Abstract
Activation of the DNA damage checkpoint causes a cell-cycle arrest through inhibition of cyclin-dependent kinases (cdks). To successfully recover from the arrest, a cell should somehow be maintained in its proper cell-cycle phase. This problem is particularly eminent when a cell arrests in G2, as cdk activity is important to establish a G2 state. Here, we identify the phosphatase Wip1 (PPM1D) as a factor that maintains a cell competent for cell-cycle re-entry during an ongoing DNA damage response in G2. We show that Wip1 function is required throughout the arrest, and that Wip1 acts by antagonizing p53-dependent repression of crucial mitotic inducers, such as Cyclin B and Plk1. Our data show that the primary function of Wip1 is to retain cellular competence to divide, rather than to silence the checkpoint to promote recovery. Our findings uncover Wip1 as a first in class recovery competence gene, and suggest that the principal function of Wip1 in cellular transformation is to retain proliferative capacity in the face of oncogene-induced stress.
Keywords: checkpoint, DNA damage, recovery, Wip1
Introduction
Cyclin–Cdk activity is low in G1 cells and gradually increases until it culminates in mitosis, constituting the basis of a eukaryotic cell-cycle oscillator. The gradual rise in Cyclin–Cdk activity promotes cell-cycle progression by ensuring sequential phosphorylation of targets that enable events such as DNA replication and entry into mitosis. The Cyclin–Cdk complexes themselves largely create this gradual rise in Cyclin–Cdk activity, as Cyclin–Cdk-dependent feedback and feedforward loops ensure production and activation of more and different Cyclin–Cdk complexes (Murray, 2004; Guardavaccaro and Pagano, 2006). In this sense, rising Cyclin–Cdk activity not only promotes cell-cycle progression, but also defines the biochemical cell-cycle state. Once a Cyclin–Cdk has been activated, the positive feedback loops enforce its activity, ensuring that the cell-cycle state is maintained. For example in G2 phase, Cyclin A–Cdk activity ensures transcription of targets that promote activation of Cyclin B–Cdk1, but also of targets promoting and maintaining Cyclin A–Cdk activity, thereby defining and maintaining a G2 state (Fung and Poon, 2005; Lindqvist et al, 2009).
Activation of the DNA damage checkpoint in G2 by ATM and ATR kinases culminates in activation of the tumour suppressor protein p53 and inhibition of the Cyclin–Cdk complexes that are essential for mitotic entry (Smits and Medema, 2001). As a consequence, cells arrest in G2, with high levels of p53 and inactive Cyclin B–Cdk1 and Cyclin A–Cdk1/2 complexes (Smits et al, 2000; Bassermann et al, 2008; Toettcher et al, 2009). In addition, the checkpoint can reduce the transcription of a large number of G2 genes through the actions of p53 and Chk1 (Taylor and Stark, 2001; Tabach et al, 2005; Spurgers et al, 2006; Jurvansuu et al, 2007; Shimada et al, 2008). Thus, the DNA damage checkpoint counteracts the cell-cycle oscillator through direct Cyclin–Cdk inhibition, but also by counteracting the transcriptional program that defines and maintains the G2 state (Toettcher et al, 2009). The question therefore arises how a cell can maintain a G2 state during an ongoing checkpoint arrest and retain competence to resume further activation of the cell-cycle oscillator to complete cell division once the checkpoint is switched off.
When DNA double-stranded breaks (DSBs) occur, the checkpoint kinase ATM (ataxia-telangiectasia mutated protein) phosphorylates both p53 and Mdm2 (Banin et al, 1998; Canman et al, 1998; Khosravi et al, 1999), resulting in stabilization of p53. Interestingly, p53 levels were shown to oscillate in response to DSB formation (Lev Bar-Or et al, 2000; Lahav et al, 2004), and these pulses were recently shown to depend on the presence of the phosphatase Wip1 (PPM1D) (Batchelor et al, 2008). Wip1 is a transcriptional target of p53 (Fiscella et al, 1997) that can antagonize ATM-dependent phosphorylation of p53 (Lu et al, 2005) and Mdm2 (Lu et al, 2007). In addition, Wip1 can dephosphorylate ATM (Shreeram et al, 2006a), as well as other components of the DNA damage checkpoint, such as p38 (Takekawa et al, 2000), Chk2 (Fujimoto et al, 2006; Oliva-Trastoy et al, 2007), Uracil DNA-glycosylase (Lu et al, 2004) and Chk1 (Lu et al, 2005). In fact, the Wip1 and the ATM consensus sequences appear to be similar, indicating that Wip1 may dephosphorylate additional proteins involved in checkpoint signalling (Yamaguchi et al, 2007). This suggests that the activation of Wip1 creates an important negative feedback mechanism that controls reversion of the DNA damage-induced arrest when the DNA damage-induced signalling cascades have to be inactivated. However, the importance of p53 pulses is currently unclear. Also, it is not known if the crucial function of Wip1 during a DNA damage response is executed during termination of the response, that is by inactivating its various targets in the DNA damage-induced signalling cascade, or rather during the ongoing arrest, by controlling p53 oscillations.
Here, we show that Wip1 is required throughout a DNA damage response in G2 to allow for eventual checkpoint recovery. Contrary to our expectations, Wip1 does not promote recovery by mere dephosphorylation of checkpoint kinases when the checkpoint is silenced, but rather exerts its effect on recovery already at earlier stages of the checkpoint response. We find that Wip1 counteracts p53-dependent repression of mitotic regulators throughout the DNA damage response in G2. This action of Wip1 is essential to retain the identity of a G2 cell and maintain its competence for further proliferation.
Results
Wip1 is required for checkpoint recovery
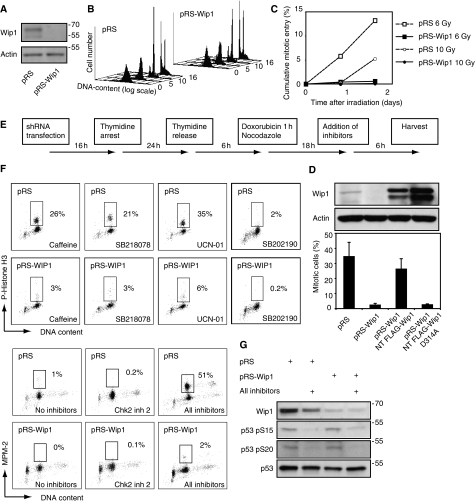
To test whether Wip1 regulates recovery from a DNA damage-induced arrest in G2, we depleted endogenous Wip1 by shRNA (Figure 1A). shRNA-mediated depletion of Wip1 only marginally affected normal kinetics of cell division (Figure 1B), indicating that Wip1 is not essential for progression through an unperturbed cell cycle. However, when Wip1-depleted cells were arrested in G2 by γ-irradiation, checkpoint recovery was strongly inhibited compared with control cells (Figure 1C). Checkpoint recovery was also inhibited when Wip1-depleted G2 cells were arrested with the DNA damaging agent doxorubicin, and subsequently stimulated to re-enter the cell cycle by treatment with caffeine, an inhibitor of ATM/ATR (Figure 1D). Similar results were obtained with another shRNA sequence (data not shown). Importantly, this phenotype could be reverted by reconstituting the cells with RNAi-insensitive FLAG-Wip1 (Figure 1D), indicating that the recovery defect is due to specific depletion of the Wip1 protein. However, rendering the RNAi-insensitive Wip1 phosphatase-deficient by mutation of D314 to alanine (Takekawa et al, 2000) resulted in a failure to revert the Wip1 RNAi phenotype, indicating that phosphatase activity of Wip1 is important for recovery from a DNA damage checkpoint (Figure 1D).
Figure 1.
Wip1 is essential for checkpoint recovery. (A) Wip1 RNAi efficiently reduces the levels of endogenous Wip1. U2OS cells, transfected with empty pRS or pRS-Wip1, were selected with puromycin and subjected to immunoblotting with the indicated antibodies. (B) Wip1 RNAi cells progress through S, G2 and M-phases in the absence of DNA-damage. U2OS cells co-transfected with GFP-Spectrin and pRS-Wip1 or empty pRS were synchronized in S-phase with a single 24 h thymidine block. The cell-cycle profile of GFP-expressing cells was assessed by FACS at different time-points after release. Y-axis: number of cells, x-axis: DNA-content (logarithmic scale), z-axis: time after thymidine release (hours). (C) Wip1 RNAi inhibits checkpoint recovery. Cells were treated as in (B), but subjected to 6 or 10 Gy γ-irradiation 7 h after release from the thymidine block. Immediately after irradiation or after 24 h incubation, taxol was added for 24 h before harvesting. The amount of mitotic GFP-positive cells was assessed by MPM-2 positivity using FACS. (D) Wip1 RNAi inhibits caffeine-induced checkpoint recovery. U2OS cells were co-transfected with GFP-spectrin and pRS, pRS-Wip1, pRS-Wip1 + RNAi insensitive FLAG-Wip1, or pRS-Wip1 + RNAi-insensitive phosphatase dead FLAG-Wip1 (D314A) and synchronized by a single 24 h thymidine block. Six hours after thymidine synchronization, cells were treated with the topoisomeraseII inhibitor doxorubicin, and nocodazole was added to trap cells entering mitosis. After 15 h, cells were treated for 6 h with caffeine (inhibitor of ATM/ATR) and scored for phosphorylated Histone H3 by FACS. Graph denotes average of three independent experiments, error bars represent standard error. Western blot shows endogenous and reconstituted Wip1 levels from a single experiment. (E) Outline of experimental setup used in (F, G) and Figure 3A. (F) A range of kinase inhibitors does not promote checkpoint recovery after Wip1 depletion. Cells were treated as in (B), but treated with a 1-h pulse of doxorubicin 6 h after release from thymidine block. After 18 h, cells were treated for 6 h with caffeine (inhibitor of ATM/ATR), SB218078 (inhibitor of Chk1), UCN-01 (inhibitor of Chk1 and MK-2), SB202190 (inhibitor of p38), Chk2 inhibitor-2 (Chk2 inhibitor), or a combination of these inhibitors. The percentage of mitotic cells was assessed by scoring GFP positive cells for P-Histone H3 or MPM2 positivity by FACS. (G) P53 is dephosphorylated on the ATM/ATR-site S15 and the Chk1/Chk2 site S20 after combined inhibitor treatment (Shieh et al, 2000; Appella and Anderson, 2001). Cells treated or not with all inhibitors as in Figure 1F were subjected to western blot with the indicated antibodies.
Wip1 has been reported to dephosphorylate several checkpoint kinases, suggesting that it could function in recovery by removing activating phosphosites from checkpoint kinases to turn off the checkpoint signal (Takekawa et al, 2000; Lu et al, 2004, 2005, 2007; Fujimoto et al, 2006; Shreeram et al, 2006a; Oliva-Trastoy et al, 2007). Therefore, we treated G2 DNA-damaged cells with inhibitors to these kinases and monitored whether cells could enter mitosis in the absence of Wip1 (Figure 1E and F). Treatment with Caffeine (ATM/ATR inhibitor), UCN-01 (Chk1 and MAPKAP2 inhibitor), or SB218078 (Chk1 inhibitor) could all mediate checkpoint recovery in control cells, whereas treatment with SB202190 (p38 inhibitor) or Chk2-inhibitor2 (Chk2 inhibitor) only marginally affected checkpoint recovery. However, none of these inhibitors could promote checkpoint recovery after Wip1 RNAi. Importantly, whereas combination of these inhibitors efficiently mediated checkpoint recovery in control cells, they did not promote checkpoint recovery in Wip1-depleted cells, indicating that the checkpoint recovery defect after Wip1 RNAi does not merely depend on sustained activation of these kinases (Figure 1F and G).
Wip1 controls recovery competence, rather than recovery itself
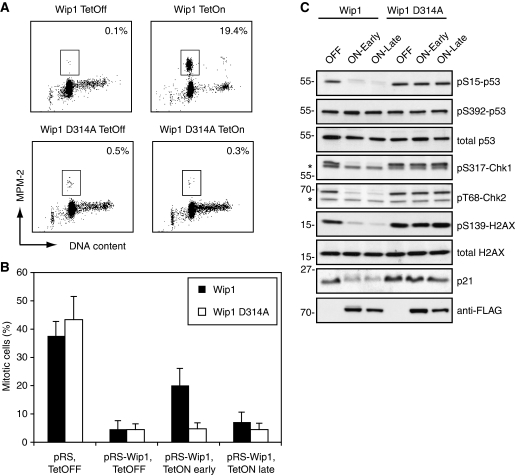
We next created stable cell lines in which we could induce the expression of RNAi-insensitive Wip1 or a phosphatase-inactive version of Wip1 (D314A). Induction of Wip1, but not Wip1 D314A, after doxorubicin treatment in G2 promoted mitotic entry (Figure 2A). This observation raises an apparent paradox, as it shows that Wip1, like caffeine and other checkpoint kinase inhibitors, acts to shut down the checkpoint, whereas enforced silencing of the checkpoint in Wip1-depleted cells fails to promote recovery. A possible explanation for this discrepancy could be that Wip1 needs to be present throughout the checkpoint response, and has an additional function that precedes its function during the recovery phase. To test whether Wip1 is required early during the DNA damage response, before actual silencing of the checkpoint, we reconstituted Wip1-depleted cells with RNAi-resistant Wip1 shortly after the DNA damage checkpoint was activated, or 18 h after initial checkpoint activation and monitored whether these cells could enter mitosis after silencing of ATM/ATR with caffeine (Figure 2B). Importantly, when reconstituted late, Wip1-depleted cells did not recover from the checkpoint, indicating that Wip1 needs to be present throughout the DNA damage checkpoint arrest to mediate checkpoint recovery. In contrast, when Wip1 expression was induced early after checkpoint activation, cells could enter mitosis after caffeine treatment, albeit not as efficiently as when Wip1 was already present during the DNA damage treatment (Figures 1D and 2B), Similar results were seen when microinjecting RNAi-resistant Wip1 early or late after DNA damage into Wip1-depleted cells (Supplementary Figure 1). Nonetheless, Wip1 was functional both when reconstituted early and late after DNA damage, as p53 S15, Chk1 S317, Chk2 T68 and H2AX S139 were dephosphorylated to similar extent, indicating that large parts of the checkpoint signalling was silenced (Figure 2C). Moreover, p21 levels were similarly reduced, indicating that p53 activity was inhibited both after early and late Wip1 reconstitution. This shows that although Wip1 can deactivate major parts of the checkpoint signalling, Wip1 needs to be present throughout the DNA damage checkpoint to mediate full checkpoint recovery. Thus, Wip1 confers checkpoint recovery competence during a DNA damage arrest.
Figure 2.
Wip1 is required throughout the checkpoint arrest to mediate recovery. (A) Forced expression of Wip1 can induce checkpoint recovery. U2TR cells stably expressing inducible wt or phosphatase-deficient Wip1 (D314A) were synchronized by thymidine and 6 h after release treated with doxorubicin for 1 h, after which the cells were treated for 24 h with Tetracyclin and taxol. The percentage of mitotic cells was assessed by scoring MPM2 positivity by FACS. (B) Wip1 is required during a G2 checkpoint arrest. U2TR cells stably expressing inducible RNAi-resistant wt or phosphatase-deficient Wip1 (D314A) were co-transfected with GFP-Spectrin and pRS or pRS-Wip1. Cells were synchronized with thymidine and 6 h after release treated with doxorubicin for 1 h, followed by taxol addition. Tetracyclin was added immediately (early) or 18 h (late) after doxorubicin washout. Caffeine was added 18 h after doxorubicin washout and incubated for 8 h. The percentage of mitotic cells was assessed by scoring GFP positive cells for MPM2 positivity by FACS. (C) Checkpoint signalling is inhibited both after early and late Wip1-induction. Cells were treated as in (B), but selected with puromycin and without caffeine addition, harvested as in (B) and subjected to immunoblotting with the indicated antibodies.
Wip1 controls recovery competence through p53
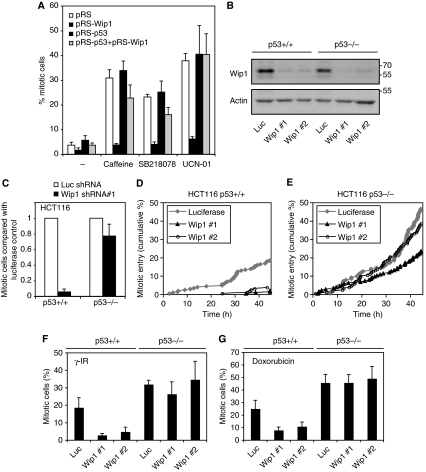
As deactivation of the DNA damage checkpoint through inhibition of checkpoint kinases could not rescue the checkpoint defect of Wip1-depleted cells, we sought to identify which proteins mediate the loss of recovery competence. A candidate target that is activated by DNA damage signalling is p53. Although p53 is not essential for induction of the G2 DNA damage checkpoint, p53 has been described to be required to sustain a long-term arrest in G2 (Bunz et al, 1998). In addition, there is considerable evidence that p53 is a major target of Wip1. Not only is p53 a direct target of Wip1 (Lu et al, 2005), but Wip1 can also dephosphorylate ATM, Chk1, Chk2, p38, and Mdm2, all direct regulators of p53 (Takekawa et al, 2000; Lu et al, 2004, 2005, 2007; Fujimoto et al, 2006; Shreeram et al, 2006a; Oliva-Trastoy et al, 2007). Moreover, Wip1 inhibition or RNAi decreases the proliferation of p53-positive, but not p53-negative cancer cell lines (Parssinen et al, 2008; Rayter et al, 2008), and Wip1 is required for APC(Min)-induced colon tumour formation and intestinal stem cell maintenance through counteracting p53 (Demidov et al, 2007b). As expected, depletion of p53 by shRNA did not interfere with the ability of cells to arrest in G2 in response to doxorubicin (Bunz et al, 1998) (Figure 3A). Importantly, co-depletion of p53 restored the ability of Wip1-depleted cells to recover after addition of caffeine, UCN01 or SB218078, indicating that the primary function of Wip1 in checkpoint recovery is to counteract the function of p53 during the ongoing arrest (Figure 3A).
Figure 3.
Wip1 is essential for checkpoint recovery by antagonizing p53. (A) p53 RNAi reverses Wip1 phenotype. U2OS cells were transfected with empty pRS, pRS + pRS-Wip1, pRS + pRS-p53 or pRS-Wip1 + pRS-p53. All samples were co-transfected with GFP-Spectrin. Checkpoint recovery was assessed as in Figure 1E and F. Graph denotes percentage of mitotic cells and error bars represent standard error of three independent experiments. (B) Knockdown of Wip1 in HCT116 cells. HCT116 p53+/+ or p53−/− cells were infected with lentiviral shRNA constructs targeting luciferase or Wip1. After selection with puromycin, harvested cells were subjected to immunoblotting with the indicated antibodies. (C) Wip1 is necessary for caffeine-induced checkpoint recovery in HCT116 cells, but not in HCT116 p53−/− cells. HCT116 and HCT116 p53−/− cells were infected with lentivirus expressing Wip1 shRNA#1 or luciferase shRNA, selected with puromycin and treated with doxorubicin for 16 h. Caffeine and taxol, to induce checkpoint recovery and to trap cells in mitosis, respectively, were added for 12 h after the doxorubicin treatment. Graph shows the ratio of mitotic Wip1 RNAi cells in relation to the amount of mitotic luciferase RNAi cells. (D, E) Wip1 is necessary for checkpoint recovery in HCT116 cells, but not in HCT116 p53−/− cells. HCT116 and HCT116 p53−/− cells were infected with lentivirus expressing Wip1 shRNA or luciferase shRNA and selected with puromycin. After treatment with 0.75 μM doxorubicin for 2 h, cells were followed by time-lapse microscopy and scored for entry into mitosis. (F, G) Wip1 is necessary for checkpoint recovery from late S/G2 in HCT116 cells, but not in HCT116 p53−/− cells. HCT116 and HCT116 p53−/− cells were infected with lentivirus expressing Wip1 shRNA or luciferase shRNA and selected with puromycin. Four hours after a BrdU-pulse, cells were treated with 6 Gy γ-irradiation or 1 h of 0.5 μM doxorubicin. Taxol was added immediately after DNA damage, and cells were harvested 24 h later. The percentage of recovering cells was assessed by scoring MPM2 positivity on the BrdU-positive population by FACS.
To validate whether Wip1 can mediate checkpoint recovery through p53 in an independent system, we next infected lentiviral Wip1 shRNA vectors in HCT116 and HCT116 p53−/− colon carcinoma cells. Two Wip1 shRNAs, independent from the shRNAs used in U2OS cells, mediated efficient knockdown in both cell lines (Figure 3B). Similar to U2OS cells, only a fraction of the Wip1-depleted HCT116 cells treated with doxorubicin entered mitosis after caffeine addition, whereas HCT116 p53−/− cells could recover in the absence of Wip1, indicating that Wip1 promotes checkpoint recovery by counteracting p53 also in HCT116 cells (Figure 3C). To see whether Wip1 is also important for spontaneous checkpoint recovery, when the checkpoint is not switched off by the addition of kinase inhibitors, we next filmed HCT116 cells after treatment with doxorubicin and monitored mitotic entry (Figure 3D and E). Importantly, whereas HCT116 p53−/− cells depleted of Wip1 entered mitosis, parental HCT116 cells depleted of Wip1 did not enter mitosis, showing that Wip1 is required also for spontaneous checkpoint recovery by counteracting p53. Using one of the Wip1 shRNAs, we reproducibly observed a slight delay in mitotic entry of HCT116 p53−/− cells, suggesting that Wip1 may have additional targets besides p53 that affect checkpoint recovery. Next, we used a BrdU-pulse to monitor whether cells that were in late S-phase or G2 during either doxorubicin addition or γ-irradiation could eventually recover from the DNA damage checkpoint and enter mitosis (Figure 3F and G). HCT116 p53−/− cells entered mitosis more efficiently than HCT116 cells, showing that p53 indeed participates in the G2 DNA damage checkpoint (Bunz et al, 1998). However, whereas Wip1-depleted HCT116 p53−/− cells recovered from both γ-irradiation and doxorubicin treatment as control-depleted cells, the checkpoint recovery of HCT116 cells depleted of Wip1 was impaired (Figure 3F and G). Thus, Wip1 counteracts p53 to confer checkpoint recovery competence to G2 cells.
Wip1 antagonizes p53-mediated repression of mitotic regulators
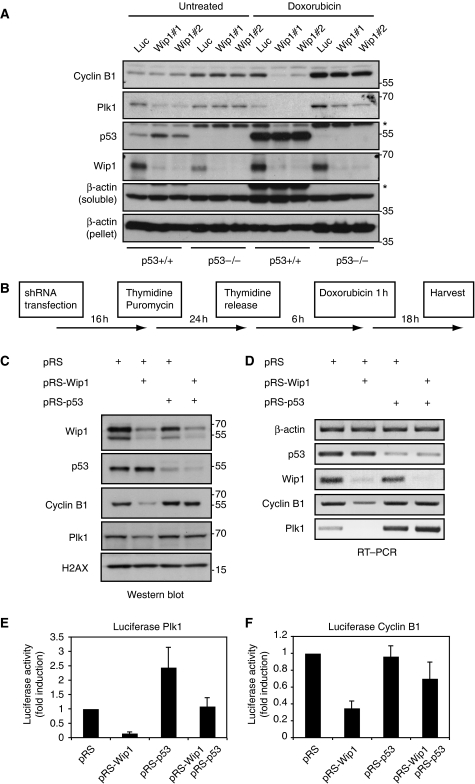
So why does Wip1 need to counteract p53, a non-essential component of the G2 DNA damage checkpoint, to mediate checkpoint recovery competence? Upon DNA damage, p53 can induce multiple target genes, including the Cdk-inhibitor p21. However, p21 is reduced to similar levels after early or late reconstitution of Wip1, whereas only cells reconstituted early could recover, arguing that p21 accumulation cannot explain the Wip1 phenotype (Figure 2C). p53 has also been described to reduce the transcription of a large number of G2 genes in response to DNA damage (Taylor and Stark, 2001; Tabach et al, 2005; Spurgers et al, 2006; Jurvansuu et al, 2007). Therefore, we analysed the levels of Cyclin B1, a key mitotic inducer, and Plk1, which is required for mitotic entry after recovery from DNA damage (van Vugt et al, 2004). The mRNA levels of Plk1 and Cyclin B1 in DNA-damaged U2OS cells were reduced compared with control G2 cells, indicating that Plk1 and Cyclin B1 are indeed targets of transcriptional repression (Supplementary Figure 2). Despite that the majority of HCT116 cells arrest in G2 after doxorubicin treatment, only a marginal increase in the expression of Cyclin B1 and a minor decrease in Plk1 levels compared with asynchronic cells was observed (Figure 4A, lanes 1 and 7). Importantly, both Plk1 and Cyclin B1 levels were dramatically reduced after DNA damage in Wip1-depleted HCT116 cells, whereas only limited effects were seen in HCT116 p53−/− cells, likely depending on DNA damage-induced degradation of Plk1 (Bassermann et al, 2008) (Figure 4A). This shows that Wip1 can counteract p53-mediated repression of Cyclin B1 and Plk1 after DNA damage. A reduction in Plk1 levels was also observed in Wip1-depleted untreated HCT116 cells, probably a consequence of low levels of intrinsic DNA damage occurring in these cells (Figure 4A; Supplementary Figure 3). A similar pattern was seen in U2OS cells, in which Wip1-depleted DNA-damaged G2 cells contained both less protein and mRNA of Plk1 and Cyclin B1 (Figure 4B–D). However, both the protein and mRNA levels of Cyclin B1 and Plk1 were restored after co-depletion of p53, indicating that Wip1 counteracts a p53-mediated reduction of Cyclin B1 and Plk1 mRNA during a DNA damage response (Figure 4B–D).
Figure 4.
Wip1 counteracts p53-mediated transcriptional repression. (A) Wip1 antagonizes p53-mediated downregulation of Plk1 and Cyclin B1 in HCT116 cells. HCT116 and HCT116 p53−/− cells were infected with lentivirus expressing Wip1 shRNA or a luciferase shRNA, selected with puromycin, treated with doxorubicin for 16 h and harvested for western blot. Whereas Wip1 was detected in the unsoluble fraction (loading control, β-actin pellet), p53, Cyclin B1 and Plk1 were detected in the soluble fraction. (B) Schematic outline of experiments in (C–F). (C, D) Wip1 antagonizes p53-mediated downregulation of Plk1 and Cyclin B1. U2OS cells were transfected as in Figure 3A, and selected by puromycin addition during a 24-h thymidine block. At 6 h after release from thymidine, cells were treated for 1 h with doxorubicin. After 18 h, at the time corresponding to addition of inhibitors in Figure 3A, cells were harvested for western blot (C) or RT–PCR (D). (E, F) Wip1 counteracts p53-mediated transcriptional repression of Plk1 and Cyclin B1. U2OS cells were transfected as in Figure 3A, with the addition of luciferase-based transcriptional reporters for Plk1 (E) and Cyclin B1 (F), and synchronized as shown in Figure 4B. Graphs show the relative luciferase expression 18 h after doxorubicin treatment.
As p53 can act as a transcriptional repressor of G2 genes (Taylor and Stark, 2001; Tabach et al, 2005; Spurgers et al, 2006; Jurvansuu et al, 2007) and p53 depletion could rescue the effect of Wip1 depletion (Figure 3), we next wondered whether the reduced Cyclin B1 and Plk1 mRNA levels after Wip1 depletion depended on p53-mediated repression of Cyclin B1 and Plk1 transcription. Therefore, we expressed transcriptional reporters driven by Cyclin B1 or Plk1 promoters in G2-arrested DNA-damaged cells. Forced expression of Wip1, but not of phosphatase dead Wip1, in DNA-damaged G2 cells elevated the expression of Plk1 and Cyclin B transcriptional reporters, indicating that Wip1 can counteract transcriptional repression in G2 (Supplementary Figure 4). Moreover, the expression of both Plk1 and Cyclin B1-transcriptional reporters were reduced after Wip1 RNAi and restored after p53 and Wip1 double RNAi, indicating that Wip1 can indeed counteract p53-dependent repression of Plk1 and Cyclin B1 transcription after DNA damage (Figure 4E and F). Taken together, these data suggest that Wip1 acts in a negative feedback loop that is activated in response to DNA damage to limit the suppressive effect of p53 on the expression of mitotic regulators during a checkpoint arrest.
Cyclin B1 becomes a rate-limiting factor for recovery in Wip1-depleted cells
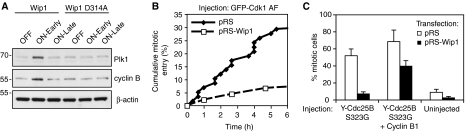
Our data show that Wip1 can antagonize p53-dependent expression of Cyclin B1 and Plk1. If true, then the recovery defect of Wip1-depleted cells should be due to a deficiency of mitotic inducers. Indeed, when re-examining the samples from early and late Wip1 reconstitution, in which no difference in phosphorylation or levels of several checkpoint components was observed, we noted that both Cyclin B1 and Plk1 levels were elevated after early Wip1 reconstitution (Figures 2C and 5A). However, late Wip1 reconstitution could not rescue the very low Cyclin B1 and Plk1 levels, indicating that the levels of mitotic inducers may determine whether cells are competent for checkpoint recovery. To test whether the recovery deficiency in Wip1-depleted cells is due to a lack of mitotic inducers, we microinjected GFP-Cdk1 AF, a form of Cdk1 that lacks inhibitory phosphorylation sites and efficiently induces premature mitotic entry in the presence of Cyclin B (Krek and Nigg, 1991). Whereas injection of GFP-CDK1 AF rapidly promoted mitotic entry in DNA damage-arrested control cells, only a fraction of Wip1-depleted cells entered mitosis, indicating that Wip1 deficient cells do not contain sufficient levels of Cyclin B to promote mitotic entry (Figure 5B). Microinjection of Plk1 did not induce mitotic entry in DNA-damaged control or Wip1-depleted cells, indicating that although Plk1 is necessary, it is not sufficient to promote checkpoint recovery (data not shown) (van Vugt et al, 2004).
Figure 5.
Cyclin B1 is rate limiting for recovery in Wip1-depleted cells. (A) Plk1 and Cyclin B1 levels are low if Wip1 is absent during the DNA damage arrest. The samples used in Figure 2C were subjected to immunoblot for Plk1 and Cyclin B1. (B) Injection of GFP-Cdk1 AF fails to induce mitotic entry in DNA-damaged Wip1-depleted cells. pRS or pRS-Wip1 transfected cells were selected with puromycin and 6 h after release from a thymidine block exposed for 1 h to 0.5 μM doxorubicin. At 18 h after doxorubicin treatment, GFP-Cdk1 AF was injected and cells were followed by time-lapse microscopy. Graph denotes cumulative mitotic entry of injected cells (C) Injection of YFP-Cdc25B3 S323G fails to induce mitotic entry in DNA-damaged Wip1 RNAi cells, unless co-injected with Cyclin B1. pRS or pRS-Wip1 transfected cells were selected with puromycin and 6 h after release from a thymidine block exposed for 1 h to 0.5 μM doxorubicin. At 18 h after doxorubicin treatment, YFP-Cdc25B3 S323G or YFP-Cdc25B3 S323G and Cyclin B1 were injected to cells in a single dish and nocodazole was added to trap cells entering mitosis. Graph denotes average of four independent experiments where 23–178 cells were counted for each injected construct, error bars represent standard error.
To test whether the levels of the key mitotic inducer Cyclin B1 was a limiting factor for checkpoint recovery in Wip1-depleted cells, we microinjected YFP-Cdc25B3 S323G, a potent activator of Cyclin B1–Cdk1 even in the presence of an active DNA damage checkpoint (Forrest and Gabrielli, 2001). Whereas injection of YFP-Cdc25B3 S323G resulted in efficient mitotic entry in DNA-damaged control cells, it failed to promote mitosis in Wip1-depleted cells. Importantly, co-injection of Cyclin B1 and YFP-Cdc25B3 S323G forced DNA-damaged Wip1 shRNA transfected cells to enter mitosis, showing that the levels of Cyclin B1 are indeed limiting for mitotic entry in DNA-damaged Wip1-depleted cells (Figure 5C). Thus, artificial activation of the remaining Cyclin B–Cdk1 failed to promote mitotic entry in Wip1-deficient DNA-damaged cells, indicating that Cyclin B–Cdk1 levels had fallen below the relatively low threshold required for mitotic entry (Lindqvist et al, 2007).
Taken together, our data show that Wip1 acts as a checkpoint recovery competence gene by counteracting p53-mediated transcriptional repression of mitotic inducers. To test whether checkpoint recovery competence is a general consequence of loss of any phosphatase that counteracts checkpoint signalling, we transfected siRNA to PPP4C (also known as PP4), a phosphatase proposed to regulate checkpoint recovery through dephosphorylation of γH2AX (Chowdhury et al, 2008; Nakada et al, 2008). PPP4C depletion impaired recovery from a DNA damage checkpoint in G2 to some extent compared with control transfected cells (Supplementary Figure 5a and b). Importantly however, PPP4C-depleted cells arrested in G2 contained high levels of Cyclin B1, indicating that PPP4C is not required to retain sufficient levels of mitotic inducers to allow checkpoint recovery (Supplementary Figure 5c). Thus, not every phosphatase that counteracts checkpoint signalling mediates checkpoint recovery competence.
Discussion
Our data show that unlimited checkpoint activation during a DNA damage-induced arrest in G2 can decrease the levels of mitotic inducers to such extent that a cell cannot enter mitosis, despite inactivation of the checkpoint. As the DNA damage checkpoint functions by suppressing Cdk activity, which in turn is necessary for stimulating the transcriptional program in G2, our data also suggest that a cell must retain sufficient levels of Cdk activity during an arrest to maintain its G2 state. A cell that has lost its G2 state will not express pro-mitotic genes, and has therefore lost its competence to recover from a G2 DNA damage checkpoint. Thus, a cell needs to actively balance the checkpoint at all stages of the DNA damage response to remain competent for eventual recovery.
Here, we show that Wip1 is the first in a novel class of proteins that is required to balance the checkpoint throughout a DNA-damage response. Rather than deactivating the G2 checkpoint to promote recovery, Wip1 counteracts p53-dependent downregulation of pro-mitotic genes during the arrest to confer checkpoint recovery competence. We could show that the defect in checkpoint recovery observed in Wip1-depleted cells, could be reverted by introduction of Cyclin B1 in combination with a checkpoint-insensitive mutant of Cdc25B. Although this experiment shows that depletion of Wip1 causes Cyclin B1 to drop below the critical level needed to initiate mitosis, it does not rule out the possibility that additional components of the checkpoint recovery pathway are also reduced below their minimal threshold. In fact, hundreds of proteins are transcriptionally upregulated during G2, and their combined status will determine the threshold of Cyclin B–Cdk1 activity needed for mitotic entry (Whitfield et al, 2002; Lindqvist et al, 2009). Indeed, here we show that the expression of Plk1, a central player in the network of proteins that regulate Cyclin B–Cdk1 activity during checkpoint recovery, is also dramatically reduced in a p53-dependent manner in Wip1-depleted cells (van Vugt et al, 2004; Lindqvist et al, 2009).
In addition to p53, DNA damage-induced release of the Wip1 target Chk1 was recently identified to mediate transcriptional repression of mitotic inducers (Shimada et al, 2008). Although our data clearly show that Wip1 mediates checkpoint recovery by counteracting p53, they do not exclude that Wip1 has an additional function by modulating Chk1-dependent transcriptional repression.
Combining modelling and experimental work, Batchelor and co-workers recently showed that Wip1 is required to control the shape of oscillations in the ATM-p53 signalling cascade, thereby ensuring that the checkpoint reaches points of very low activity during the arrest (Batchelor et al, 2008). Our data suggest that these breathing pauses during the checkpoint could allow for short pulses of transcription that prevent a cell from losing its G2 identity.
Several studies have linked Wip1 to tumour formation (Bulavin et al, 2002, 2004; Nannenga et al, 2006; Shreeram et al, 2006b; Demidov et al, 2007a, 2007b). Here, we show that Wip1 limits the suppression of mitotic inducers during a prolonged checkpoint arrest to remain cells competent for eventual checkpoint recovery. Interestingly, both early during tumour development and after genotoxic anti-cancer treatment a DNA damage checkpoint is activated, which cells must overcome to continue to proliferate (Bartkova et al, 2006; Di Micco et al, 2006). On the basis of our observations we propose that Wip1's function in tumourigenesis is to retain the competence for cell division during a DNA damage response.
Interestingly, loss of Wip1 was recently shown to induce a p53-dependent G2 arrest in neural stem/progenitor cells (Zhu et al, 2009). It therefore remains a possibility that retaining checkpoint recovery competence is required also to maintain a neural stem cell population.
Materials and methods
Plasmids and antibodies
pRETROSUPER plasmids were targeting GACTCCAGTGGTAATCTAC (nt 775–793), GCAATGGATGATTTGATGC (nt 115–133) and CTACATGTGTAACAGTTCC (nt 705–723) in p53, and GGATGACTTTGTCAGAGCT (nt 612–630) and GTACATGGAGGACGTTACT (nt 57–75) in Wip1. Non-targetable Wip1 was constructed by introducing the silent mutations T615C and C618T in FLAG-Wip1 (Lu et al, 2005) cDNA, and phosphatase dead Wip1 (Takekawa et al, 2000) was constructed by introducing the D314A mutations using Quikchange site-directed mutagenesis (Stratagene). Both Wip1 and Wip1 D314A were subcloned to pCDN4TO plasmids (Invitrogen). YFP-Cdc25B3 S323G was from (Lindqvist et al, 2004). PS10 Histone H3, pS139 H2AX, H2AX and Plk1 antibodies were from Upstate, pT68 Chk2, pS317 Chk1, pS392 p53, pS20 p53 and pS15 p53 antibodies were from Cell Signalling, Wip1 antibodies were from R&D systems and Santa Cruz. Actin, p53, p21 and Cyclin B1 antibodies were from Santa Cruz. BrdU and PPP4C antibodies were from Abcam and FLAG antibodies were from Sigma.
Cell culture, transfection and microinjection
U2OS and HCT116 cells were kept in DMEM supplemented with 6% foetal calf serum and antibiotics. Monoclonal U2TR cell lines, containing tetracycline inducible Wip1 or Wip1 D314A were constructed as in Macurek et al (2008), and kept in DMEM supplemented with 10% foetal calf serum and antibiotics. Thymidine, Caffeine, Puromycin, Paclitaxel, Nocodazole and Chk2 inhibitorII were from Sigma and used at 2.5 mM, 5 mM, 2 μg/ml, 1 μM, 250 ng/ml and 10 μM, respectively. SB202190 and SB218078 were from Calbiochem and used at 6 μM and 2.5 μM, respectively. UCN01 was a kind gift from Floris Foijer and used at 0.3 μM. Transfection with calcium phosphate was performed using the standard calcium phosphate technique. Cells were microinjected in HEPES-buffered medium with 0.1 μg/μl expression plasmid together with 0.01 μg/μl pEGFP-C1 (clontech) using an Eppendorf Micromanipulator 5171 coupled to a Transjector 5246.
Lentiviral transduction
HCT116 cells were transduced either by control lentivirus targeting luciferase, TGACCAGGCATTCACAGAAAT or by lentivirus targeting Wip1 gtggacaatcagggaaacttt (nt1135–1155) or cgagagaatgtccaaggtgta (nt 1372–1392). Lentiviral particles were generated in COS7 cells by cotransfection (using fuGENE 6 Transfection Reagent; Roche Diagnostics, Mannheim, Germany) of plasmids encoding the lentiviral gag and pol elements (pMDLgpRRE), the rev protein (pRSV Rev), the viral envelope (pMD2G; all kindly provided by D. Trono). After 72 h, the supernatant was collected, and cells were transduced for 48 h in the presence of hexadimethrine bromide (4 μg/ml; Aldrich, Steinheim, Germany). Stable knock down was performed by puromycin selection.
FACS, RT–PCR and microscopy
FACS was performed as in Smits et al (2000). For double BrdU and MPM2 staining, cells were harvested in 70% ethanol, incubated in 2 M HCl, 0.1% Triton X-100, and neutralized with 0.1 M Borate buffer pH 8.5. The percentage of MPM2-positive cells was assessed on the BrdU-positive population. For RT–PCR, total RNA was extracted using Trizol and converted to cDNA using SuperscriptII (Invitrogen). Standard PCR were run at several dilutions to ensure detection below saturation. Immunofluorescence was performed as described (Lindqvist et al, 2007). Images were acquired on a Zeiss LSM510 META microscope, or a Deltavision imaging system using NA 1.4 objectives. For time-lapse microscopy, cells were imaged with DIC on a Zeiss Axiovert 200 M using NA 0.75 objectives.
Luciferase assay and western blot
Luciferase activity was determined 48 h after transfection, using the Dual luciferase kit (Promega) according to the manufacturer's instructions. Relative luciferase activity was expressed as a ratio of firefly luciferase activity to control Renilla luciferase activity. Plk1-luciferase reporter (Laoukili et al, 2005) and Cyclin B1-luciferase reporter (Evans et al, 2007) were described earlier. Western blots were either performed on a whole cell lysate (U2OS), or on cells solubilized with 1% NP-40.
siRNA transfection and automated image analysis
For siRNA experiments (Supplementary Figure 5), cells were grown in 96-well plates (Viewplate-96, Perkin Elmer) and transfected with 20–30 nM siRNA using HiPerFect (Qiagen) according to manufacturer's recommendations. ON-Targetplus SMARTpools of four siRNAs targeting Wip1 (J-004554) or PPP4C (J-008486) were from Dharmacon. Cells were fixed by addition of equal volume of an 8% formaldehyde solution to the medium to prevent loss of mitotic cells, permeabilized with methanol and stained with DAPI and Cyclin B1 and pHistone H3 antibodies. Image acquisition was performed using a Cellomics ArrayScan VTI (Thermo Scientific) using a 20 × 0.40 NA objective. Image analysis was performed using Cellomics ArrayScan HCS Reader (Thermo Scientific). In short, cells were identified based on DAPI staining and they were scored as mitotic if the intensity of pHistoneH3 staining reached a pre-set threshold. Cyclin B intensity was measured by quantifying the average fluorescence of a five-pixel wide region surrounding the DAPI staining. All images and automated image quantifications were subsequently checked manually. Image analysis was performed on at least 500 cells per condition.
Supplementary Material
Supplementary Figures
Review Process File
Acknowledgments
We thank the members of the Medema laboratory for discussions and comments on the paper, LA Donehower for FLAG-Wip1, R Bernards for pRS-Wip1, R Wolthuis for GFP-Cdk1 AF and D Trono for lentiviral packaging constructs. AL was supported by a fellowship from the Wenner-Gren foundation and by the Netherlands Organization for Scientific Research (NWO) (ZonMw 916.86.083). AB is funded by the Ramon y Cajal Program of the MEC. MB and LM were funded by grants from the AICR (MB 07-0552 and LM 08-0172). RHM and LM were funded by the Netherlands Genomics Initiative of NWO and the Dutch Cancer Society (UU2006-3579).
Footnotes
The authors declare that they have no conflict of interest.
References
- Appella E, Anderson CW (2001) Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem 268: 2764–2772 [DOI] [PubMed] [Google Scholar]
- Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y (1998) Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281: 1674–1677 [DOI] [PubMed] [Google Scholar]
- Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M (2008) The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 134: 256–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LVF, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J et al. (2006) Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444: 633–637 [DOI] [PubMed] [Google Scholar]
- Batchelor E, Mock CS, Bhan I, Loewer A, Lahav G (2008) Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Mol Cell 30: 277–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA, Ambrosino C, Sauter G, Nebreda AR, Anderson CW, Kallioniemi A, Fornace AJ, Appella E (2002) Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet 31: 210–215 [DOI] [PubMed] [Google Scholar]
- Bulavin DV, Phillips C, Nannenga B, Timofeev O, Donehower LA, Anderson CW, Appella E, Fornace AJ (2004) Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nat Genet 36: 343–350 [DOI] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282: 1497–1501 [DOI] [PubMed] [Google Scholar]
- Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD (1998) Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281: 1677–1679 [DOI] [PubMed] [Google Scholar]
- Chowdhury D, Xu X, Zhong X, Ahmed F, Zhong J, Liao J, Dykxhoorn DM, Weinstock DM, Pfeifer GP, Lieberman J (2008) A PP4-phosphatase complex dephosphorylates gamma-H2AX generated during DNA replication. Mol Cell 31: 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidov ON, Kek C, Shreeram S, Timofeev O, Fornace AJ, Appella E, Bulavin DV (2007a) The role of the MKK6/p38 MAPK pathway in Wip1-dependent regulation of ErbB2-driven mammary gland tumorigenesis. Oncogene 26: 2502–2506 [DOI] [PubMed] [Google Scholar]
- Demidov ON, Timofeev O, Lwin HN, Kek C, Appella E, Bulavin DV (2007b) Wip1 phosphatase regulates p53-dependent apoptosis of stem cells and tumorigenesis in the mouse intestine. Cell Stem Cell 1: 180–190 [DOI] [PubMed] [Google Scholar]
- Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, di Fagagna FD (2006) Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444: 638–642 [DOI] [PubMed] [Google Scholar]
- Evans PM, Zhang W, Chen X, Yang J, Bhakat KK, Liu C (2007) Kruppel-like factor 4 is acetylated by p300 and regulates gene transcription via modulation of histone acetylation. J Biol Chem 282: 33994–34002 [DOI] [PubMed] [Google Scholar]
- Fiscella M, Zhang HL, Fan SJ, Sakaguchi K, Shen SF, Mercer WE, VandeWoude GF, Oconnor PM, Appella E (1997) Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci USA 94: 6048–6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest A, Gabrielli B (2001) Cdc25B activity is regulated by 14-3-3. Oncogene 20: 4393–4401 [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Onishi N, Kato N, Takekawa M, Xu X, Kosugi A, Kondo T, Imamura M, Oishi I, Yoda A, Minami Y (2006) Regulation of the antioncogenic Chk2 kinase by the oncogenic Wip1 phosphatase. Cell Death Differ 13: 1170–1180 [DOI] [PubMed] [Google Scholar]
- Fung TK, Poon RY (2005) A roller coaster ride with the mitotic cyclins. Semin Cell Dev Biol 16: 335–342 [DOI] [PubMed] [Google Scholar]
- Guardavaccaro D, Pagano M (2006) Stabilizers and destabilizers controlling cell cycle oscillators. Mol Cell 22: 1–4 [DOI] [PubMed] [Google Scholar]
- Jurvansuu J, Fragkos M, Ingemarsdotter C, Beard P (2007) Chk1 instability is coupled to mitotic cell death of p53-deficient cells in response to virus-induced DNA damage signaling. J Mol Biol 372: 397–406 [DOI] [PubMed] [Google Scholar]
- Khosravi R, Maya R, Gottlieb T, Oren M, Shiloh Y, Shkedy D (1999) Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl Acad Sci USA 96: 14973–14977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W, Nigg EA (1991) Mutations of p34cdc2 phosphorylation sites induce premature mitotic events in HeLa cells: evidence for a double block to p34cdc2 kinase activation in vertebrates. EMBO J 11: 3331–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, Elowitz MB, Alon U (2004) Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat Genet 36: 147–150 [DOI] [PubMed] [Google Scholar]
- Laoukili J, Kooistra MRH, Bras A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH (2005) FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol 7: 126–U134 [DOI] [PubMed] [Google Scholar]
- Lev Bar-Or R, Maya R, Segel LA, Alon U, Levine AJ, Oren M (2000) Generation of oscillations by the p53-Mdm2 feedback loop: a theoretical and experimental study. Proc Natl Acad Sci USA 97: 11250–11255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Kallstrom H, Rosenthal CK (2004) Characterisation of Cdc25B localisation and nuclear export during the cell cycle and in response to stress. J Cell Sci 117: 4979–4990 [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Roudriguez-Bravo V, Medema RH (2009) The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol 185: 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, van Zon W, Rosenthal CK, Wolthuis RMF (2007) Cyclin B1-Cdk1 activation continues after centrosome separation to control mitotic progression. PLoS Biol 5: 1127–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Ma O, Nguyen TA, Jones SN, Oren M, Donehower LA (2007) The Wip1 phosphatase acts as a gatekeeper in the p53-Mdm2 autoregulatory loop. Cancer Cell 12: 342–354 [DOI] [PubMed] [Google Scholar]
- Lu XB, Bocangel D, Nannenga B, Yamaguchi H, Appella E, Donehower LA (2004) The p53-induced oncogenic phosphatase PPM1D interacts with uracil DNA glycosylase and suppresses base excision repair. Mol Cell 15: 621–634 [DOI] [PubMed] [Google Scholar]
- Lu XB, Nannenga B, Donehower LA (2005) PPM1D dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints. Genes Dev 19: 1162–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB, Medema RH (2008) Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 455: 119–123 [DOI] [PubMed] [Google Scholar]
- Murray AW (2004) Recycling the cell cycle: cyclins revisited. Cell 116: 221–234 [DOI] [PubMed] [Google Scholar]
- Nakada S, Chen GI, Gingras AC, Durocher D (2008) PP4 is a gamma H2AX phosphatase required for recovery from the DNA damage checkpoint. EMBO Rep 9: 1019–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannenga B, Lu XB, Dumble M, Van Maanen M, Nguyen TA, Sutton R, Kumar TR, Donehower LA (2006) Augmented cancer resistance and DNA damage response phenotypes in PPM1D null mice. Mol Carcinog 45: 594–604 [DOI] [PubMed] [Google Scholar]
- Oliva-Trastoy M, Berthonaud V, Chevalier A, Ducrot C, Marsolier-Kergoat MC, Mann C, Leteurtre F (2007) The Wip1 phosphatase (PPM1D) antagonizes activation of the Chk2 tumour suppressor kinase. Oncogene 26: 1449–1458 [DOI] [PubMed] [Google Scholar]
- Parssinen J, Alarmo EL, Karhu R, Kallioniemi A (2008) PPM1D silencing by RNA interference inhibits proliferation and induces apoptosis in breast cancer cell lines with wild-type p53. Cancer Genet Cytogenet 182: 33–39 [DOI] [PubMed] [Google Scholar]
- Rayter S, Elliott R, Travers J, Rowlands MG, Richardson TB, Boxall K, Jones K, Linardopoulos S, Workman P, Aherne W, Lord CJ, Ashworth A (2008) A chemical inhibitor of PPM1D that selectively kills cells overexpressing PPM1D. Oncogene 27: 1036–1044 [DOI] [PubMed] [Google Scholar]
- Shieh SY, Ahn J, Tamai K, Taya Y, Prives C (2000) The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev 14: 289–300 [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Niida H, Zineldeen DH, Tagami H, Tanaka M, Saito H, Nakanishi M (2008) Chk1 is a histone H3 threonine 11 kinase that regulates DNA damage-induced transcriptional repression. Cell 132: 221–232 [DOI] [PubMed] [Google Scholar]
- Shreeram S, Demidov ON, Hee WK, Yamaguchi H, Onishi N, Kek C, Timofeev ON, Dudgeon C, Fornace AJ, Anderson CW, Minami Y, Appella E, Bulavin DV (2006a) Wip1 phosphatase modulates ATM-dependent signaling pathways. Mol Cell 23: 757–764 [DOI] [PubMed] [Google Scholar]
- Shreeram S, Hee WK, Demidov ON, Kek C, Yamaguchi H, Fornace AJ, Anderson CW, Appella E, Bulavin DV (2006b) Regulation of ATM/p53-dependent suppression of myc-induced lymphomas by Wip1 phosphatase. J Exp Med 203: 2793–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits VAJ, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH (2000) Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol 2: 672–676 [DOI] [PubMed] [Google Scholar]
- Smits VAJ, Medema RH (2001) Checking out the G2/M transition. Biochim Biophys Acta 1519: 1–12 [DOI] [PubMed] [Google Scholar]
- Spurgers KB, Gold DL, Coombes KR, Bohnenstiehl NL, Mullins B, Meyn RE, Logothetis CJ, McDonnell TJ (2006) Identification of cell cycle regulatory genes as principal targets of p53-mediated transcriptional repression. J Biol Chem 281: 25134–25142 [DOI] [PubMed] [Google Scholar]
- Tabach Y, Milyavsky M, Shats I, Brosh R, Zuk O, Yitzhaky A, Mantovani R, Domany E, Rotter V, Pilpel Y (2005) The promoters of human cell cycle genes integrate signals from two tumor suppressive pathways during cellular transformation. Mol Syst Biol 1: 2005.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekawa M, Adachi M, Nakahata A, Nakayama I, Itoh F, Tsukuda H, Taya Y, Imai K (2000) p53-inducible Wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J 19: 6517–6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Stark GR (2001) Regulation of the G2/M transition by p53. Oncogene 20: 1803–1815 [DOI] [PubMed] [Google Scholar]
- Toettcher JE, Loewer A, Ostheimer GJ, Yaffe MB, Tidor B, Lahav G (2009) Distinct mechanisms act in concert to mediate cell cycle arrest. Proc Natl Acad Sci USA 106: 785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt M, Bras A, Medema RH (2004) Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol Cell 15: 799–811 [DOI] [PubMed] [Google Scholar]
- Whitfield ML, Sherlock G, Saldanha AJ, Murray JI, Ball CA, Alexander KE, Matese JC, Perou CM, Hurt MM, Brown PO, Botstein D (2002) Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell 13: 1977–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Durell SR, Chatterjee DK, Anderson CW, Appella E (2007) The Wip1 phosphatase PPM1D dephosphorylates SQ/TQ motifs in checkpoint substrates phosphorylated by PI3K-like kinases. Biochemistry 46: 12594–12603 [DOI] [PubMed] [Google Scholar]
- Zhu YH, Zhang CW, Lu L, Demidov ON, Sun L, Yang L, Bulavin DV, Xiao ZC (2009) Wip1 regulates the generation of new neural cells in the adult olfactory bulb through p53-dependent cell cycle control. Stem Cells 27: 1433–1442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures
Review Process File