Malaria parasites have a complex life cycle that requires invasion of several different cell types in both vertebrate and mosquito hosts. In Plasmodium falciparum, the merozoite must attach to and invade a new erythrocyte in order to continue parasite development in the blood of an infected host. Merozoite entry into erythrocytes is a multi-step process requiring merozoite adhesion, re-orientation, junction formation, and invasion [1]. Each step is thought to be mediated by the coordinated interactions of numerous specific merozoite ligands and erythrocyte surface receptors [2]. A number of mediators involved in the invasion process are positioned on the merozoite surface and in the organelles of the apical complex. However, the functions of molecules involved in this interaction are still poorly characterized.
Understanding the complex process of P. falciparum merozoite invasion requires identification and characterization of numerous potential parasite ligands and their potential interactions. MAEBL is a paralogue of the DBL-EBP family (Duffy binding like-erythrocyte binding protein), but has a chimerical structure and shares similarity with AMA1 [3]. M1 and M2 are tandem cysteine-rich regions with similarity to AMA1 and are present in the N-terminal portion of the MAEBL ectodomain. MAEBL was identified in P. yoelii and P. falciparum blood stage parasites as a minor membrane protein with erythrocyte binding activity expressed in the apical organelles and on the surface of invasive merozoites [3–6]. Expressed abundantly in sporozoites, MAEBL appears to be important for sporozoite invasion into the mosquito salivary glands and in establishing exoerythrocytic schizonts [6–12]. It has been reported that sera from infected individuals living in a malaria endemic region of western Kenya recognized M2 recombinant antigen and had the ability to inhibit M2-erythrocyte binding [6]. However, whether MAEBL is essential or even has a significant role for erythrocytic stage growth is unclear.
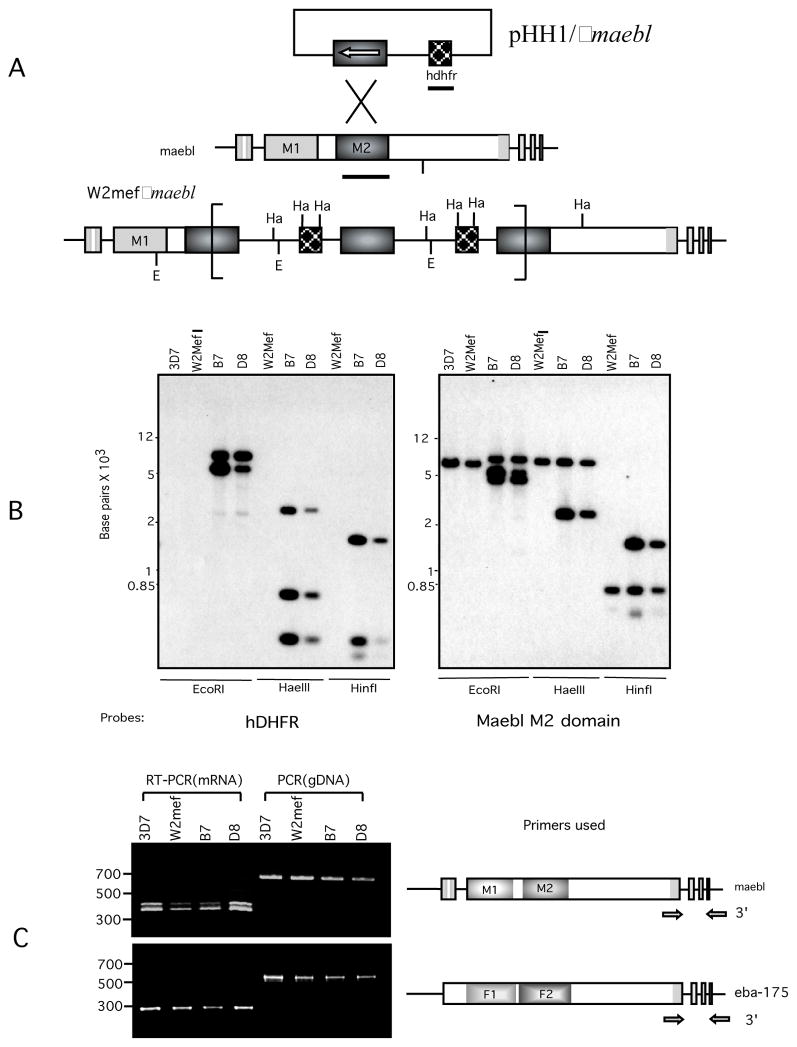
To evaluate MAEBL expression in P. falciparum erythrocyte stages and its potential role in merozoite invasion of erythrocytes, maebl disrupted parasite lines were created and examined for possible importance of MAEBL in erythrocytic invasion pathways (Fig. 1A). Homologous integration into the maebl locus was carried out by using the plasmid pHH1/Δmaebl containing the selection marker hDHFR and targeting sequence that disrupted maebl coding sequence (CDS) after the second ligand domain (M2). A cloned parasite line of the P. falciparum W2mef isolate was transfected with this construct and two independent clones, B7 and D8, were obtained through intermittent selection with WR99210. In order to confirm that pHH1/hDHFRΔmaebl had integrated through single crossover homologous recombination into the maebl locus, genomic DNA from parental wild-type W2mef and W2mef Δmaebl clones were analyzed by Southern blot hybridization probed with hDHFR and maebl M2 CDS (Fig. 1B). Integration of multiple copies of the whole plasmid into the maebl locus was observed in B7 and D8 clones.
Figure 1.
(A) The region of maebl encoding the M2 domain was cloned into the XhoI site of plasmid pHH1/hDHFR and introduced into P. falciparum W2mef parasites by electroporation as described previously [21]. Parasites were cultivated using standard techniques in the presence of 10 nM WR99210 in order to select for parasites carrying the plasmid and resulted in two independent maebl knockout clones designated B7 and D8. EcoRI(E) and HaeIII (Ha) restriction sites used in mapping the plasmid integration events are shown. Solid bars indicate the probes from maebl M2 region and hdhfr used for Southern blot analysis in B. (B) Southern blot hybridizations were performed using standard protocols to confirm integration in the maebl locus [5]. 2 μg genomic DNA from P. falciparum 3D7(reference clone), w2mef, and Δmaebl clones was digested with EcoRI, HaeIII or HinfI. The restricted fragments were separated by 0.8% agarose gel, transferred to nylon membranes, and hybridized with 32P-labeled probes of hdhfr and maebl M2 domain. Clones B7 and D8 showed disruption of the endogenous maebl locus. (C) Products from PCR and RT-PCR reaction in P. falciparum clones (3D7, W2mef) and W2mefΔmaebl clones (B7, D8) were analyzed by agarose gel electrophoresis. Approximate locations of each primer pair are shown as arrows.
RT-PCR analysis in parental and maebl clones indicated that maebl continued to be actively transcribed down stream to the disruption site (Fig. 1C). In order to determine if normal mRNA splicing occurred we analyzed primary transcript in the region at the 3′ end of maebl spanning introns 2–4. Comparable RT-PCR analysis of eba-175 was included as a control. Similarly spliced transcripts were detected in both wild-type (3D7, W2mef) and Δmaebl clones (B7, D8) of P. falciparum. Standard PCR was performed as a reference for the size of the unspliced primary transcript or genomic DNA. We detected both the canonical ORF1 mRNA and alternative ORF2 mRNA of splicing patterns of the maebl 3′ exons (Fig. 1C), which were verified by sequence analysis (data not shown).
Despite the gene disruption, splicing of the 3′ exons was indistinguishable from that of the intact gene of wild-type parasites. The 3′ end of the gene was still actively transcribed and the mRNA processed in a manner indistinguishable from that in the wild-type parasites. Orientation of the hDHFR selection cassette within the plasmid is inverse to the ORF of the maebl targeting sequence [13], so the 5′ CAM regulatory region lies adjacent to the 3′ CDS of the disrupted maebl locus. This strong bidirectional promoter is likely driving mRNA synthesis of the 3′ end of maebl after the disruption instead of the resident maebl promoter, which would be over 20,000 nt upstream [14]. In higher eukaryotes, transcription and pre-mRNA processing are functionally linked so analogous experimental changes in promoter structure have been shown to alter splicing patterns, presumably through altering associated co-factors responsible for splicing [15–18]. Our results show that definition of exon structure, including recognition of suboptimal alternative splicing junctions, occurs independently of promoter usage. This suggests that maebl pre-mRNA processing is an inherent property of the RNA.
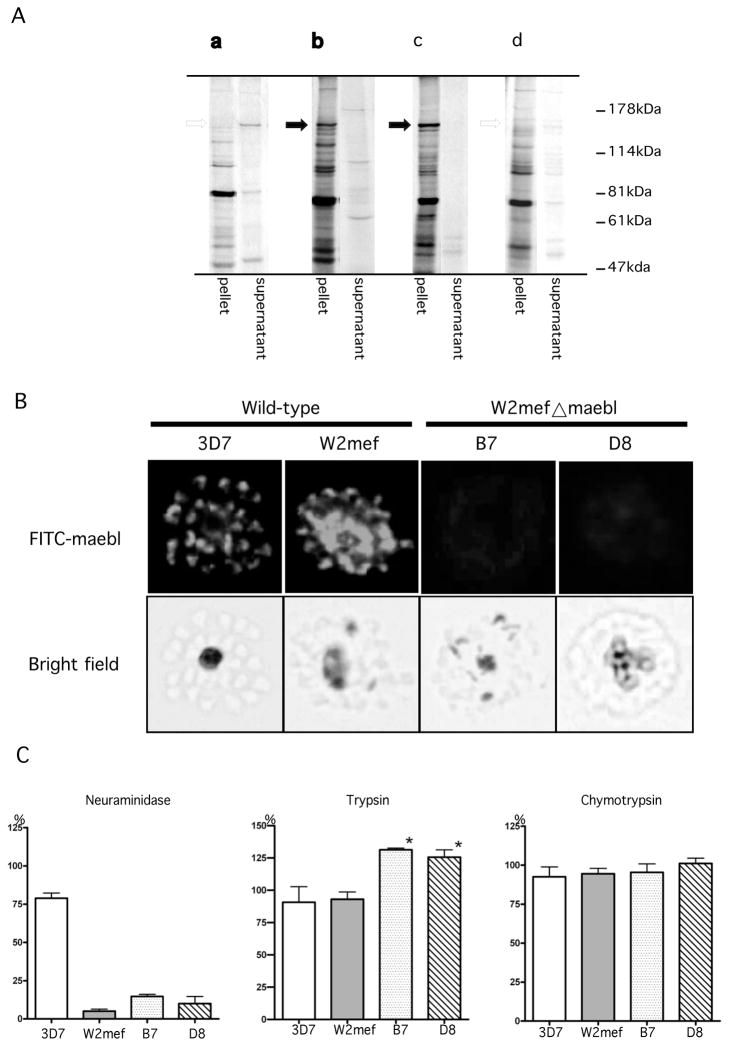
We confirmed expression of MAEBL in P. falciparum erythrocytic stages using a newly developed monoclonal antibody (MAB) 2C11 against the M2 domain. Anti-M2 MAB 2C11 identified MAEBL as a 140~155 kDa protein present in 3D7 and W2mef, but this protein was absent in the Δmaebl parasite clones (Fig. 2A). MAB 2C11 identified a MAEBL protein migrating at 140–155 kDa (Fig. 2Ab), which was immunoprecipitated from the SDC-extracted parasite fraction but not the TX-100-extracted parasite fraction (Fig. 2Aa). This result was consistent with previous studies that identified the mature form of MAEBL as a 140/150-kDa protein in erythrocyte stages of P. falciparum and P. yoelii, using antisera to the M2 or C-CYS regions, respectively [6, 19]. These MAEBL products in schizonts were soluble in ionic detergent, but not non-ionic detergent. Targeted disruption of maebl in P. falciparum caused a total loss of this 140~155 kDa protein expressed in erythrocytic stages as determined by immunoprecipitation using MAB 2C11. Indirect immunofluorescence assays using MAB 2C11 against MAEBL in wild-type parasites showed punctate apical fluorescence in free merozoites and in late stage schizonts of 3D7 and W2mef, but not B7 and D8 clones (Fig. 2B). No reactivity was observed in either ring stages or trophozoite-infected erythrocytes (data not shown). The insertion of the plasmid in the maebl locus disrupted its coding sequence after the M2 ligand domain and so a truncated form MAEBL was expected. However, unlike a similar disruption of eba-175 [20–22], such a truncated MAEBL was not detected. There is a significant difference in transcript abundance as well as the time of expression between maebl and eba175, which may account for the total loss of the MAEBL protein [12, 23].
Figure 2.
(A) Immunoprecipitation of 35S-Met/Cys metabolically labeled 3D7 parasites with MAB 2C11(a, b). The MAB 2C11 precipitated MAEBL only from the detergent extract containing the ionic detergent sodium deoxycholate (SDC)(b). MAB 2C11 immunoprecipitated the 140~155 kDa MAEBL protein from the SDC-extracted fraction of W2mef parasites (c), but not from the W2mef maebl clone D8 (d). (B) Indirect Immunofluoresent assay (IFA). Schizont-enriched P. faciparum infected erythrocytes were smeared on glass slides and air-dried. Slides were fixed in 1% formaldehyde, preincubated with 3% BSA 1%Triton in PBS, and then reacted with primary antibody (MAB 2C11). Secondary antibody was FITC-conjugated goat anti-mouse IgG antibody. The slides were mounted and viewed by fluorescent microscopy. Anti-MAEBL MAB 2C11 reacted with late stage schizonts in wild-type 3D7 and W2mef, demonstrating a punctuate apical localization pattern. Consistent with the immunoprecipitation results the MAB did not react with the Δmaebl clones. (C) Comparison of parasite growth rates in untreated and enzyme-treated erythrocytes between wild-type and Δmaebl clones. Prior to the assay, parasites were propagated in 25 cm2 flasks in 5 ml cultures using standard methods in RPMI 1640 with 5% hematocrit. A+ human erythrocytes were treated with either 1mg/ml trypsin (TPCK treated), 1mg/ml chymotrypsin type-VII, or 0.5 Unit/ml neuraminidase type II for 1 hr at 37°C, followed by treatment with the appropriate protease inhibitor for 10 min, and washed with RPMI-1640. Parasites were grown at 37°C in 96 well culture plates placed in a sealed environmental chamber gassed daily with a mix of 5% CO2/5% O2/90% N2. The parasitemia was counted microscopically after 60 hours of incubation with the treated erythrocytes. The percentage of growth represents the average of three independent assays. Asterisk (*) indicates the treatment groups for Δmaebl clones B7 and D8 that had a significant difference (p<0.05 ANOVA) in growth in trypsin-treated erythrocytes compared to W2mef and 3D7.
The ability to disrupt P. falciparum maebl demonstrated that MAEBL is not required for parasite survival in blood-stage development under ideal culture conditions, but MAEBL may have a conditional role in P. falciparum blood-stage growth evident under certain conditions. Erythrocyte invasion by P. falciparum is a very complex process that requires multiple ligand-receptor interactions, with apparent redundancies at different steps and with different pathways, so phenotypes may not be evident except under restrictive growth conditions. To explore the possible role of MAEBL in P. falciparum blood-stage development, we determined the invasion phenotype of the W2mef Δmaebl clones by an in vitro assay that measured their short-term growth rates in intact and enzyme-treated human erythrocytes (Fig. 2C). Neuraminidase (N), trypsin (T), and chymotrypsin (C) were used to treat A+ human erythrocytes. W2mef, the parent clone of the Δmaebl, and clone 3D7 of P. falciparum were included for reference. Invasion rates were inferred from the growth rates, which were determined relative to growth rates in intact untreated erythrocytes. Assay data were averaged from results of at least three experiments and standard error was calculated for each (Fig. 2C). Although growth rates of B7 and D8 in neuraminidase-treated erythrocytes (14.8%, 10.0%, respectively) were consistently higher than W2mef (5.1%), these differences were not significant. Surprisingly, the growth of Δmaebl clones B7 and D8 in trypsin-treated erythrocytes was higher compared to W2mef (131%, 126%, respectively). These differences in B7 and D8 growth rates for trypsin-treated erythrocytes were significant compared to W2mef and 3D7 (p<0.05 ANOVA). Similar results were found when using the 3H-hypoxanthine incorporation method [24] to measure the growth of parasites (data not shown). There was no significant difference in any of these four clones when grown in chymotrypsin-treated erythrocytes.
The enzyme treatments employed in this study commonly are used as a tool to characterize Plasmodium merozoite invasion pathways: sialic acid-dependent/trypsin-sensitive (associated with glycophorin A); sialic acid-dependent/trypsin-insensitive (glycophorin B); and sialic acid-independent/trypsin-sensitive (receptor X)[24]. Using these defined invasion pathways, the Δmaebl clones exhibited a minor erythrocyte invasion phenotype compared to their parental clone W2mef. Erythrocyte invasion by both W2mef and Δmaebl clones were almost eliminated by treatment with neuraminidase, indicating that invasion is dependent on sialic acid residues on the erythrocyte surface (Fig. 2C left). However, invasion into erythrocytes treated with trypsin was significantly enhanced in the parasites with the disrupted maebl gene (Fig. 2C middle). This invasion pathway was different from the glycophorin B pathway, because treatment by chymotrypsin (which removes glycophorin B) had no effect on invasion in these parasites (Fig. 2C right). This result showed that loss of MAEBL expression in merozoites alters the W2mef invasion pathway to a novel alternative pathway, which is sialic acid dependent and trypsin insensitive. This pathway may be similar to several isolates of P. falciparum (e.g. Indochina I) that invade erythrocytes through a sialic acid dependent/trypsin-resistant pathway independent of glycophorin B [24]. The enhanced invasion of MAEBL null parasites by this alternative pathway presents the possibility that some ligands such as MAEBL have a function to control or restrict invasion mechanisms or processes of other invasion-related proteins.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (R01 AI33656). Peter Blair was supported by a NIH Predoctoral Fellowship in Experimental Parasitology and Vector Biology (T32 A10703018). Fabian Saenz is supported in part by a Fulbright/Western Hemisphere Fellowship and a Kellogg Institute Graduate Fellowship Award from the University of Notre Dame.
References
- 1.Aikawa M, Miller LH. Structural alteration of the erythrocyte membrane during malarial parasite invasion and intraerythrocytic development. Ciba Found Symp. 1983;94:45–63. doi: 10.1002/9780470715444.ch4. [DOI] [PubMed] [Google Scholar]
- 2.Cowman AF, Crabb BS. The Plasmodium falciparum genome--a blueprint for erythrocyte invasion. Science. 2002;298:126–8. doi: 10.1126/science.1078169. [DOI] [PubMed] [Google Scholar]
- 3.Kappe SHI, Noe AR, Fraser TS, Blair PL, Adams JH. A family of chimeric erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA. 1998;95:1230–35. doi: 10.1073/pnas.95.3.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kappe SHI, Curley GP, Noe AR, Dalton JP, Adams JH. Erythrocyte binding protein homologues of rodent malaria parasites. Mol Biochem Parasitol. 1997;89:137–48. doi: 10.1016/s0166-6851(97)00113-8. [DOI] [PubMed] [Google Scholar]
- 5.Blair PL, Kappe SH, Maciel JE, Balu B, Adams JH. Plasmodium falciparum MAEBL is a unique member of the ebl family. Mol Biochem Parasitol. 2002;122:35–44. doi: 10.1016/s0166-6851(02)00067-1. [DOI] [PubMed] [Google Scholar]
- 6.Ghai M, Dutta S, Hall T, Freilich D, Ockenhouse CF. Identification, expression, and functional characterization of MAEBL, a sporozoite and asexual blood stage chimeric erythrocyte-binding protein of. Mol Biochem Parasitol. 2002;123:35–45. doi: 10.1016/s0166-6851(02)00123-8. [DOI] [PubMed] [Google Scholar]
- 7.Kappe SH, Gardner MJ, Brown SM, et al. Exploring the transcriptome of the malaria sporozoite stage. Proc Natl Acad Sci USA. 2001;98:9895–900. doi: 10.1073/pnas.171185198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kariu T, Yuda M, Yano K, Chinzei Y. MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. J Exp Med. 2002;195:1317–23. doi: 10.1084/jem.20011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preiser PR, Renia L, Singh N, et al. Antibodies Against MAEBL Ligand Domains M1 and M2 Inhibit Sporozoite Development In Vitro. Infection and Immunity. 2004 doi: 10.1128/IAI.72.6.3604-3608.2004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan P, Abraham EG, Ghosh AK, et al. Analysis of the Plasmodium and Anopheles transcriptomes during oocyst differentiation. J Biol Chem. 2004;279:5581–7. doi: 10.1074/jbc.M307587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Florens L, Washburn MP, Raine JD, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–6. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 12.Le Roch KG, Zhou Y, Blair PL, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–8. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 13.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–9. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 14.Wickham ME, Thompson JK, Cowman AF. Characterization of the merozoite surface protein-2 promoter using stable and transient transfection in Plasmodium falciparum. Mol Biochem Parasitol. 2003;129:147–56. doi: 10.1016/s0166-6851(03)00118-x. [DOI] [PubMed] [Google Scholar]
- 15.Cramer P, Pesce CG, Baralle FE, Kornblihtt AR. Functional association between promoter structure and transcript alternative splicing. Proc Natl Acad Sci USA. 1997;94:11456–60. doi: 10.1073/pnas.94.21.11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts GC, Gooding C, Mak HY, Proudfoot NJ, Smith CW. Co-transcriptional commitment to alternative splice site selection. Nucleic Acids Res. 1998;26:5568–72. doi: 10.1093/nar/26.24.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cramer P, Caceres JF, Cazalla D, et al. Coupling of transcription with alternative splicing: RNA pol II promoters modulate SF2/ASF and 9G8 effects on an exonic splicing enhancer. Mol Cell. 1999;4:251–8. doi: 10.1016/s1097-2765(00)80372-x. [DOI] [PubMed] [Google Scholar]
- 18.Kadener S, Fededa JP, Rosbash M, Kornblihtt AR. Regulation of alternative splicing by a transcriptional enhancer through RNA pol II elongation. Proc Natl Acad Sci USA. 2002;99:8185–90. doi: 10.1073/pnas.122246099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noe AR, Adams JH. Plasmodium yoelii YM MAEBL protein is coexpressed and colocalizes with rhoptry proteins. Mol Biochem Parasitol. 1998;96:27–35. doi: 10.1016/s0166-6851(98)00084-x. [DOI] [PubMed] [Google Scholar]
- 20.Duraisingh MT, Maier AG, Triglia T, Cowman AF. Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. Proc Natl Acad Sci USA. 2003;100:4796–801. doi: 10.1073/pnas.0730883100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed MB, Caruana SR, Batchelor AH, et al. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid-independent pathway of invasion. Proc Natl Acad Sci USA. 2000;97:7509–14. doi: 10.1073/pnas.97.13.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko O, Fidock DA, Schwartz OM, Miller LH. Disruption of the C-terminal region of EBA-175 in the Dd2/Nm clone of Plasmodium falciparum does not affect erythrocyte invasion. Mol Biochem Parasitol. 2000;110:135–46. doi: 10.1016/s0166-6851(00)00263-2. [DOI] [PubMed] [Google Scholar]
- 23.Blair PL, Witney A, Haynes JD, et al. Transcripts of developmentally regulated Plasmodium falciparum genes quantified by real-time RT-PCR. Nucleic Acids Res. 2002;30:2224–31. doi: 10.1093/nar/30.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolan SA, Proctor JL, Alling DW, et al. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol Biochem Parasitol. 1994;64:55–63. doi: 10.1016/0166-6851(94)90134-1. [DOI] [PubMed] [Google Scholar]