Abstract
Pulmonary vascular remodeling occurs in patients with chronic thromboembolic pulmonary hypertension (CTEPH). One factor contributing to this vascular wall thickening is the proliferation of pulmonary artery smooth muscle cells (PASMC). Store-operated Ca2+ entry (SOCE) and cytosolic free Ca2+ concentration ([Ca2+]cyt) in PASMC are known to be important in cell proliferation and vascular remodeling in pulmonary hypertension. Rapamycin is widely known for its antiproliferative effects in injured coronary arteries. Although several reports have suggested favorable effects of rapamycin in animal models of pulmonary hypertension, no reports have been published to date in human tissues. Here we report that rapamycin has an inhibitory effect on SOCE and an antiproliferative effect on PASMC derived from endarterectomized tissues of CTEPH patients. Cells were isolated from endarterectomized tissues obtained from patients undergoing pulmonary thromboendarterectomy (PTE). Immunohistochemical analysis indicated high deposition of platelet-derived growth factor (PDGF) in tissue sections from PTE tissues and increased PDGF receptor expression. PDGF transiently phosphorylated Akt, mammalian target of rapamycin (mTOR), and p70S6 kinase in CTEPH cells from CTEPH patients. Acute treatment (30 min) with rapamycin (10 nM) slightly increased cyclopiazonic acid (10 μM)-induced Ca2+ mobilization and significantly reduced SOCE. Chronic treatment (24 h) with rapamycin reduced Ca2+ mobilization and markedly inhibited SOCE. The inhibitory effects of rapamycin on SOCE were less prominent in control cells. Rapamycin also significantly reduced PDGF-stimulated cell proliferation. In conclusion, the data from this study indicate the importance of the mTOR pathway in the development of pulmonary vascular remodeling in CTEPH and suggest a potential therapeutic benefit of rapamycin (or inhibition of mTOR) in these patients.
Keywords: proliferation, rapamycin, therapy
chronic thromboembolic pulmonary hypertension (CTEPH) is a disease in which unresolved clots lodge in the central pulmonary arteries, eventually resulting in increased pulmonary vascular resistance and elevated pulmonary arterial pressure. Formation of a fibrotic occlusion by unresolved clots in central pulmonary arteries is a major cause for the elevated pulmonary arterial pressure in patients with CTEPH. In most patients, vascular remodeling in the distal pulmonary vascular bed has been shown to be an important component in the development of pulmonary hypertension in this disease (10, 26). Our previous studies (12, 47) demonstrate that the fibrotic clot tissues excised during pulmonary thromboendarterectomy (PTE) from the occluded region in the central and proximal pulmonary arteries consist of smooth muscle cells, endothelial cells, and myofibroblasts. In cell cultures prepared from these endarterectomized tissues of CTEPH patients, we found that majority of the cells resembled myofibroblasts staining for vimentin and smooth muscle α-actin (SMαA). Although the clinical characterization of the disease has been well documented, the cellular and molecular mechanisms involved in the formation of a central vascular fibrotic occlusion and downstream vascular remodeling are still unclear. Deciphering such pathways will increase our understanding of the disease, potentially enabling better diagnosis and improved treatment for patients with CTEPH.
Excessive proliferation of smooth muscle cells is one factor contributing to the vascular wall thickening and remodeling in the pulmonary arteries in patients with pulmonary hypertension. Store-operated Ca2+ entry (SOCE), or capacitative Ca2+ entry (CCE), and transient receptor potential (TRP) channels are known to be important in cell proliferation and vascular remodeling in pulmonary hypertension (13, 18, 24, 43, 48). Our laboratory has previously demonstrated (48) that upregulated TRP channels and augmented SOCE are involved in excessive proliferation and contraction of pulmonary artery smooth muscle cells (PASMC) isolated from patients with idiopathic pulmonary arterial hypertension. In addition to the Ca2+/protein kinase C (PKC) pathway, many other signaling pathways are involved in proliferation of vascular smooth muscle and endothelial cells; one such example is the Akt pathway. Akt is a member of the serine/threonine-specific kinase family known for potentiating cell survival via inhibition of apoptotic pathways. It possesses a pleckstrin homology domain that binds to phosphoinositides phosphatidylinositol 3,4,5-trisphosphate (PIP3) and phosphatidylinositol 3,4-bisphosphate after conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) by phosphatidylinositol 3-kinase (PI3K) (21). PTEN, a lipid phosphatase that dephosphorylates PIP3, reverts this pathway by converting PIP3 back to PIP2 (40, 46). Akt promotes cell survival directly by phosphorylation of proapoptotic proteins such as BAD, a member of the Bcl-2 family (41), and members of the forkhead transcription factor family FOXO1a and 3a (7, 29). Cell survival may also be promoted by Akt via indirect actions to enhance the degradation of IκB, promoting NF-κB-mediated transcriptional activation (39).
Mammalian target of rapamycin (mTOR) is an important signaling protein involved in cancer cell proliferation via the Akt pathway (15). Blockade of mTOR with rapamycin has been well documented to inhibit cell proliferation in a variety of cell types; it has primarily been studied in cancer cells (2, 8), although other cell types include coronary arterial smooth muscle cells (14), embryonic stem cells (22), and vascular progenitor cells (27, 34). It is currently unknown whether the Akt/mTOR pathway is involved in growth factor-mediated SOCE in human PASMC.
The PI3K-Akt pathway is fundamental in the communication of mitogenic signals to mTOR. Protein synthesis is decreased when mTOR is inhibited; this can be due to inactivation of p70S6 kinase (p70S6K) (9) or activation of the eukaryotic initiation factor 4E binding protein 1 (4E-BP1) (42). The Akt-mTOR-p70S6K pathway is central to the regulation of cell growth and proliferation; in response to mitogenic stimuli, p70S6K acts as a major effector of mTOR phosphorylation at Ser2448. 4E-BP1 is a small translation repressor molecule that modulates the availability of eukaryotic translation initiation factor 4E (eIF4E) for the formation of the eukaryotic translation initiation factor 4F (eIF4F) complex. eIF4F mediates the binding of the 40S ribosomal subunit to the 5′ end of capped mRNA; the 5′ cap refers to a modified guanosine on eukaryotic mRNA. The complex comprises three proteins: the cap binding subunit, eIF4E; an RNA-dependent ATPase/ATP-dependent RNA helicase, eIF4A; and eIF4G, a high-molecular-weight protein that acts as a scaffold for binding eIF4E and eIF4A (38).
Rapamycin is widely known for its antiproliferative effects in injured coronary arteries (33, 34, 37). Importantly, upregulation of p70S6K and hyperphosphorylation of 4E-BP1 were observed in growth factor-stimulated quiescent coronary artery smooth muscle cells and after balloon injury in rat carotid arteries (6). Furthermore, in vascular smooth muscle cells, rosiglitazone has been shown to inhibit cell proliferation via this pathway (36). Although several reports have suggested favorable effects of rapamycin in animal models of pulmonary hypertension (32, 35, 52), no reports have been published to date in human tissues. We hypothesized that rapamycin would have an inhibitory effect on SOCE and an antiproliferative effect on cells isolated from endarterectomized tissues from patients with CTEPH.
MATERIALS AND METHODS
Tissue collection and cell isolation.
Endarterectomized tissue was obtained from patients with CTEPH during PTE. All experiments were carried out after the approval of our protocol by the Institutional Review Board of the University of California, San Diego. Written informed consent was obtained from all patients before the procedure. Endarterectomized tissues were obtained directly from the operating room, and pieces of tissue were isolated from regions distal to the occlusion. For cell preparation, tissue was cut into ∼2-mm2 pieces and incubated in Hanks' balanced salt solution (HBSS) containing 2.5 mg/ml collagenase (Worthington Biochemical), 0.5 mg/ml elastase (Sigma), and 1.0 mg/ml bovine serum albumin (BSA; Sigma) for 55 min at 37°C with frequent agitation to create a cell suspension. After the incubation, the cells were washed in Dulbecco's modified Eagle's medium (DMEM) supplemented with 20% fetal bovine serum (FBS, Mediatech) and centrifuged at 1,500 rpm for 5 min. Cells were then resuspended in smooth muscle growth medium (SmGM, Lonza) composed of smooth muscle basal medium (SmBM, Lonza), 10% FBS, human epidermal growth factor, human fibroblast growth factor-B, and insulin. The cells were incubated in a humidified 5% CO2 atmosphere at 37°C for 1 wk. After reaching confluence, the cells were subcultured by trypsinization with 0.05% trypsin-EDTA (Lonza). Human PASMC and pulmonary artery endothelial cells (PAEC) from normal subjects were purchased from Lonza. PAEC were cultured in endothelial growth medium (Lonza).
Immunohistochemistry.
Endarterectomized tissues were embedded in optimal cutting temperature (OCT) compound (Sakura Tissue Tek), frozen, and cut on a cryostat into 10-μm sections. For basic characterization, standard hematoxylin and eosin (H & E) staining or SMαA antibody staining was performed. For subsequent studies, the sections were stained with PDGF-A antibody and the ABC Staining System (Santa Cruz Biotechnology) according to the manufacturer's instructions. Samples were then counterstained in hematoxylin (Ricca Chemical). Images were taken on an Olympus IX70 microscope and captured with an Olympus U-CMT camera at ×10 or ×20 magnification.
Immunofluorescence staining.
Cells on 25-mm coverslips were fixed with 4% paraformaldehyde in PBS and incubated in blocking solution (PBS containing 2% BSA, 0.1% Triton X-100, and 2% FBS). Cells were then exposed to the primary antibodies in blocking solution for 2 h, washed in PBS, and incubated for 45 min at room temperature with secondary antibody diluted in blocking solution. Cells were counterstained with DAPI (0.3 μM, Invitrogen) and mounted in antifade mounting solution. The imaging was performed with a Deltavision RT Deconvolution Microscope (Applied Precision) and softWoRx software (Applied Precision). The images were processed by Image J (National Institutes of Health software).
Western blot analysis.
Cells were washed with ice-cold PBS, suspended into lysis buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 100 μg/ml phenylmethylsulfonyl fluoride, phosphatase inhibitors, and protease inhibitors), and incubated for 30 min on ice. The cell lysates were then sonicated and centrifuged at 12,000 rpm for 10 min, and the supernatant was collected. Protein concentrations were determined by Dc Protein assay (Bio-Rad Laboratories) using BSA as a standard. Samples were applied on SDS-PAGE (4–20%), and proteins were transferred onto nitrocellulose membranes by electroblot. Membranes were blocked in 5% nonfat milk and incubated overnight at 4°C with primary antibodies and then with secondary antibody. Blots were developed with the SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology).
Measurement of cytoplasmic Ca2+ concentration.
Cells on 25-mm coverslips were placed in a recording chamber on the stage of an inverted Nikon Eclipse/TE 200 microscope with the TE-FM epifluorescence attachment. Cytosolic Ca2+ concentration ([Ca2+]cyt) was measured in each cell with the membrane-permeant Ca2+-sensitive fluorescent indicator fura-2 AM (Invitrogen). The cells were incubated at room temperature for 30 min in modified Krebs solution (MKS) containing 4 μM fura-2 AM. The loaded cells were then washed with MKS for 30 min to remove excess extracellular dye and allow intracellular esterases to cleave cytosolic fura-2 AM into active fura-2. Fura-2 fluorescence was observed as 510-nm-wavelength light emission with excitation wavelengths of 340 and 380 nm by the use of the digital fluorescence imaging system from Intracellular Imaging. In all experiments, multiple cells were imaged in a single field, and one arbitrarily chosen peripheral cytosolic area from each cell was spatially averaged. [Ca2+]cyt was expressed as fura-2 fluorescence emission ratio excited at 340 and 380 nm (F340/F380). Resting [Ca2+]cyt was determined by the average F340/F380 during the initial MKS recording. Changes in [Ca2+]cyt were calculated as the peak F340/F380 minus the averaged steady-state F340/F380 in the 20 s preceding the initiation of the rise to peak. To ensure consistency all time periods in each condition were kept constant between experiments.
Proliferation assay.
Cell number was determined by using Trypan blue to stain viable cells, which were counted with a hemocytometer. Cell count in each of the four 1-mm2 corner squares in the hemocytometer was averaged to calculate total cell number per milliliter in cell suspension. Cell number, normalized by the surface area of the wells (cells/cm2), was used to compare cell growth rate. [3H]thymidine uptake assay was also performed to assess DNA synthesis. PASMC were seeded in 12-well plates and treated for each experiment. Twenty-four hours before treatment was finished, 1 μCi of [3H]thymidine was added to each conditioned medium. One day later, cells were washed with cold PBS once and washed twice with cold 7.5% trichloroacetic acid and then lysed with 0.5 M NaOH. Radioactivity was measured in a liquid scintillation counter.
mTOR small interfering RNA.
Four small interfering RNA (siRNA) sequences, control siRNA (fluorescein-conjugated and unconjugated SignalSilence control siRNAs), mTOR siRNA-I, and mTOR-siRNA II, were purchased from Cell Signaling Technology. PASMC were grown in six-well plates and transfected with siRNA for 24 h with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. In brief, siRNA was incubated in Opti-MEM (Invitrogen) for 5 min at room temperature before addition of Lipofectamine 2000 (7.5 μl/well). The siRNA/Opti-MEM mix was incubated for a further 30 min before addition to the cells. siRNA transfection efficiency was determined with fluorescein-conjugated siRNA. Both mTOR siRNA-I and mTOR siRNA-II significantly reduced mTOR protein expression.
Chemicals, antibodies and materials.
PDGF-BB, human α-thrombin, and rapamycin were purchased from Sigma, Enzyme Research Laboratories, and Calbiochem, respectively. Antibody to SMαA was purchased from Sigma. Antibodies to PDGF-A, PDGF receptor (PDGF-R)α and PDGF-Rβ, as well as FITC-conjugated anti-rabbit IgG and horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibodies, were all purchased from Santa Cruz Biotechnology. HRP-conjugated donkey anti-mouse IgG antibody was purchased from Millipore. Antibodies to GAPDH were purchased from Abcam and Millipore. Antibodies to mTOR and phospho-mTOR (Ser2448), p70S6K and phospho-p70S6K (Thr389), Akt and phospho-Akt (Ser473), eIF4E and phospho-eIF4E (Ser209), and 4E-BP1 and phospho-4E-BP1 (Ser65) were purchased from Cell Signaling Technology.
Statistical analysis.
Data are expressed as means ± SE. Differences between groups were examined for statistical significance with Student's t-test. Differences were considered to be statistically significant when P < 0.05.
RESULTS
Characterization of cells isolated from endarterectomized tissues of CTEPH patients.
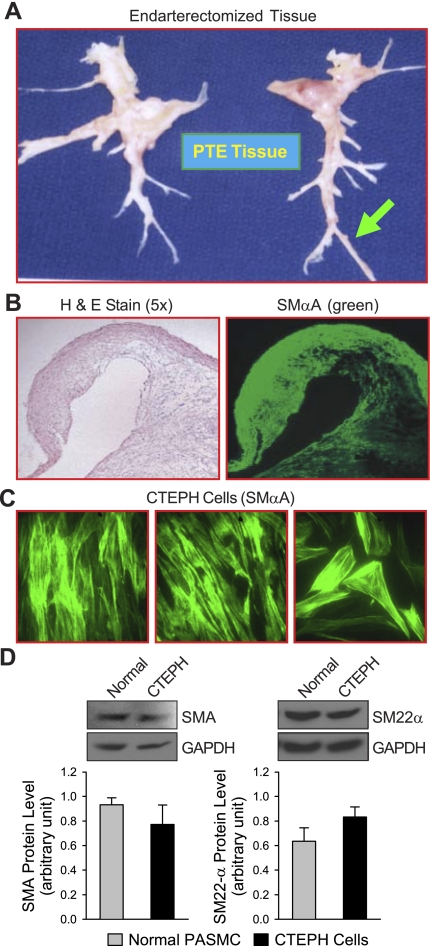
As previously reported by Moser and Bloor (28), the tissues surgically removed by PTE from patients with CTEPH are mainly composed of occluded materials in the central pulmonary artery as well as a thin layer of media and fibrotic intima in the central pulmonary artery and downstream branches (Fig. 1A) (28). Cross section of the distal artery (arrow in Fig. 1A) shows that most of the cells stained positively with SMαA (Fig. 1, B and C). Cultured cells from endarterectomized tissues (referred to as CTEPH cells) show some morphological heterogeneity. Most of the cells, however, did stain for and have strong protein expression of SMαA and SM-22α, indicative of smooth muscle cell types (Fig. 1, C and D). Many cells also stained for vimentin, which may be suggestive of the presence of myofibroblasts (30, 44).
Fig. 1.
Characterization of tissues removed from chronic thromboembolic pulmonary hypertension (CTEPH) patients during pulmonary thromboendarterectomy (PTE). A: endarterectomized tissue from a patient with CTEPH; tissue samples were taken from the distal regions, as indicated by the arrow. B: sections from PTE tissues were stained with hematoxylin and eosin (H & E) to study tissue morphology (left, ×5 magnification) and with smooth muscle α-actin (SMαA; green) to indicate the presence of smooth muscle-type cells (right). C: immunofluorescent images of CTEPH cells (prepared from 3 different CTEPH patients) stained with antibody against SMαA. D: representative Western blot images (top) and summarized data (bottom; means ± SE) of SMαA (SMA; left) and SM-22α (right) protein expression in normal pulmonary artery smooth muscle cells (PASMC) (normal) and CTEPH cells; graphs show protein expression normalized to internal control GAPDH. There is no significant difference in protein expression levels of SMαA and SM-22α between normal PASMC and CTEPH cells.
Higher expression levels of PDGF and PDGF receptors in endarterectomized tissues of CTEPH patients.
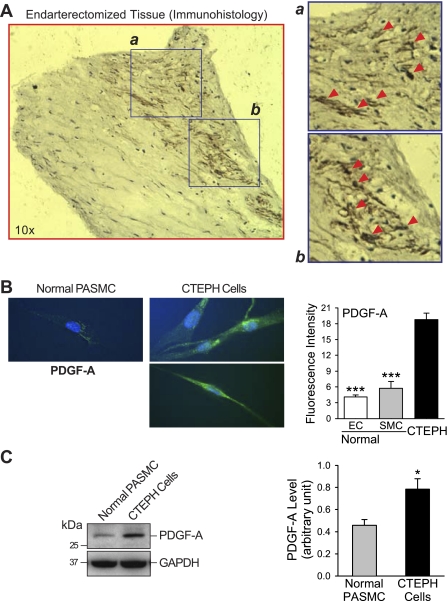
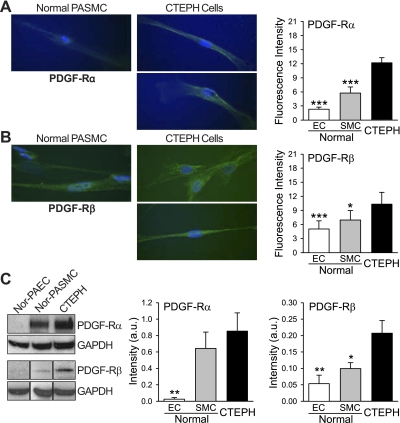
Immunohistochemical analysis of the endarterectomized tissue sections indicated that many cells contained a high level of PDGF-A, as indicated by the brown regions in Fig. 2A and highlighted in the insets (Fig. 2A, a and b). Compared with PASMC from normal subjects, CTEPH cells expressed a higher level of PDGF-A, indicated by increased immunofluorescence and protein expression in Fig. 2, B and C. In addition, the cells also expressed a higher level of the PDGF receptors PDGF-Rα and PDGF-Rβ (Fig. 3, A and B). Fluorescence intensity was determined by measuring the mean intensity in each cell and averaging the intensity in all cells, and protein expression was directly measured by Western blot (Fig. 3C). Previously published data from our laboratory (49) showed that PDGF can increase expression of STAT3 and c-Jun, an AP-1 transcription activator. PDGF is also known to potently activate the Akt/mTOR pathway (39, 51); therefore experiments were designed to decipher the role of the Akt/mTOR pathway in CTEPH cell proliferation.
Fig. 2.
Upregulated PDGF in PTE tissues and cells derived from PTE tissues in CTEPH patients. A: representative section of PTE tissue stained with hematoxylin (blue) and anti-PDGF-A antibody (brown). Arrowheads in magnified sections (a and b) indicate regions with high PDGF-A accumulation. B: example of immunostaining of cells showing PDGF-A expression (left); quantitative data compare normal pulmonary artery endothelial cells (PAEC) (EC), PASMC (SMC), and CTEPH cells (right). C: representative data (left) showing Western blot analysis of PDGF-A expression in normal PASMC and CTEPH cells; data are normalized to GAPDH expression for semiquantitation (means ± SE, right). *P < 0.05 vs. normal PASMC; ***P < 0.001 vs. CTEPH cells.
Fig. 3.
Upregulated PDGF receptors in cells derived from PTE tissues in CTEPH patients. A and B: representative immunofluorescent images (left) of normal PASMC and CTEPH cells showing expression of PDGF receptors PDGF-Rα (A) and PDGF-Rβ (B); quantitative comparisons (means ± SE) are made between normal PASMC, PAEC, and CTEPH cells (right). C: representative data (left) showing Western blot analysis of PDGF-Rα and PDGF-Rβ expression in normal PAEC (nor-PAEC), normal PASMC (nor-PASMC), and CTEPH cells (CTEPH); data are normalized to GAPDH expression for semiquantitation (means ± SE, center and right). Western blot result for PDGF-Rβ was obtained from different experiments. a.u., Arbitrary unit. *P < 0.05, **P < 0.01, ***P < 0.001 vs. CTEPH cells.
PDGF activates Akt/mTOR pathways in CTEPH cells from patients.
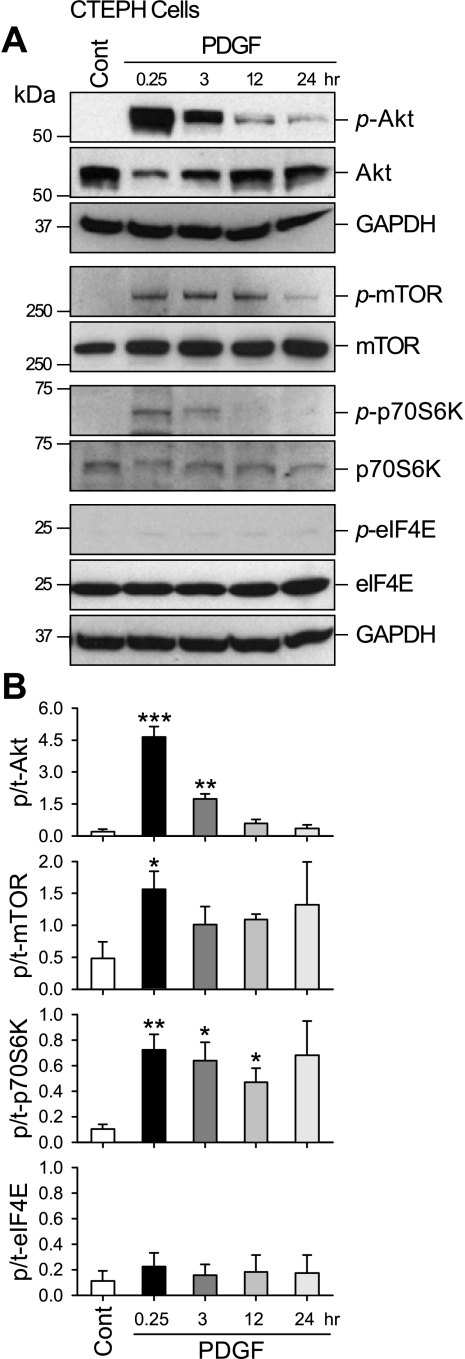
For these experiments, cells were incubated in SmBM supplemented with PDGF (10 ng/ml) for 24 h. Figure 4 shows representative Western blots (Fig. 4A) indicating that PDGF treatment of CTEPH cells transiently increased phosphorylation of Akt, which subsequently caused marked increase in phosphorylated mTOR and p70S6K. Interestingly, no changes in phosphorylated eIF4E were detected in the CTEPH cells (Fig. 4B). These results indicate that PDGF activates PASMC, at least in part, via activation of Akt/mTOR pathway, and PDGF-induced mTOR activation only leads to phosphorylation of p70S6K (with no effect on eIF4E) in CTEPH cells.
Fig. 4.
PDGF phosphorylates Akt, mammalian target of rapamycin (mTOR), and p70S6 kinase (p70S6K), but not eukaryotic translation initiation factor 4E (eIF4E), in CTEPH cells. A: representative Western blots showing time-dependent changes in phosphorylated (p-Akt, p-mTOR, p-p70S6K and p-eIF4E) components and total proteins (Akt, mTOR, p70S6K, and eIF4E) of the mTOR signaling on PDGF stimulation. Protein expression was assessed at 0.25, 3, 12, and 24 h after PDGF treatment. GAPDH was used as an internal/loading control. Cont, control. B: summary data (means ± SE) were quantitated and compared at each time point, with the control being without PDGF treatment. p/t, Phosphorylated/total. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control (Cont).
PDGF increases store-operated Ca2+ entry in CTEPH cells from patients.
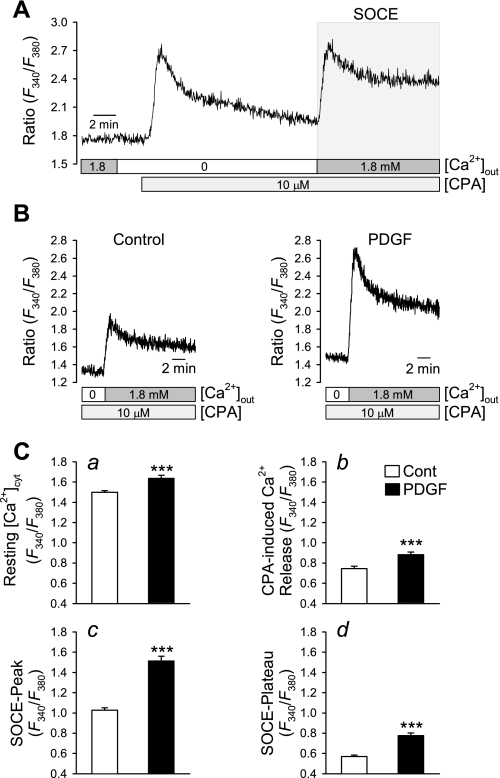
We showed previously (49) that PDGF stimulated increases in STAT3 and c-Jun and in the expression of canonical TRP channel 6 (TRPC6) increased SOCE, previously referred to as CCE, and proliferation in PASMC. In the next set of experiments, we compared the increases in [Ca2+]cyt due to Ca2+ mobilization from the sarcoplasmic reticulum (SR) and to SOCE in CTEPH cells cultured in SmBM (control) and PDGF-containing medium. The amplitude of SOCE was measured by a standard protocol shown in Fig. 5A : extracellular application of cyclopiazonic acid (CPA, 10 μM), a sarco(endoplasmic) reticulum Ca2+-Mg2+-ATPase (SERCA) inhibitor, to a cell in the absence of extracellular Ca2+ induced a transient increase in [Ca2+]cyt, which was due to Ca2+ mobilization from the SR. After 10 min, when the transient rise in [Ca2+]cyt declined to a steady state, restoration of extracellular Ca2+ induced a second increase in [Ca2+]cyt, which was due to SOCE (Fig. 5A).
Fig. 5.
PDGF treatment enhances store-operated Ca2+ entry (SOCE) in PASMC. A: representative trace showing changes in cytosolic Ca2+ concentration ([Ca2+]cyt), expressed as the ratio of 340- and 380-nm fluorescence (F340/F380), in response to cyclopiazonic acid (CPA)-induced store depletion (indicated by 1st transient increase in [Ca2+]cyt) in the absence of extracellular Ca2+ ([Ca2+]out) and subsequent SOCE (shaded box) on replenishment of extracellular Ca2+. B: representative records showing the amplitude of SOCE in control cells (left) and PDGF-treated CTEPH cells (right). C: quantitative comparison of basal [Ca2+]cyt (a), CPA-induced rise in [Ca2+]cyt due to Ca2+ release or leakage (b), the peak of store-operated Ca2+ entry (SOCE-peak, c), and the SOCE plateau phase (d) in control (n = 93) and PDGF-treated (n = 73) PASMC. Data are expressed as means ± SE. ***P < 0.001 vs. control.
In CTEPH cells growth arrested in SmBM, passive depletion of the intracellular stores by inhibition of SERCA with CPA (10 μM) in Ca2+-free solution before replacement of 1.8 mM extracellular Ca2+ induced SOCE. Treatment with PDGF significantly increased the resting [Ca2+]cyt (Fig. 5Ca) and the amplitude of SOCE (Fig. 5, B and C); Fig. 5C, c and d, indicate increases in SOCE peak and steady-state [Ca2+]cyt (P < 0.001). In addition, the CPA-induced increases in [Ca2+]cyt due to Ca2+ mobilization from the intracellular stores were enhanced after PDGF stimulation (Fig. 5Cb). These results indicate that PDGF treatment significantly enhances SOCE in CTEPH cells.
Inhibition of mTOR with rapamycin significantly inhibits store-operated Ca2+ entry in cells from CTEPH patients.
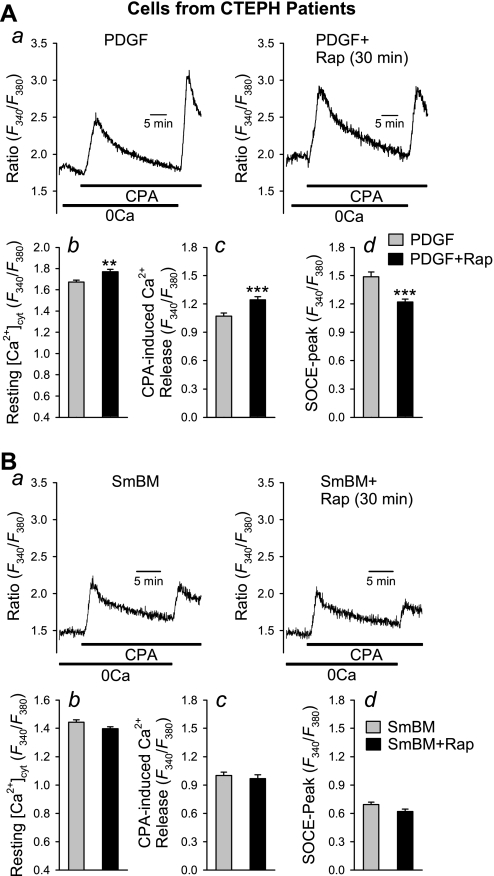
In proliferating CTEPH cells (cultured in PDGF-containing medium), acute treatment with rapamycin (10 nM for 30 min) significantly decreased the amplitude of SOCE (Fig. 6A, a and d). Conversely, acute treatment with rapamycin increased the resting [Ca2+]cyt (P < 0.01, Fig. 6Ab) and the amplitude of [Ca2+]cyt rise due to Ca2+ leakage from the intracellular stores, indicating that acute blockade of mTOR did not reduce the capacity of Ca2+ in the SR (Fig. 6Ac), although it inhibited the amplitude of SOCE. In growth-arrested CTEPH cells (maintained in SmBM), acute treatment with rapamycin had no significant effect on CPA-induced elevation of [Ca2+]cyt, SOCE, or resting [Ca2+]cyt (Fig. 6B). These results imply that the acute inhibitory effect of rapamycin on SOCE is associated with PDGF stimulation.
Fig. 6.
Acute inhibition of mTOR with rapamycin attenuates CPA-mediated SOCE in PDGF-stimulated CTEPH cells. A, a: representative traces showing changes in [Ca2+]cyt in PDGF-stimulated cells with (right) or without (left) acute treatment with rapamycin (Rap; 10 nM for 30 min). Summary data (means ± SE) show basal [Ca2+]cyt (b), amplitude of CPA-induced rise in [Ca2+]cyt due to Ca2+ release or leakage (c), and peak amplitude of SOCE (d) in PDGF-treated cells (n = 43 cells) and PDGF-treated cells with rapamycin (n = 45 cells). B, a: representative traces showing changes in [Ca2+]cyt in nonstimulated cells [in smooth muscle basal medium (SmBM)] with (right) or without (left) acute treatment with rapamycin (10 nM for 30 min). Summary data (means ± SE) show basal [Ca2+]cyt (b), amplitude of CPA-induced rise in [Ca2+]cyt due to Ca2+ release or leakage (c), and peak amplitude of SOCE (d) in control (SmBM) cells (n = 74 cells) and PDGF-treated cells with rapamycin (n = 67 cells). **P < 0.01, ***P < 0.001 vs. PDGF-treated cells.
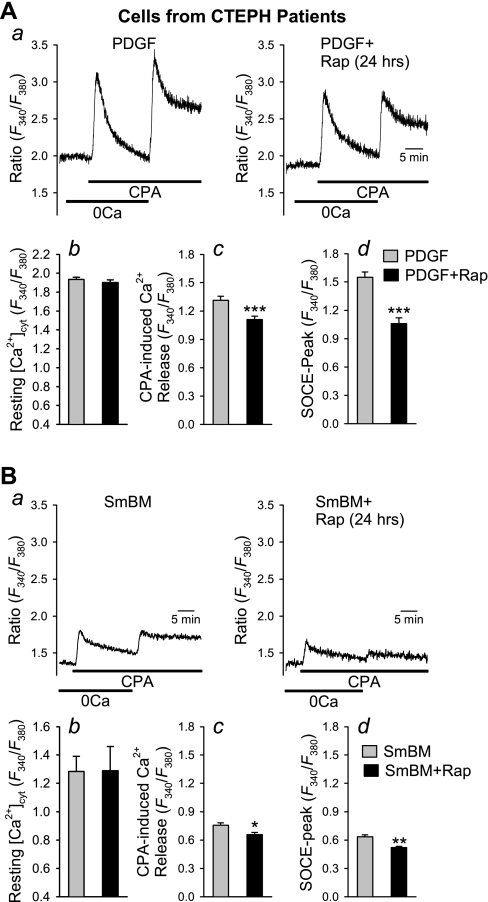
Prolonged treatment of PDGF-stimulated CTEPH cells with rapamycin (24 h) not only inhibited SOCE but also decreased the amplitude of CPA-induced Ca2+ release or leakage (Fig. 7A). Furthermore, under basal/growth-arrested conditions, chronic (or prolonged) rapamycin treatment also significantly decreased the amplitude of CPA-induced Ca2+ release and SOCE (Fig. 7B). No changes in resting [Ca2+]cyt were observed between PDGF-alone- and PDGF + rapamycin-treated cells after chronic treatment (Fig. 7, Ab and Bb). These results indicate that prolonged inhibition of mTOR may reduce the concentration of Ca2+ in the SR as a result of inhibited SOCE.
Fig. 7.
Prolonged (or chronic) inhibition of mTOR with rapamycin attenuates CPA-mediated SOCE in CTEPH cells. A, a: representative traces showing changes in [Ca2+]cyt in PDGF-stimulated cells with (right) or without (left) chronic treatment with rapamycin (10 nM for 24 h). Summary data (means ± SE) show basal [Ca2+]cyt (b), amplitude of CPA-induced rise in [Ca2+]cyt due to Ca2+ release or leakage (c), and peak amplitude of SOCE (d) in PDGF-treated cells (n = 38) and PDGF-treated cells with 24 h of rapamycin (n = 40). B, a: representative traces showing changes in [Ca2+]cyt in nonstimulated (or growth arrested) cells (in basal media) with (right) or without (left) chronic treatment with rapamycin (10 nM for 24 h). Summary data (means ± SE) show basal [Ca2+]cyt (b), amplitude of CPA-induced rise in [Ca2+]cyt due to Ca2+ release or leakage (c), and peak amplitude of SOCE (d) in control cells (SmBM, n = 60 cells) and PDGF-treated cells with 24 h of rapamycin (n = 55 cells).*P < 0.05, **P < 0.01, ***P < 0.001 vs. PDGF-treated cells.
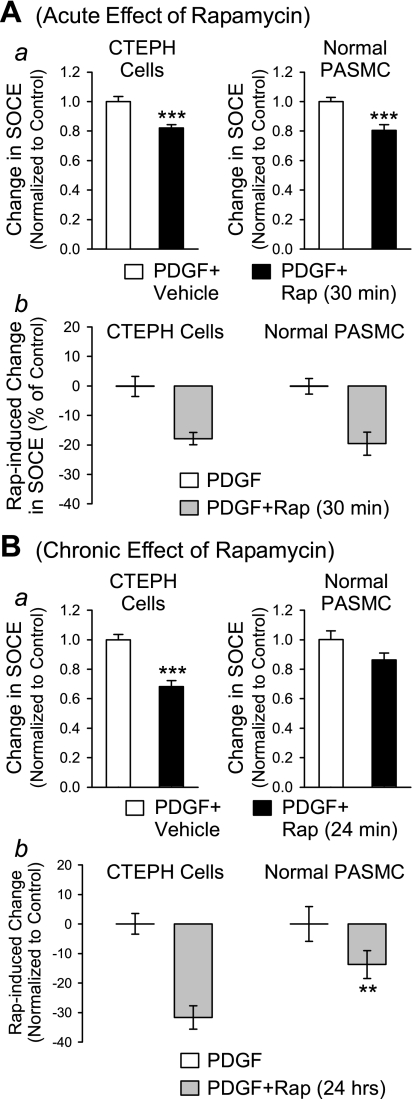
Comparison of effects of rapamycin on [Ca2+]cyt in CTEPH cells from patients and normal PASMC.
Using human PASMC prepared from normal subjects as a control, we found that the rapamycin-mediated inhibition of SOCE also occurred in normal PASMC (Fig. 8). Acute inhibition of mTOR with rapamycin similarly decreased the amplitude of SOCE in normal PASMC (Fig. 8A). Interestingly, a 24-h chronic inhibition of mTOR by rapamycin showed a significantly greater inhibition of SOCE in CTEPH cells compared with normal PASMC (Fig. 8B). These observations indicate that blockade of the mTOR pathway seems to have a significantly greater inhibitory effect on SOCE (activated by passive depletion of intracellular stores with CPA) in CTEPH cells.
Fig. 8.
Comparison of inhibitory effect of rapamycin on SOCE in normal PASMC and CTEPH cells. A, a: acute (30 min) effect of rapamycin (10 nM) on SOCE in PDGF-stimulated CTEPH cells (left) and normal PASMC (right) expressed as change in SOCE induced by acute rapamycin treatment compared with vehicle. b: Direct comparison of SOCE in CTEPH cells and normal PASMC induced by rapamycin compared with vehicle. B, a: chronic (24 h) effect of rapamycin (10 nM) on SOCE in PDGF-stimulated CTEPH cells (left) and normal PASMC (right) expressed as change in SOCE induced by rapamycin compared with vehicle. b: Direct comparison of SOCE in CTEPH cells and PASMC induced by chronic rapamycin treatment compared with vehicle. **P < 0.01, ***P < 0.001 vs. PDGF+vehicle.
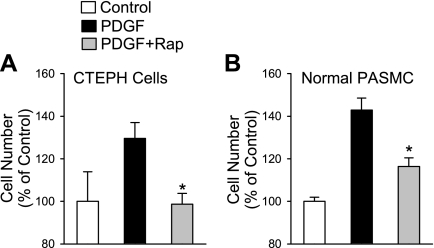
Rapamycin inhibits PDGF-mediated proliferation of CTEPH cells from patients.
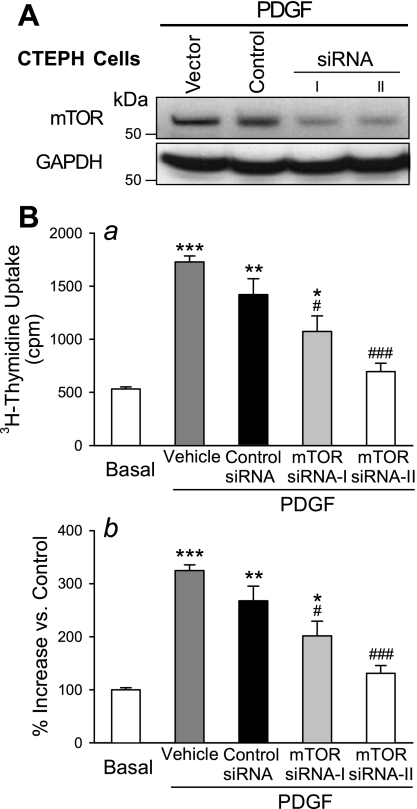
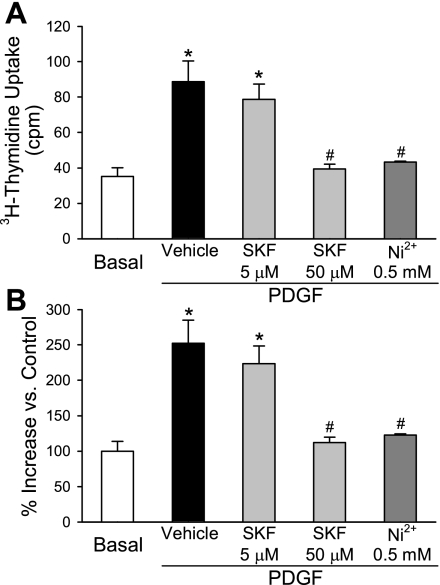
In concert with the inhibitory effects on SOCE and [Ca2+]cyt, inhibition of mTOR with chronic rapamycin treatment (24 h) also completely prevented PDGF-induced proliferation of CTEPH cells (Fig. 9A). In normal PASMC the proliferation was also significantly (P < 0.05) inhibited although not completely blocked (Fig. 9B). To further investigate the role of mTOR in CTEPH cell proliferation, two siRNAs targeting mTOR were assessed. As shown in Fig. 10, inhibition of mTOR expression by both of the siRNAs markedly reduced the protein level of mTOR (Fig. 10A) and significantly decreased the PDGF-stimulated proliferation of CTEPH cells (Fig. 10B) compared with control cells (which were not treated with siRNA) and vehicle siRNA-treated cells (Fig. 10B, [3H]thymidine uptake in Ba and hemocytometer-based cell counting in Bb). Similarly, inhibition of SOCE with recognized inhibitors, such as SK&F-96365 (50 μM) and Ni2+, significantly inhibited PDGF-mediated cell proliferation in CTEPH cells, determined by [3H]thymidine incorporation (Fig. 11A) and hemocytometer-based cell counting (Fig. 11B). These results indicate that inhibition of mTOR expression with siRNA and inhibition of SOCE with pharmacological blockers of store-operated Ca2+ channels both attenuate PDGF-mediated cell proliferation in CTEPH cells.
Fig. 9.
Rapamycin prevents PDGF-stimulated proliferation of CTEPH cells. Comparison of chronic effect of rapamycin (10 nM for 24 h) on PDGF-stimulated proliferation of CTEPH cells (A) and normal PASMC (B). Data are expressed as means ± SE. Experiments were repeated 3 times. *P < 0.05 vs. PDGF-treated cells.
Fig. 10.
Inhibition of mTOR by small interfering RNA (siRNA) attenuates PDGF-induced cell proliferation in CTEPH cells. A: representative Western blot showing mTOR and GAPDH in CTEPH cells treated with vehicle, control siRNA (Control), and siRNA (siRNA-I and siRNA-II) targeting mTOR in the presence of PDGF. B: summarized data (means ± SE) show [3H]thymidine incorporation (a) and increase in cell number (b) in CTEPH cells before (basal) and after (PDGF) incubation in PDGF-containing medium in the presence of vehicle, control siRNA, or siRNA for mTOR (siRNA-I and siRNA-II). *P < 0.05, **P < 0.01, ***P < 0.001 vs. nonstimulated basal conditions; #P < 0.05, ##P < 0.01 vs. vehicle and control siRNA.
Fig. 11.
Inhibition of SOCE significantly inhibits PDGF-stimulated cell proliferation in CTEPH cells. Summarized data (means ± SE) show [3H]thymidine incorporation (A) and increase in cell number (B) in CTEPH cells before (basal) and after (PDGF) incubation in PDGF-containing medium in the presence of vehicle, SK&F-96365 (SKF, 5 and 50 μM), or Ni2+ (0.5 mM). *P < 0.05 vs. nonstimulated basal conditions; #P < 0.05 vs. vehicle.
DISCUSSION
This study carefully examined the effect of rapamycin, and thus the role of the Akt/mTOR signaling pathway and SOCE and the impact this pathway has on proliferation of cells isolated from endarterectomized tissue from CTEPH patients. It is crucial to fully define such mechanisms in order to improve both the diagnosis and treatment outcome of these patients because the cellular and molecular determinants of the susceptibility, progression, and success of PTE surgery are currently unknown (10, 26). The University of California, San Diego Medical Center is a major referral center for more than 50% of the world's cases of CTEPH, therefore proffering unique access to tissues surgically removed from patients during PTE (10, 26). This study describes a prominent role for SOCE in the proliferation of cells isolated from endarterectomized tissue of CTEPH patients and an inherent involvement of the Akt/mTOR signaling pathway in SOCE and cell proliferation.
With smooth muscle cell proliferation and hypertrophy being important contributors to the remodeling that occurs in patients with pulmonary hypertension, it is important to understand the mechanisms that contribute to the abnormal cell regulation. Increased expression and release of growth factors, e.g., PDGF, vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and other mitogenic factors (endothelin-1, 5-HT), in lung tissue and in PASMC/PAEC have been well documented in patients with pulmonary hypertension and animal models of pulmonary hypertension. Increased [Ca2+]cyt due to Ca2+ entry through TRP channels (24, 31, 43, 48) has been demonstrated to cause excessive proliferation of PASMC in patients (and animal models) with pulmonary hypertension (4, 5, 17), while abnormal intracellular Ca2+ sequestration is thought to be linked to skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease (20).
CTEPH is a specific form of pulmonary hypertension that arises because of 1) formation of fibrotic occlusion in large pulmonary arteries, 2) downstream pulmonary vascular remodeling (characterized by significantly thickened arterial wall), and 3) potential pulmonary vascular remodeling in small vessels (observed in some patients). The mechanisms pertaining to the persistence of the fibrotic occlusion and the development of pulmonary arterial remodeling remain undetermined.
PDGF is a potent growth factor regulating cell growth and is widely associated with angiogenesis. In this study, we demonstrate a significant presence of PDGF in tissue sections and pulmonary vascular cells from CTEPH patients and an upregulation of both of the PDGF receptors (PDGF-Rα and PDGF-Rβ) in CTEPH cells from CTEPH patients compared with normal human PASMC. Previous work in our laboratory (49) has shown that PDGF stimulates PASMC cell proliferation and augments store depletion-mediated Ca2+ entry or SOCE/CCE in experiments monitoring changes in [Ca2+]cyt. An associated c-Jun/STAT3-induced upregulation of TRPC6 expression was found to cause this augmented SOCE/CCE and proliferation (49). In addition to its well-characterized signaling via JAK and STAT pathway, the PDGF receptor can also stimulate PI3K-dependent signaling through phosphorylation of the intracellular receptor tyrosine kinase domains, activating PI3K to convert PIP2 to PIP3. PIP3 can phosphorylate Akt and stimulate its signaling pathway via mTOR, and subsequently p70S6K and/or eIF4E.
The Akt/mTOR pathway is most frequently associated with the enhanced growth potential of tumors (50), and the regulation of this pathway is proving influential in determining the pathogenesis and aggressiveness of cancers and helpful in selecting favorable therapeutics. Indeed, detection of phospho-p70S6K in hepatocellular carcinomas is a potential selection tool for patients who may benefit from a therapeutic approach targeting the mTOR pathway (3). In the pulmonary circulation, Akt/mTOR signaling has been shown to be a key pathway in hypoxia-induced adventitial fibroblast proliferation (16), angiotensin IV-mediated PAEC proliferation (23), serotonin-induced growth of PASMC (25), and VEGF signaling in PAEC (1). Rapamycin has been shown to have significant beneficial effects in monocrotaline-induced pulmonary hypertension and chronic hypoxia-induced pulmonary hypertension in animal models, both preventing or reversing pulmonary vascular remodeling (32–34, 52).
PDGF-induced PI3K-dependent activation of Akt and p70S6K has been shown to enhance the proliferation and migration of PASMC and proposed to play a role in pulmonary vascular remodeling (19). Our study confirmed this and demonstrated that rapamycin was able to decrease Ca2+ influx into CTEPH cells to a significantly greater extent than in normal human PASMC. The data in the present investigation therefore highlight the potential for inhibition of the Akt/mTOR pathway in CTEPH patients. Interestingly, silencing of proline rich protein 5, a component of mTOR complex 2, was recently shown to decrease cell proliferation via inhibition of Akt and p70S6K1 phosphorylation, which also correlated with a decreased expression of PDGF-Rβ (45). This is thus supportive of our findings in PASMC in which increased PDGF signaling increases phosphorylation of Akt and p70S6K and enhances proliferation. Under our experimental conditions, no PDGF-stimulated phosphorylation of eIF4E was observed. The activity of both the p70S6K1 and 4E-BP1/eIF4E pathways, however, is required for independently and additively mediating mTOR-dependent control of cell cycle progression (11). It is worth mentioning here that we chose human PASMC and PAEC maintained at low passage (<6) as controls for our CTEPH cells. Although there is no ideal control, because only CTEPH patients develop such persistent occluding tissues, we believe that the CTEPH cells must originate either from the circulation or from the PASMC and PAEC in the surrounding pulmonary artery. We have investigations ongoing to confirm the origin of the CTEPH cells.
In conclusion, our data highlight the importance of the mTOR pathway in the development of pulmonary vascular remodeling in CTEPH and suggest a potential therapeutic benefit of rapamycin in these patients.
GRANTS
This work was supported, in part, by National Heart, Lung, and Blood Institute Grants HL-054043 and HL-066012. A. L. Firth is supported by a Postdoctoral Training Fellowship from the California Institute of Regenerative Medicine.
ACKNOWLEDGMENTS
We thank the team of surgeons and physicians at the Univ. of California, San Diego Medical Center, who contributed to collecting the tissue specimens from which the cells in this study were derived.
REFERENCES
- 1.Abid MR, Guo S, Minami T, Spokes KC, Ueki K, Skurk C, Walsh K, Aird WC. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler Thromb Vasc Biol 24: 294–300, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Altomare DA, Wang HQ, Skele KL, De Rienzo A, Klein-Szanto AJ, Godwin AK, Testa JR. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene 23: 5853–5857, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Baba HA, Wohlschlaeger J, Cicinnati VR, Hilgard P, Lang H, Sotiropoulos GC, Takeda A, Beckebaum S, Schmitz KJ. Phosphorylation of p70S6 kinase predicts overall survival in patients with clear margin-resected hepatocellular carcinoma. Liver Int 29: 399–405, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Barst RJ. PDGF signaling in pulmonary arterial hypertension. J Clin Invest 115: 2691–2694, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black SM, DeVol JM, Wedgwood S. Regulation of fibroblast growth factor-2 expression in pulmonary arterial smooth muscle cells involves increased reactive oxygen species generation. Am J Physiol Cell Physiol 294: C345–C354, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun-Dullaeus RC, Mann MJ, Seay U, Zhang L, von Der Leyen HE, Morris RE, Dzau VJ. Cell cycle protein expression in vascular smooth muscle cells in vitro and in vivo is regulated through phosphatidylinositol 3-kinase and mammalian target of rapamycin. Arterioscler Thromb Vasc Biol 21: 1152–1158, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Carraway H, Hidalgo M. New targets for therapy in breast cancer: mammalian target of rapamycin (mTOR) antagonists. Breast Cancer Res 6: 219–224, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem 280: 25485–25490, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med 345: 1465–1472, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol 24: 200–216, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firth AL, Yao W, Platoshyn O, Remillard CV, Sacks RS, Auger WR, Madani M, Thistlethwaite PA, Jamieson SW, Yuan JX. Vast morphological diversity exists in cells isolated from endarterectomized tissue from patients with chronic thromboembolic pulmonary hypertension (CTEPH) (Abstract). Am J Respir Crit Care Med 177: A772, 2008 [Google Scholar]
- 13.Firth AL, Remillard CV, Yuan JX. TRP channels in hypertension. Biochim Biophys Acta 1772: 895–906, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francy JM, Nag A, Conroy EJ, Hengst JA, Yun JK. Sphingosine kinase 1 expression is regulated by signaling through PI3K, AKT2, and mTOR in human coronary artery smooth muscle cells. Biochim Biophys Acta 1769: 253–265, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, Liu KJ, Shi X, Jiang BH. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol 287: C281–C291, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Gerasimovskaya EV, Tucker DA, Stenmark KR. Activation of phosphatidylinositol 3-kinase, Akt, and mammalian target of rapamycin is necessary for hypoxia-induced pulmonary artery adventitial fibroblast proliferation. J Appl Physiol 98: 722–731, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 328: 1732–1739, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JXJ. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 280: H746–H755, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, Krymskaya VP. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283: L354–L363, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Green HJ, Burnett M, Duhamel TA, D'Arsigny C, O'Donnell DE, Webb KA, Ouyang J. Abnormal sarcoplasmic reticulum Ca2+-sequestering properties in skeletal muscle in chronic obstructive pulmonary disease. Am J Physiol Cell Physiol 295: C350–C357, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Klippel A, Kavanaugh WM, Pot D, Williams LT. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol 17: 338–344, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SH, Lee MY, Han HJ. Short-period hypoxia increases mouse embryonic stem cell proliferation through cooperation of arachidonic acid and PI3K/Akt signalling pathways. Cell Prolif 41: 230–247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YD, Block ER, Patel JM. Activation of multiple signaling modules is critical in angiotensin IV-induced lung endothelial cell proliferation. Am J Physiol Lung Cell Mol Physiol 283: L707–L716, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Fanburg BL. Serotonin-induced growth of pulmonary artery smooth muscle requires activation of phosphatidylinositol 3-kinase/serine-threonine protein kinase B/mammalian target of rapamycin/p70 ribosomal S6 kinase 1. Am J Respir Cell Mol Biol 34: 182–191, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manecke GR, Jr, Wilson WC, Auger WR, Jamieson SW. Chronic thromboembolic pulmonary hypertension and pulmonary thromboendarterectomy. Semin Cardiothorac Vasc Anesth 9: 189–204, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Miriuka SG, Rao V, Peterson M, Tumiati L, Delgado DH, Mohan R, Ramzy D, Stewart D, Ross HJ, Waddell TK. mTOR inhibition induces endothelial progenitor cell death. Am J Transplant 6: 2069–2079, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Moser K, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 103: 685–692, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Moylan JS, Smith JD, Chambers MA, McLoughlin TJ, Reid MB. TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am J Physiol Cell Physiol 295: C986–C993, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakatani T, Honda E, Hayakawa S, Sato M, Satoh K, Kudo M, Munakata H. Effects of decorin on the expression of alpha-smooth muscle actin in a human myofibroblast cell line. Mol Cell Biochem 308: 201–207, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Ng LC, Kyle BD, Lennox AR, Shen XM, Hatton WJ, Hume JR. Cell culture alters Ca2+ entry pathways activated by store depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 294: C313–C323, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Nishimura T, Faul JL, Berry GJ, Veve I, Pearl RG, Kao PN. 40-O-(2-hydroxyethyl)-rapamycin attenuates pulmonary arterial hypertension and neointimal formation in rats. Am J Respir Crit Care Med 163: 498–502, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Nuhrenberg TG, Langwieser N, Schwarz JB, Hou Y, Frank P, Sorge F, Matschurat S, Seidl S, Kastrati A, Schomig A, Clauss MA, Zohlnhofer D. EMAP-II downregulation contributes to the beneficial effects of rapamycin after vascular injury. Cardiovasc Res 77: 580–589, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Nuhrenberg TG, Voisard R, Fahlisch F, Rudelius M, Braun J, Gschwend J, Kountides M, Herter T, Baur R, Hombach V, Baeuerle PA, Zohlnhofer D. Rapamycin attenuates vascular wall inflammation and progenitor cell promoters after angioplasty. FASEB J 19: 246–248, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Paddenberg R, Stieger P, von Lilien AL, Faulhammer P, Goldenberg A, Tillmanns HH, Kummer W, Braun-Dullaeus RC. Rapamycin attenuates hypoxia-induced pulmonary vascular remodeling and right ventricular hypertrophy in mice. Respir Res 8: 15, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S, Lim S, Chang W, Song H, Lee S, Song BW, Kim HJ, Cha MJ, Choi E, Jang Y, Chung N, Cho SY, Hwang KC. The inhibition of insulin-stimulated proliferation of vascular smooth muscle cells by rosiglitazone is mediated by the Akt-mTOR-P70S6K pathway. Yonsei Med J 49: 592–600, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramzy D, Rao V, Tumiati LC, Xu N, Miriuka S, Delgado D, Ross HJ. Role of endothelin-1 and nitric oxide bioavailability in transplant-related vascular injury: comparative effects of rapamycin and cyclosporine. Circulation 114: I214–I219, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433: 477–480, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401: 86–90, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95: 29–39, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA 96: 6199–6204, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 65: 7052–7058, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 166: 1321–1332, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woo SY, Kim DH, Jun CB, Kim YM, Haar EV, Lee SI, Hegg JW, Bandhakavi S, Griffin TJ. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling. J Biol Chem 282: 25604–25612, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA 95: 15587–15591, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao W, Firth AL, Sacks RS, Ogawa A, Auger WR, Fedullo PF, Madani MM, Lin GY, Sakakibara N, Thistlethwaite PA, Jamieson SW, Rubin LJ, Yuan JX. Identification of putative endothelial progenitor cells (CD34+CD133+Flk-1+) in endarterectomized tissue of patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 296: L870–L878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JXJ. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101: 13861–13866, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284: C316–C330, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene 27: 5497–5510, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Bajraszewski N, Wu E, Wang H, Moseman AP, Dabora SL, Griffin JD, Kwiatkowski DJ. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest 117: 730–738, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou H, Liu H, Porvasnik SL, Terada N, Agarwal A, Cheng Y, Visner GA. Heme oxygenase-1 mediates the protective effects of rapamycin in monocrotaline-induced pulmonary hypertension. Lab Invest 86: 62–71, 2006 [DOI] [PubMed] [Google Scholar]