Abstract
Background
The present study was designed to test the hypothesis that dexmedetomidine added to ropivacaine would increase the duration of antinociception to a thermal stimulus in a dose-dependent fashion in a rat model of sciatic nerve blockade.
Methods
Fifty adult Sprague Dawley rats (10 rats/group) received unilateral sciatic nerve blocks with 0.2 ml of 0.5% ropivacaine or 0.2 ml of 0.5% ropivacaine plus dexmedetomidine (2.7 μM [0.5 μg/kg], 11.7 μM [2 μg/kg], 34.1 μM [6 μg/kg], or 120.6 μM [20 μg/kg]) in a randomized, blinded fashion. Time to paw withdrawal latency to a thermal stimulus for both paws and an assessment of motor function were measured every 30 min after the nerve block until a return to baseline.
Results
Dexmedetomidine added to ropivacaine increased the duration of dense sensory blockade and time for return to normal sensory function in a dose-dependent fashion (p < 0.005). There was a significant time (p < 0.005), dose (p < 0.005), and time by dose effect (p < 0.005) on paw withdrawal latencies of the operative paws. There were no significant differences in paw withdrawal latencies of the control paws, indicating little systemic effect of the dexmedetomidine. The duration of motor blockade was also increased with dexmedetomidine. High-dose dexmedetomidine (120.6 μM) was not neurotoxic.
Conclusion
This is the first study showing that dexmedetomidine added to ropivacaine increases the duration of sensory blockade in a dose-dependent fashion in rat. The findings are an essential first step encouraging future efficacy studies in humans.
Introduction
Peripheral nerve blocks are used frequently in a variety of surgical procedures for surgical anesthesia and postoperative pain. Long-acting local anesthetics alone can provide analgesia for 9-14 h.1-4 If the block is performed in the morning or early afternoon, patients commonly report postoperative pain during nighttime hours. The need for opioids leads to the potential for opioid-induced side effects, including the inhibition of restorative sleep5 and the potential for airway obstruction and desaturation.6-8 Ideally, single shot peripheral nerve blocks would provide analgesia throughout the first postoperative night.
Many additives to local anesthetics have been investigated in an attempt to increase the duration of the block in order to improve postoperative pain. The efficacy of clonidine, an ∝2-adrenoceptor agonist, has been established in a variety of regional anesthesia techniques.9 Clonidine has been shown in many clinical studies to prolong the duration of anesthesia and analgesia in peripheral nerve blocks, although results with long acting local anesthetics have been somewhat less impressive.10,11 Some studies have found no beneficial effect with the addition of clonidine.11
Dexmedetomidine (trade name Precedex®, Hospira, Inc., Lake Forest, IL) is a selective ∝2-adrenoceptor agonist with US Food and Drug Administration approval for continuous intravenous sedation in the intensive care setting and procedural sedation in non-intubated patients. A previous study showed that high-dose dexmedetomidine enhanced the duration of sensory and motor blockade when added to bupivacaine in a sciatic nerve block model in rat.12 The doses of dexmedetomidine used were between 28-40 μg/kg and did not induce neurotoxicity alone or when mixed with 0.5% bupivacaine. These high doses, however, far exceed that which is proposed in humans. In addition, many anesthesiologists have changed their practice and now prefer the use of ropivacaine (trade name Naropin®, APP Pharmaceuticals, LLC, Schaumburg, IL) in lieu of bupivacaine for peripheral nerve blocks. This preference is based on evidence that local anesthetic-induced cardiac arrest with ropivacaine is more likely to respond to resuscitation efforts than with bupivacaine.13-16 Ropivacaine is known to have vasoconstrictive properties,17,18 which may alter the analgesic effects of additives.
The present study tested the hypothesis that dexmedetomidine added to ropivacaine, when compared with ropivacaine alone, enhances the duration of sensory blockade to a heat stimulus in a dose-dependent fashion. Additional analysis determined whether progressively higher doses of perineural dexmedetomidine provide systemic analgesia as measured by sensory response to a heat stimulus to an unblocked control paw.
Materials and Methods
This study adhered to American Physiological Society and National Institutes of Health guidelines and was approved by the University of Michigan Committee for the Use and Care of Animals (Ann Arbor, Michigan). All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory AnimalsΨ and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research.Φ
Drug preparation
Commercially available 0.75% ropivacaine (Naropin®, AstraZeneca LP, Wilmington, DE) was mixed with preservative free normal saline or dexmedetomidine (Precedex®) to make final solutions. The control group received 0.5% ropivacaine alone. In the experimental groups, ropivacaine was mixed with dexmedetomidine to make solutions based on the individual rat’s weight 24 h prior to experimental testing (μg/kg). All experimental groups had a final concentrations of 0.5% ropivacaine plus 2.7 ± 0.2 μM (mean concentration dexmedetomidine ± SD [0.5 μg/kg]), 11.7 ± 0.8 μM (2 μg/kg), 34.1 ± 3.0 μM (6 μg/kg), or 120.6 ± 6.4 μM (20.0 μg/kg) dexmedetomidine (table 1). The pH of ropivacaine (5.69 ± 0.05) was used as the standard to which all solutions were maintained.
Table 1.
Study Groups.
| Group Name | Drug Concentration |
|---|---|
| Group 1 (n = 10) |
Ropivacaine |
| Group 2 (n = 10) |
Ropivacaine + 2.7 ± 0.2 μM (0.5 μg/kg) DEX |
| Group 3 (n = 10) |
Ropivacaine + 11.7 ± 0.8 μM (2.0 μg/kg) DEX |
| Group 4 (n = 10) |
Ropivacaine + 34.1 ± 3.0 μM (6.0 μg/kg) DEX |
| Group 5 (n = 10) |
Ropivacaine + 120.6 ± 6.4 μM (20.0 μg/kg) DEX |
The five different study groups are noted above. Groups 2-5 represent the experimental doses that evaluate the dose-dependent effects of dexmedetomidine, in combination with ropivacaine, on the duration of analgesia from a sciatic nerve block. Dexmedetomidine doses were calculated based on weight (μg/kg). The ropivacaine concentration (0.5%) and total volume injected (0.2 ml) were constant between all groups. Group 1 represents the control group. DEX = dexmedetomidine.
Paw Withdrawal Latency Testing
The IITC Life Sciences Inc. Plantar Analgesia Meter (Series 8 Model 336T, IITC Life Science Inc., Woodland Hills, CA) was used to test paw withdrawal latency (PWL).19 The analgesia meter used a test unit containing a heat source that radiated a light beam. An angled mirror on the test unit was used to locate the correct target on the paw. The meter was set with an active intensity of 40%, an idle intensity of 10%, and a cut-off time for the heat source of 15 s. The time to paw withdrawal from the heat stimulus comprised the PWL measure (reaction time was measured to 0.01 s). An acrylic six-chamber container was used to separate the rats that were placed on the glass (Model 400, IITC Life Science Inc.) heated base. In order to decrease the level of variance in PWL measurements, the temperature of the base was set to 30°C five min prior to and throughout each round of PWL testing.20
Fifty male Sprague Dawley rats (Crl:CD [SD]IGS BR) weighing 250-350 g were purchased from Charles River Laboratories (Wilmington, MA). Rats without any signs of neurobehavioral impairment were maintained throughout the experiment in 12:12 light-dark cycles with lights on from 6:00 AM to 6:00 PM. For the three days prior to surgery and neurobehavioral monitoring, rats were conditioned to the paw withdrawal chambers for one h per day. Each rat was placed in the same position of the six-chamber container during the conditioning and neurobehavioral testing. On each of the three days prior to testing, both paws of the rat were exposed to the heat stimulus as a portion of the conditioning process. The day before surgery and testing, five PWL baseline measurements were obtained on both the operative and control paws. The mean value of the five measures was recorded as the rat’s baseline value.
Subfascial Sciatic Nerve Injection
An investigator (CMB) blinded to the drug condition carried out the sciatic nerve injections. Rats were assigned using simple random sampling without replacement. The laboratory assistants responsible for drug preparation were not involved with the surgery, neurobehavioral monitoring, PWL measures, data collection, or data analysis.
Rats were anesthetized and maintained with 2.5% isoflurane. For the surgical procedure, rats were placed in the right lateral decubitus position. The sciatic nerve of the left hind extremity was exposed using a lateral incision over the thigh and division of the superficial fascia as previously described.12,21-23 Following the dissection, the sciatic nerve was clearly identified at a point proximal its bifurcation. All rats received unilateral injections of 0.2 ml of drug into the perineural space below the clear fascia covering the nerve and proximal to the bifurcation of the sciatic nerve. Injections were made using a tuberculin syringe and a 30-gauge needle. The time of the injection was recorded and deemed the zero time point. A nonabsorbable muscle fascia suture was placed at the midpoint of the injection site as a marker for subsequent nerve removal. The suture was placed in the muscle fascia of the biceps femoris below the subcutaneous tissue and was neither directly touching nor surrounding the nerve.12 The incisions were closed and isoflurane was discontinued.
Neurobehavioral Examination and PWL Testing
Following the sciatic nerve injection, the incision was closed and the anesthetized rat was returned to its cage and placed supine. The time to the return to a prone position (resumption of righting reflex [RoRR]) was recorded to the nearest minute. After righting, rats were placed in the chamber for PWL testing. Three PWL measures from both the operative and control paw were obtained every 30 min from the time of the injection. The mean value of the three measures at each time point was calculated. Measurements were taken every 30 min until three consecutive PWL values at or below the baseline measurement were obtained. All rats were monitored for at least 210 min. In addition, the motor function of the surgical hind paw was assessed every 30 min by observation as either a curled paw (motor score = 1, indicates motor blockade) or normal paw position (motor score = 0, no motor blockade).12,24,25 Once the rat had returned to baseline sensory and motor function, it was returned to its home cage. The next morning, five PWL measurements were obtained prior to nerve removal for future analysis. In rats scheduled for nerve collection at 14 days postinjection, an additional five PWL measurements were taken immediately prior to nerve removal and euthanasia.
Histopathological Evaluation
Following the neurobehavioral examination, rats were assigned to one of two groups for sciatic nerve removal and pathologic evaluation. Nerves were removed under general anesthesia at 24 h or 14 days. Approximately 1.5 cm of nerve was removed with the injection site at the midpoint as marked by the fascial suture in the muscle directly above. In order to avoid any trauma-induced artifacts, care was taken not to stretch the nerves during the removal process. Nerves were placed in 2.5% glutaraldehyde for 24-72 h, then washed three times and placed in a phosphate buffer. Seven nerves in the 120.6 μM (20 μg/kg) dexmedetomidine group (three at 24 h and four at 14 days) were analyzed. Nerves were cut into 5-micron sections and stained with hemotoxylin and eosin and Luxol fast blue. Those nerves not analyzed were stored at 4°C.
A pathologist, blinded to experimental treatment, analyzed the slides using previously established scales for perineural inflammation (0 = no inflammation, 1 = small focal areas of mild edema and/or cellular infiltrate, 2 = locally extensive areas of moderate edema/cellular infiltrate, 3 = diffuse areas of moderate to marked edema/cellular infiltrate) and signs of nerve damage (0 = no lesions, 1 = 0-2% of the fibers with lesions in axons or myelin, 2 = 2-5% with lesions, 3 = >5% with lesions).12,26,27
Statistics
Sensory time-course data and RoRR are presented as means ± SEM. Motor data are presented as medians and interquartile ranges. Data were analyzed using SAS 9.2 (SAS Institute Inc., Cary, NC). A proportional hazards survival analysis (Cox Model) was used to compare the duration of a dense sensory blockade across doses (defined as the time when PWL went below 14 s for 3 consecutive time periods), the time to return to normal (defined as PWL < 6.59 s [baseline PWL plus 1 standard deviation for all 50 rats]), the time to return to normal motor function (time period at which the paw was seen to be normal, motor score = 0), and the RoRR (time period in minutes after discontinuation of isoflurane to the rat turning from a supine to prone position).28 Post-hoc tests were used to compare PWL measures at different drug doses, with a Bonferroni correction for multiple comparisons (α = 0.005 was used for each of the 10 post-hoc comparisons). A repeated-measures ANOVA evaluated the effects of dose, time, and dose by time on PWL of the operative and control paws. Post-hoc tests were completed for between group comparisons at time points 90-210 min using a Bonferroni test for multiple comparisons (α = 0.005 was used for each of the 10 post-hoc comparisons).
Results
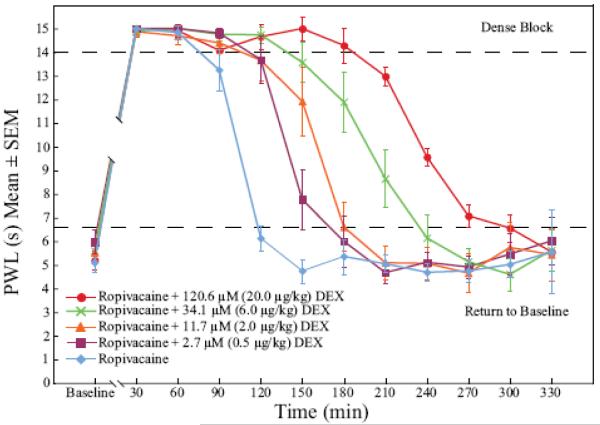
Dexmedetomidine added to ropivacaine increased the duration of analgesia to a heat stimulus in a dose-dependent fashion (fig. 1). The duration of dense sensory blockade (defined as PWL ≥ 14 s) was increased in a dose-dependent fashion when the ropivacaine group was compared with all dexmedetomidine groups (p < 0.005). Dense sensory blockade was significantly longer when highest dose dexmedetomidine group (120.6 μM) was compared with all other dexmedetomidine groups (p < 0.005). The time to return to baseline sensory function (defined as PWL < 6.59 s) was significantly longer in the 11.7, 34.1, and 120.6 μM dexmedetomidine groups when compared with ropivacaine alone (p < 0.005). Intergroup increases in time to return to normal sensory function were also seen when 120.6 μM was compared with 2.7 and 11.7 μM dexmedetomidine (p < 0.0001 and p = 0.0006 respectively) and when 34.1 μM was compared with 2.7 μM dexmedetomidine (p = 0.0005).
Figure 1.
Dexmedetomidine added to ropivacaine enhanced the duration of dense sensory blockade (p < 0.005) and time to return to normal sensory function (p < 0.005) in response to a thermal stimulus in a dose-dependent fashion when compared to the control group, ropivacaine alone. The graph shows the time-course of paw withdrawal latency values of the baseline taken 24 h before surgery (Baseline; mean value of all rats = 5.46 ± 1.13 seconds) and at 30-min time-points after the sciatic nerve block. DEX = dexmedetomidine; PWL = paw withdrawal latency.
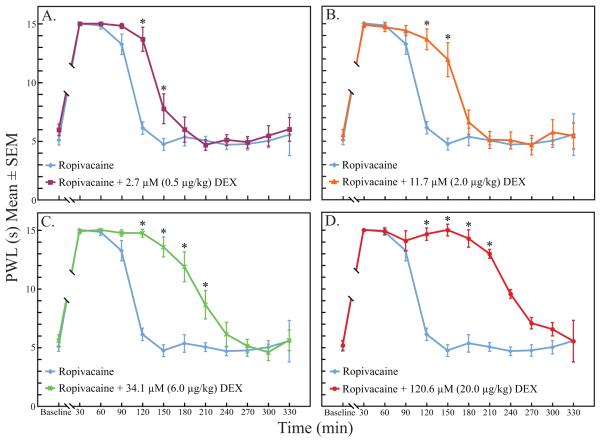
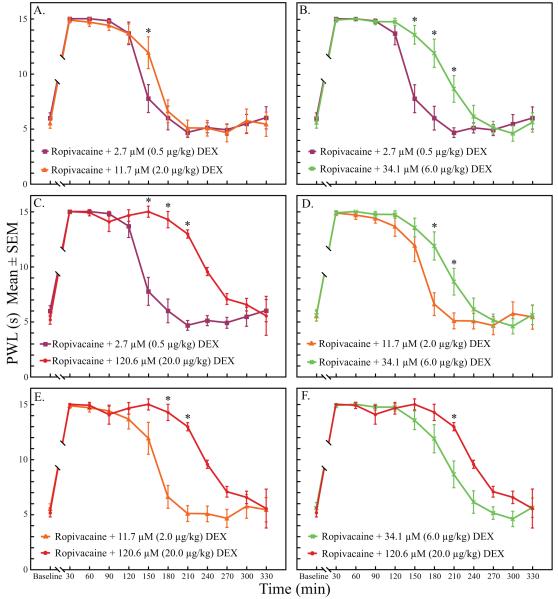
There was a significant time (p < 0.005), dose (p < 0.005), and time by dose effect (p < 0.005) on the PWL of the operative paws. There were missing PWL measurements at time points 30 and 60 min in some groups due to increased righting times (See RoRR results below; Results section, paragraph 5). Missing PWL measures also occurred after 240 min due to a return to normal sensory function in some rats. Therefore, intergroup analyses by time points were restricted to the 90-210 min interval (table 2). PWL at time points 120 and 150 min were significantly longer in all dexmedetomidine groups when compared with the ropivacaine group (p < 0.005, fig. 2). At time points 180 and 210 min, PWL measures in the two highest dexmedetomidine groups (34.1 and 120.6 μM) were significantly longer than when ropivacaine was administered alone (p < 0.005, fig. 2). There were also multiple time point differences when comparing between the dexmedetomidine groups (p < 0.005, fig. 3).
Table 2.
Paw withdrawal latency values at time points 90-210 min.
| 90 min | 120 min | 150 min | 180 min | 210 min | |
|---|---|---|---|---|---|
|
Ropivacaine Mean PWL values (sec) |
13.25 | 6.13 | 4.74 | 5.35 | 5.05 |
| SEM | 0.87 | 0.53 | 0.50 | 0.74 | 0.38 |
|
Ropivacaine + 2.7 μM (0.5 μg/kg) DEX Mean PWL values (sec) |
14.81 | 13.68 | 7.76 | 5.99 | 4.67 |
| SEM | 0.22 | 1.02 | 1.27 | 1.07 | 0.44 |
|
Ropivacaine + 11.7 μM (2.0 μg/kg) DEX Mean PWL values (sec) |
14.40 | 13.66 | 11.93 | 6.60 | 5.09 |
| SEM | 0.44 | 0.88 | 1.45 | 1.04 | 0.72 |
|
Ropivacaine + 34.1 μM (6.0 μg/kg) DEX Mean PWL values (sec) |
14.75 | 14.74 | 13.56 | 11.89 | 8.65 |
| SEM | 0.27 | 0.34 | 0.85 | 1.28 | 1.21 |
|
Ropivacaine + 120.6 μM (20.0 μg/kg) DEX Mean PWL values (sec) |
14.08 | 14.65 | 15.00 | 14.28 | 12.98 |
| SEM | 0.73 | 0.38 | 0.00 | 0.71 | 1.21 |
Figure 2A-D.
Increasing doses of dexmedetomidine prolonged the duration of paw withdrawal latency to a heat stimulus when compared to the ropivacaine control group. Between group comparisons from 90-210 min found multiple significant differences at individual time points. DEX = dexmedetomidine; PWL = paw withdrawal latency.
* indicates statistical significance, p < 0.005.
Figure 3A-F.
Between group comparisons from 90-210 min for the dexmedetomidine groups also found multiple significant differences at individual time points. DEX = dexmedetomidine; PWL = paw withdrawal latency.
* indicates statistical significance, p < 0.005.
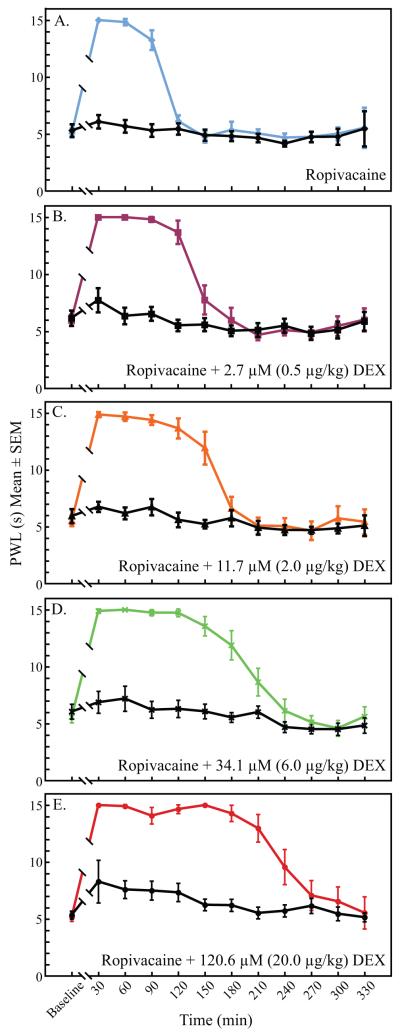
All rats showed significantly longer PWL measurements for the operative paw when compared with the control paw (fig. 4). PWL for control paws between all groups were also analyzed between 90-210 min. The highest dose dexmedetomidine group (120.6 μM) had significantly longer PWL of the control paw at 90 min when compared with the ropivacaine control (p = 0.0016). Although the mean PWL for the control paws in all of the dexmedetomidine groups were higher than the ropivacaine group, there were no other significant differences in the PWL of the control paws between groups.
Figure 4A-E.
Paw withdrawal latency values of the operative paw (color) versus the unblocked control paw (black) for each drug dose. The analgesic effects of ropivacaine (A) and ropivacaine plus different doses of dexmedetomidine (B-E) were significantly greater in the operative paws. There was little systemic analgesic effect of dexmedetomidine as measured by paw withdrawal latencies of the unblocked control paws. DEX = dexmedetomidine; PWL = paw withdrawal latency.
Motor blockade was significantly longer in all dexmedetomidine groups compared with ropivacaine alone (p < 0.005, fig. 5). Intergroup increases in time to return to normal motor function was seen when the 120.6 μM dexmedetomidine group was compared with 2.7 and 11.7 μM groups (p = 0.0006 and p = 0.0018, respectively). Otherwise, there were no significant differences in the duration of motor blockade between the different dexmedetomidine groups.
Figure 5.
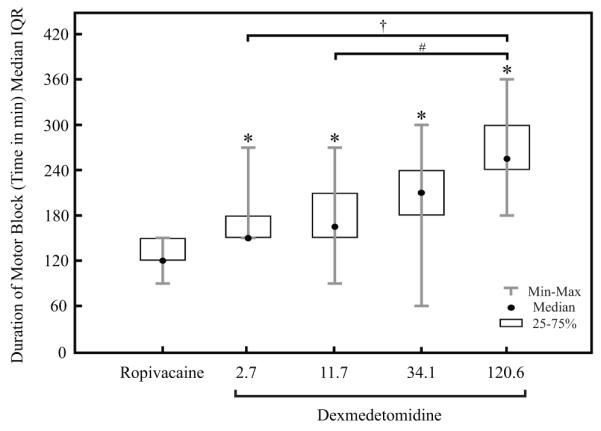
There was a direct increase in the duration of motor blockade with increasing doses of dexmedetomidine added to ropivacaine for sciatic nerve blockade. A motor block was identified by the observation of a curled paw (motor score = 1). A return to normal paw posture was given a motor score of 0.
*All doses containing dexmedetomidine were significantly different from ropivacaine administered alone (p < 0.005). † and # indicate significant differences between the highest dose dexmedetomidine group and the 2.7 μM (0.5 μg/kg) and 11.7 μM (2.0 μg/kg) groups, respectively (p < 0.0001 and p = 0.0006). IQR = Interquartile Range.
There were no between group differences in the total anesthesia times or isoflurane levels (p = 0.24) for anesthesia maintenance (p = 0.31). There were no differences in RoRR times between the ropivacaine group and the 2.7, 11.7 and 34.1 μM dexmedetomidine groups. The 120.6 μM dexmedetomidine group had significantly longer RoRR times when compared with all other groups (p < 0.005, fig. 6).
Figure 6.
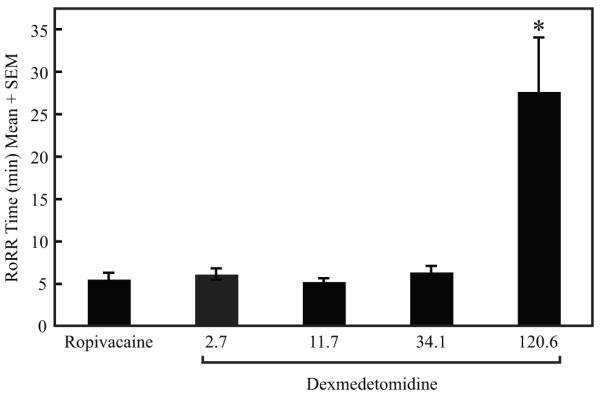
The highest dose of dexmedetomidine (120.6 μM [20 μg/kg]) studied caused sedative effects immediately following anesthesia that prolonged the time to resumption of righting reflex (RoRR) when compared with all other groups (p < 0.005). There were no other significant differences in RoRR. Total anesthesia time and percent isoflurane were not different between groups (Data not shown).
* indicates statistical significance, p < 0.005.
Histopathologic analysis revealed normal axons and myelin in all six of the nerves analyzed in the 120.6 μM dexmedetomidine group (Patholgy score = 0, no nerve lesions). The three nerves analyzed at 24 h showed mild to moderate, locally extensive to diffuse, perineural congestion and lymphocytic/plasmacytic inflammatory cell infiltrates (Inflammation score = 1-2). The pathology did not, however, extend to the nerves. There was no significant perineural inflammation in the four nerves evaluated at 14 days (Inflammation score = 0).
Discussion
This is the first study showing that dexmedetomidine added to ropivacaine increases the duration of sensory motor blockade to a thermal stimulus in rat. The time of dense sensory blockade and time to recovery of normal sensory function were increased in a dose-dependent manner with progressively higher doses of dexmedetomidine (fig. 1). At multiple time points between 90-210 min, there were significant differences between the ropivacaine control group and all dexmedetomidine groups (fig. 2), as well as differences between the different dexmedetomidine doses (fig. 3).
The present study indicates that clinically relevant doses of dexmedetomidine (2.7, 11.7 and 34.1 μM [0.5, 2.0, and 6.0 μg/kg, respectively]) enhanced blockade when added to ropivacaine. Previous work showed enhanced sensory and motor blockade when high-dose dexmedetomidine (211.2 μM [28-40 μg/kg]) was added to bupivacaine in sciatic nerve blocks in rat.12 The US Food and Drug Administration approved dose for intravenous sedation of mechanically ventilated patients in the intensive care unit is a bolus dose of dexmedetomidine (1 μg/kg) over 10 min, followed by an infusion of 0.2-0.7 μg/kg/hr.γ Significantly higher intravenous infusion doses have been described without ill effect.29 Although it is not possible to predict the potential systemic absorption of dexmedetomidine from the perineural space, the doses used in the present study approach approved intravenous doses and would not likely have significant systemic side effects.
Dexmedetomidine provides analgesia and sedation without respiratory depression when given intravenously,29-32 and the centrally mediated analgesia and sedation could alter sensory perception. Unlike the previous study in which all rats received bilateral sciatic nerve blocks with either bupivacaine alone or bupivacaine plus dexmedetomidine,12 rats in the present study displayed unilateral blocks with an unblocked control paw, providing an index of systemic analgesia (fig. 4). The data show that the effects of dexmedetomidine were predominately at the peripheral nerve level (fig. 4). The highest dose of dexmedetomidine (120.6 μM [20 μg/kg]) had the greatest systemic effects with significantly longer RoRR (Fig. 6). RoRR times in other dexmedetomidine groups (2.7, 11.7 and 34.1 μM [0.5, 2.0, and 6.0 μg/kg, respectively]), however, were not significantly different when compared with the ropivacaine control group.
The duration of motor blockade was also increased in the dexmedetomidine groups when compared with ropivacaine (fig. 5). Median times for return to normal motor function were higher with increasing doses of dexmedetomidine, however, this was only significant with the highest dose of dexmedetomidine (120.6 μM [20 μg/kg]). Otherwise, there were no significant differences in motor function between the (2.7, 11.7 and 34.1 μM [0.5, 2.0, and 6.0 μg/kg, respectively]) doses of dexmedetomidine.
The efficacy of clonidine, another ∝2-adrenoceptor agonist, added to local anesthetics in peripheral nerve blocks in humans has been described,10,11 along with a demonstrated mechanism of action. Clonidine enhances activity-dependent hyperpolarization by inhibiting the Ih current.33-36 In the central and peripheral nervous systems, the Ih current plays a key role in cell excitability, especially the firing frequency.37 The Ih current is activated during the hyperpolarization phase of an action potential and normally acts to reset a nerve for subsequent action potentials. Therefore, by blocking the Ih current, clonidine enhances hyperpolarization and inhibits subsequent action potentials. Whether the effects of dexmedetomidine are similar to those of clonidine can be addressed by future investigation. Clonidine was found to have a concentration-dependent inhibition on A∝ and C fiber compound action potentials in an in vitro rat sciatic nerve model.33 Clinical studies adding clonidine to mepivacaine found 0.5 μg/kg as the optimal dose for anesthesia and analgesia when compared with 0.1, 0.2, 0.3, 0.4, 1.0, and 1.5 μg/kg.38 There are no available data comparing clonidine and dexmedetomidine in a perineural model. Whether higher doses of dexmedetomidine will safely enhance postoperative analgesia in humans is yet to be determined.
The use of dexmedetomidine in the perineural space is not approved by the US Food and Drug Administration and has never been reported in humans. Although there was no neurotoxicity noted at 24 h or 14 days in the seven nerves in the high-dose dexmedetomidine group (120.6 μM [20 μg/kg]), an Investigational New Drug application must be approved by the Food and Drug Administration prior to use in humans in the United States. The present report does not advocate human administration of dexmedetomidine in the perineural space without prior approval from the US Food and Drug Administration. Previous preclinical data showed no neurotoxicity caused by high-dose dexmedetomidine administered alone or when combined with approved concentrations of bupivacaine in sciatic nerve blocks in rat.12 The combination of dexmedetomidine with bupivacaine was associated with significantly less perineural inflammation at 24 h when compared with bupivacaine alone. These findings were consistent with the antiinflammatory properties of clonidine found in previous work.39-42 Dexmedetomidine is associated with hypotension and bradycardia,43 and its future use in patients with significant cardiovascular disease or prone to hypotension would be cautioned against.
Although ropivacaine and bupivacaine are both long-acting local anesthetics, ropivacaine has unique pharmacologic properties and has replaced bupivacaine for peripheral nerve blocks in many institutions throughout the world. The predominate reason for the change is the belief that ropivacaine is more likely to respond to resuscitation efforts in the event of cardiac arrest from intravascular injection when compared with bupivacaine.13-16 In addition to a likely safer cardiac profile, some studies have shown that ropivacaine is associated with less motor blockade when compared with bupivacaine.44-47 Improved motor function while maintaining analgesia allows patients to participate in physical therapy and improves postoperative function. Selectivity for C-and A-delta fibers compared with A-alpha fibers has been demonstrated with clonidine.33,48 Whether the same is true with dexmedetomidine is not yet determined; however, the combination of dexmedetomidine and ropivacaine may prove to have a favorable relative sensory to motor blockade.
Limitations
The primary outcome measure of this study was limited to latency of paw withdraw to a thermal stimulus. The results encourage future studies aiming to determine the degree to which increasing doses of dexmedetomidine alter responses to other nociceptive modalities. The assessment of motor blockade used was limited to a subjective assessment of the intrinsic paw musculature based on paw positioning and toe curling. Whereas a similar subjective measure has been previously described,12,24,25 it does not provide objective data concerning motor blockade. The paw thrust measures that have been used in similar studies21-23 are dependent upon the positioning and level of consciousness of the rat and are also subject to variability. The value and practice of motor assessment using paw thrust against a measured balance is, therefore questioned by some investigators. In order to obtain accurate and consistent PWL measures, it was essential that the rat not be removed from its PWL chamber. Rats were acclimated to a specific chamber with the same rats in neighboring chambers. Any perturbance to allow for a better motor assessment would have drastically altered the sensory measures. We acknowledge that the relative degree of motor to sensory blockade is clinically significant, and future studies will address the impact of dexmedetomidine on motor blockade.
Conclusion
Dexmedetomidine added to ropivacaine increased the duration of sensory blockade to a heat stimulus in rat in a dose-dependent fashion. Increasing doses of dexmedetomidine were associated with longer times of dense sensory blockade and time to return of normal sensory function. Nociceptive testing of the control paw revealed no significant change in PWL caused by dexmedetomidine. This finding supports the interpretation that the analgesic effects of dexmedetomidine on the operated paw resulted from actions at the level of the sciatic nerve. The finding that clinically relevant doses of dexmedetomidine added to ropivacaine enhance peripheral nerve blocks in rat encourages future studies designed to determine whether dexmedetomidine added to ropivacaine can prolong peripheral nerve blocks in humans.
Summary Statement: Dexmedetomidine (2.7, 11.7, 34.1, or 120.6 μM) added to ropivacaine increased the duration of sensory blockade in a dose-dependent fashion in sciatic nerve block in rat. Dexmedetomidine did not provide analgesia to the unblocked paw.
Acknowledgements
We thank Kevin K. Tremper, M.D., Ph.D. (Professor, Department of Anesthesiology, University of Michigan, Ann Arbor, Michigan) for support and guidance. For technical support, we thank members of research laboratory at the University of Michigan, Department of Anesthesiology: Allison M. Janda, Student at Bucknell University (Research Assistant), Mary Norat, B.S. (Laboratory Manager), Christopher Watson, Ph.D. (Postdoctoral Fellow), Sarah Watson, B.S. (Research Associate), and Courtney R. Baracy, B.S. (Research Assistant).
Financial Support: Supported by the Department of Anesthesiology, University of Michigan, Ann Arbor, Michigan. Ralph Lydic, Ph.D. is supported by National Institutes of Health grants HL-40881 and HL-57120 (Bethesda, Maryland).
Footnotes
National Academies Press, Washington D.C., 1996, http://www.csupomona.edu/~research/acuc/docs/GuidetoUseCareLabAnimals.pdf, Last date accessed March 26, 2009
National Academies Press, Washington, D.C., 2003, www.national-academies.org/ilar, Last date accessed March 26, 2009
Dexmedetomidine summary, US Food and Drug Administration, http://www.fda.gov/cder/foi/label/1999/21038lbl.pdf, Last accessed on 2/28/09
Study performed at: Department of Anesthesiology, University of Michigan, Ann Arbor, Michigan
These data have not been previously been presented in any format.
Disclosures: The University of Michigan has filed for a patent application covering the subject matter of this publication.
References
- 1.Casati A, Fanelli G, Albertin A, Deni F, Anelati D, Antonino FA, Beccaria P. Interscalene brachial plexus anesthesia with either 0.5% ropivacaine or 0.5% bupivacaine. Minerva Anestesiol. 2000;66:39–44. [PubMed] [Google Scholar]
- 2.Hickey R, Hoffman J, Ramamurthy S. A comparison of ropivacaine 0.5% and bupivacaine 0.5% for brachial plexus block. Anesthesiology. 1991;74:639–42. doi: 10.1097/00000542-199104000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Hickey R, Rowley CL, Candido KD, Hoffman J, Ramamurthy S, Winnie AP. A comparative study of 0.25% ropivacaine and 0.25% bupivacaine for brachial plexus block. Anesth Analg. 1992;75:602–6. doi: 10.1213/00000539-199210000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Vaghadia H, Chan V, Ganapathy S, Lui A, McKenna J, Zimmer K. A multicentre trial of ropivacaine 7.5 mg × ml(−1) vs bupivacaine 5 mg × ml(−1) for supra clavicular brachial plexus anesthesia. Can J Anaesth. 1999;46:946–51. doi: 10.1007/BF03013129. [DOI] [PubMed] [Google Scholar]
- 5.Lydic R, Baghdoyan HA. In: Neurochemical mechanisms mediating opioid-induced REM sleep disruption, Sleep and Pain. Lavigne G, Sessle BJ, Choinire M, Soja PJ, editors. Internation Association for the Study of Pain (IASP) Press; Seattle: 2007. pp. 99–122. [Google Scholar]
- 6.Bonafide CP, Aucutt-Walter N, Divittore N, King T, Bixler EO, Cronin AJ. Remifentanil inhibits rapid eye movement sleep but not the nocturnal melatonin surge in humans. Anesthesiology. 2008;108:627–33. doi: 10.1097/ALN.0b013e3181684bc3. [DOI] [PubMed] [Google Scholar]
- 7.Bowdle TA. Nocturnal arterial oxygen desaturation and episodic airway obstruction after ambulatory surgery. Anesth Analg. 2004;99:70–6. doi: 10.1213/01.ANE.0000125113.03812.2A. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg J, Rosenberg-Adamsen S, Kehlet H. Post-operative sleep disturbance: Causes, factors and effects on outcome. Eur J Anaesthesiol Suppl. 1995;10:28–30. [PubMed] [Google Scholar]
- 9.Eisenach JC, De Kock M, Klimscha W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995) Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Murphy DB, McCartney CJ, Chan VW. Novel analgesic adjuncts for brachial plexus block: A systematic review. Anesth Analg. 2000;90:1122–8. doi: 10.1097/00000539-200005000-00023. [DOI] [PubMed] [Google Scholar]
- 11.McCartney CJ, Duggan E, Apatu E. Should we add clonidine to local anesthetic for peripheral nerve blockade? A qualitative systematic review of the literature. Reg Anesth Pain Med. 2007;32:330–8. doi: 10.1016/j.rapm.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–11. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chazalon P, Tourtier JP, Villevielle T, Giraud D, Saissy JM, Mion G, Benhamou D. Ropivacaine-induced cardiac arrest after peripheral nerve block: Successful resuscitation. Anesthesiology. 2003;99:1449–51. doi: 10.1097/00000542-200312000-00030. [DOI] [PubMed] [Google Scholar]
- 14.Polley LS, Santos AC. Cardiac arrest following regional anesthesia with ropivacaine: Here we go again! Anesthesiology. 2003;99:1253–4. doi: 10.1097/00000542-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Ohmura S, Kawada M, Ohta T, Yamamoto K, Kobayashi T. Systemic toxicity and resuscitation in bupivacaine-, levobupivacaine-, or ropivacaine-infused rats. Anesth Analg. 2001;93:743–8. doi: 10.1097/00000539-200109000-00039. [DOI] [PubMed] [Google Scholar]
- 16.Klein SM, Pierce T, Rubin Y, Nielsen KC, Steele SM. Successful resuscitation after ropivacaine-induced ventricular fibrillation. Anesth Analg. 2003;97:901–3. doi: 10.1213/01.ANE.0000075839.18073.37. [DOI] [PubMed] [Google Scholar]
- 17.Cederholm I, Evers H, Lofstrom JB. Skin blood flow after intradermal injection of ropivacaine in various concentrations with and without epinephrine evaluated by laser Doppler flowmetry. Reg Anesth. 1992;17:322–8. [PubMed] [Google Scholar]
- 18.Dahl JB, Simonsen L, Mogensen T, Henriksen JH, Kehlet H. The effect of 0.5% ropivacaine on epidural blood flow. Acta Anaesthesiol Scand. 1990;34:308–10. doi: 10.1111/j.1399-6576.1990.tb03092.x. [DOI] [PubMed] [Google Scholar]
- 19.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 20.Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods. 1997;76:183–91. doi: 10.1016/s0165-0270(97)00097-6. [DOI] [PubMed] [Google Scholar]
- 21.Gerner P, Luo SH, Zhuang ZY, Djalali AG, Zizza AM, Myers RR, Wang GK. Differential block of N-propyl derivatives of amitriptyline and doxepin for sciatic nerve block in rats. Reg Anesth Pain Med. 2005;30:344–50. doi: 10.1016/j.rapm.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Hung YC, Kau YC, Zizza AM, Edrich T, Zurakowski D, Myers RR, Wang GK, Gerner P. Ephedrine blocks rat sciatic nerve in vivo and sodium channels in vitro. Anesthesiology. 2005;103:1246–52. doi: 10.1097/00000542-200512000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Kau YC, Hung YC, Zizza AM, Zurakowski D, Greco WR, Wang GK, Gerner P. Efficacy of lidocaine or bupivacaine combined with ephedrine in rat sciatic nerve block. Reg Anesth Pain Med. 2006;31:14–8. doi: 10.1016/j.rapm.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Dyhre H, Soderberg L, Bjorkman S, Carlsson C. Local anesthetics in lipid-depot formulations--neurotoxicity in relation to duration of effect in a rat model. Reg Anesth Pain Med. 2006;31:401–8. doi: 10.1016/j.rapm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Soderberg L, Dyhre H, Roth B, Bjorkman S. Ultralong peripheral nerve block by lidocaine:prilocaine 1:1 mixture in a lipid depot formulation: Comparison of in vitro, in vivo, and effect kinetics. Anesthesiology. 2006;104:110–21. doi: 10.1097/00000542-200601000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Kytta J, Heinonen E, Rosenberg PH, Wahlstrom T, Gripenberg J, Huopaniemi T. Effects of repeated bupivacaine administration on sciatic nerve and surrounding muscle tissue in rats. Acta Anaesthesiol Scand. 1986;30:625–9. doi: 10.1111/j.1399-6576.1986.tb02488.x. [DOI] [PubMed] [Google Scholar]
- 27.Pere P, Watanabe H, Pitkanen M, Wahlstrom T, Rosenberg PH. Local myotoxicity of bupivacaine in rabbits after continuous supraclavicular brachial plexus block. Reg Anesth. 1993;18:304–7. [PubMed] [Google Scholar]
- 28.Allison PD. In: Estimating Cox Regression Models with PROC PHREG, Survival Analysis Using SAS: A Practical Guide. Allison PD, Cary ND, editors. SAS Publishing; 1995. pp. 111–84. [Google Scholar]
- 29.Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004;101:787–90. doi: 10.1097/00000542-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Martin E, Ramsay G, Mantz J, Sum-Ping ST. The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J Intensive Care Med. 2003;18:29–41. doi: 10.1177/0885066602239122. [DOI] [PubMed] [Google Scholar]
- 31.Ramsay MA, Saha D, Hebeler RF. Tracheal resection in the morbidly obese patient: The role of dexmedetomidine. J Clin Anesth. 2006;18:452–4. doi: 10.1016/j.jclinane.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: Patient and clinician perceptions. Br J Anaesth. 2001;87:684–90. doi: 10.1093/bja/87.5.684. [DOI] [PubMed] [Google Scholar]
- 33.Butterworth JF, Strichartz GR. The alpha 2-adrenergic agonists clonidine and guanfacine produce tonic and phasic block of conduction in rat sciatic nerve fibers. Anesth Analg. 1993;76:295–301. [PubMed] [Google Scholar]
- 34.Dalle C, Schneider M, Clergue F, Bretton C, Jirounek P. Inhibition of the I(h) current in isolated peripheral nerve: a novel mode of peripheral antinociception? Muscle Nerve. 2001;24:254–61. doi: 10.1002/1097-4598(200102)24:2<254::aid-mus110>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Gaumann DM, Brunet PC, Jirounek P. Hyperpolarizing afterpotentials in C fibers and local anesthetic effects of clonidine and lidocaine. Pharmacology. 1994;48:21–9. doi: 10.1159/000139158. [DOI] [PubMed] [Google Scholar]
- 36.Starke K, Wagner J, Schumann HJ. Adrenergic neuron blockade by clonidine: Comparison with guanethidine and local anesthetics. Arch Int Pharmacodyn Ther. 1972;195:291–308. [PubMed] [Google Scholar]
- 37.Pape HC. Queer current and pacemaker: The hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- 38.Singelyn FJ, Gouverneur JM, Robert A. A minimum dose of clonidine added to mepivacaine prolongs the duration of anesthesia and analgesia after axillary brachial plexus block. Anesth Analg. 1996;83:1046–50. doi: 10.1097/00000539-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 39.Lavand’homme PM, Eisenach JC. Perioperative administration of the alpha2-adrenoceptor agonist clonidine at the site of nerve injury reduces the development of mechanical hypersensitivity and modulates local cytokine expression. Pain. 2003;105:247–54. doi: 10.1016/s0304-3959(03)00221-5. [DOI] [PubMed] [Google Scholar]
- 40.Romero-Sandoval A, Eisenach JC. Perineural clonidine reduces mechanical hypersensitivity and cytokine production in established nerve injury. Anesthesiology. 2006;104:351–5. doi: 10.1097/00000542-200602000-00022. [DOI] [PubMed] [Google Scholar]
- 41.Romero-Sandoval A, Eisenach JC. Clonidine reduces hypersensitivity and alters the balance of pro- and anti-inflammatory leukocytes after local injection at the site of inflammatory neuritis. Brain Behav Immun. 2007;21:569–80. doi: 10.1016/j.bbi.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero-Sandoval EA, McCall C, Eisenach JC. Alpha2-adrenoceptor stimulation transforms immune responses in neuritis and blocks neuritis-induced pain. J Neurosci. 2005;25:8988–94. doi: 10.1523/JNEUROSCI.2995-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: A review of clinical applications. Curr Opin Anaesthesiol. 2008;21:457–61. doi: 10.1097/ACO.0b013e328305e3ef. [DOI] [PubMed] [Google Scholar]
- 44.Dyhre H, Lang M, Wallin R, Renck H. The duration of action of bupivacaine, levobupivacaine, ropivacaine and pethidine in peripheral nerve block in the rat. Acta Anaesthesiol Scand. 1997;41:1346–52. doi: 10.1111/j.1399-6576.1997.tb04656.x. [DOI] [PubMed] [Google Scholar]
- 45.Simpson D, Curran MP, Oldfield V, Keating GM. Ropivacaine: A review of its use in regional anaesthesia and acute pain management. Drugs. 2005;65:2675–717. doi: 10.2165/00003495-200565180-00013. [DOI] [PubMed] [Google Scholar]
- 46.Hansen TG. Ropivacaine: A pharmacological review. Expert Rev Neurother. 2004;4:781–91. doi: 10.1586/14737175.4.5.781. [DOI] [PubMed] [Google Scholar]
- 47.Zink W, Graf BM. Benefit-risk assessment of ropivacaine in the management of postoperative pain. Drug Saf. 2004;27:1093–114. doi: 10.2165/00002018-200427140-00003. [DOI] [PubMed] [Google Scholar]
- 48.Gaumann DM, Brunet PC, Jirounek P. Clonidine enhances the effects of lidocaine on C-fiber action potential. Anesth Analg. 1992;74:719–25. doi: 10.1213/00000539-199205000-00017. [DOI] [PubMed] [Google Scholar]