Abstract
An advantage in zebrafish is that we can identify spatial and temporal patterns of protein expression using whole-mount immunohistochemistry. To allow primary antibodies to interact with their targets, most tissues must undergo some type of antigen retrieval. Many retrieval techniques have utilized protein-digesting enzymes to access antigens. Here we investigate the use of phospholipase A2 (PLA2) as the sole enzyme for antigen retrieval as well as in combination with low concentrations of proteinase K. Concentrations of proteinase K used with PLA2 are unable to expose the antigen when used as the sole enzyme. We demonstrate that PLA2 is useful for both nuclear and cytoplasmic antigens but not for extracellular matrix components.
Introduction
Whole-mount immunohistochemistry (IHC) is a valuable tool for investigation of protein expression during zebrafish embryonic development. Immunohistochemistry is also an important complement to gene expression, as translation and transcription may not be concurrent. However, there are relatively few zebrafish antibodies available. Instead, immunohistochemical procedures are often accomplished by first identifying antibodies raised in other species that cross-react with the zebrafish antigen. Typically, a number of different antibodies are tested for cross-reactivity using a number of different fixatives and antigen retrieval techniques. This can become expensive because of the number of antibodies tested. As a result, it is advantageous to have a number of permeabilization options and combinations to determine whether an antibody will cross-react with the zebrafish antigen before investigating another antibody.
Often, crosslinking fixatives such as paraformaldehyde results in better tissue preservation, creating a wider range of accessible antigens compared with precipitating fixatives such as methanol or acetone.1 To detect the antigen, especially with older embryos in the second half of embryogenesis, either proteinase K or collagenase are applied before adding the primary antibody.2,3 Required concentrations of either enzyme are dependent on the age of the embryo. A combination of trypsin and hyaluronidase has also been used to retrieve antigens such as collagen within denser tissues such as cartilage.4
To develop a broader range of antigen retrieval options, we investigated the use of secreted phospholipase A2 (sPLA2) from bee venom as a permeabilization agent. There are many roles for secreted phospholipases from venom components to inflammation,5 but bee sPLA2 falls within the IIIA class of phospholipases and specifically cleaves the sn-2-acyl chain to create free fatty acids and lysophospholipids.6 Whole bee venom contains additional peptides that digest extracellular matrix and initiates cell membrane lysis.7 Membrane lysis induced by PLA2 in whole bee venom is stimulated by melittin,8 another venom component, but the enzyme also functions in the purified state.9,10 sPLA2 by itself is unable to lyse erythrocytes,11 suggesting that enzymatic activity may permeabilize membranes but not disrupt cellular architecture.
The use of sPLA2 was investigated both as the sole enzyme for permeabilization or in combination with proteinase K. We hypothesized that use of sPLA2 for whole-mount IHC will permeabilize tissues without substantial antigen disruption by affecting membranes rather than proteins. The effectiveness of PLA2 was assessed using antibodies to a number of different cellular components, including nuclear, cytoplasmic, and extracellular. For each antibody, concentrations of PLA2 were titrated to determine optimal levels of enzyme.
Materials and Methods
Fish stocks
Fish maintenance and matings were performed as previously described.12 AB wild-type fish were used for all procedures.12
IHC
Embryos were fixed in 4% paraformaldehyde for 2 h at room temperature or overnight at 4°C. Whole-mount IHC was performed on these embryos (rather than first peeling skin) for more consistent results and to prevent disturbance of tissue in subsequent washes if skin is removed. Embryos were washed in (phosphate buffered saline with Tween-20 [PBST]; 8 g NaCl, 0.2 g KCL, 1.44 g Na2HPO4, and 0.96 g KH2PO4 in 1 L dH2O, pH 7.4), followed by methanol, and stored at −20°C for at least 1 h for initial permeabilization. Embryos were rehydrated and placed in 1 mL of PBST. Stock solutions of PLA2 (Cat# P9279; Sigma, St. Louis, MO) (0.01 mg/mL) and proteinase K (Cat# P2308; Sigma) (0.5 mg/mL) were created by dissolving in dH2O. The volume was kept constant at 500 μL, and the amount of PLA2 alone or in combination with proteinase K was varied for a 20 min digestion on a rocking platform (volume of enzyme stock added is low enough to not dramatically alter osmolarity of PBST). After permeabilization, embryos were washed three times in PBST for 45 min and placed overnight in primary antibody with 4% goat serum diluted (Cat# S-1000; Vector Laboratories, Burlingame, CA) in PBST. Primary antibodies (summarized in Table 1)are anti-smooth muscle myosin heavy chain [SMMHC] (1:100 dilution; Biomedical Technologies, Stoughton, MA; Cat# BT-562), anti-laminin (1:100 dilution; Sigma; Cat# L9393), anti-cadherin (1:100 dilution; Sigma; Cat# C7638), anti-serotonin (1:500 dilution; Sigma; Cat# S5545), anti-beta-catenin (1:100 dilution; Sigma; Cat# C2206), anti-BrdU (1:100 dilution; Sigma; Cat# B8434), anti-desmin (1:100 dilution; Sigma; Cat# D8281), anti-Phospho-Smad (1:100 dilution; Cell Signaling Technology, Boston, MA; Cat# 9511), anti-Hu C/D (ELAV4) (1:200 dilution; Invitrogen, Carlsbad, CA; Cat# A21271), and anti-acetylated tubulin (1:100 dilution; Sigma; Cat# T7451). Embryos were then washed three times in PBST for 45 min. Secondary antibody (1:500 dilution goat anti-rabbit 488 Alexa fluor Cat# A-11008 and goat anti-mouse 594Alexa Fluor Cat# A11020; Molecular Probes–Invitrogen, Carlsbad, CA) was diluted in 4% goat serum/PBST and added for 2 h. Embryos were washed for 45 min in PBST and observed on an inverted Nikon Eclipse 2000. Images were recorded using IP Lab software.
Table 1.
Antibodies Analyzed for Phospholipase A2 Permeabilization
| Antibody | Vendor | PLA2 treatment |
|---|---|---|
| Smooth muscle myosin heavy chain | Biomedical Technologies, Stoughton, MA | 0.3 μg PLA2/2.5 μg Pro K |
| Laminin | Sigma, St. Louis, MO | Does not work |
| Serotonin | Sigma, St. Louis, MO | 0.18 μg PLA2/2.5 μg Pro K |
| Desmin | Sigma, St. Louis, MO | 0.2 μg PLA2/2.5 μg Pro K |
| Phospho-Smad | Cell Signaling Technology, Boston, MA | 0.3 μg PLA2 |
| Hu C/D (ELAV4) | Invitrogen, Carlsbad, CA | No additional permeabilization |
| BrdU | Sigma, St. Louis, MO | 0.2 μg PLA2 |
| Beta-catenin | Sigma, St. Louis, MO | 0.1 μg PLA2 |
| Cadherin | Sigma, St. Louis, MO | No additional permeabilization |
| Acetylated tubulin | Sigma, St. Louis, MO | No additional permeabilization |
Summary of antibodies tested for use with PLA2. Enzyme treatment used for each of the antibodies is listed in the right column. Antibodies to Hu C/D (ELAV4), cadherin, and acetylated tubulin do not require additional permeabilization for antigen binding. Laminin does not work with any PLA2 treatment.
PLA2, phospholipase A2.
Digestive system observation
The digestive system was observed by dissecting and mounting it as a separate unit. The skin was first peeled and the yolk removed with Dumont #5 forceps (Cat#14095; World Precision Instruments, Sarasota, FL). The pharynx is pulled away from the body of the embryo with the esophagus and intestine, and mounted in Vectashield (Cat# H-1200; Vector Laboratories). The tissue was observed as before.
5-Bromo-2′-deoxyuridine incorporation
BrdU (30 mM, Cat# B5002; Sigma) was injected into the yolk of 53 hpf embryos and incubated for 1 h. Embryos were then fixed in 4% paraformaldehyde for 2 h at room temperature to overnight at 4°C. Embryos were washed in PBST and dehydrated in methanol for at least 1 h at −20°C. Embryos were permeabilized as in the IHC section. The one difference was the incubation of the embryos with 3 N HCL for 30 min (according to the suppliers recommendations) after phospholipase permeabilization.
Histology
Embryos were prepared for histology by infiltration and embedding in glycol methacrylate (JB4 plus; Polysciences, Warrington, PA). Embryos embedded in JB4 were cut in 5 μm cross sections on a Leica RM2135 microtome using a glass knife. Fluorescence was observed on an inverted Nikon Eclipse 2000.
Results
PLA2 unmasks cytoplasmic targets
Enzymes, such as proteinase K, collagenase, and trypsin, are commonly used to unmask antigens.2,4 While digestion of proteins allows antibodies to access the tissue, this can also alter the integrity of cellular components or even cleave the target antigen. As an alternative, we used PLA2 primarily to disrupt membranes to gain access to intracellular antigens.
We used primary antibodies to a number cytoplasmic and nuclear targets to determine what range of targets can be retrieved by PLA2. As embryogenesis progresses, embryos become more difficult to permeabilize, requiring higher concentrations of enzyme for antigen retrieval. We chose larvae at 96 hpf, well into the later half of embryogenesis to better evaluate the usefulness of PLA2.
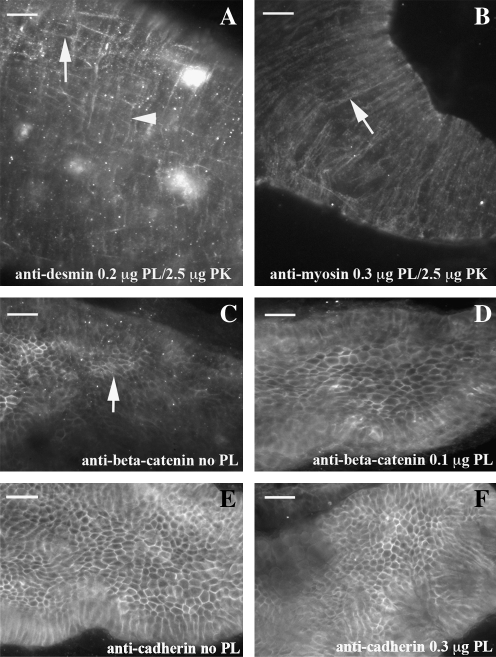
We chose a group of antigens (cadherin, beta-catenin, SMMHC, and desmin) located in the cytoplasm (summarized in Table 1).For each of these antigens, cross-reacting antibodies have been previously identified.2,13 The primary antibody was added without additional retrieval techniques to determine the level of antigen availability. SMMHC and desmin did not show any staining (data not shown). Beta-catenin has partial staining (Fig. 1C), while the cadherin is completely observed (Fig. 1E).
FIG. 1.
PLA2 retrieves cytoplasmic antigens. Patterns within the dissected intestine are shown with either a combination of PLA2 and proteinase K or PLA2 alone using 96 hpf embryos. Desmin IHC (A) reveals a robust and reproducible pattern with a combination of PLA2 and a low concentration of proteinase K. Both longitudinal muscle (arrow) and circular (arrow head) are observed. Smooth muscle myosin heavy chain IHC (B) shows strong staining within circular muscle (arrow) with a combination of PLA2 and proteinase K. Both beta-catenin (C) and cadherin (E) IHC display part or all of the pattern without antigen retrieval. Addition of an intermediate concentration of PLA2 creates an even pattern of beta-catenin (D), while high levels of PLA2 do not result in distortion of the cadherin pattern (F). In all panels, anterior is left and posterior is right. Scale bar is 20 μM at top left corner. PLA2, phospholipase A2; IHC, immunohistochemistry.
PLA2 alone and in combination with low concentrations of proteinase K were used with each antibody. Desmin is not adequately viewed with PLA2 alone; however, in combination with low concentrations of proteinase K (relative to the concentration that would permeabilize the antigen if used as the only enzyme), there is a robust and reproducible staining (Fig. 1A). Addition of PLA2 before primary antibody incubation allows SMMHC staining, while addition of low-concentration proteinase K results in more consistent staining (Fig. 1B). Addition of PLA2 before beta-catenin IHC results in an even pattern throughout the intestine (Fig. 1D). PLA2 used at high concentrations with cadherin IHC does not change the staining pattern (Fig. 1F). To test for background staining, PLA2 was used followed by secondary antibody incubation (without primary antibody). We did not observe background in these conditions. We, however, have not had success using PLA2 for permeabilization before RNA in situ hybridization (data not shown).
PLA2 unmasks nuclear targets
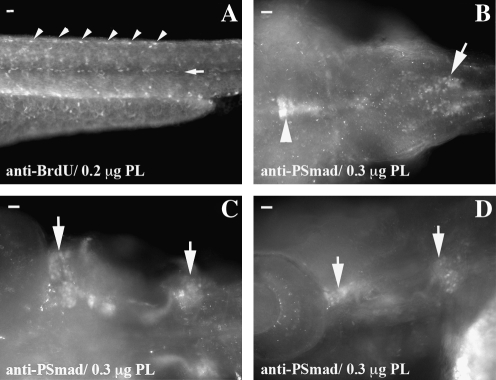
To determine whether PLA2 can permeabilize additional membranes to access the nucleus, we chose a number of antigens with a nuclear component (Hu C/D or ELAV4, serotonin, phospho-Smad, BrdU, and phospo-H3) (summarized in Table 1).Cross-reacting antibodies to each of these antigens have also been previously used.14–17 Primary antibodies were added without any additional retrieval techniques, and only Hu C/D-ELAV4 and phospho H3 produced a full staining pattern. Addition of PLA2 before primary antibody incubation is able to retrieve each of the other antigens (Figs. 2 and 3). Anti-BrdU IHC reveals the complete staining pattern with additional treatment of 3 M HCL (Fig. 2A), while treatment of embryos with only HCL does not produce any of the staining pattern (data not shown). Phospho-Smad also displays the complete staining pattern with PLA2 (Fig. 2B–D).
FIG. 2.
PLA2 retrieves nuclear antigens. Patterns within whole-mount embryos are revealed with PLA2 as the sole enzyme. The pattern of proliferation is identified in 54 hpf embryos injected with BrdU (A). Repeating patterns are observed at somite boundaries (arrowheads) and at somite midline (arrow). Phospho-Smad IHC demonstrates strong nuclear staining in the midbrain (arrowhead B) and hindbrain (arrow B). There is also strong staining in the developing ear (arrows in C and D). In all panels, anterior is left and posterior is right. Scale bar is 20 μM at top left corner.
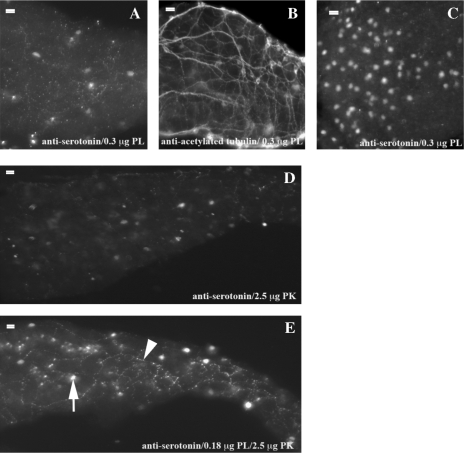
FIG. 3.
Concentrations of PLA2 are different for nuclear and cytoplasmic serotonin antigen retrieval. High concentrations of PLA2 are required for retrieval of nuclear serotonin within the intestine (A) and pharynx (C) but disrupt serotonin within the axons (A). Overall axon structure is not disrupted at this PLA2 concentration as shown by acetylated tubulin IHC (B). Low concentrations of proteinase K partially permeabilize the tissue (D), while in combination with lower concentrations of PLA2, the full pattern of nuclear (arrow) and axon (arrowhead) staining is present (E). In all panels, anterior is left and posterior is right. Scale bar is 20 M at top left corner.
Staining with serotonin after PLA2 treatment reveals the nuclear pattern in developing neurons (Fig. 3A) and pharyngeal endocrine cells (Fig. 3C) but disrupts a portion of the axon staining. This disruption, however, does not appear to affect overall cell integrity as exposure to equivalent concentrations of PLA2 before incubation with anti-acetylated tubulin does not alter the pattern of axon projections (Fig. 3B). Lower PLA2 concentrations before addition of anti-serotonin did not reveal either the axon or nuclear staining. Serotonin is the only antigen of the group with some disruption of the staining pattern.
To avoid disruption of serotonin axonal staining, we lowered the PLA2 concentration and included a low concentration of proteinase K (2.5 μg). Sole use of proteinase K at this concentration reveals only a small portion of the pattern (Fig. 3D). In contrast, combination of PLA2 and proteinase K reveals the entire pattern of serotonin localization in both the nucleus and axons (Fig. 3E).
PLA2 does not retrieve the extracellular antigen laminin
To determine whether PLA2 is able to retrieve antigens in the extracellular matrix, we used an antibody to laminin (summarized in Table 1).Permeabilization with PLA2 alone was not able to produce any of the laminin IHC. We were only able to view the pattern with concentrations of proteinase K that were used in previous experiments (not shown). Addition of PLA2 in combination with any of the proteinase K concentrations did not demonstrate any changes in the staining pattern that we observed with cellular antigens.
Discussion
Embryos in the later half of embryogenesis often require some type of antigen retrieval for whole-mount IHC. Many previous methods rely on digestion of proteins to allow antibodies access to the antigen. While enzymes such as proteinase K are effective in this process, retrieval of some antigens require digestion conditions that begin to affect tissue integrity or may begin to cleave the antigen. As an alternative, we used PLA2 as a way to permeabilize membranes. We find that this technique is effective for gaining access to both cytoplasmic and nuclear antigens. After IHC is performed on whole-mount embryos, they can also easily be embedded and sectioned to observe the arrangement of antigen-containing cells within organs.
The use of detergents such as Triton X-100 and Tween-20 also function to permeabilize or remove cellular membranes. While these detergents have the ability to expose some antigens, during the later half of embryogenesis these effects are not consistent and often develop background staining. The enzymatic activity of PLA2 may result in more of a partial membrane removal to produce consistent IHC with little background staining.
One important question was whether treatment of tissues with PLA2 would distort the architecture of the cells. We began with a number of antibodies that had previously been demonstrated to cross-react with zebrafish antigens. A group of these antibodies were able to bind their target without further antigen retrieval. With this group we found that the antigen distribution was nearly identical with and without concentrations of PLA2 used to permeabilize the other antigens. While this demonstrates that antibodies are already able to penetrate most of the cell before additional permeabilization, it also suggests that PLA2 treatment does not dramatically change cell architecture, making this a useful retrieval technique.
With some of the antigens, PLA2 was used as the sole enzyme for antigen retrieval. Nuclear antigens were more consistently retrieved when PLA2 was used at levels between 0.2 μg and 0.3 μg. In contrast, with cytoplasmic antigens PLA2 may not be adequate as a sole enzyme and require the addition of another permeabilization technique. Cytoplasmic antigens often required proteinase K or worked better in combination with the two enzymes. The concentrations of proteinase K used in combination with PLA2 do not expose the antigen when used as the sole enzyme. We find that the combination of PLA2 and proteinase K creates highly reproducible antigen retrieval for the cytoplasmic antigens.
The serotonin antibody was the one staining that produced some disrupted pattern within the axons at concentrations of PLA2 used to retrieve the nuclear pattern. Axons, however, do not appear to be generally disrupted, as the concentration of PLA2 used with anti-serotonin does not alter the pattern of acetylated tubulin. Secreted phospholipases have different membrane affinities due to composition,6 and bee phospholipase may have greater affinity for vesicle membranes containing serotonin as opposed to other cellular membranes. The disruption, however, is not so severe that lower concentrations of both PLA2 and proteinase K can produce robust and highly reproducible stainings equal to or better than using proteinase K alone.
While PLA2 is useful for retrieval of cellular antigens, it may not be useful for extracellular antigens. Anti-laminin is unique among the antibodies tested in that there was no pattern without additional permeabilization, and PLA2 did not affect the staining with or without proteinase K. Each of the other antibodies either stain with either PLA2 alone, in combination with proteinase K, or without additional permeabilization. This would be expected because there are no membranes to disrupt in the extracellular matrix. Exposure of antigens in the extracellular matrix seems to be more suited for previously developed techniques using trypsin and hyaluronidase.4
Overall, PLA2 can be used for the retrieval of a variety of cytoplasmic and nuclear antigens in whole-mount immunohistochemistry. This technique is a useful alternative for antigens requiring high levels of protein digestion enzymes affecting tissue integrity and architecture. PLA2 on its own does not cause significant disruption of cellular architecture allowing for use at high concentrations and in combination with other digestion methods to produce consistent staining patterns.
Acknowledgment
This work was supported by the NIH Grant 1R15DK 074416-01A1 to K.N.W.
Disclosure Statement
No competing financial interests exist.
References
- 1.Larsson L-I. Immunocytochemistry: Theory and Practice. Boca Raton, FL: CRC Press; 1988. [Google Scholar]
- 2.Wallace KN. Pack M. Unique and conserved aspects of gut development in zebrafish. Dev Biol. 2003;255:12–29. doi: 10.1016/s0012-1606(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 3.Novak AE. Ribera AB. Immunocytochemistry as a tool for zebrafish developmental neurobiology. Methods Cell Sci. 2003;25:79–83. doi: 10.1023/B:MICS.0000006894.43940.b1. [DOI] [PubMed] [Google Scholar]
- 4.Nüsslein-Volhard C. Dahm R. Zebrafish: a Practical Approach. 1st. Oxford, NY: Oxford University Press; 2002. [Google Scholar]
- 5.Valentin E. Lambeau G. Increasing molecular diversity of secreted phospholipases A(2) and their receptors and binding proteins. Biochim Biophys Acta. 2000;1488:59–70. doi: 10.1016/s1388-1981(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 6.Berg OG. Gelb MH. Tsai MD. Jain MK. Interfacial enzymology: the secreted phospholipase A(2)-paradigm. Chem Rev. 2001;101:2613–2654. doi: 10.1021/cr990139w. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez JM. Ownby CL. Skeletal muscle degeneration induced by venom phospholipases A2: insights into the mechanisms of local and systemic myotoxicity. Toxicon. 2003;42:915–931. doi: 10.1016/j.toxicon.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Mingarro I. Perez-Paya E. Pinilla C. Appel JR. Houghten RA. Blondelle SE. Activation of bee venom phospholipase A2 through a peptide-enzyme complex. FEBS Lett. 1995;372:131–134. doi: 10.1016/0014-5793(95)00964-b. [DOI] [PubMed] [Google Scholar]
- 9.Alix SN. Woodbury DJ. Phospholipase A2 action on planar lipid bilayers generates a small, transitory current that is voltage independent. Biophys J. 1997;72:247–253. doi: 10.1016/S0006-3495(97)78663-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ameratunga RV. Hawkins R. Prestidge R. Marbrook J. A high efficiency method for purification and assay of bee venom phospholipase A2. Pathology. 1995;27:157–160. doi: 10.1080/00313029500169782. [DOI] [PubMed] [Google Scholar]
- 11.Watala C. Kowalczyk JK. Hemolytic potency and phospholipase activity of some bee and wasp venoms. Comp Biochem Physiol. 1990;97:187–194. doi: 10.1016/0742-8413(90)90191-b. [DOI] [PubMed] [Google Scholar]
- 12.Westerfield M. The zebrafish book: a Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) Eugene, OR: M. Westerfield; 1993. [Google Scholar]
- 13.Wallace KN. Dolan AC. Seiler C. Smith EM. Yusuff S. Chaille-Arnold L, et al. Mutation of smooth muscle myosin causes epithelial invasion and cystic expansion of the zebrafish intestine. Dev Cell. 2005;8:717–726. doi: 10.1016/j.devcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Bisgrove BW. Raible DW. Walter V. Eisen JS. Grunwald DJ. Expression of c-ret in the zebrafish embryo: potential roles in motoneuronal development. J Neurobiol. 1997;33:749–768. [PubMed] [Google Scholar]
- 15.Olden T. Akhtar T. Beckman SA. Wallace KN. Differentiation of the zebrafish enteric nervous system and intestinal smooth muscle. Genesis. 2008;46:484–498. doi: 10.1002/dvg.20429. [DOI] [PubMed] [Google Scholar]
- 16.Pietsch J. Delalande JM. Jakaitis B. Stensby JD. Dohle S. Talbot WS, et al. Lessen encodes a zebrafish trap100 required for enteric nervous system development. Development. 2006;133:395–406. doi: 10.1242/dev.02215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tucker JA. Mintzer KA. Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]