell but that it requires consideration of a broad-spectrum phenotypic change resulting from Golgi dysfunction, dysfunctional anterograde and retrograde intracellular trafficking, as well as the interplay between Golgi structure and entry into mitosis (Table 1).
Table 1.
Similar and dissimilar subcellular changes in PAECs exposed to MCTP or NO scavenging with c-PTIO
| Feature | MCTP | c-PTIO |
|---|---|---|
| Cell morphology | enlarged, angular | enlarged, cuboidal |
| Entry into mitosis | inhibited (inhibits that of c-PTIO) | enhanced |
| Nuclear size | increased | increased, reduced postmitosis |
| Cell surface NO (DAF2-DA) | inhibited | inhibited |
| S-nitrosylation* | ||
| Caveolin-1 | inhibited | inhibited |
| Clathrin heavy chain | inhibited | inhibited |
| NSF | inhibited | inhibited |
| eNOS | inhibited | inhibited |
| Golgi (giantin, GM130, p115) | increased, enlarged | increased, dispersed |
| Protein localizations | ||
| caveolin-1 | reduced plasma membrane, some in Golgi (GM130) | reduced plasma membrane, some in Golgi (GM130) |
| eNOS | reduced plasma membrane, increased cell-centric | increased at intercellular junctions, cell-centric |
| PECAM-1 (CD31) | loss from intercellular contacts | loss from intercellular contacts |
| β-actin | increased stress fibers | cell-periphery |
| Live-cell secretion (ssHRP)† | initial inhibition, then enhanced, long-lived | initial inhibition, then enhanced, long-lived |
| Live-cell compartment labeling† | ||
| C5 ceramide (Golgi) | enhanced | inhibited |
| LysoTracker | unchanged | unchanged |
| ER-Tracker | unchanged | unchanged |
| MitoTracker | unchanged | unchanged |
| Live-cell ligand uptake2 | ||
| AcLDL | inhibited | inhibited |
| Transferrin | inhibited | inhibited |
| CTB | inhibited | inhibited |
| Surface-accessible receptor† | ||
| LDLR | reduced | unchanged |
| Transferrin-R | reduced | small reduction |
| PECAM-1 (CD31) | reduced | reduced |
| BMPR-2 | reduced | small reduction |
| Tie-2 | reduced | small reduction |
c-PTIO, (4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide; MCTP, monocrotaline pyrrole; DAF-2DA, 4-5 diaminofluorescein diacetate; HRP, horseradish perioxidase; NSF, N-ethylmaleimide-sensitive factor; PECAM-1, platelet endothelial cell adhesion molecule-1; AcLDL, acetyl low density lipoprotein; CTB, cholera toxin subunit; BMPR-2, bone morphogenetic receptor type-2.
From Ref. 38.
Compared with untreated PAECs on a per unit cell surface basis.
The morphological megalocytosis produced by c-PTIO in endothelial cells (an enlarged cuboidal cell phenotype) is virtually identical to that produced by small-interfering RNA-mediated downregulation of dynamin-2 (see Fig. 1B, top, in Ref. 22). Dynamin-2 is a GTPase involved in several critical aspects of intracellular membrane trafficking and one whose activity appears to require S-nitrosylation (22). Moreover, there are prior reports (21) showing that NO scavenging by c-PTIO, but not the inactive c-PTI, increases cell cycle traverse in endothelial cell cultures with increased accumulation of cells in G2/M. Additionally, c-PTIO (20 μM) increased cell proliferation (increased cell number) in cultures of human salivary gland neoplastic cells without any observable apoptosis (64). Conversely, various NO donors have been reported to inhibit cell cycle traverse, including that of vascular smooth muscle cells (56). The present data confirm the role of NO in regulating endothelial cell proliferation and entry into mitosis.
While NO scavenging increased the entry of PAECs into mitosis, MCTP had the opposite effect. MCTP, and other pyrrolizidine alkaloids, produce a unique block at a checkpoint that lies subsequent to the Golgi enlargement/dispersal stage but before nuclear dissolution and entry into mitosis (39, 57, 61, 66). MCTP-treated PAECs showed a loss of cdc2, the kinase required for entry into mitosis (39). Thus while many of the effects of MCTP on trafficking can be replicated by NO scavenging, MCTP generates a unique premitosis block despite stimulating continued DNA synthesis.
The relationships among eNOS expression, NO, and the pathogenesis and progression of PAH are confusing (reviewed in Ref. 23). On the one hand, idiopathic PAH in humans has been represented as an NO-deficiency disease state with the growing use of sildenafil, NO inhalation, and nebulized nitrites as therapeutic approaches in this disease (4, 10, 11, 13, 23, 29, 62, 68). On the other hand, varying data have been reported on the relationships between eNOS expression levels and NO activity (23 and citations therein). Nevertheless, we note that previous studies of eNOS expression in PAH (discussed in Ref. 23. and citations therein) dealt with eNOS expression in assays at the whole lung tissue or whole cell levels and not with respect to the exact subcellular localization of the eNOS protein. There is a similar controversy with respect to the role of NO in the MCT/rat model. Roberts et al. (50) reported that NO inhalation ameliorated MCT-induced PAH and vascular changes in 8-day-old rat pups. Hill and Pearl (17) also reported attenuation of PAH by NO inhalation in the MCT/adult rat model. Moreover Stewart and colleagues (7, 69) reported amelioration of MCT-induced PAH by genetically engineered cell-based overexpression of eNOS in the pulmonary vascular bed. In contrast, other investigators (19, 31) have reported little or no effect of NO inhalation on PAH in the juveline or adult rat administered MCT. Nevertheless, Maruyama et al. (31) did report that short-term inhalation of NO at least partially ameliorated PAH and that chronic 19-day inhalation of NO (at 40 ppm) did cause a statistically significant reduction in the percentage of muscularized arteries at the alveolar wall (Table 4 in Ref. 31). In our experiments, the NO donor NONOate delayed and reduced MCTP-induced megalocytosis but did not completely reverse it (Supplemental Figs. 1 and 2).
The sustained secretory phenotype of PAECs exposed to MCTP or c-PTIO (Fig. 4) is relevant to discussions of the pathogenesis of PAH in that many investigators have posited the ability of PAECs in this disease to secrete proproliferative growth factors and cytokines in a paracrine manner that then affect adjacent vascular cells, such as smooth muscle cells and pericytes, to migrate and transdifferentiate (2, 36, 46, 47, 62). The endogenous proteins (candidate elastases, proteases, cytokines, and growth factors) thus secreted over several days by megalocytotic PAECs exposed to MCTP or c-PTIO in cell culture remain to be identified. Moreover, while the focus of the present investigation was on defining changes and mechanisms in a key pulmonary cell substrate that is thought to contribute to vascular remodeling, the pulmonary arterial endothelial cell, the lung stroma is also likely to be an active participant in the progression of this disease. We note that Rai et al. (47) have previously drawn attention to the pseudotumor or carcinoma-like aspects of vascular cell proliferation in PAH. In parallel with the emerging understanding of cancer progression that includes the bilateral contributions of the tumor cell and of the host/IL-6/STAT3 axis (5, 52), we posit a similar contribution of stromal/perivascular cell-types including infiltrating macrophages in the ultimate pathogenesis of PAH (30, 41).
To summarize, MCTP and NO scavenging produced a broad spectrum of subcellular functional and structural alterations in PAECs in culture (Table 1). These included not only the enlargement and dispersal of the Golgi but functional changes in anterograde and retrograde trafficking and in diverse vasorelevant cell surface receptors. In a discrete distinction, NO scavenging but not MCTP enhanced entry of PAECs into mitosis. The questions emanating from the present work are 1) is the Golgi dysfunction syndrome reported here of any relevance in human idiopathic PAH, and 2) is there a causal relationship between Golgi dysfunction and vasculopathies of PAH? The related submission (54a) addresses these questions.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-077301 and HL-087176 (to P. B. Sehgal).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Somshuvra Mukhopadhyay for numerous helpful discussions and Kirit Patel for technical assistance.
REFERENCES
- 1.Afzelius BA, Schoental R. The ultrastructure of the enlarged hepatocytes induced in rats with a single oral dose of retrorsine, a pyrrolozidine (Senecio) alkaloid. J Ultrastruct Res 20: 328–345, 1967 [DOI] [PubMed] [Google Scholar]
- 2.Arcot SS, Lipke DW, Gillespie MN, Olson JW. Alterations of growth factor transcripts in rat lungs during development of monocrotaline-induced pulmonary hypertension. Biochem Pharmacol 46: 1086–1091, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Bonifacino JS, Glick BS. The mechanism of vesicle budding and fusion. Cell 116: 153–166, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bloch KD, Ichinose F, Roberts JD, Jr, Zapol WM. Inhaled NO as a therapeutic agent. Cardiovasc Res 75: 339–348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell 15: 79–80, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bull LA. The histological evidence of liver damage from pyrrolizidine alkaloids. Aust Vet J 31: 33–40, 1955 [Google Scholar]
- 7.Campbell AIM, Kuliszewski MA, Stewart DJ. Cell-based gene transfer to the pulmonary vasculature: endothelial nitric oxide synthase overexpression inhibits monocrotaline-induced pulmonary hypertension. Am J Respir Cell Mol Biol 21: 567–575, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol 27: 1877–1885, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Connolly CN, Futter CE, Gibson A, Hopkins CR, Cutler DF. Transport into and out of the Golgi complex studied by transfecting cells with cDNAs encoding horseradish peroxidase. J Cell Biol 127: 641–652, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med 173: 1186–1193, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in lungs of patients with pulmonary hypertension. N Engl J Med 333: 214–221, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Gillis CN, Huxtable RJ, Roth RA. Effects of monocrotaline pretreatment of rats on removal of 5-hydroxytryptamine and noradrenaline by perfused lung. Br J Pharmacol 63: 435–443, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girgis RE, Champton HC, Diette GB, Johns RA, Permutt S, Sylvester JT. Decreased exhaled nitric oxide in pulmonary arterial hypertension: response to bosentan therapy. Am J Respir Crit Care Med 172: 352–357, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Golemski SM, West J, Tada Y, Fagan KA. Interleukin-6 causes mild pulmonary hypertension and augments hypoxia-induced pulmonary hypertension in mice. Chest 128: 572S–573S, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Hagen M, Fagan K, Steudel W, Carr M, Kirk K, Rodman DM, West J. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol 292: L1473–L1479, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Harris PN, Anderson RC, Chen KK. The action of senecionine, integerrimine, jacobine, longilobine, and spartiodine, especially on the liver. J Pharmacol Exp Ther 75: 60–77, 1942 [Google Scholar]
- 17.Hill LL, Pearl RG. Combined inhaled nitric oxide and inhaled prostacyclin during experimental chronic pulmonary hypertension. J Appl Physiol 86: 1160–1164, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Hoorn CM, Roth RA. Monocrotaline pyrrole alters DNA, RNA and protein synthesis in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 262: L740–L747, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Horstman DJ, Frank DU, Rich GF. Prolonged inhaled NO attenuates hypoxic, but not monocrotaline-induced, pulmonary vascular remodeling in rats. Anesth Analg 86:74–81, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Huxtable RJ. Activation and pulmonary toxicity of pyrrolizidene alkaloids. Pharmac Ther 47: 371–389, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Jansenn YM, Soultanakis R, Steece K, Heerdt E, Singh RJ, Joseph J, Kalyanaraman B. Depletion of nitric oxide causes cell cycle alterations, apoptosis and oxidative cells in pulmonary cells. Am J Physiol Lung Cell Mol Physiol 275: L1100–L1109, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Kang-Decker N, Cao S, Chatterjee S, Yao J, Egan LJ, Semela D, Mukhopadhyay D, Shah V. Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. J Cell Sci 120: 492–501, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Klinger JR. The nitric oxide/cGMP signaling pathway in pulmonary hypertension. Clin Chest Med 28: 143–167, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Lalich JJ, Merkow L. Pulmonary arteritis produced in rat by feeding Crotalaria spectabilis. Lab Invest 10: 744–750, 1961 [PubMed] [Google Scholar]
- 25.Lamé MW, Jones AD, Wilson DW, Dunston SK, Segall HJ. Protein targets of monocrotaline pyrrole in pulmonary arterial endothelial cells. J Biol Chem 275: 29091–29099, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Lamé MW, Jones AD, Wilson DW, Segall HJ. Monocrotaline pyrrole targets proteins with and without cysteine residues in the cytosol and membrane of human pulmonary arterial endothelial cells. Proteomics 5: 4398–43413, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lappin PB, Ross KL, King LE, Fraker PJ, Roth RA. The response of pulmonary vascular endothelial cells to monocrotaline pyrrole: cell proliferation and DNA synthesis in vitro and in vivo. Toxicol Appl Pharmacol 150: 37–48, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Lappin PB, Roth RA. Hypertrophy and prolonged DNA synthesis in smooth muscle cells characterize pulmonary arterial wall thickening after monocrotaline pyrrole administration to rats. Toxicol Pathol 25: 372–380, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Malerba M, Radaeli A, Ragnoli B, Airo P, Corradi M, Ponticiello A, Zambruni A, Grassi V. Exhaled nitric oxide levels in systemic sclerosis with and without pulmonary hypertension. Chest 132: 575–580, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Mathew R, Huang J, Shah M, Patel K, Gewitz M, Sehgal PB. Disruption of endothelial-cell caveolin-1α/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation 110: 1499–1506, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Maruyama J, Maruyama K, Mitani Y, Kitabatake M, Yamauchi T, Miyasaka K. Continuous low-dose NO inhalation does not prevent monocrotaline-induced pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol 272: H517–H524, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Mattocks AR, Jukes R, Brown J. Simple procedures for preparing putative toxic metabolites of pyrrolizidine alkaloids. Toxicon 27: 561–567, 1989 [DOI] [PubMed] [Google Scholar]
- 33.Mattocks AR, Legg RF. Antimitotic activity of dehydroretrorcine, a pyrrolizidine alkaloid metabolite, and some analogous compounds, in a rat liver parenchymal cell line. Chem Biol Interact 30: 325–336, 1980 [DOI] [PubMed] [Google Scholar]
- 34.Merkow L, Kleinerman J. An electron microscopic study of pulmonary vasculitis induced by monocrotaline. Lab Invest 15: 547–564, 1966 [PubMed] [Google Scholar]
- 35.Meyrick BO, Gamble W, Reid LM. Development of Crotolaria pulmonary hypertension. Am J Physiol Heart Circ Physiol 239: H692–H702, 1980 [DOI] [PubMed] [Google Scholar]
- 36.Meyrick BO, Reid LM. Crotalaria-induced pulmonary hypertension: uptake of 3H-thymidine by the cells of the pulmonary circulation and alveolar walls. Am J Pathol 106: 84–94, 1982 [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee S, Shields D. Nuclear import is required for the pro-apoptotic function of the Golgi protein p115. J Biol Chem 284: 1709–1717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukhopadhyay S, Lee J, Sehgal PB. Depletion of the ATPase NSF from Golgi membranes with hypo-S-nitrosylation of vasorelevatnt proteins in endothelial cells exposed to monocrotaline pyrrole. Am J Physiol Heart Circ Physiol 295: H1943–H1955, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukhopadhyay S, Sehgal PB. Discordant regulatory changes in monocrotaline-induced megalocytosis of lung arterial endothelial and alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 290: L1216–L1226, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Mukhopadhyay S, Shah M, Patel K, Sehgal PB. Monocrotaline pyrrole-induced megalocytosis of lung and breast epithelial cells: disruption of plasma membrane and Golgi dynamics and an enhanced unfolded protein response. Toxicol Appl Pharmacol 211: 209–220, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhay S, Shah M, Xu F, Patel K, Tuder RM, Sehgal PB. Cytoplasmic provenance of STAT3 and PY-STAT3 in endolysosomal compartments in pulmonary arterial endothelial and smooth muscle cells: implications in PAH. Am J Physiol Lung Cell Mol Physiol 294: L449–L468, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Mukhopadhyay S, Xu F, Sehgal PB. Aberrant cytoplasmic sequestration of eNOS in endothelial cells after monocrotaline, hypoxia and senescence: subcellular eNOS localization and live-cell caveolar and cytoplasmic NO imaging studies. Am J Physiol Heart Circ Physiol 293: H77–H85, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Nam S, Buettner R, Turkson J, Kim D, Cheng JQ, Muehlbeyer S, Hippe F, Vatter S, Merz KH, Eisenbrand G, Jove R. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc Natl Acad Sci USA 102: 5998–6003, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GJ, Shi L, Qiu D, Benson G, Pearl RG, Kao PN. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation 108: 1640–1645, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Pagano RE, Sleight RG. Defining lipid transport pathways in animal cells. Science 229: 1051–1057, 1985 [DOI] [PubMed] [Google Scholar]
- 46.Rabinovitch M. Molecular pathogenesis of pulmonary hypertension. J Clin Invest 118: 2372–2379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rai PR, Cool CD, King JAC, Stevens T, Burns N, Winn RA, Kasper M, Voelkel NF. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 178: 558–564, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reindel JF, Hoorn CM, Wagner JG, Roth RA. Comparison of response of bovine and porcine pulmonary arterial endothelial cells to monocrotaline pyrrole. Am J Physiol Lung Cell Mol Physiol 261: L406–L414, 1991 [DOI] [PubMed] [Google Scholar]
- 49.Reindel JF, Roth RA. The effects of monocrotaline pyrrole on cultured bovine pulmonary artery endothelial and smooth muscle cells. Am J Pathol 138: 707–719, 1991 [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts JD, Jr, Chiche JD, Weiman J, Steudel W, Zapol WM, Bloch KD. Nitric oxide inhalation decreases pulmonary artery remodeling in the injured lungs of rat pups. Circ Res 87: 140–145, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Sarkar R, Gordon D, Stanley JC, Webb RC. Cell cycle effecte of nitric oxide on vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 272: H1810–H1818, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Sehgal PB. Interleukin 6 in infection and cancer. Proc Soc Exp Biol Med 195: 183–191, 1990 [DOI] [PubMed] [Google Scholar]
- 53.Sehgal PB, Mukhopadhyay S. Dysfunctional intracellular trafficking in the pathobiology of pulmonary arterial hypertension. Am J Respir Cell Mol Biol 37: 31–37, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sehgal PB, Mukhopadhyay S. Pulmonary hypertension: a disease of tethers, SNAREs and SNAPs? Am J Physiol Heart Circ Physiol 293: H77–H85, 2007 [DOI] [PubMed] [Google Scholar]
- 54a.Sehgal PB, Mukhopadhyay S, Patel K, Xu F, Almodovar S, Tuder RM, Flores SC. Golgi dysfunction is a common feature in idiopathic human pulmonary hypertension and vascular lesions in SHIV-nef-infected macaques. Am J Physiol Lung Cell Mol Physiol (July31, 2009). doi: 10.1152/ajplung.00087.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sehgal PB, Mukhopadhyay S, Xu F, Patel K, Shah M. Dysfunction of Golgi tethers, SNAREs and SNAPs in monocrotaline-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 292: L1526–L1542, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Shah M, Patel K, Mukhopadhyay S, Xu F, Guo G, Sehgal PB. Membrane associated STAT3 and PY-STAT3 in the cytoplasm. J Biol Chem 281: 7302–7308, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Shah M, Patel K, Sehgal PB. Monocrotaline pyrrole-induced endothelial cell megalocytosis involves a Golgi blockade mechanism. Am J Physiol Cell Physiol 288: C850–C862, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Shao C, Furusawa Y, Aoki Sper/NO-induced reversible proliferation inhibition and cycle arrests associated with a micronucleus induction in HSG cells. Nitric Oxide 8: 83–88, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nat Rev Immunol 6: 919–929, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Sutterlin C, Hsu P, Mallabiabarrena A, Malhotra V. Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell 109: 359–369, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Thomas HC, Lamé MW, Wilson DW, Segall HJ. Cell cycle alterations associated with covalent binding of monocrotaline pyrrole to pulmonary artery endothelial cell DNA. Toxicol Appl Pharmacol 141: 319–329, 1996 [DOI] [PubMed] [Google Scholar]
- 62.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 28: 23–42, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tyler RC, Muramatsu M, Abman SH, Stelzner TJ, Rodman DM, Bloch KD, McMurtry IF. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 276: L297–L303, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Wang Z, Newman WH. Smooth muscle cell migration stimulated by interleukin 6 is associated with cytoskeletal reorganization. J Surg Res 111: 261–266, 2003 [DOI] [PubMed] [Google Scholar]
- 65.White RJ, Meoli DF, Swarthout RF, Kallop DY, Galaria II, Harvey JL, Miller CM, Blaxall BC, Hall CM, Pierce RA, Cool CD, Taubman MB. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L583–L590, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Wilson DW, Lamé MW, Dunston SK, Segall HJ. DNA damage cell checkpoint activities are altered in monocrotaline pyrrole-induced cell cycle arrest in human pulmonary artery endothelial cells. Toxicol Appl Pharmacol 166: 69–80, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Xu H, Shields D. Prohormone processing in the trans-Golgi network: endoproteolytic cleavage of prostomatin and formation of nascent secretory vesicles in permeabilized cells. J Cell Biol 122: 1169–1184, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zamanian RT, Haddad F, Doyle RL, Weinacker AB. Management strategies for patients with pulmonary hypertension in the intensive care unit. Crit Care Med 35: 2037–2050, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Zhao YD, Courtman DW, Ng DS, Robb MJ, deng YP, Trogadis J, Han RNN, Stewart DJ. Microvascular regeneration in established pulmonary hypertension by angiogeneic gene transfer. Am J Respir Cell Mol Biol 35: 182–189, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.