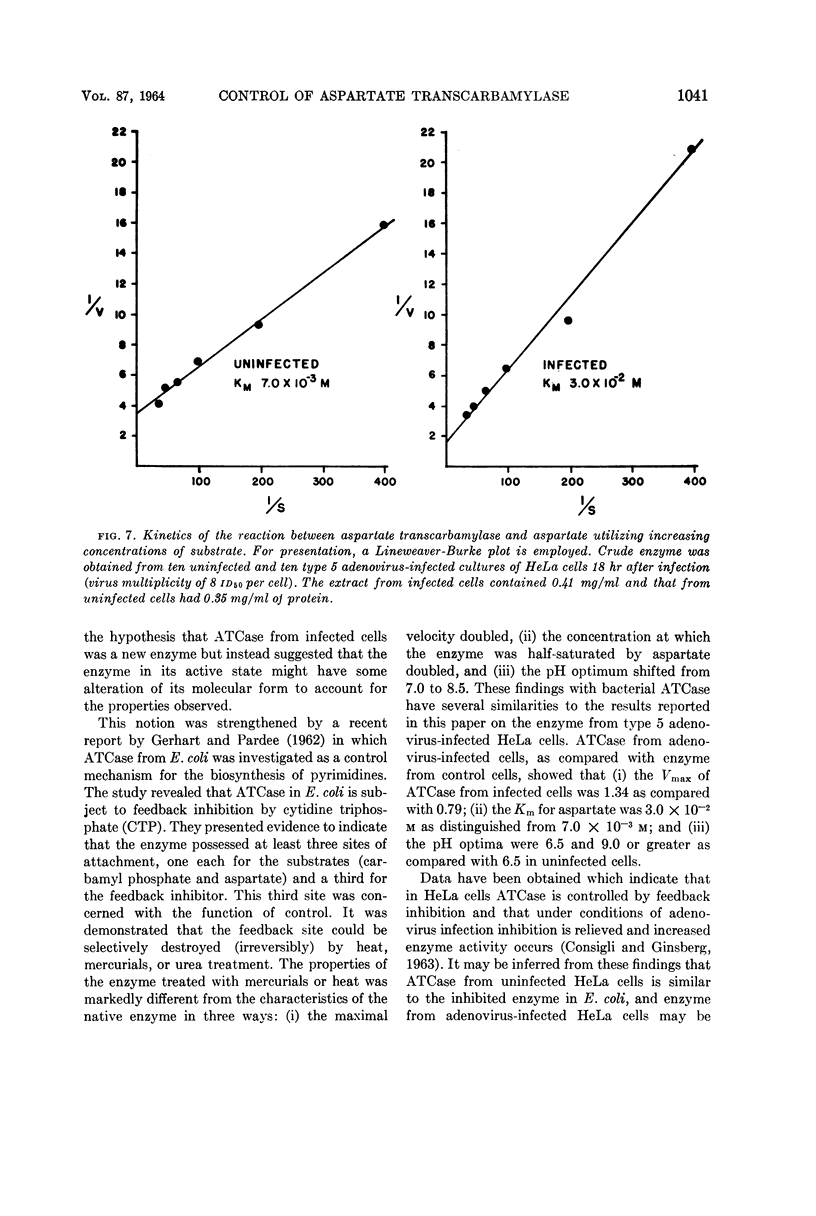
Abstract
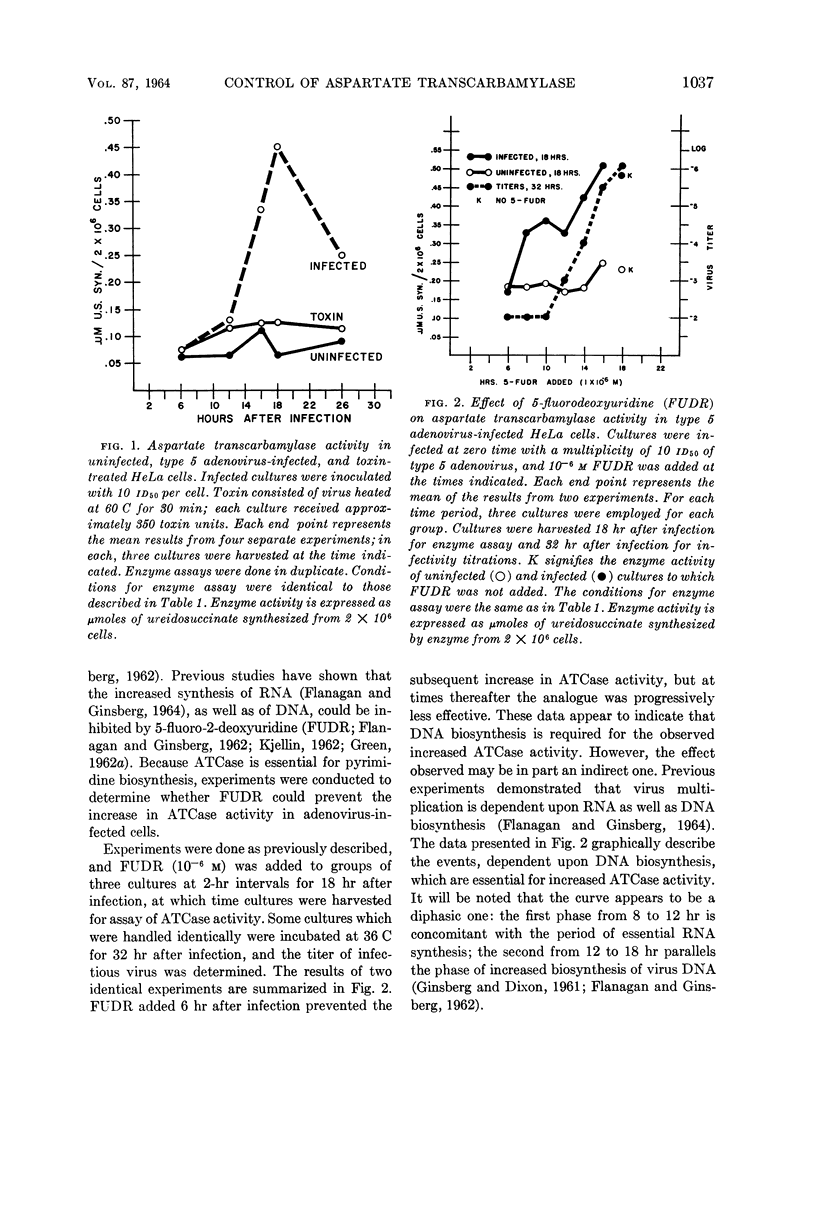
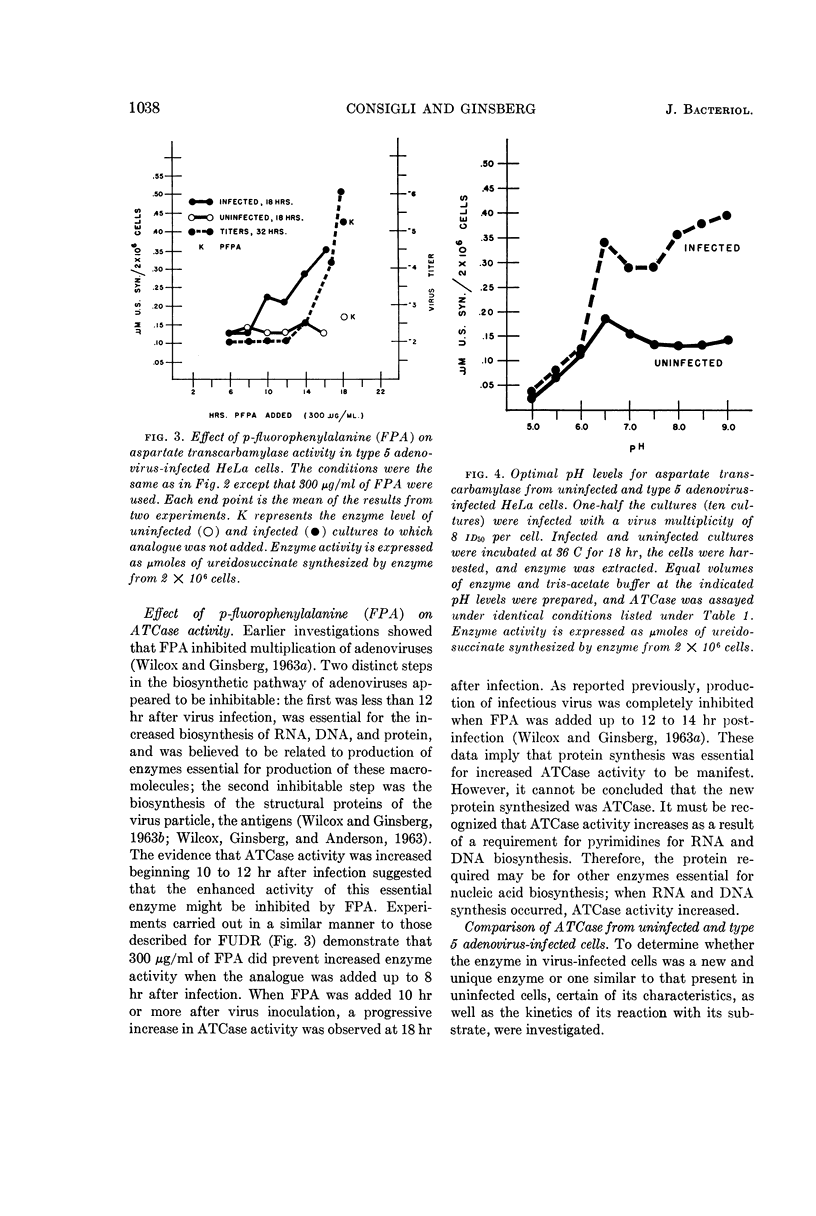
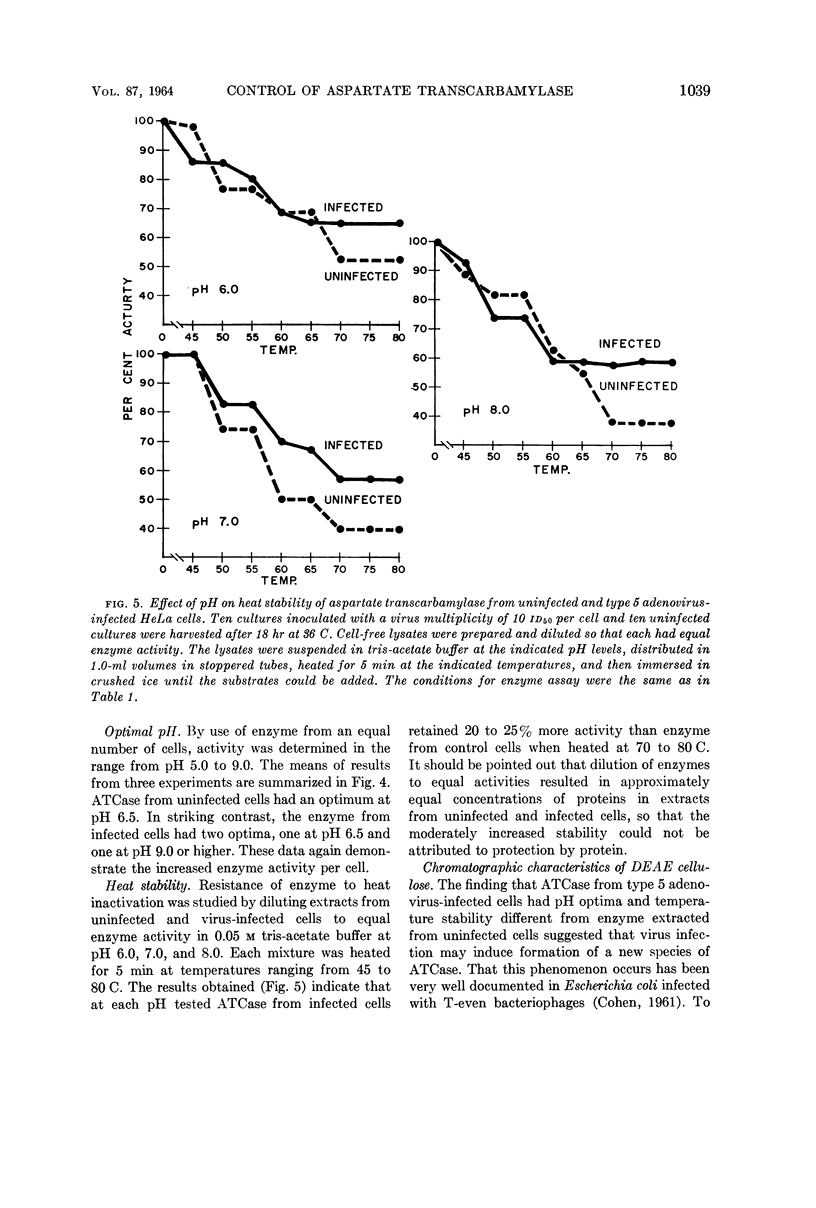
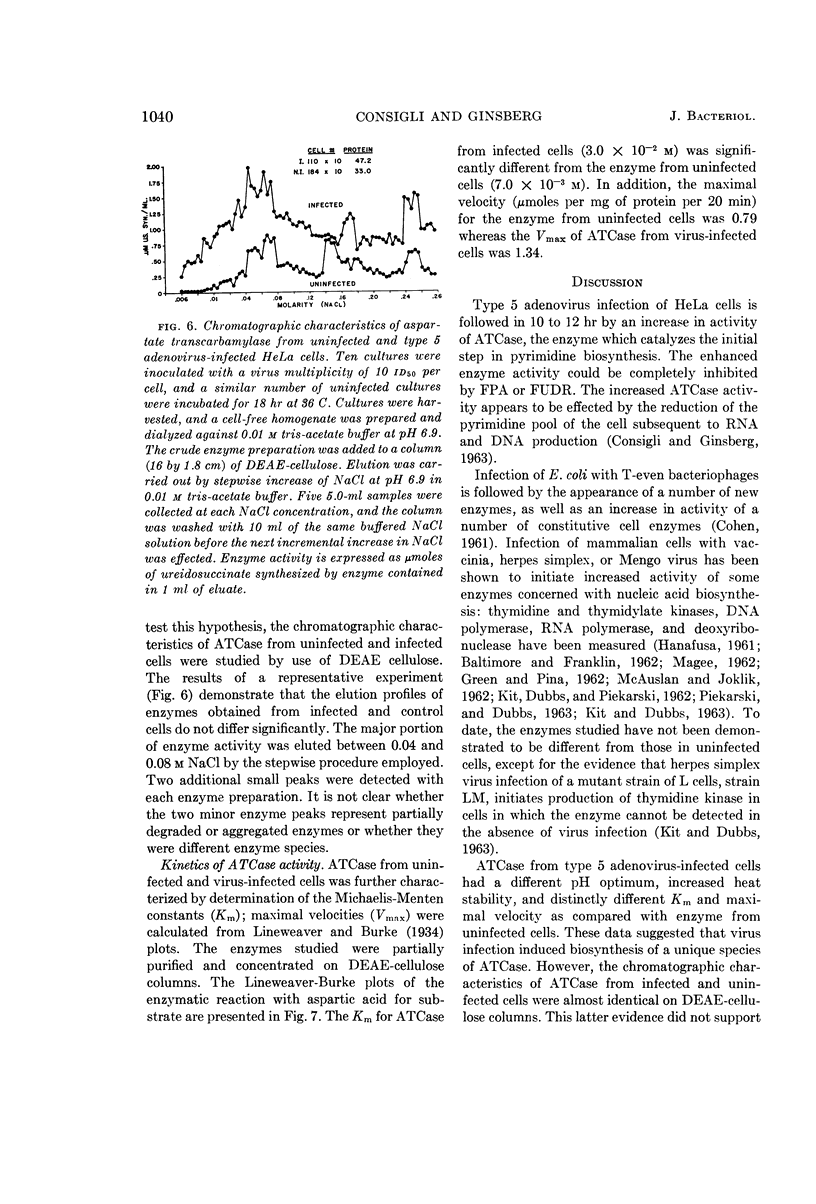
Consigli, Richard A. (University of Pennsylvania, Philadelphia), and Harold S. Ginsberg. Activity of aspartate transcarbamylase in uninfected and type 5 adenovirus-infected HeLa cells. J. Bacteriol. 87:1034–1043. 1964.—A two- to three-fold increase in aspartate transcarbamylase (ATCase) activity was observed in type 5 adenovirus-infected HeLa cells 18 hr after infection. The enhanced enzyme activity was virus-specific and dependent on biosynthesis of deoxyribonucleic acid and protein. When various characteristics as well as the kinetics of the enzymes from uninfected and infected cells were compared, ATCase from adenovirus-infected cells was shown to have an altered pH optimum, greater heat stability, increased maximal velocity, and increased Km value for aspartate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALTIMORE D., FRANKLIN R. M. The effect of Mengovirus infection on the activity of the DNA-dependent RNA polymerase of L-cells. Proc Natl Acad Sci U S A. 1962 Aug;48:1383–1390. doi: 10.1073/pnas.48.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER G. S., LEUCHTENBERGER C., GINSBERG H. S. Cytological and cytochemical studies of HeLa cells infected with adeno-viruses. J Exp Med. 1957 Mar 1;105(3):195–216. doi: 10.1084/jem.105.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. S. Virus-induced acquisition of metabolic function. Fed Proc. 1961 Jul;20:641–649. [PubMed] [Google Scholar]
- EVERETT S. F., GINSBERG H. S. A toxin-like material separable from type 5 adenovirus particles. Virology. 1958 Dec;6(3):770–771. doi: 10.1016/0042-6822(58)90123-5. [DOI] [PubMed] [Google Scholar]
- FLANAGAN J. F., GINSBERG H. S. Synthesis of virus-specific polymers in adenovirus-infected cells; effect of 5-fluorodeoxyuridine. J Exp Med. 1962 Aug 1;116:141–157. doi: 10.1084/jem.116.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. F., Ginsberg H. S. Role of ribonucleic acid biosynthesis in multiplication of type 5 adenovirus. J Bacteriol. 1964 May;87(5):977–987. doi: 10.1128/jb.87.5.977-987.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- GINSBERG H. S., BADGER G. F., DINGLE J. H., JORDAN W. S., Jr, KATZ S. Etiologic relationship of the RI-67 agent to acute respiratory disease (ARD). J Clin Invest. 1955 Jun;34(6):820–831. doi: 10.1172/JCI103137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBERG H. S., DIXON M. K. Deoxyribonucleic acid (DNA) and protein alterations in HeLa cells infected with type 4 adenovirus. J Exp Med. 1959 Apr 1;109(4):407–422. doi: 10.1084/jem.109.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBERG H. S., DIXON M. K. Nucleuc acid synthesis in types 4 and 5 adenovirus-infected HeLa cells. J Exp Med. 1961 Feb 1;113:283–299. doi: 10.1084/jem.113.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBERG H. S., GOLD E., JORDAN W. S., Jr Tryptose phosphate broth as supplementary factor for maintenance of HeLa cell tissue cultures. Proc Soc Exp Biol Med. 1955 May;89(1):66–71. doi: 10.3181/00379727-89-21718. [DOI] [PubMed] [Google Scholar]
- GREEN M. Biochemical studies on adenovirus multiplication. 1. Stimulation of phosphorus incorporation into deoxyribonucleic acid and ribouncleic acid. Virology. 1959 Nov;9:343–358. doi: 10.1016/0042-6822(59)90127-8. [DOI] [PubMed] [Google Scholar]
- GREEN M. Biochemical studies on adenovirus multiplication. III. Requirement for DNA synthesis. Virology. 1962 Dec;18:601–613. doi: 10.1016/0042-6822(62)90063-6. [DOI] [PubMed] [Google Scholar]
- GREEN M., DAESCH G. E. Biochemical studies on adenovirus multiplication. II. Kinetics of nucleic acid and protein synthesis in suspension cultures. Virology. 1961 Feb;13:169–176. doi: 10.1016/0042-6822(61)90051-4. [DOI] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. Acquisition of thymidine kinase activity by herpes simplex-infected mouse fibroblast cells. Biochem Biophys Res Commun. 1963 Apr 2;11:55–59. doi: 10.1016/0006-291x(63)90027-5. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J. Enhanced thymidine phosphorylating activity of mouse fibroblasts (strain LM) following vaccinia infection. Biochem Biophys Res Commun. 1962 Jun 19;8:72–75. doi: 10.1016/0006-291x(62)90238-3. [DOI] [PubMed] [Google Scholar]
- KIT S., PIEKARSKI L. J., DUBBS D. R. Induction of thymidine kinase by vaccinia-infected mouse fibroblasts. J Mol Biol. 1963 Jan;6:22–33. doi: 10.1016/s0022-2836(63)80078-9. [DOI] [PubMed] [Google Scholar]
- KJELLEN L. Effect of 5-halogenated pyrimidines on cell proliferation and adenovirus multiplication. Virology. 1962 Sep;18:64–70. doi: 10.1016/0042-6822(62)90177-0. [DOI] [PubMed] [Google Scholar]
- KORITZ S. B., COHEN P. P. Colorimetric determination of carbamylamino acids and related compounds. J Biol Chem. 1954 Jul;209(1):145–150. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGEE W. E. DNA polymerase and deoxyribonucleotide kinase activities in cells infected with vaccinia virus. Virology. 1962 Aug;17:604–607. doi: 10.1016/0042-6822(62)90167-8. [DOI] [PubMed] [Google Scholar]
- PEREIRA H. G. A protein factor responsible for the early cytopathic effect of adenoviruses. Virology. 1958 Dec;6(3):601–611. doi: 10.1016/0042-6822(58)90109-0. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., ROIZMAN B., LEVY H. B. Characterization of a factor formed in the course of adenovirus infection of tissue cultures causing detachment of cells from glass. J Exp Med. 1958 Nov 1;108(5):713–729. doi: 10.1084/jem.108.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S., ANDERSON T. F. STRUCTURE OF TYPE 5 ADENOVIRUS. II. FINE STRUCTURE OF VIRUS SUBUNITIS. MORPHOLOGIC RELATIONSHIP OF STRUCTURAL SUBUNITS TO VIRUS-SPECIFIC SOLUBLE ANTIGENS FROM INFECTED CELLS. J Exp Med. 1963 Aug 1;118:307–314. doi: 10.1084/jem.118.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. Protein synthesis in type 5 adenovirus-infected cells. Effect of p-flourophenylalanine on synthesis of protein. nucleic acids, and infectious virus. Virology. 1963 Jun;20:269–280. doi: 10.1016/0042-6822(63)90115-6. [DOI] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. STRUCTURE OF TYPE 5 ADENOVIRUS. I. ANTIGENIC RELATIONSHIP OF VIRUS-STRUCTURAL PROTEINS TO VIRUS-SPECIFIC SOLUBLE ANTIGENS FROM INFECTED CELLS. J Exp Med. 1963 Aug 1;118:295–306. doi: 10.1084/jem.118.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]


