Abstract
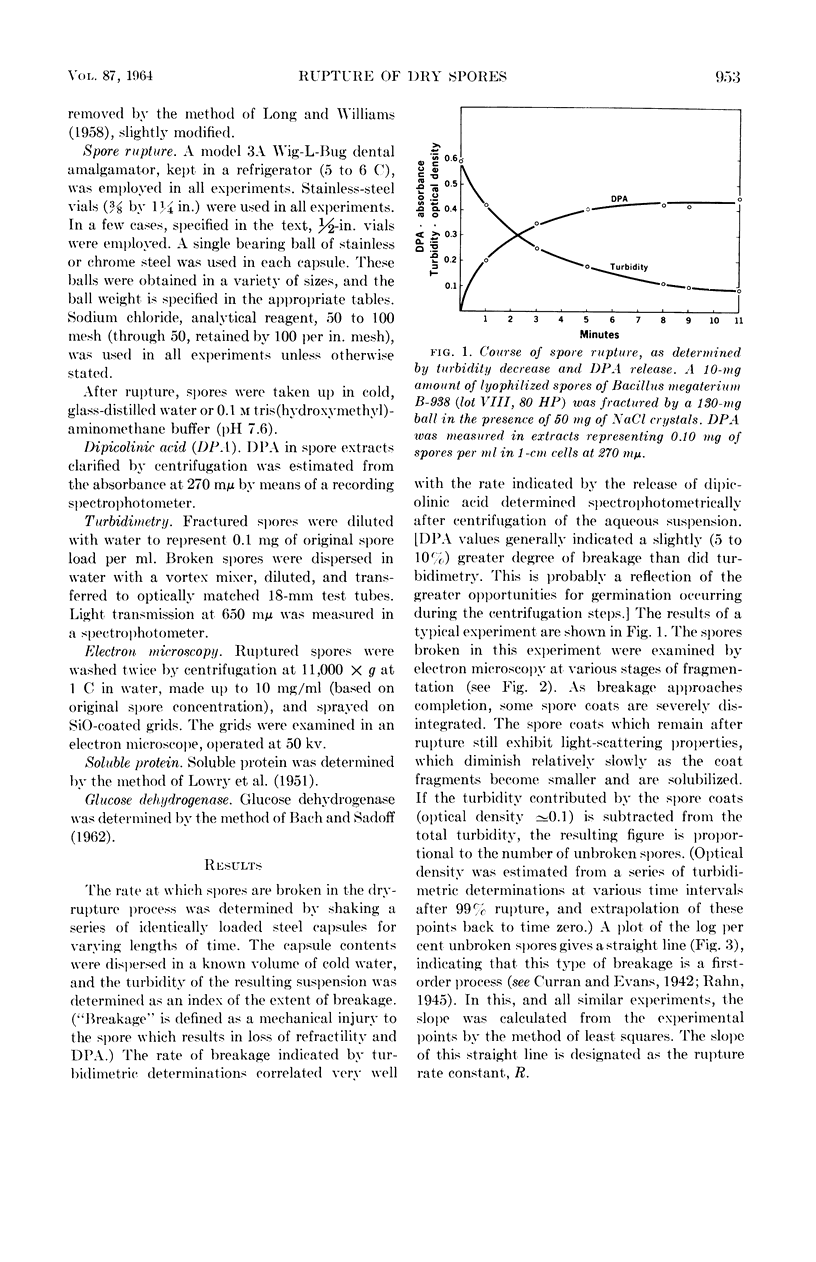
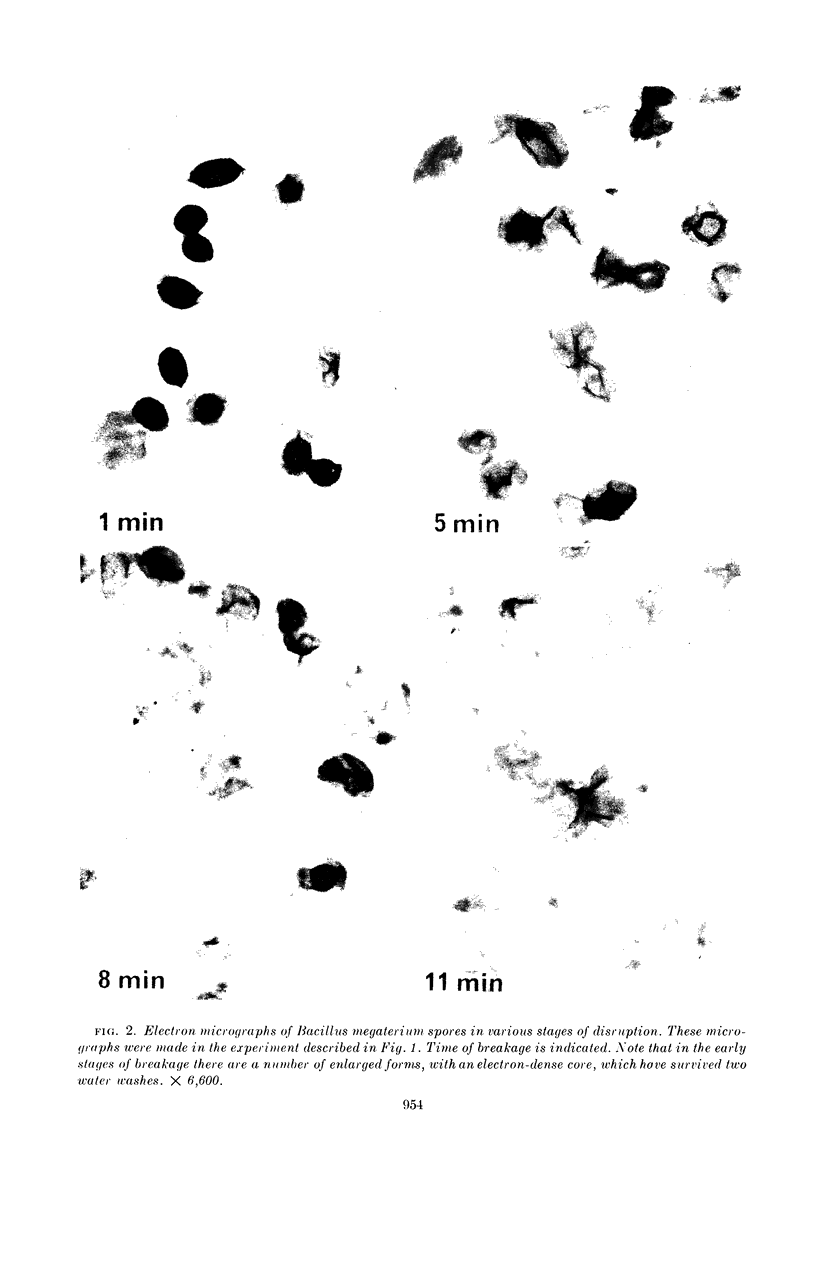
Sacks, L. E. (U.S. Department of Agriculture, Albany, Calif.), Peter B. Percell, Richard S. Thomas, and Glen F. Bailey. Kinetics of dry rupture of bacterial spores in the presence of salt. J. Bacteriol. 87:952–960. 1964.—The kinetics of breaking spores in the dry state by use of an excess of sodium chloride and a steel ball in a shaking device were investigated. Under most conditions, disruption is a first-order process. The disruption-rate constant varies directly with the weight of the ball and inversely with the weight of the capsule contents (spores plus salt). Different spore batches differ somewhat in susceptibility to dry rupture. The dry-rupture process is highly reproducible and it is relatively simple to obtain preparations in which exactly 50%, or 90%, of the spores are broken. The procedure is uniquely suited to the disruption of small (5 to 20 mg) samples, but 150 mg of spores have been handled with conventional equipment. Apparently, the chief function of the salt is to separate the spores from one another with a relatively hard, energy-nonabsorbing matrix, preventing aggregation and consequent cushioning of the ball's impact. However, under certain conditions (small ball, high salt, large crystals) appreciable breakage results from collisions of spores with the salt crystals. The minimal salt-spore ratio for efficient breakage depends on the spore batch, but is usually greater than 3:1. Fine glass beads or inorganic salts other than sodium chloride will also serve as the matrix. Electron micrographs of the spores in various stages of disruption are shown, as are electron micrographs of the spore coats of Bacillus macerans, B. megaterium, B. cereus, B. coagulans, and Clostridium bifermentans. Prolonged agitation disintegrates spore coats. The spore coats of B. macerans exhibit a characteristic ribbed structure, previously detected only by carbon replicas of intact spores. Possible application to other biological materials is considered.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACH J. A., SADOFF H. L. Aerobic sporulating bacteria. I. Glucose dehydrogenase of Bacillus cereus. J Bacteriol. 1962 Apr;83:699–707. doi: 10.1128/jb.83.4.699-707.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN W. L., ORDAL Z. J., HALVORSON H. O. Production and cleaning of spores of putrefactive anaerobe 3679. Appl Microbiol. 1957 May;5(3):156–159. doi: 10.1128/am.5.3.156-159.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran H. R., Evans F. R. The Killing of Bacterial Spores in Fluids by Agitation with Small Inert Particles. J Bacteriol. 1942 Feb;43(2):125–139. doi: 10.1128/jb.43.2.125-139.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRECZ N., ANELLIS A., SCHNEIDER M. D. Procedure for cleaning of Clostridium botulinum spores. J Bacteriol. 1962 Sep;84:552–558. doi: 10.1128/jb.84.3.552-558.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONG S. K., WILLIAMS O. B. Method for removal of vegetative cells from Bacterial spore preparations. J Bacteriol. 1958 Sep;76(3):332–332. doi: 10.1128/jb.76.3.332-332.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- POWELL J. F., STRANGE R. E. Biochemical changes occurring during sporulation in Bacillus species. Biochem J. 1956 Aug;63(4):661–668. doi: 10.1042/bj0630661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn O. PHYSICAL METHODS OF STERILIZATION OF MICROORGANISMS. Bacteriol Rev. 1945 Mar;9(1):1–47. doi: 10.1128/br.9.1.1-47.1945_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L. J., Foster J. W. MECHANICAL GERMINATION OF BACTERIAL SPORES. Proc Natl Acad Sci U S A. 1960 Jan;46(1):118–128. doi: 10.1073/pnas.46.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACKS L. E., ALDERTON G. Behavior of bacterial spores in aqueous polymer two-phase systems. J Bacteriol. 1961 Sep;82:331–341. doi: 10.1128/jb.82.3.331-341.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACKS L. E., BAILEY G. F. DRY RUPTURE OF BACTERIAL SPORES. J Bacteriol. 1963 Mar;85:720–721. doi: 10.1128/jb.85.3.720-721.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMALL G. A. REPORT OF A CASE. RESPIRATORY PARALYSIS AFTER A LARGE DOSE OF INTRAPERITONEAL POLYMYXIN B. AND BACITRACIN. Anesth Analg. 1964 Mar-Apr;43:137–139. [PubMed] [Google Scholar]
- STEWART B. T., HALVORSON H. O. Studies on the spores of aerobic bacteria. I. The occurrence of alanine racemase. J Bacteriol. 1953 Feb;65(2):160–166. doi: 10.1128/jb.65.2.160-166.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]




