Abstract
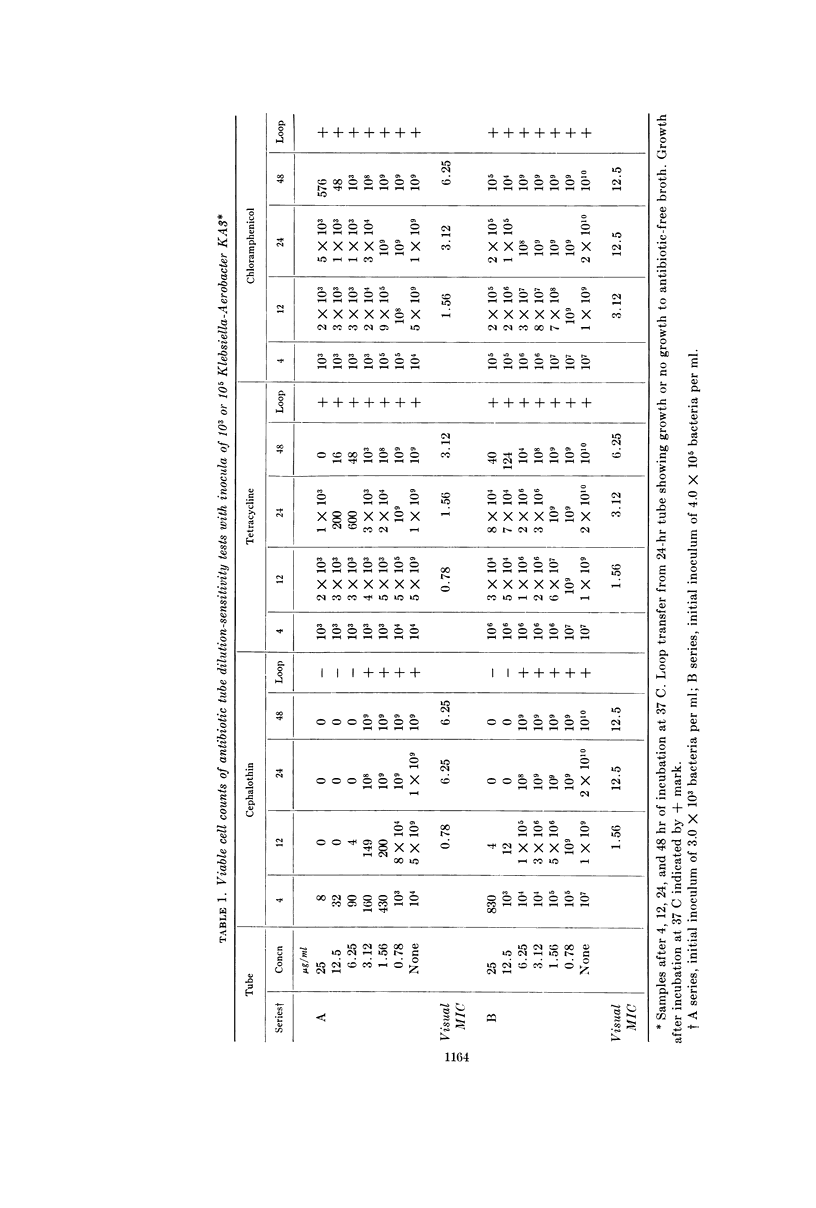
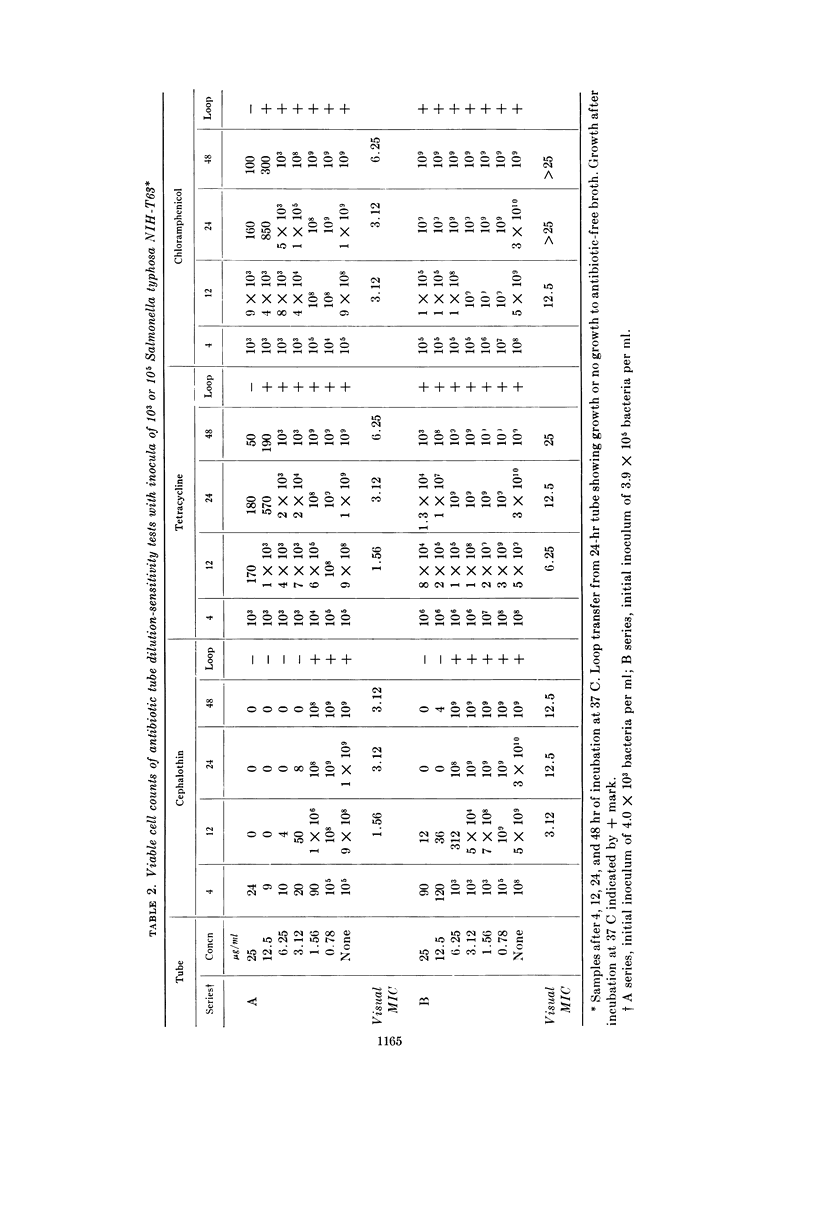
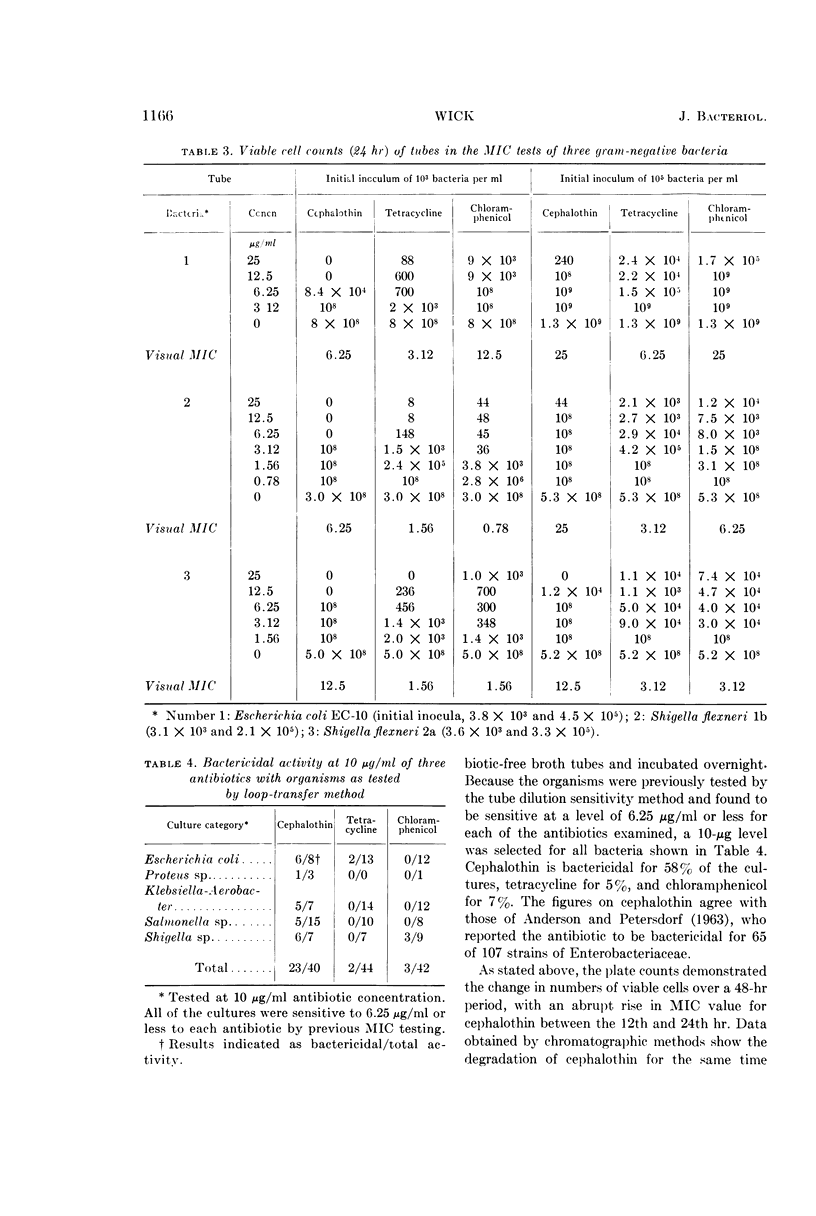
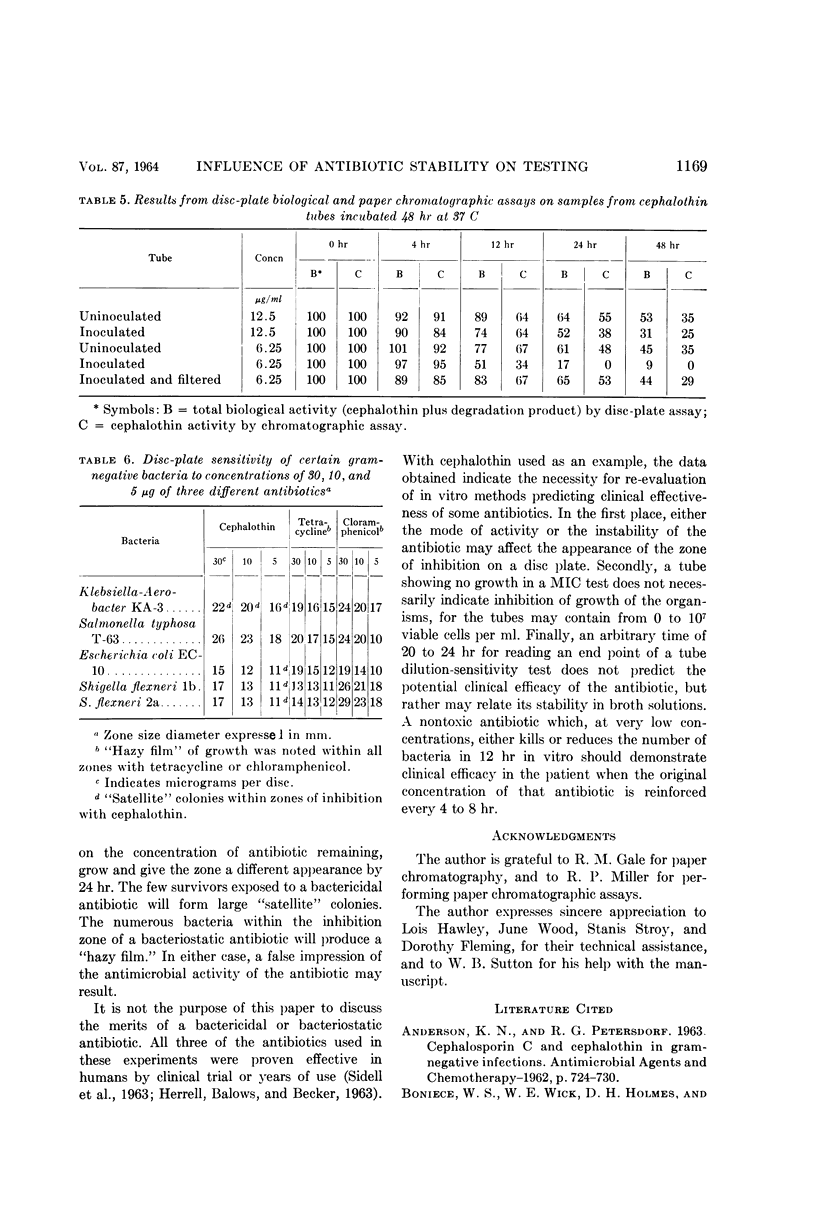
Wick, Warren E. (The Lilly Research Laboratories, Indianapolis, Ind.). Influence of antibiotic stability on the results of in vitro testing procedures. J. Bacteriol. 87:1162–1170. 1964.—Certain antibiotics undergo at least partial degradation under the conditions of in vitro testing procedures. With cephalothin used as an example, experimental evidence is presented to indicate the necessity for re-evaluation of results obtained from in vitro sensitivity testing methods for some antibiotics. The in vitro activity of cephalothin, tetracycline, and chloramphenicol against a variety of gram-negative bacteria is described. Plate counts demonstrate changes in the viable cell population over a 48-hr period in tubes of minimal inhibitory concentration (MIC) tests, with an abrupt rise in MIC value for cephalothin between the 12th and 24th hr. Data obtained by chromatographic methods, showing the degradation of cephalothin for the same time interval, indicated that instability of the antibiotic between the 12th and 24th hr might adversely affect the results obtained from standard 20- to 24-hr in vitro antibiotic sensitivity testing methods. Because repeated administration of an antibiotic to a patient at 4- to 8-hr intervals reinforces the original concentration, a more accurate estimate of antibacterial activity of that antibiotic might preferably be related to this time interval.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONIECE W. S., WICK W. E., HOLMES D. H., REDMAN C. E. In vitro and in vivo laboratory evaluation of cephalothin, a new broad spectrum antibiotic. J Bacteriol. 1962 Dec;84:1292–1296. doi: 10.1128/jb.84.6.1292-1296.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMAIN A. L., WALTON R. B., NEWKIRK J. F., MILLER I. M. MICROBIAL DEGRADATION OF CEPHALOSPORIN C. Nature. 1963 Aug 31;199:909–910. doi: 10.1038/199909a0. [DOI] [PubMed] [Google Scholar]
- FLEMING P. C., GOLDNER M., GLASS D. G. Observations on the nature, distribution, and significance of cephalosporinase. Lancet. 1963 Jun 29;1(7296):1399–1401. doi: 10.1016/s0140-6736(63)92051-8. [DOI] [PubMed] [Google Scholar]
- HERRELL W. E., BALOWS A., BECKER J. SOME EXPERIMENTAL AND CLINICAL STUDIES ON CEPHALOTHIN. Clin Pharmacol Ther. 1963 Nov-Dec;4:709–719. doi: 10.1002/cpt196346709. [DOI] [PubMed] [Google Scholar]
- ROBERTS C. E., Jr, ALLEN J. D., KIRBY W. M. Laboratory and clinical studies of penicillin X-1497. Clin Pharmacol Ther. 1961 Jan-Feb;2:70–79. doi: 10.1002/cpt19612170. [DOI] [PubMed] [Google Scholar]
- SIDELL S., BURDICK R. E., BRODIE J., BULGER R. J., KIRBY W. M. New antistaphylococcal antibiotics. Comparative in vitro and in vivo activity of cephalothin, nafcillin, cloxacillin, oxacillin, and methicillin. Arch Intern Med. 1963 Jul;112:21–28. doi: 10.1001/archinte.1963.03860010067005. [DOI] [PubMed] [Google Scholar]
- STEWART G. T. Microbiological studies on sodium 6-(2, 6 dimethoxybenzamido) penicillanate monohydrate (BRL 1241) in vitro and in patients. Br Med J. 1960 Sep 3;2(5200):694–699. doi: 10.1136/bmj.2.5200.694. [DOI] [PMC free article] [PubMed] [Google Scholar]



