Abstract
Parathyroid hormone-related protein (PTHrP), a paraneoplastic protein expressed by two-thirds of human non-small cell lung cancers, has been reported to slow progression of lung carcinomas in mouse models and to lengthen survival of patients with lung cancer. This study investigated the effects of ectopic expression of PTHrP on proliferation and cell cycle progression of two human lung adenocarcinoma cell lines that are normally PTHrP negative. Stable transfection with PTHrP decreased H1944 cell DNA synthesis, measured by thymidine incorporation, bromodeoxyuridine uptake, and MTT proliferation assay. A substantial fraction of PTHrP-positive cells was arrested in or slowly progressing through G1. Cyclin D2 and cyclin A2 protein levels were 60–70% lower in PTHrP-expressing cells compared with control cells (P < 0.05, N = 3 independent clones per group), while expression of p27Kip1, a cyclin-dependent kinase inhibitor, was increased by 35 ± 9% (mean ± SE, P < 0.05) in the presence of PTHrP. Expression of other cyclins, including cyclins D1 and D3, and cyclin-dependent kinases was unaffected by PTHrP. PTHrP did not alter the phosphorylation state of Rb, but decreased cyclin-dependent kinase (CDK) 2-cyclin A2 complex formation. Ectopic expression of PTHrP stimulated ERK phosphorylation. In MV522 cells, PTHrP had similar effects on DNA synthesis, cyclin A2 expression, pRb levels, CDK2-cyclin A2 association, and ERK activation. In summary, PTHrP appears to slow progression of lung cancer cells into S phase, possibly by decreasing activation of CDK2. Slower cancer cell proliferation could contribute to slower tumor progression and increased survival of patients with PTHrP-positive lung cancer.
Keywords: cyclin-dependent kinase inhibitor proteins, extracellular signal-regulated mitogen-activated protein kinases, G1 phase
parathyroid hormone-related protein (PTHrP) is a paraneoplastic protein that is expressed in a wide variety of malignant and normal tissues. Its predominant functions relate to development, growth, smooth muscle tone, and calcium transport. In cancer, PTHrP is best known as the mediator of the syndrome of hypercalcemia of malignancy. PTHrP and parathyroid hormone (PTH) share primary and secondary sequence elements in their amino termini (19). Consequently, the two hormones bind to a common receptor, the type I PTH/PTHrP receptor (PTH1R), and elicit indistinguishable responses in cells and tissues bearing that receptor, including bone resorption by osteoclasts and decreased clearance of calcium by the kidney (33). PTH1R is a G protein-coupled receptor that exerts effects through cAMP, protein kinase A, protein kinase C, intracellular calcium, and nitric oxide pathways (1, 18, 26).
In addition to regulating calcium homeostasis, PTHrP also exerts effects on cell proliferation, apoptosis, motility, and invasiveness (6, 10, 31, 34), processes that contribute to the aggressive characteristics of malignancies and modify the propensity for a cancer to metastasize. These effects vary with cancer type and context and can alternatively augment or diminish the malignant phenotype. PTHrP expression results in more rapid progression in animal models of Leydig cell tumors and prostate cancer (6, 31) and is associated with worse outcomes in patients with gliomas (28). In contrast, the protein has been associated with improved survival in women with breast cancer (15, 16) and reduced tumor recurrence in renal cell carcinoma (17). PTHrP is produced in roughly two-thirds of human non-small cell lung carcinomas (NSCLC) (12) and could play a role in tumor progression or prognosis for that disease. In fact, we have demonstrated that PTHrP has autocrine effects in reducing lung carcinoma growth in mice (11), and that it is associated with longer survival in patients with NSCLC (12).
The mechanisms that PTHrP could engage to regulate lung cancer progression have not been established. PTHrP causes a dose-dependent reduction in proliferation of BEN lung carcinoma cells (11), so an antimitogenic effect could be involved. The effect is small in BEN cells, however, probably because the cells produce significant quantities of PTHrP, limiting the impact of exogenous treatment with the hormone. Recently, we discovered that human H1944 lung adenocarcinoma cells express PTH1R but not PTHrP. Thus experimental manipulations of PTHrP can be compared with a background state with no endogenous hormone, providing an ideal model for studying the effects of PTHrP. In this paper, we have tested the growth effects, evaluated the signal transduction pathways, and investigated the growth modulators that are regulated by PTHrP in H1944 and MV522 lung carcinoma cells.
MATERIALS AND METHODS
Materials.
Primary antibodies for CDK1 (cyclin-dependent kinase 1), CDK2 (sc-163 and sc-6248), CDK4, cyclin A2 (sc-53230 and E67.1), cyclin B1, cyclin D1, cyclin D2, cyclin D3, cyclin E2, extracellular signal-regulated kinase 1 (ERK1), phosphorylated ERK1/2, p27, phosphorylated and total retinoblastoma protein (Rb), p130 and p107, secondary antibodies, small interfering RNA (siRNA) for human p27 (sc-29429), nonsilencing siRNA, protein A/G plus-agarose bead, RIPA lysis buffer, and immunocytochemical reagents came from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for phosphorylated and total Src were purchased from Cell Signaling Technology (Danvers, MA). The PTH1R antibody was obtained from Covance, (Princeton, NJ). [3H]thymidine was a product of Perkin Elmer (Waltham, MA). The University of California San Diego (UCSD) Cell Culture Facility provided cell culture media and reagents.
Cell culture.
NCI-H1944 human lung adenocarcinoma cells were obtained from ATCC (Manassas, VA). MV522 human lung adenocarcinoma cells were a gift of Nissi Vaarki (UCSD). The cells were grown in RPMI-1640 plus 10% fetal bovine serum in an atmosphere of 5% carbon dioxide. To induce PTHrP expression, cells were transfected with a pciNeo-PTHrP 1-87 expression plasmid with a cationic liposome method, as previously described (27). Control cells received empty vector. Nontransfected cells were eliminated by incubation with a lethal dose of G418. Colonies were isolated by pipette tip picking, expanded, and screened for PTHrP by immunoassay of media for PTHrP 1-34.
We chose to transfect PTHrP 1-87 rather than the full-length protein, PTHrP 1-141, to focus on the effects of the amino terminal portion of the molecule containing the ligand for PTH1R. The PTHrP 1-87 expression plasmid drives production of high levels of protein, while shorter plasmids coding for peptides containing PTHrP 1-34 express very little PTHrP (5). Details concerning plasmid construction have been published (5).
PTHrP immunoassay.
PTHrP was assayed in conditioned media and lysates of lung cancer cells by our own radioimmunoassays, using antibodies directed against PTHrP 1-34, PTHrP 38-64, and PTHrP 109-141, as described previously (13, 24).
RT-PCR.
RNA was isolated from lung cancer cells with the RNeasy mini kit (Qiagen, Valencia, CA) and used as template to produce cDNA with murine moloney leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA). A sequence for PTH1R was amplified from the cDNA by Hot Star Taq DNA Polymerase (Qiagen) using primers 5′-GACAGCAATGGCAGCTGG-3′ (forward) and 5′-GAGTAGAGCACAGCGTCC-3′ (reverse). The PCR protocol consisted of initial Taq activation at 95°C for 15 min, followed by denaturing at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min over 40 cycles. The protocol concluded with an additional extension cycle at 72°C for 10 min. The amplification yielded a product of 300 nucleotides, as expected.
Quantitative PCR.
For quantitative PCR, cDNA was amplified on an ABI Prism 7000 real-time PCR machine using SYBR green I dye detection. The relative cDNA ratios were calculated from threshold cycles by the Pfaffl method (30) using the value of threshold cycles. The threshold for cDNA from control cells was the comparator, and the reference gene was β-actin. Primers (RealTimePrimers.com) were forward 5′-ACA GTA AAC AGC CTG CGT TC-3′, reverse 5′-AAGAGGGACCAATGGTTTTC-3′ for cyclin A2; forward 5′-TGATGGGGAAGGAGTACAAA-3′, reverse 5′-TTGGAAGAAGAAGCAGGATG-3′ for cyclin D2; and forward 5′-TCAAACGTGCGA GTGTCTAA-3′, reverse 5′-CCACTCGTACTTGCCCTCTA-3′ for p27.
Thymidine incorporation assay.
Cells were plated at 4 × 105 cells/well in 24-well plates and studied at 60–70% confluency. After 24-h synchronization in media + 0.2% BSA, cells were washed and then exposed for 4–24 h to growth media + 0.5 μCi [3H]thymidine/well. Finally, cells were washed, precipitated in trichloroacetic acid, lysed in NaOH, and counted.
Flow cytometry.
Unsynchronized cells at 30–50% confluency were trypsinized, harvested, washed, and fixed in 100% ethanol. DNA was stained with 50 μg/ml propidium iodide with RNase and measured on a Coulter Elite flow cytometer (Beckman Coulter Electronics, Miami, FL). The percentage of cells in the different cell cycle phases was determined with Multicycle software (Phoenix Flow Systems, San Diego, CA). To assay bromodeoxyuridine (BrdU) incorporation, cells plated at 1 × 106 in 100-mm cell culture plates were incubated with a final concentration of 10 μM BrdU (BD Pharmingen, San Diego, CA) for 2 or 24 h, trypsinized, and fixed with BD Cytofix/Cytoperm buffer per tube for 15 min at room temperature. The BrdU epitope was exposed by treatment with 30 μg of DNAse/tube for 1 h at 37°C and stained with anti-BrdU-allophycocyanin (1:50) for 20 min at room temperature. BrdU immunofluorescence was measured together with propidium iodide fluorescence on the Coulter Elite flow cytometer. Background BrdU immunofluorescence was determined in cells not exposed to BrdU, but stained with the BrdU antibody. Cells exposed to BrdU were judged to contain BrdU, if their immunofluorescence was greater than the background level.
MTT assay.
Cells were plated at 5,000 cells/well in 96-well plates in growth media, allowed to adhere for 18 h, and replenished with fresh media. At 1, 2, and 4 days after plating, assays were stopped, and cell quantity was assessed based on staining with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolim bromide (MTT) using a kit (Chemicon, Temecula, CA). Optical absorbance was measured at 570 nm with a Victor 1420 Multilabel plate reader (Perkin Elmer). Cell numbers were calculated based on a standard curve obtained by staining a known numbers of cells.
siRNA transfection.
Cells were plated in 24-well plates at 40–50% confluence in 500-μl growth medium without antibiotics. For each transfection, 20 pmol of siRNA oligomer in 50 μl of serum-free cell medium was mixed with 1 μl Lipofectamine 2000, diluted 1:50, and incubated for 20 min at room temperature. The complex was then added to the well with cells and medium and incubated cells at 37°C 48 h before measuring gene knockdown.
Immunoblots.
Equal quantities of cell lysate protein were applied to Criterion 4–12% Bis-Tris gels (Bio-Rad, Hercules, CA), separated by electrophoresis, and transferred to polyvinylidene fluoride membranes. Membranes were blocked with 10% nonfat dry milk for 1 h at room temperature, probed with primary antibodies overnight at 4°C, and exposed to goat anti-mouse or goat anti-rabbit IgG-horseradish peroxidase secondary antibodies for 1 h at room temperature. Chemiluminescence was elicited by treatment with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL), and recorded with the UVP BioSpectrum 410 imaging system (Upland, CA). Band densities were analyzed with ImageJ software (National Institutes of Health).
Immunoprecipitation.
Pelleted cells were lysed in 2 ml RIPA lysis buffer by barbottage back and forth through a 22-gauge needle and syringe. Nuclear debris was pelleted, and the lysate was precleared by adding 20 μl protein A/G plus agarose beads with 1 μg control IgG, corresponding to host species of primary antibody. Sample protein concentration was determined by bicinchoninic acid protein assay to aliquot 500 μg cellular protein for immunoprecipitation. This quantity of protein was mixed with 10 μl primary antibody and 20 μl protein A/G plus agarose beads, placed on shaker, and incubated overnight at 4°C. Beads were pelleted, washed 4× with PBS, and boiled for 3 min in 40 μl electophoresis buffer. Beads were then pelleted, and 20 μl of supernatant were used for Western immunoblot analysis.
Statistics.
Mean measurement values for experimental and control groups were compared by t-test or ANOVA (StatView 4.5, Abacus Concepts). Statistical significance was accepted at P < 0.05.
RESULTS
PTHrP and PTH1R expression.
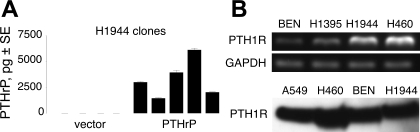
PTHrP levels in conditioned media and lysates from wild-type H1944 cells were below the limit of detection, roughly 70 pg/ml for a 100-μl sample, of our PTHrP immunoassays. No PTHrP could be detected in conditioned media, even after 72-h incubation with nearly confluent H1944 cells. Using standard lipofection methods and selection with G418, we produced several stable clones of H1944 cells that expressed high levels of PTHrP and control clones transfected with empty vector that produced no measurable hormone (Fig. 1A). PTHrP production over 24 h averaged 3,300 ± 800 pg (range 900–6,100 pg) in five clones transfected with the PTHrP plasmid. For comparison, the output of BEN squamous lung carcinoma cells, which are considered high producers of PTHrP (2), was only 800 ± 100 pg under similar conditions and confluency over the same time period (P < 0.01). The PTHrP-transfected H1944 clones have continued to express high levels of PTHrP over several months and passages.
Fig. 1.
Parathyroid hormone-related protein (PTHrP) and type I parathyroid hormone/PTHrP receptor (PTH1R) expression. A: immunoassay with anti-PTHrP 1-34 antibody detected PTHrP in G418-resistant colonies of human H1944 lung adenocarcinoma cells stably transfected with PTHrP 1-87, but not in cells transfected with empty vector. The PTHrP-transfected clones made, on average, four times as much PTHrP in 24 h as BEN squamous lung carcinoma cells (see text), which are considered to produce high levels of PTHrP (2). Data in the graphs represent means ± SE for 3–5 replicate wells. B: RT-PCR (top) and immunoblots (bottom) revealed that H1944 cells expressed the receptor for PTHrP, PTH1R. Protein and RNA from BEN squamous lung cancer cells, A549 adenocarcinoma cells, H1395 lung adenocarcinoma cells, and H460 lung large-cell carcinoma cells were used as positive controls for PTH1R.
Although they do not make PTHrP, H1944 cells express the receptor for PTHrP, PTH1R. The cells contain PTH1R mRNA and protein by PCR and immunoblot, respectively (Fig. 1B). Thus the cells could potentially respond to PTHrP as a paracrine growth factor.
Effect of PTHrP on cell proliferation.
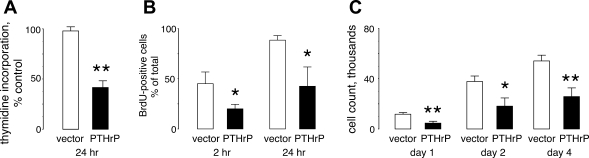
We used 24-h thymidine incorporation to screen the PTHrP-negative and PTHrP-positive lung cancer clones for effects of PTHrP on DNA synthesis. The PTHrP-producing clones incorporated thymidine into DNA at a slower rate than the PTHrP-negative H1944 preparations, ranging from 29 to 63% of the control rate. On average, thymidine incorporation in the PTHrP-positive cells was 43 ± 7% as fast as incorporation in control clones (Fig. 2A, P < 0.001). PTHrP also slowed the rate of incorporation of BrdU into cellular DNA. After either 2 or 24 h of incubation with BrdU, the percentage of PTHrP-expressing lung cancer cells that were BrdU-positive was less than one-half the percentage of control cells that had taken up BrdU in the same time period (Fig. 2B). Close to 100% of the PTHrP-negative H1944 cells contained BrdU in their DNA at 24 h, compared with <50% of the PTHrP-positive cells. To corroborate the results of the DNA synthesis assays, we also measured cell proliferation with the MTT assay. After plating at 4,000 cells/well on day 0, cell numbers rose progressively at day 1 and day 2, ultimately reaching 57,000 ± 4,000 cells/well at day 4 (n = 5 clones) for the PTHrP-negative vector clones and 28,000 ± 4,000 cells/well for the PTHrP-positive cells (P < 0.01 vs. vector controls, n = 4 clones) (Fig. 2C). Cell numbers for PTHrP-positive clones were no greater than 50% of the numbers for the controls at each of the time points.
Fig. 2.
PTHrP effects on DNA synthesis and proliferation. A: stable clones of PTHrP-positive and PTHrP-negative H1944 cells were assayed for rate of DNA synthesis by incubation with [3H]thymidine. The PTHrP-positive cells incorporated thymidine into DNA at 43 ± 7% of the rate demonstrated by PTHrP-negative clones. **P < 0.01. N = 5 PTHrP clones and 4 vector-transfected clones. B: cells were incubated with 10 μM bromodeoxyuridine (BrdU) for 2 or 24 h and analyzed for BrdU content by flow cytometry. PTHrP expression reduced the percentage of cells that were BrdU positive by at least one-half at both time points. Data represent results from 3 clones each for the control and experimental groups. *P < 0.05 vs. vector. C: growth was assessed by MTT assay in PTHrP-producing and nonproducing H1944 cells plated in 96-well plates at 4,000 cells/well. The PTHrP-positive cells grew at ∼50% of the rate of the vector-transfected cells. The data represent results from 5 vector clones and 4 PTHrP clones. *P < 0.05 and **P < 0.01 vs. vector.
Effect of PTHrP on cell cycle distributions.
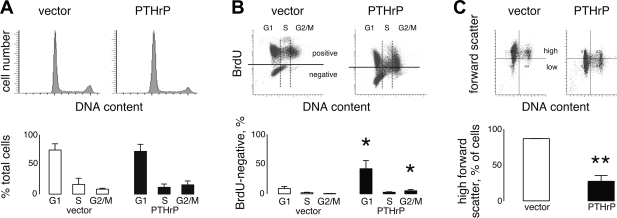
By flow cytometry, PTHrP-negative and PTHrP-positive clones exhibited roughly the same percentages of cells in G0/G1, S, and G2/M phases (Fig. 3A). Thus PTHrP did not slow proliferation by changing the distribution of cells among cell cycle phases. A subdiploid plateau, indicating apoptosis, was not present for either experimental group.
Fig. 3.
Effects of PTHrP on cell cycle distribution, progression, and cell size by flow cytometry. A: cell cycle distributions for H1944 cells at 50% confluency were similar between PTHrP-expressing clones and vector clones. Plots show representative distributions, while the graphs below represent average results from 5 PTHrP-positive clones and 3 PTHrP-negative clones (P = not significant). B: bivariate plots of 24-h BrdU vs. DNA content found a high percentage of BrdU-negative cells in the PTHrP-expressing clones. In contrast, nearly all of the vector-transfected cells had taken up BrdU by 24 h. The bivariate plots show representative results from the control and experimental groups, while the graphs show averages from 3 clones per treatment. *P < 0.05 vs. vector control. C: forward scatter, a flow cytometry marker of cell size, was significantly greater for PTHrP-negative cells compared with PTHrP-transfected cells. Bar graphs represent mean data from 3 PTHrP-negative clones and 4 PTHrP-positive clones. **P < 0.01.
To gather information about the effects of PTHrP on progression through cell cycle phases, we incubated cells with BrdU for 24 h and compared BrdU content vs. DNA content. As already noted, over 90% of the cells from the vector control clones incorporated BrdU over 24 h, demonstrating that they were actively progressing through the cell cycle. The BrdU-negative cells (horizontal lines on bivariate plots) represented a small fraction of the total, and most of these were found in G1 (Fig. 3B). In contrast, a substantial population of cells was BrdU negative for the PTHrP-expressing clones, indicating that the cells had not synthesized DNA during the test period. Again, the BrdU-negative cells were predominantly in G1 phase, but at a much higher percentage than seen with the control clones, roughly 42% of the total (P < 0.05). BrdU-negative cells were also slightly more frequent in G2/M, apparently blocked in that phase, for the PTHrP group compared with controls.
Flow cytometry revealed another difference between the PTHrP-negative and PTHrP-positive H1944 cells. The PTHrP-positive cells demonstrated significantly less forward scatter than the control cells (Fig. 3C), suggesting that PTHrP-expressing cells were smaller than the PTHrP-negative controls.
Effect of PTHrP on cell cycle proteins.
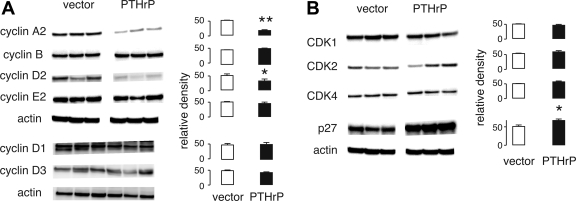
Because PTHrP expression appeared to be associated with a block or slower transition through G1, we next investigated whether the protein affected expression of CDK and associated proteins that regulate cell cycle progression. Cyclin D2 and cyclin A protein levels were 60–70% lower in PTHrP-expressing cells compared with control cells (P < 0.05, N = 3 independent clones per group), but cyclins B1, D1, D3, and E2 were unchanged (Fig. 4A). Expression of p27Kip1 was increased by 35 ± 9% (mean ± SE, P < 0.05) in the presence of PTHrP (Fig. 4B). CDK1, CDK2, and CDK4 were unaffected by PTHrP. Although PTHrP affected protein levels for cyclin A2, cyclin D2, and p27, we did not observe similar effects on mRNA measured by PCR. The relative abundance of cyclin A2, cyclin D2, and p27 transcripts in PTHrP-positive cells compared with controls was 1.4 (0.9–2.1), 1.1 (0.7–1.9), and 0.9 (0.8–1.1) [mean (95% confidence interval)], respectively, not significantly different than 1.
Fig. 4.
Effects of PTHrP on cell cycle proteins. PTHrP transfection decreased levels of cyclin D2 and cyclin A2 (A), while increasing expression of the p27Kip1 cyclin-dependent kinase (CDK) inhibitor (B). The CDKs, cyclin B1, cyclin D1, cyclin D3, and cyclin E2, were unaffected by PTHrP. Representative immunoblots appear on the left, and bar graphs of relative density, normalized by the densities of the actin bands, on the right. Data represent means ± SE from 3 independent clones per experimental group. *P < 0.05, **P < 0.01 vs. vector control. Blots here and in other figures were performed multiple times with reproducible results.
Cyclin D may regulate the activity of CDK4 and CDK6, while p27 may inhibit activity. Therefore, we measured the effects of PTHrP on phosphorylation of Rb, a regulator of G1 progression that is a target CDK4 and CDK6 (22). However, ectopic expression of PTHrP had no effect on the phosphorylation state of Rb in H1944 cells, nor did it alter expression of the related pocket protein family members, p130 or p107 (data not shown). Since cyclin A can activate CDK2, we evaluated the effects of PTHrP on association of those two proteins. PTHrP caused a 25–75% decrease in the quantity of cyclin A2 that could be immunoprecipitated with CDK2 (Fig. 5), but had no effect on cyclin E2-CDK2 association (data not shown).
Fig. 5.
PTHrP alters association of cyclin A2 with CDK2. Cell lysates from PTHrP-positive and -negative H1944 cells were immunoprecipitated (IP) with a rabbit polyclonal antibody for CDK2, then immunoblotted (IB) for cyclin A2 or with mouse monoclonal CDK2 antibody. The PTHrP-positive cells contained significantly less cyclin A2 associated with CDK2 than did the vector controls. *P < 0.05. N = 3 clones/group.
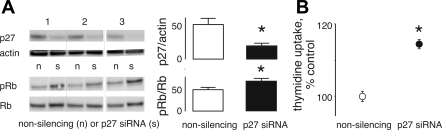
We treated PTHrP-positive H1944 cells with siRNA against p27 to investigate whether p27 had a role in the reduced proliferation due to PTHrP. SiRNA treatment resulted in a 70% knockdown of p27 levels. Decreased p27 expression was associated with a 60 ± 15% increase in the Rb phosphorylation and a 15 ± 1% increase in thymidine incorporation (Fig. 6).
Fig. 6.
Interaction of p27 with H1944 cell proliferation. A: PTHrP-positive H1944 cells contained 67% less p27 after treatment with anti-p27 lentiviral small interfering RNA (siRNA) particles compared with nonsilencing particles. The p27 knockdown resulted in a 60% increase in Rb phosphorylation and a 15% increase in thymidine incorporation over 24 h (B) compared with control cells treated with nonsilencing siRNA. *P < 0.05.
PTHrP signaling effects.
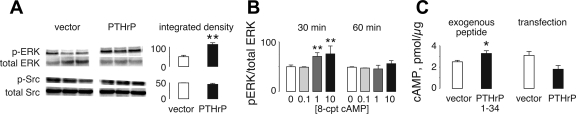
We evaluated H1944 cells for activation of Src and ERK signaling, two pathways known to regulate proliferation, with immunoblots for the phosphorylated forms of those kinases. PTHrP-positive cells contained higher levels of phosphorylated ERK than did the PTHrP-negative controls (Fig. 7A), but the extent of Src phosphorylation was unaffected. We investigated whether cAMP, a potential second messenger for PTHrP, regulated ERK phosphorylation by treating cells with 8-(4-chlorophenylthio)-cAMP, a long-acting cell-permeant cAMP mimetic. The compound caused a dose-dependent increase in levels of phosphorylated ERK with short-term exposure, but the effect was lost by 60 min (Fig. 7B) and 24 h (data not shown). Finally, we measured cAMP levels after exogenous PTHrP treatment and compared levels in the vector and PTHrP-transfected clones. Acute exposure to PTHrP 1-34 significantly increased cAMP levels in H1944 cells, but levels in cells with elevated chronic production of the hormone were lower than control levels (P = 0.065) in vector-transfected cells not exposed to the hormone (Fig. 7C).
Fig. 7.
Effects of PTHrP on signaling pathways. A: PTHrP-positive H1944 clones demonstrated increased levels of extracellular signal-regulated kinase (ERK) phosphorylation (p-ERK) compared with the PTHrP-negative vector controls, but Src activation was unaffected. The bar graphs show average values from 3 independent clones for each group. **P < 0.01 vs. vector. B: wild-type H1944 cells were treated with 8-(4-chlorophenylthio)-cAMP (8-CPT-cAMP), a cell-permeant, long-acting cAMP mimetic. The compound increased ERK phosphorylation in dose-dependent fashion acutely, but the effect was gone by 60 min of treatment. **P < 0.01 vs. vector. n = 3 wells/time point. C: treatment with exogenous PTHrP 1-34 stimulated an acute increase in cAMP levels in wild-type H1944 cells. *P < 0.05 vs. vehicle treatment. n = 3 wells/group. However, PTHrP-producing cells did not differ in cAMP content from vector-transfected cells (n = 3 clones per group tested in duplicate). Units are pmol/μg cell protein.
PTHrP effects in MV522 cells.
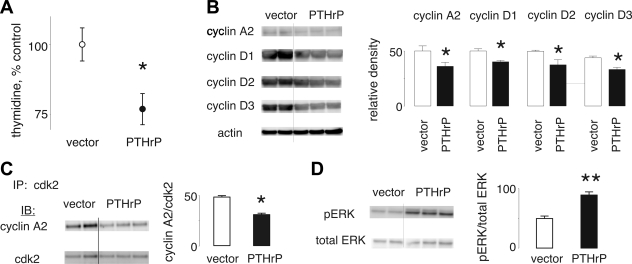
Like H1944 cells, wild-type MV522 cells make no PTHrP, but do express PTH1R (data not shown). We generated two stable clones of MV522 cells transfected with the empty expression plasmid and three clones stably expressing PTHrP. These clones produced 500 ± 183 pg/24 h compared with none in wild-type and vector-transfected cells. The PTHrP-producing MV522 cells incorporated thymidine significantly slower than the vector-transfected cells by ∼25% (Fig. 8A). The PTHrP-positive MV522 cells demonstrated 30% less cyclin A2 and 25% less cyclin D1, cyclin D2, and cyclin D3 than the PTHrP-negative control cells (Fig. 8B, P < 0.05). The effects of PTHrP on measures of CDK4/6 activity and CDK2 activity were similar to what we observed in H1944 cells. Thus PTHrP-expressing cells and PTHrP-null MV522 cells did not differ in the extent of phosphorylation of Rb (data not shown). Less cyclin A2 was immunoprecipitated with CDK2 from lysates of PTHrP-producing MV522 cells than from the control cells (Fig. 8C). However, the amount of cyclin E2 associated with CDK2 was unaffected by PTHrP expression (data not shown). Finally, PTHrP-positive MV522 cells demonstrated greater levels of ERK phosphorylation than did the PTHrP-negative cells (Fig. 8D).
Fig. 8.
PTHrP effects in MV522 lung adenocarcinoma cells. A: 24-h thymidine uptake was reduced by 25% in PTHrP-producing MV522 cells compared with vector controls. B: cyclin A2, cyclin D1, cyclin D2, and cyclin D3 levels were reduced in PTHrP-producing clones compared with controls. C: less cyclin A2 was associated with CDK2 in PTHrP-positive cells than in the PTHrP-null line. D: PTHrP-expressing cells demonstrated greater levels of ERK phosphorylation than did the controls. *P < 0.05. **P < 0.01.
DISCUSSION
This project extends previous work, suggesting that exogenous PTHrP 1-34 inhibited lung cancer cell proliferation (11). The study presented here compared proliferation of two lines of lung adenocarcinoma cells that lacked PTHrP production with independent clones of the same cell type transfected with a PTHrP expression plasmid. We found evidence for reduced mitogenesis in the PTHrP-positive H1944 cell clones based on three separate measures: decreased incorporation of labeled thymidine into cellular DNA, reduced immunoreactivity for BrdU after 2- and 24-h incubations, and decreased cell numbers at 1, 2, and 4 days of incubation. The reduction in size, based on change in forward scatter, of the PTHrP-positive cells vs. the vector-transfected control cells is also consistent with more indolent growth, although other factors could also affect size. Transfection with PTHrP also inhibited DNA synthesis by MV22 cells, another lung adenocarcinoma line, and caused changes in cell cycle protein expression and activation similar to those in H1944 cells, supporting a common mechanism. Our earlier experiments demonstrated that PTHrP 1-34 inhibited growth of BEN cells, a squamous lung carcinoma cell line, in a dose-dependent fashion (11). Anti-proliferative effects of PTHrP in three NSCLC lines and across two different histologies suggest that these actions could be a general response in NSCLC and not cell or histology specific.
The flow cytometry and immunoblotting experiments provided insight into the mechanisms that PTHrP could activate to reduce proliferation. The bivariate comparisons of BrdU uptake and DNA content suggested that PTHrP induces a block in G1. A small fraction of cells in G2/M also failed to take up BrdU and appeared to be blocked in that phase. The lower levels of cyclin D2 and higher levels of p27Kip1 found on immunoblots in PTHrP-expressing cells compared with control cells suggest that PTHrP might decrease activity of CDK4 and/or CDK6. However, this mechanism does not hold, because ectopic PTHrP production did not reduce phosphorylation of Rb, the main substrate of CDK4 and CDK6 (22). The effects on cyclin D2 may be inconsequential: cyclin D1 appears to be important for regulating proliferation in lung cancer cells (7), but it was not altered by PTHrP in H1944 cells.
PTHrP also mediated decreases in H1944 cell and MV522 cell cyclin A2 levels. Cyclin A2 is necessary for both the G1/S and G2/M transitions and has a role in stimulating DNA synthesis (32). It can complex with and activate CDK2 and CDK1 (cdc2). In fact, we found decreased association between cyclin A2 and CDK2 in the PTHrP-positive lung cancer cell clones, suggesting that the hormone decreased activity of CDK2. This mechanism could explain the more sluggish cell cycle progression of PTHrP-expressing cells.
We were curious whether the increase in p27 induced by PTHrP had an impact on lung cancer cell proliferation. Knocking down levels of the CDK inhibitor in H1944 cells caused increased Rb phosphorylation and a slight increase in the rate of DNA synthesis. The results suggest that PTHrP might have a minor effect on cell growth rate through its effect on p27. p27 has complex effects on cell cycle kinetics. Some data suggest that the protein stabilizes CDK4/6-cyclin D complex formation and mediates complex translocation to the nucleus, actions that could stimulate rather than inhibit cell growth (29). Thus increases in p27, as observed with PTHrP expression, could lead to opposing actions on Rb phosphorylation and unpredictable net results.
PTHrP 1-34 reduces proliferation in other cell types through mechanisms that involve the same cyclins and CDK inhibitors. In vascular smooth muscle cells, for example, PTHrP arrests cells in G1 by increasing p27Kip and decreasing phosphorylation of Rb (8, 35). The process involves protein kinase A. PTHrP signaling reduces osteoblast proliferation by reducing cyclin D1 expression, reducing CDK4/6 activity, and inhibiting CDK1. The PTHrP signaling pathways for osteoblasts can involve cAMP, protein kinase C, and ERK (4). Our laboratory has shown previously that PTHrP 1-34 stimulates cAMP in lung cancer cells (10). In addition, we find that ectopic expression of PTHrP activates ERK in H1944 cells, similar to its effects in osteoblasts. ERK is normally considered to be a positive proliferative signaling agent, but its effect may vary, depending on the duration of the signal. Sustained activation of ERK, in particular, has been reported to exert anti-proliferative effects (3). In the H1944 cells, activation of ERK due to stable PTHrP transfection would fall into the category of a sustained process. Further work will be necessary to determine whether ERK phosphorylation contributes to the growth inhibition due to PTHrP.
PTHrP is a multifunctional protein. Its amino terminus acts through PTH1R, but biological effects have also been ascribed to midmolecule and carboxy terminal portions of the protein (26). These effects are presumably receptor mediated, although receptors have yet to be identified. PTHrP also contains a nuclear localizing sequence (14, 20, 25). The molecule can escape the secretory pathway and enter the nucleus under direction of this sequence (14, 20, 25). The nuclear actions of PTHrP, known as intracrine effects, regulate cell function and growth. Interestingly, the intracrine effects of PTHrP oppose the autocrine/paracrine effects on proliferation of vascular smooth muscle cells (8, 21). The receptor-mediated effects of PTHrP 1-34 inhibit growth of the smooth muscle cells, while the intracrine effects are promitogenic.
Arguments could be made that the PTHrP effects we report in this study represent autocrine actions of amino terminal PTHrP acting on PTH1R to generate cAMP. Carboxy terminal sequences are excluded as mediators of the growth inhibition, because we transfected the lung cancer cells with a plasmid coding for PTHrP 1-87. This truncated protein lacks the nuclear localizing sequence, between residues 87 and 106, making intracrine effects unlikely. However, our results also show that chronic production of PTHrP does not lead to a chronic increase in intracellular cAMP in H1944 cells, presumably through receptor downregulation and other homeostatic mechanisms. Thus we cannot implicate PTH1R in the mechanism. For example, a midmolecule peptide within PTHrP 35-87 could be a growth inhibitor. In addition, PTHrP 1-87 could enter the nucleus passively without a nuclear localizing sequence (9), because its size is <60 kDa (23). Further work will be necessary to establish whether the growth inhibitory effects of PTHrP in lung cancer depend on autocrine or paracrine vs. intracrine mechanisms and to identify the protein domains that are involved.
Our laboratory's previous studies have suggested that PTHrP slows lung carcinoma progression and modifies outcome in patients with lung cancer. In one investigation, we gave mice with PTHrP-expressing orthotopic lung carcinomas neutralizing antibodies against PTHrP 1-34 or isotype control antibody (11). The antibodies reduced tumor PTHrP levels and accelerated tumor growth, suggesting that PTHrP produced by the carcinoma restrained its growth. In a patient study, we discovered that women with lung carcinomas that made PTHrP survived close to 3 yr longer, on average, than women whose tumors did not make the protein (12). Men, however, did not appear to enjoy the same benefit from PTHrP. The results of the present study provide a potential explanation for our previous findings on tumor growth and survival. Slower proliferation in the presence of PTHrP could represent the explanation for slower growth of PTHrP-positive lung carcinomas. In turn, slower tumor progression could contribute to longer survival of patients whose carcinomas express PTHrP. Thus PTHrP would appear to bear a causal relationship with slower lung carcinoma progression and long survival. Additional work is in progress to investigate the etiology for the sex dependence.
In summary, this study demonstrates that stable expression of PTHrP inhibits growth of two different lung carcinoma cell lines. The growth inhibition is associated with decreased expression of cyclin D2 and cyclin A2, increased expression of p27, decreased association of cyclin A2 and CDK2, and increased activation of ERK. The changes in the cyclins and p27 appeared to be posttranscriptional, because transcripts did not change while protein levels did. The implications of PTHrP-mediated growth inhibition could be slower progression of tumors expressing PTHrP and prolonged patient survival compared with PTHrP-negative lung carcinomas. Work is necessary to explore the pathways(s) by which PTHrP inhibits lung cancer cell proliferation and investigate whether changes in cyclin A2, p27Kip1, CDK2 activity and phosphorylated ERK play a role in this process.
GRANTS
A Department of Veterans Affairs Merit Review Award (R. H. Hastings and L. J. Deftos), the Flight Attendants Medical Research Institute (R. H. Hastings and L. J. Deftos), National Cancer Institute CA23100 (L. J. Deftos), an American Lung Association/LUNGevity Lung Cancer Discovery Award (R. H. Hastings), and a Joan's Legacy grant (R. H. Hastings) supported the work for this project.
ACKNOWLEDGMENTS
The authors thank Kathy Smith and Cheryl Chalberg for performing the PTHrP immunoassays, Dr. Paul Insel at UCSD for assaying cAMP, and the team of Terrell Huang and Jon Sin for assisting with immunoblots and PCR. Laura Ditmer designed the PTHrP expression plasmid, while Doug Burton performed the transformation and isolated the plasmid.
REFERENCES
- 1.Abou-Samra AB, Juppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JJ, et al. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA 89: 2732–2736, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt DW, Wachsman W, Deftos LJ. Parathyroid hormone-like protein: alternative messenger RNA splicing pathways in human cancer cell lines. Cancer Res 54: 850–853, 1994 [PubMed] [Google Scholar]
- 3.Clark JA, Black AR, Leontieva OV, Frey MR, Pysz MA, Kunneva L, Wolszynska-Read A, Roy D, and Black JD. Involvement of the ERK signaling cascased in protein kinase C-mediated cell cycle arrest in intestinal epithelial cells. J Biol Chem 279: 9233–9247, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Datta NS, Chen C, Berry JE, McCauley LK. PTHrP signaling targets cyclin D1 and induces osteoblastic cell growth arrest. J Bone Miner Res 20: 1051–1064, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Ditmer LS, Burton DW, Deftos LJ. Elimination of the carboxy-terminal sequences of parathyroid hormone-related protein 1-173 increases production and secretion of the truncated forms. Endocrinology 137: 1608–1617, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Dougherty KM, Blomme EA, Koh AJ, Henderson JE, Pienta KJ, Rosol TJ, McCauley LK. Parathyroid hormone-related protein as a growth regulator of prostate carcinoma. Cancer Res 59: 6015–6022, 1999 [PubMed] [Google Scholar]
- 7.Driscoll B, Wu L, Buckley S, Hall FL, Anderson KD, Warburton D. Cyclin D1 antisense RNA destabilizes pRb and retards lung cancer cell growth. Am J Physiol Lung Cell Mol Physiol 273: L941–L949, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Fiaschi-Taesch NM, Takane KK, Masters S, Lopez-Talavera JC, Stewart AF. Parathyroid hormone-related protein as a regulator of pRb and the cell cycle in arterial smooth muscle. Circulation 110: 177–185, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Gujral A, Burton DW, Terkeltaub RA, Deftos LJ. PTHrP induces interleukin 8 production by prostate cancer cells via a novel intracrine mechanism not mediated by its classical nuclear localization sequence. Cancer Res 61: 2282–2288, 2001 [PubMed] [Google Scholar]
- 10.Hastings RH, Araiza F, Burton DW, Zhang L, Bedley M, Deftos LJ. Parathyroid hormone-related protein ameliorates death receptor-mediated apoptosis in lung cancer cells. Am J Physiol Cell Physiol 285: C1429–C1436, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Hastings RH, Burton DW, Quintana RA, Biederman E, Gujral A, Deftos LJ. Parathyroid hormone-related protein regulates the growth of orthotopic human lung tumors in athymic mice. Cancer 92: 1402–1410, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Hastings RH, Laux AM, Casillas A, Xu R, Lukas Z, Ernstrom K, Deftos LJ. Sex-specific survival advantage with PTHrP in non-small cell lung carcinoma patients. Clin Cancer Res 12: 499–506, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Hastings RH, Quintana RA, Sandoval R, Burton DW, Deftos LJ. Amino-terminal and mid-molecule parathyroid hormone-related protein, phosphatidylcholine, and type II cell proliferation in silica lung injury. Am J Physiol Lung Cell Mol Physiol 285: L1312–L1322, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Henderson JE, Amizuka N, Warshawsky H, Biasotto D, Lanske BM, Goltzman D, Karaplis AC. Nucleolar localization of parathyroid hormone-related peptide enhances survival of chondrocytes under conditions that promote apoptotic cell death. Mol Cell Biol 15: 4064–4075, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson M, Danks J, Moseley J, Slavin J, Harris T, McKinlay M, Hopper J, Martin T. Parathyroid hormone-related protein production by breast cancers, improved survival, and reduced bone metastases. J Natl Cancer Inst 93: 234–237, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Henderson MA, Danks JA, Slavin JL, Byrnes GB, Choong PF, Hopper JL, Martin TJ. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res 66: 2250–2256, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Iwamura M, Wu M, Muramoto M, Ohori M, Egawa S, Uchida T, Baba S. Parathyroid hormone-related protein is an independent prognostic factor for renal cell carcinoma. Cancer 86: 1028–1034, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Kalinowski L, Dobrucki LW, Malinski T. Nitric oxide as a second messenger in parathyroid hormone-related protein signaling. J Endocrinol 170: 433–440, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Kemp BE, Moseley JM, Rodda CP, Ebeling PR, Wettenhall RE, Stapleton D, Diefenbach-Jagger H, Ure F, Michelangeli VP, Simmons HA, Raisz LG, Martin TJ. Parathyroid hormone-related protein of malignancy: active synthetic fragments. Science 238: 1568–1570, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Lam MH, Thomas RJ, Martin TJ, Gillespie MT, Jans DA. Nuclear and nucleolar localization of parathyroid hormone-related protein. Immunol Cell Biol 78: 395–402, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Massfelder T, Dann P, Wu TL, Vasavada R, Helwig JJ, Stewart AF. Opposing mitogenic and anti-mitogenic actions of parathyroid hormone-related protein in vascular smooth muscle cells: a critical role for nuclear targeting. Proc Natl Acad Sci USA 94: 13630–13635, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushime H, Quelle DE, Shurtleff SA, Shibuya M, Sherr CJ, Kato JY. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol 14: 2066–2076, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattaj IW, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem 67: 265–306, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Montgrain PR, Quintana RA, Burton DW, Deftos LJ, Casillas A, Rascon YM, Hastings RH. Parathyroid hormone-related protein varies with sex and androgen status in nonsmall cell lung cancer. Cancer 110: 1313–1320, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Nguyen M, He B, Karaplis A. Nuclear forms of parathyroid hormone-related peptide are translated from non-AUG start sites downstream from the initiator methionine. Endocrinology 142: 694–703, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Orloff JJ, Reddy D, de Papp AE, Yang KH, Soifer NE, Stewart AF. Parathyroid hormone-related protein as a prohormone: posttranslational processing and receptor interactions. Endocr Rev 15: 40–60, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Pache JC, Burton DW, Deftos LJ, Hastings RH. A carboxyl leucine-rich region of parathyroid hormone-related protein is critical for nuclear export. Endocrinology 147: 990–998, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Pardo FS, Lien WW, Fox HS, Efird JT, Auilera JA, DW B, Deftos LJ. Parathyroid hormone-related protein expression is correlated with clinical course in patients with glial tumors. Cancer 101: 2622–2628, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Paternot S, Arsenijevic T, Coulonval K, Bockstaele L, Dumont JE, Roger PP. Distinct specificities of pRb phosphorylation by cdk4 activated by cyclin D1 or cyclin D3. Cell Cycle 5: 61–70, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabbani SA, Gladu J, Liu B, Goltzman D. Regulation in vivo of the growth of Leydig cell tumors by antisense ribonucleic acid for parathyroid hormone-related peptide. Endocrinology 136: 5416–5422, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Sanchez I, Dynlacht BD. New insights into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol 16: 311–321, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Segre G. Receptors for parathyroid hormone and parathyroid hormone-related protein. In: Principles of Bone Biology, edited by Bilezikian JP, Raisz LG, Rodan GA. New York: Academic, 1996 [Google Scholar]
- 34.Shen X, Falzon M. PTH-related protein enhances LoVo colon cancer cell proliferation, adhesion, and integrin expression. Regul Pept 125: 17–27, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Stuart WD, Maeda S, Khera P, Fagin JA, Clemens TL. Parathyroid hormone-related protein induces G1 phase growth arrest of vascular smooth muscle cells. Am J Physiol Endocrinol Metab 279: E60–E67, 2000 [DOI] [PubMed] [Google Scholar]