Abstract
Among the chemokines, members of the CXC family include IP-10 (Interferon-gamma induced protein of 10Kda). Elevated serum IP-10 levels have been shown in diabetes. However, there is a paucity of data examining the sources and regulation of IP-10 under hyperglycemic conditions and this was the overall aim of the study. Type 1 diabetes (T1DM) is a pro-inflammatory state. We previously demonstrated increased toll like receptor (TLR) 2 and 4 activation in monocytes of T1DM patients. Thus, we also examined the role of the TLR pathway in modulating IP-10 release from human monocytes under hyperglycemia. Also, circulating and monocytic levels of IP-10 in patients with T1DM with and without microvascular complications (T1DM-MV and T1DM) and controls (C ) was assessed. Under HG, IP-10 mRNA and protein were significantly increased compared to normoglycemia. Incubation of monocytes with dominant negative Ikb but not control vector significantly abrogated HG-induced IP-10 release. Furthermore, both TLR 2 siRNA as well as TLR 4 siRNA, either alone or in combination significantly abrogated HG-induced IP-10 release. Serum and monocytic levels of IP-10 were significantly increased in T1DM and T1DM-MV compared to matched controls. Thus, we demonstrate increased circulating and monocytic IP-10 in T1DM. Down-regulation of TLR 2 and TLR 4 abrogates HG-induced IP-10 release via NF-κB inhibition.
Keywords: Chemokine, IP-10, macrophage, toll like receptors, transcription
1. Introduction
Type 1 diabetes (T1DM) is associated with increased microvascular complications and inflammation plays a pivotal role (1). Recent studies have shown that T1DM is a pro-inflammatory state as evidenced by increased circulating levels of CRP, sCD40L, and proinflammatory chemokines and cytokines (2–5). Also, we have shown that monocytes from T1DM diabetic patients secrete increased levels of IL-6 and IL-1b and that the latter is increased significantly in those with microvascular complications (3,4). Among the chemokines, members of the CXC family include IL-8 and IP-10 (Interferon-gamma induced protein of 10Kda)(6–10).
We have previously shown that IL-8 levels are increased in T1DM with and without microvascular complications (4). IP-10 (interferon-gamma-inducible protein-10), another member of the CXC chemokine superfamily, is a highly inducible chemoattractant and also modulates adhesion molecule expression after stimulation with IFN-gamma (10,11). IP-10 is increased in the carotid artery of rats after angioplasty and it is expressed by cultured vascular smooth muscle cells (VSMC) (12). Deficiency of IP-10 or its receptor, CXCR3 reduces lesion formation in Apo E−/− mice (13). Elevated serum IP-10 levels have been shown in diabetes but it is unclear if the patients had complications (14). Also, there is a paucity of data examining cellular sources of IP-10 in Type 1 diabetes and its regulation under hyperglycemic conditions. Thus, we examined the effect of hyperglycemia on IP-10 mRNA and protein in human monocytes and also examined levels of IP-10 in patients with T1DM with and without microvascular complications. Furthermore, recently, we have reported increased toll like receptor 2 and 4 activation in monocytes of patients with T1DM (15). Previously, TLR 4 agonists, such as lipopolysaccharide (LPS) have been shown to induce IP-10 production (10). Thus, we examined the effect of the TLR pathway in modulating IP-10 release from human monocytes under hyperglycemia.
2.Methods
For the in vitro studies, human monocytes were isolated from healthy subjects. For the in vivo study, T1DM patients with and without microvascular complications and healthy controls (n=39, 36 and 40 respectively) were recruited following informed consent. The selection criteria for the subjects have been described by us previously (3,4). All had duration of diabetes at least > 1 year since onset of disease and were on no other hypoglycemic agents other than insulin.
Microvascular complications included retinopathy (22%), nephropathy (68%) and neuropathy (5%). All human investigation was conducted according to the principles expressed in the Declaration of Helsinki and approved by the Institutional Review Board of the UC Davis Medical Center. Human monocytes were isolated from peripheral blood by negative magnetic separation as described previously (3).Serum and monocyte IP-10 levels were measured using a commercially available ELISA (R&D Biosystems) which had an intra-assay CV of <7% and lower detection limit of 1.8 pg/mL.
For all in vitro studies, monocytes were incubated with glucose (5.5mM-NG or 15mM –HG) or mannitol (9.5mM) as hyperosmolar control, for a period of 48 hours. Supernatants were collected for measurement of IP-10 by ELISA and cells were analyzed for protein content by the method of Lowry et al (3). Also expression of IP-10 in intact cells was analyzed by flow cytometry using phycoerythrin-labeled anti-human IP-10 antibodies and isotype controls. mRNA for IP-10 was examined by RT-PCR using primers from R&D as described previously (15).
Transfection with dominant negative IκB vector or control vector was performed for 48 hours as described previously (16). Transfection efficiency, as monitored by luciferase activity was 66%. Also, siRNA to TLR2 or TLR 4 or control siRNA, obtained from Ambion (Austin, TX) were transfected using siPORTamine transfection reagents as described previously (17). Transfection efficiency as measured by mRNA by RT-PCR was at least 65%.
All experiments were performed at least three times in duplicate. The comparisons between group means were analyzed using ANOVA followed by paired t-tests for uniformly distributed data and Tukey’s post-hoc tests for skewed data. The experimental results are presented as the means ± SD. Paired t tests were used to compute differences in the variables, and the level of significance was set at P<0.05.
3.Results
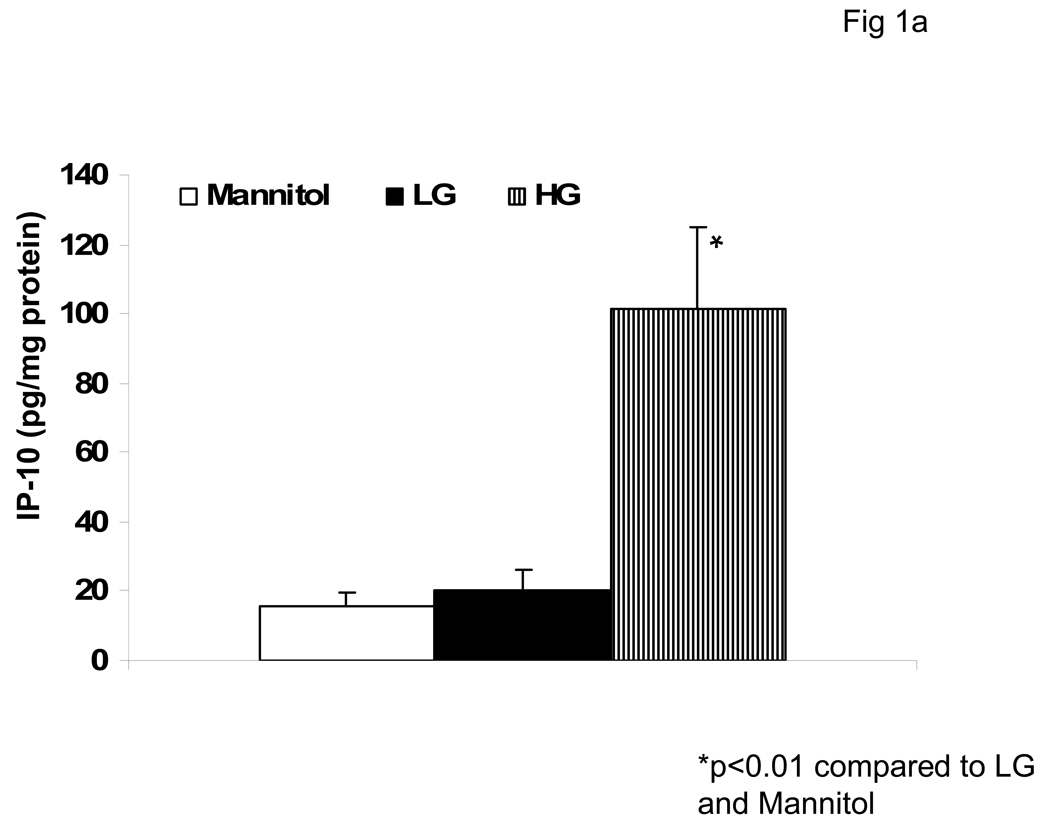
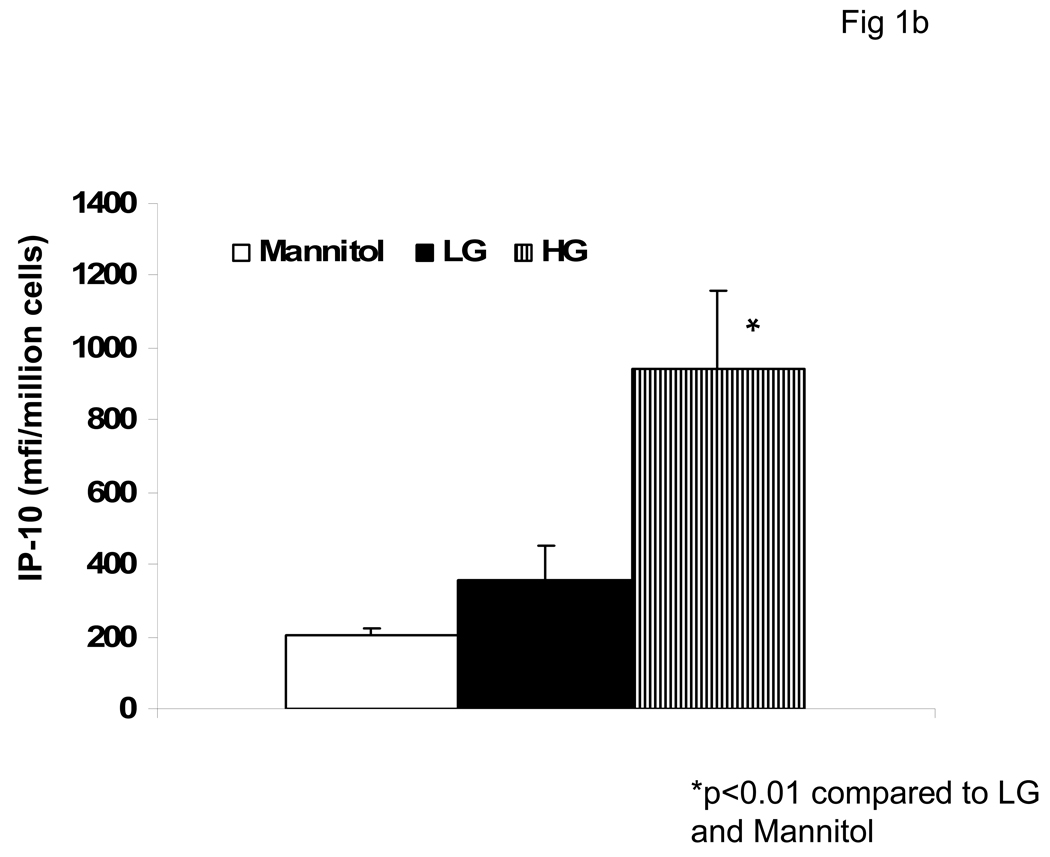
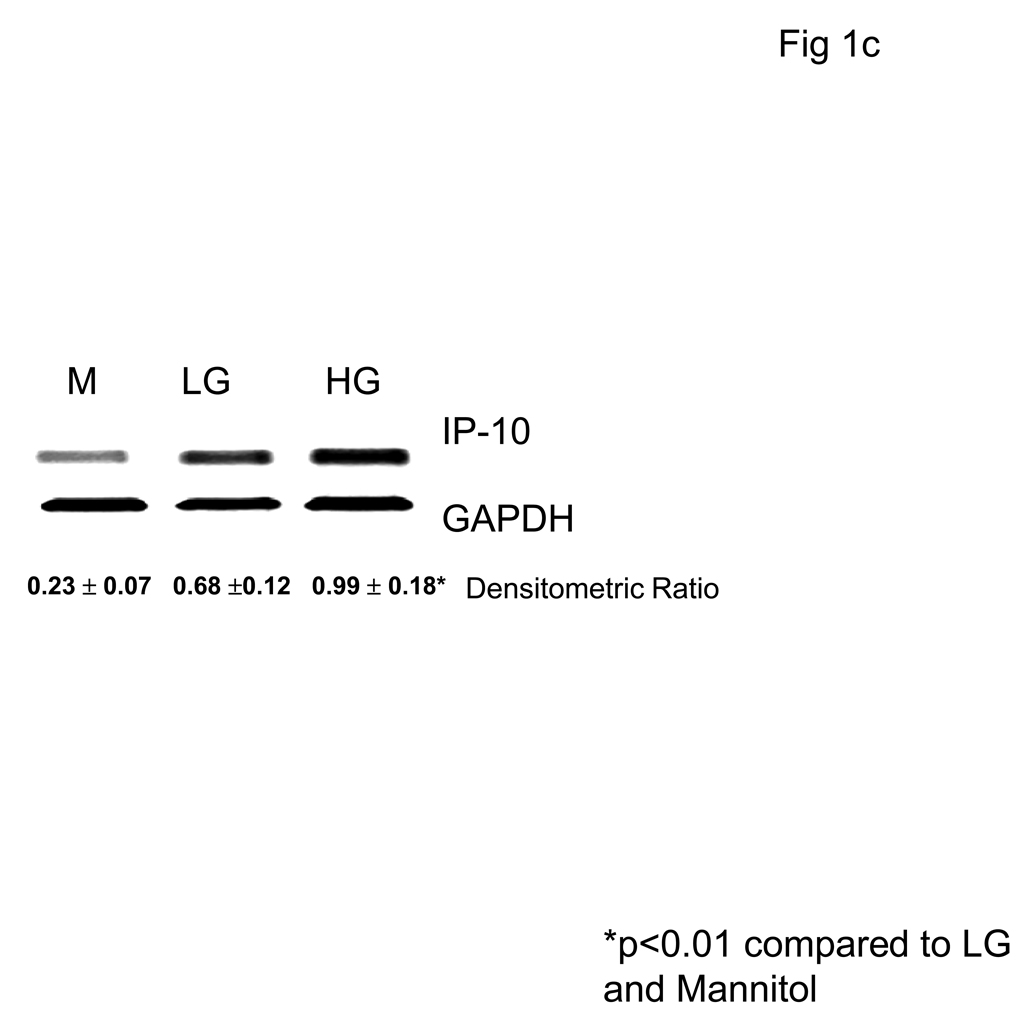
We first examined the effect of hyperglycemia (HG, 15mM glucose) compared to normoglycemia (NG, 5.5mM) on IP-10 release from monocytes by ELISA and flow cytometry (Fig 1a and Fig 1b). Under HG, IP-10 release was significantly increased compared to NG. Mannitol was used as hyperosmolar control and did not significantly affect IP-10 release. Furthermore, HG induced IP-10 mRNA (Fig 1c).
Fig 1. Hyperglycemia Induces IP-10 release from Human monocytes.
Monocytes were isolated and incubated with mannitol as hyperosmolar control or NG (5.5mM, NG) or HG (15mM, HG). IP-10 levels in supernatants was assessed by ELISA (Fig 1A) and cellular IP-10 was examined by flow cytometry (Fig 1B) as described in Methods. Data are expressed as mean ± S.D. Also, mRNA for IP-10 from human monocytes was examined by RT-PCR as described in Methods. (Fig 1C). Densitometric ratio for IP-10/GAPDHmRNA is provided. *p<0.01 compared to Mannitol and LG, n=5 experiments.
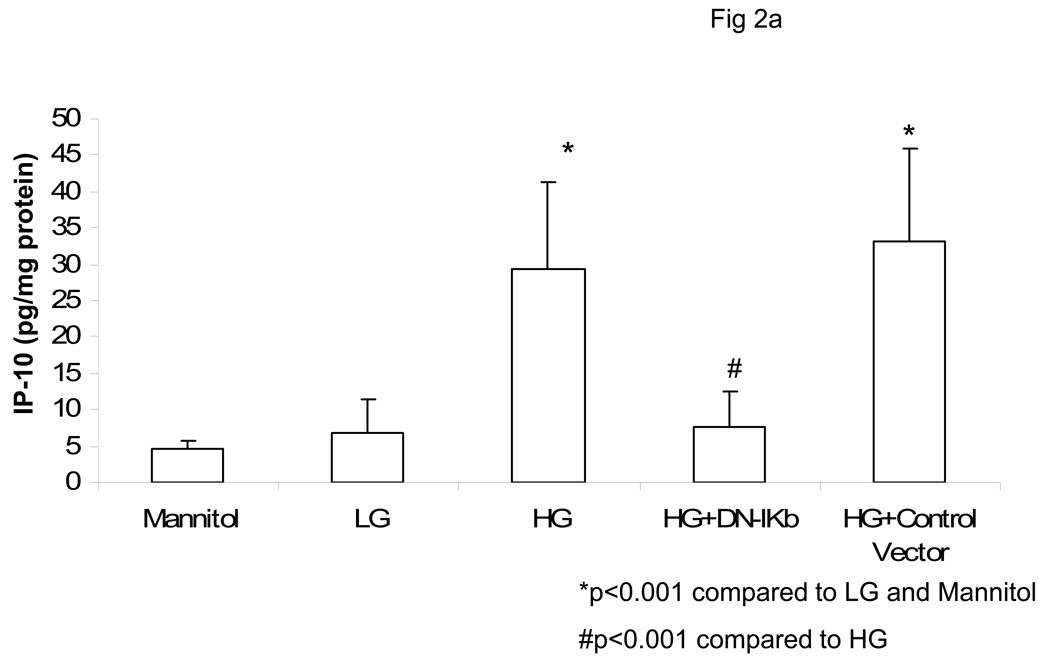
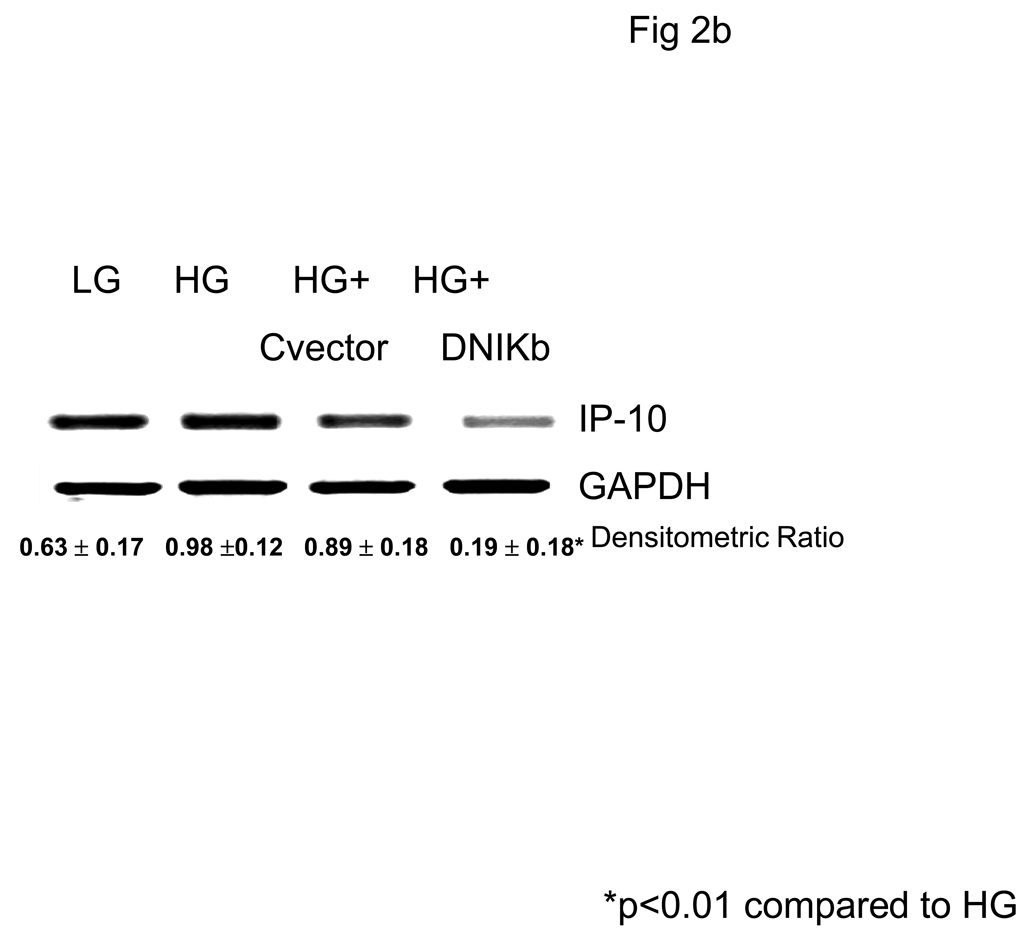
Previously, we have shown that HG induces monocytic release of IL-1b and IL-6 via activation of NF-κB (17,19). Thus, we tested the effect of NF-κB inhibition on IP-10 release. Incubation of monocytes with DN-IKb but not control vector significantly abrogated HG-induced IP-10 mRNA and release (Fig 2a and Fig 2B).
Fig 2. Hyperglycemia Induces IP-10 release from Human monocytes via Upregulation of NFKB.
Monocytes were isolated and incubated with mannitol as hyperosmolar control or NG (5.5mM, NG) or HG (15mM, HG). Some cells were transfected with either Control vector of dominant negative IKb. IP-10 levels in supernatants was assessed by ELISA (Fig 2A). Data are expressed as mean ± S.D. Cellular mRNA for IP-10 from human monocytes was examined by RTPCR as described in Methods. Densitometric ratio for IP-10/GAPDHmRNA is provided. (Fig 2B). *p<0.001 compared to Mannitol and LG, #p<0.001 compared to HG, n=5 experiments.
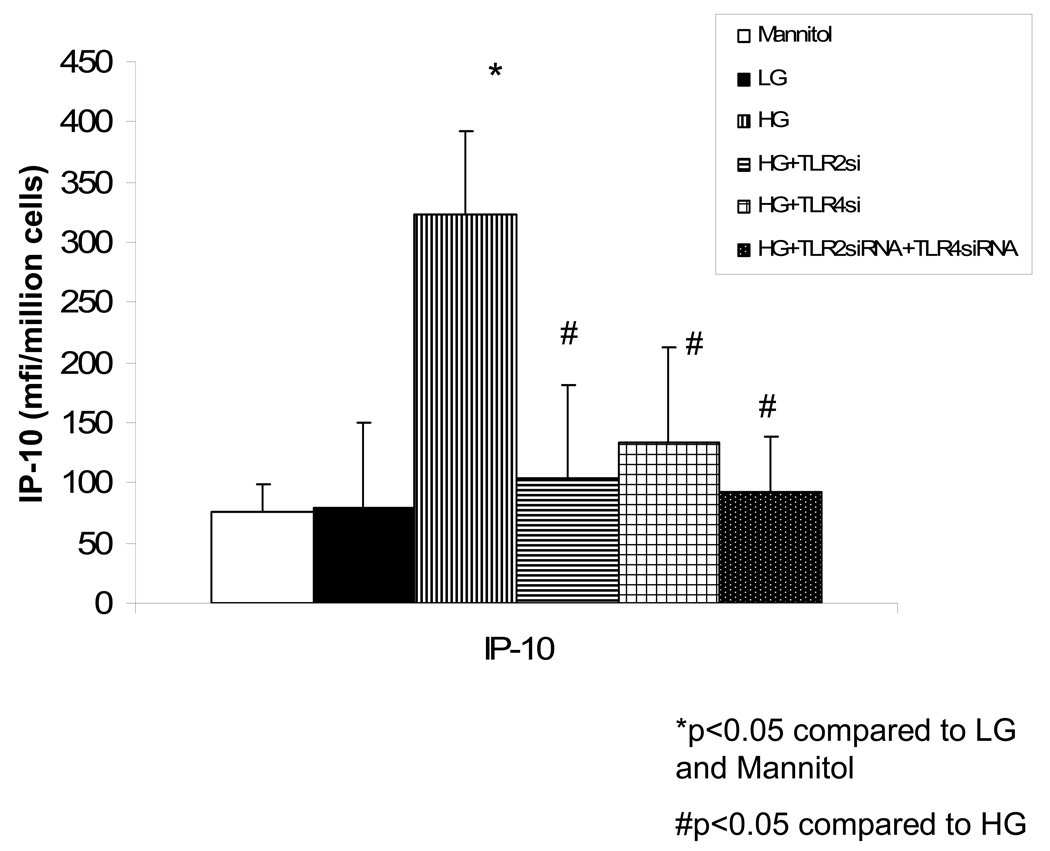
Recently, we showed increased TLR2 and TLR 4 expression and downstream signaling in T1DM monocytes compared to matched controls (15). Also, increased TLR 2 and TLR 4 activity was significantly correlated to increased NF-κB activity in T1DM patients (15). In addition, HG has been shown to upregulate TLR 2 and TLR 4 expression resulting in increased transcriptional activity of NF-κB (17), presumably leading to increased IP-10 release. Thus, we tested the role of TLR2 and TLR 4, 2 TLRs that are increased in T1DM and important in atherosclerosis. Both TLR2 siRNA as well as TLR 4 siRNA, either alone or in combination significantly abrogated HG-induced IP-10 mRNA and release and the combination of TLR 2 and TLR 4 siRNA was not additive to either alone (Fig 3). Scrambled control siRNA failed to have any significant effects (data not shown).
Fig 3. Hyperglycemia- Induced IP-10 release from Human monocytes is Inhibited by Blockade of TLR 2 and/or TLR4.
Monocytes were isolated and incubated with mannitol as hyperosmolar control or NG (5.5mM, NG) or HG (15mM, HG). Some cells were transfected with either TLR 2 siRNA or TLR4 siRNA or the combination. IP-10 levels in supernatants was assessed by ELISA (Fig 3A).Data are expressed as mean ± S.D. Alsocellular mRNA for IP-10 from human monocytes was examined by RT-PCR as described in Methods. (Fig 3B). *p<0.001 compared to Mannitol and LG, #p<0.01 compared to HG, n=4 experiments.
For the in vivo studies, baseline subject characteristics are depicted in Table 1. There were no significant differences in age, BMI, and lipid profile between Control and T1DM groups with and without complications. Also, glucose, HbA1c levels and hsCRP were significantly higher in both T1DM groups compared to controls.
Table 1.
Baseline Subject Characteristics
| Controls (n=40) | T1DM (n=36) | T1DM-MV (n=39) | |
|---|---|---|---|
| Age (years) | 33 ± 11 | 32 ± 16 | 37 ± 17 |
| Gender (M/F) | 17/23 | 14/22 | 16/23 |
| BMI (kg/sq.m) | 25 ± 5 | 23 ± 9 | 28 ± 14 |
| Glucose (mg/dL) | 88 ± 13 | 163 ± 77* | 154 ± 83* |
| Duration of Diabetes (yrs) | 0 | 10.3 (5–16) | 17 (9–22) |
| HbA1C(%) | 5.2 ± 0.4 | 7.9 ± 1.3* | 8.4 ± 1.9* |
| CRP (mg/L) | 1.3 (0.7,1.8) | 1.8 (1.0,2.5)* | 2.4 (0.9,2.9)*a |
| Total Cholesterol (mg/dL) | 179 ± 29 | 188 ± 39 | 190 ± 39 |
| Triglycerides (mg/dL) | 83 ± 33 | 86 ± 44 | 81 ± 57 |
| LDL Cholesterol (mg/dL) | 110 ± 19 | 116 ± 33 | 114 ± 38 |
| HDL Cholesterol (mg/dL) | 47 ± 15 | 48 ± 21 | 51 ± 18 |
Data are expressed as mean ± S.D and median and interquartile range for CRP.
p<0.05 compared to Controls.
p<0.05 compared to T1DM
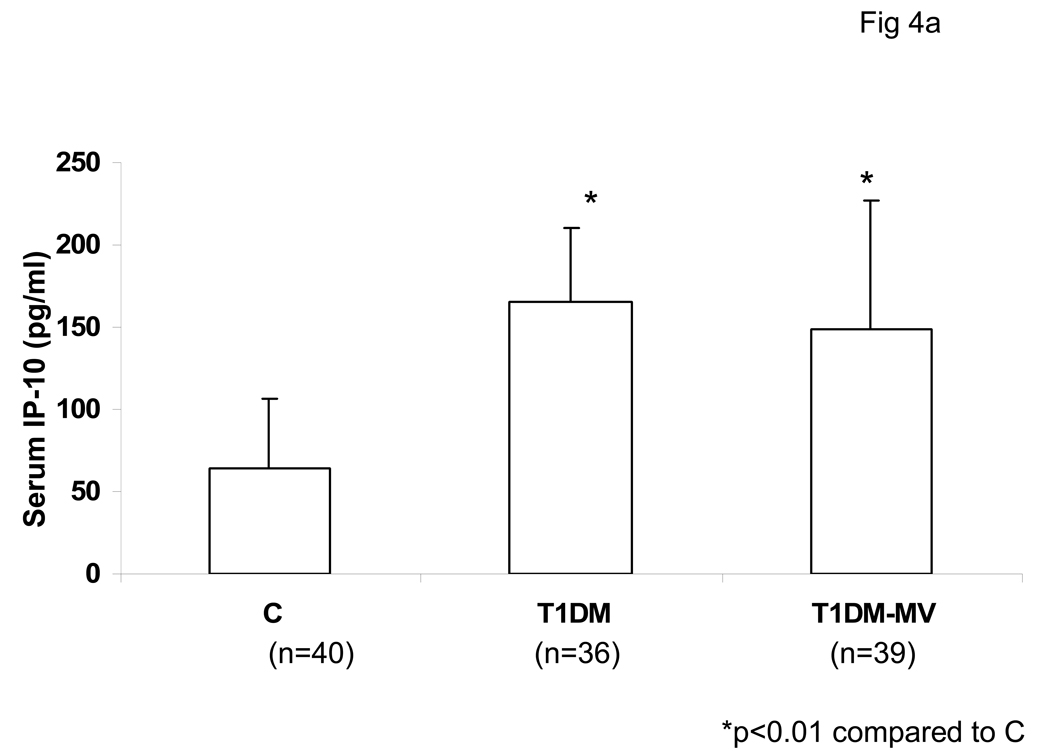
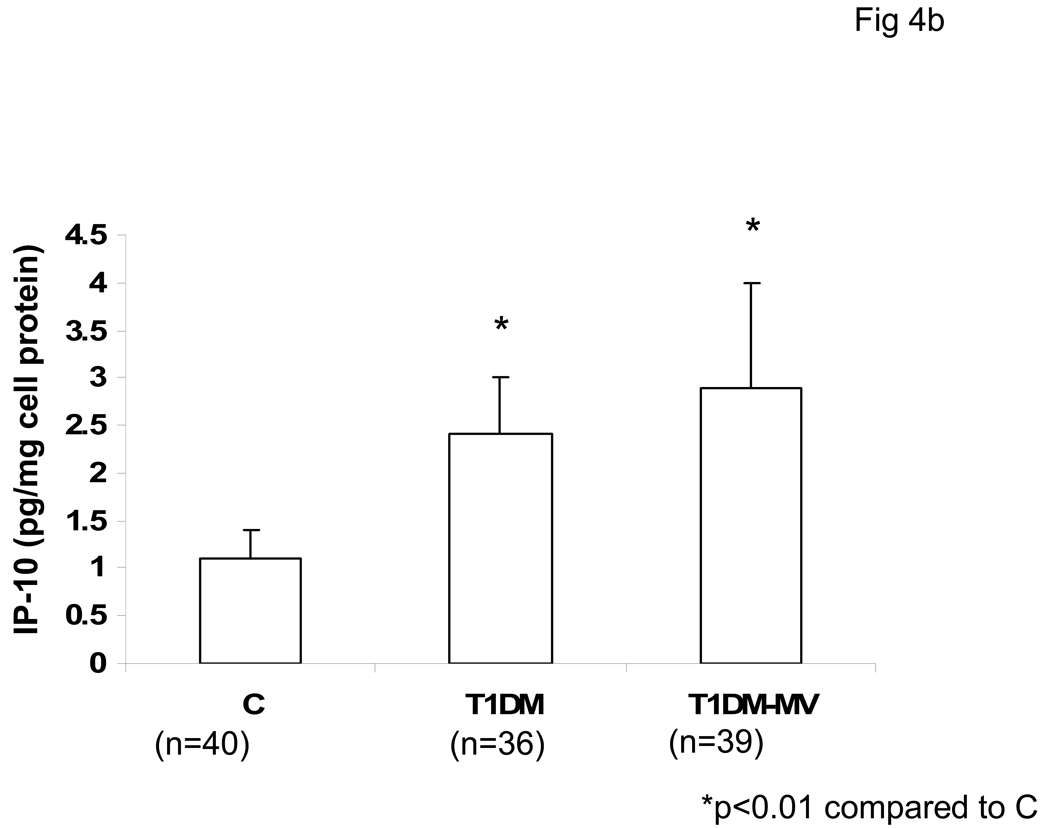
Serum levels of IP-10 were significantly increased in both T1DM with and without microvascular complications compared to matched controls (Fig 4a). Also, secreted IP-10 levels were significantly increased from monocytes of both T1DM without and with microvascular complications as compared to controls, in resting and LPS-activated state (Fig 4b). From our data, it appears that monocytic IP-10 contributes to 70% of IP-10 in circulation. Also, there was no significant correlation between HbA1C levels and IP-10 release (r=0.44, p=0.38).
Fig 4. Serum IP-10 levels are Elevated in T1DM.
Healthy controls (C ), T1DM without and with microvascular complications (T1DM-MV) were recruited following informed consent as described in Methods. Circulating IP-10 levels were analyzed in serum by ELISA as described in Methods (Fig 4A). Also, monocytic IP-10 release were assessed by ELISA (Fig 4B). Data are expressed as mean ± S.D. *p<0.01 compared to controls.
4.Discussion
The presence of chemokines in the atherosclerotic lesion contributes to the recruitment of inflammatory cells such as macrophages and T-lymphocytes (6–11). There is growing recognition that the chemokine IP-10 plays important roles in chronic inflammatory conditions such as atherosclerosis. IP-10 is a member of the CXC chemokine family, which includes other factors such as MIG, I-TAC, IL-8, and MIP-2, and is produced by a variety of cell types, including inflammatory cells such as monocytes, macrophages, and lymphocytes (6–11). Human serum levels of IP-10 range from 20–400 pg/ml, with the higher values found most commonly among individuals with chronic inflammatory conditions such as rheumatoid arthritis, HIV infections, and atherosclerosis. In human atheroma, there is abundant expression of IP-10 (18). Recent studies have also described increased plasma levels of IP-10 in the early and subclinical stages of Types 1 and 2 diabetes (14, 19–22). Although generally recognized as an important chemoattractant for lymphocytes and monocytes, IP-10 also enhances adherence of inflammatory cells to endothelial cells and suppresses angiogenesis (6–11). There is thus a growing appreciation for the potential role of IP-10 in a variety of chronic inflammatory conditions, including diabetes and atherosclerosis.
Circulating IP-10 levels are increased in T1DM patients (14,23,24). IP-10 could also be relevant to diabetic nephropathy, since neutralizing antibodies to IP-10 significantly decreased number of infiltrating T-cells in tubulointerstitium and improved renal function (19). Also, levels of IP-10 have been reported to be significantly increased in vitreous samples from patients with proliferative diabetic retinopathy (21) and since it is angiostatic, this possibly denotes proliferative diabetic retinopathy involution since most of these patients had undergone laser photocoagulation (22). In this study, we confirm increased circulating levels as well as monocytic levels of IP-10 in T1DM with and without microvascular complications compared to controls. Furthermore, incubation of human monocytes with 15mM glucose results in a 5-fold induction of IP-10 release. Previously, Shanmugam et al (23) have shown increased IP-10 mRNA in T1DM (N=4) and the induction by RAGE ligands such as S100 B was via increased mRNA stability and not at the transcriptional level. Thus, the increase in IP-10 from monocytes could be via NF-κB (present report) or interaction of AGEs with RAGE resulting in increased mRNA (secreted IP-10 was not reported in that study) (23).
NF-κB activation has been implicated in the pathogenesis of atherosclerosis (25). Furthermore, we and others have shown increased NF-κB activation under HG conditions and in patients with T1DM and T2DM (3,4, 15, 17). The presence of NF-κB binding sites has been found in the 5' regulatory regions of gene promoters such as MCP-1 and IP-10 (10). In cultured rat VSMC, atorvastatin has been shown to inhibit mRNA expression of chemokines MCP-1 and IP-10, correlating with the normalization of NF-κB activity (26). Previously, under HG, monocytes have been shown to secrete increased levels of IL-8 via upregulation of NF κB (27). In this study, we demonstrate that NF-κB is pivotal in HG-induced IP-10 release since downregulation of NF-κB activity results in abrogation of HG-induced IP-10 release.
TLR2 and 4 expression are upregulated in atherosclerotic plaque macrophages and in animal models of atherosclerosis (28,29). TLR 4 binds to the lipopolysaccharide (LPS; endotoxin) of the outer membrane of Gram negative bacteria. TLR2 recognizes and signals bacterial lipoproteins, peptidoglycans and lipoteichoic acid from Gram positive bacterial cell walls. Total genetic deficiency of TLR4 is associated with reduction in lesion size, lipid content, and macrophage infiltration in hypercholesterolemic apoE−/− mice (29). Also, TLR2/LDLR−/−, and in a recent paper, TLR2 /Apo E−/− mice are protected from the development of atherosclerosis (29). Furthermore, 2 groups have demonstrated that deficiency of MyD88 (myeloid differentiation factor 88), one of the downstream TLR intracellular signaling molecules results in reduction in plaque size, lipid content, expression of proinflammatory genes, and systemic expression of proinflammatory cytokines and chemokines (29). Furthermore, Mohammad et al (30) recently showed increased TLR2 and TLR4 expression in Type 1 diabetic NOD mice and this correlated with increased NF-κB activation in response to the TLR 4 ligand, LPS, resulting in increased pro-inflammatory cytokines (30). We have also recently demonstrated increased TLR2 and TLR 4 activation in diabetes and in HG (15). Insulin infusion suppresses several TLRs (31). Furthermore, HG appears to induce TLR activation via upregulation of NF-κB (17). Also, human, but not murine macrophages substantially upregulate IP-10 expression in response to TLR4-mediated stimuli such as LPS. Also, IFN-gamma has been shown to augment LPS induced IP-10 through up-regulation of TLR-4, and the signal pathways of NF-κB /ERK1. In the present study, we describe increased production of IP-10 by human monocytes exposed to hyperglycemia, and demonstrates that this activation requires TLR2 and TLR 4 upregulation and appears to be mediated via NF-κB activation, since blockade of either TLR 2 or TLR 4 or in combination decreased HG-induced IP-10 release Since blocking both TLR 2 and TLR4 was not additive, it suggests that they both activate IP-10 via a common downstream pathway, possibly NFKb.
In conclusion, we demonstrate increased circulating and monocytic levels of IP-10 in T1DM and implicate an important role of TLR 2 and TLR 4 pathway in augmenting IP-10 release under HG via activation of NF-κB.
Acknowledgements
NIH K24AT00596, JDF (IJ); NIH DK 69801 (SD)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diehl C. National Heart, Lung, and Blood Institute; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111:3489–3493. doi: 10.1161/CIRCULATIONAHA.104.529651. [DOI] [PubMed] [Google Scholar]
- 2.Cipolletta C, Ryan KE, Hanna EV, Trimble ER. Activation of peripheral blood CD14+monocytes occurs in diabetes. Diabetes. 2005;54:2779–2786. doi: 10.2337/diabetes.54.9.2779. [DOI] [PubMed] [Google Scholar]
- 3.Devaraj S, Cheung AT, Jialal I, Griffen SC, Nguyen D, Glaser N, Aoki T. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes. 2007;56(11):2790–2796. doi: 10.2337/db07-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55(3):774–779. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- 5.Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD EURODIAB Prospective Complications Study Group. Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes--the EURODIAB Prospective Complications Study. Diabetologia. 2005;48(2):370–378. doi: 10.1007/s00125-004-1628-8. [DOI] [PubMed] [Google Scholar]
- 6.Ruster C, Wolf G. The role of chemokines and chemokine receptors in diabetic nephropathy. Front Biosci. 2008;13:944–955. doi: 10.2741/2734. [DOI] [PubMed] [Google Scholar]
- 7.Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97(5):714–721. [PubMed] [Google Scholar]
- 8.Christen U, Von Herrath MG. IP-10 and type 1 diabetes: a question of time and location. Autoimmunity. 2004;37(5):273–282. [PubMed] [Google Scholar]
- 9.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61(3):246–257. [PubMed] [Google Scholar]
- 10.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86(2):515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 11.Luster AD, Unkeless JC, Ravetch JV. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Yue TL, Ohlstein EH, Sung CP, Feuerstein GZ. Interferon-inducible protein-10 involves vascular smooth muscle cell migration, proliferation, and inflammatory response. J Biol Chem. 1996;271(39):24286–24293. doi: 10.1074/jbc.271.39.24286. [DOI] [PubMed] [Google Scholar]
- 13.Heller EA, Liu E, Tager AM, Yuan Q, Lin AY, Ahluwalia N, Jones K, Koehn SL, Lok VM, Aikawa E, Moore KJ, Luster AD, Gerszten RE. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation. 2006;113(19):2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 14.Shigihara T, Oikawa Y, Kanazawa Y, Okubo Y, Narumi S, Saruta T, Shimada A. Significance of serum CXCL10/IP-10 level in type 1 diabetes. J Autoimmun. 2006;26(1):66–71. doi: 10.1016/j.jaut.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93(2):578–583. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devaraj S, Venugopal SK, Singh U, Jialal I. Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta} Diabetes. 2005;54(1):85–91. doi: 10.2337/diabetes.54.1.85. [DOI] [PubMed] [Google Scholar]
- 17.Dasu MR, Devaraj S, Ling Z, Hwang DH, Jialal I. High glucose induces Toll-like receptor expression in human monocytes: Mechanism of activation. Diabetes. 2008 Jul 23; doi: 10.2337/db08-0564. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest. 1999;104(8):1041–1050. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolkow PP, Niewczas MA, Perkins B, Ficociello LH, Lipinski B, Warram JH, Krolewski AS. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol. 2008;19(4):789–797. doi: 10.1681/ASN.2007050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruster C, Wolf G. The role of chemokines and chemokine receptors in diabetic nephropathy. Front Biosci. 2008;13:944–955. doi: 10.2741/2734. [DOI] [PubMed] [Google Scholar]
- 21.Abu El-Asrar AM, Struyf S, Kangave D, Geboes K, Van Damme J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur Cytokine Netw. 2006;17(3):155–165. [PubMed] [Google Scholar]
- 22.Elner SG, Strieter R, Bian ZM, Kunkel S, Mokhtarzaden L, Johnson M, Lukacs N, Elner VM. Interferon-induced protein 10 and interleukin 8. C-X-C chemokines present in proliferative diabetic retinopathy. Arch Ophthalmol. 1998;116(12):1597–1601. doi: 10.1001/archopht.116.12.1597. [DOI] [PubMed] [Google Scholar]
- 23.Shanmugam N, Ransohoff RM, Natarajan R. Interferon-gamma-inducible protein (IP)-10 mRNA stabilized by RNA-binding proteins in monocytes treated with S100b. J Biol Chem. 2006;281(42):31212–31221. doi: 10.1074/jbc.M602445200. [DOI] [PubMed] [Google Scholar]
- 24.Nicoletti F, Conget I, Di Mauro M, Di Marco R, Mazzarino MC, Bendtzen K, Messina A, Gomis R. Serum concentrations of the interferon-gamma-inducible chemokine IP-10/CXCL10 are augmented in both newly diagnosed Type I diabetes mellitus patients and subjects at risk of developing the disease. Diabetologia. 2002 Aug;45(8):1107–1110. doi: 10.1007/s00125-002-0879-5. [DOI] [PubMed] [Google Scholar]
- 25.Brand K, Page S, Walli AK, Neumeier D, Baeuerle PA. Role of nuclear factor-kappa B in atherogenesis. Exp Physiol. 1997;82(2):297–304. doi: 10.1113/expphysiol.1997.sp004025. [DOI] [PubMed] [Google Scholar]
- 26.Ortego M, Bustos C, Hernández-Presa MA, Tuñ ón J, Díaz C, Hernández G, Egido J. Atorvastatin reduces NF-kappaB activation and chemokine expression in vascular smooth muscle cells and mononuclear cells. Atherosclerosis. 1999;147(2):253–261. doi: 10.1016/s0021-9150(99)00193-8. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan S, Bolick DT, Hatley ME, Natarajan R, Reilly KB, Yeh M, Chrestensen C, Sturgill TW, Hedrick CC. Glucose regulates interleukin-8 production in aortic endothelial cells through activation of the p38 mitogen-activated protein kinase pathway in diabetes. J Biol Chem. 2004;279(30):31930–31936. doi: 10.1074/jbc.M400753200. [DOI] [PubMed] [Google Scholar]
- 28.Uematsu S. Akira S Toll-like receptors and innate immunity. J Mol Med. 2006;84(9):712–725. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 29.Mullick AE, Tobias PS, Curtiss LK. Toll-like receptors and atherosclerosis: key contributors in disease and health? Immunol Res. 2006;34(3):193–209. doi: 10.1385/IR:34:3:193. [DOI] [PubMed] [Google Scholar]
- 30.Mohammad MK, Morran M, Slotterbeck B, Leaman DW, Sun Y, Grafenstein H, Hong SC, McInerney MF. Dysregulated Toll-like receptor expression and signaling in bone marrow-derived macrophages at the onset of diabetes in the non-obese diabetic mouse. Int Immunol. 2006;18(7):1101–1113. doi: 10.1093/intimm/dxl045. [DOI] [PubMed] [Google Scholar]
- 31.Ghanim H, Mohanty P, Deopurkar R, Sia CL, Korzeniewski K, Abuaysheh S, Chaudhuri A, Dandona P. Acute modulation of toll-like receptors by insulin. Diabetes Care. 2008;31(9):1827–1831. doi: 10.2337/dc08-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]