Abstract
The Invader® assay uses a structure-specific flap endonuclease (FEN) to cleave a three-dimensional complex formed by hybridization of allele-specific overlapping oligonucleotides to target DNA containing a single nucleotide polymorphism (SNP) site. Annealing of the oligonucleotide complementary to the SNP allele in the target molecule triggers the cleavage of the oligonucleotide by cleavase, a thermostable FEN. Cleavage can be detected by several different approaches. Most commonly, the cleavage product triggers a secondary cleavage reaction on a fluorescence resonance energy transfer (FRET) cassette to release a fluorescent signal. Alternatively, the cleavage can be detected directly by use of fluorescence polarization (FP) probes, or by mass spectrometry. The invasive cleavage reaction is highly specific, has a low failure rate, and can detect zeptomol quantities of target DNA. While the assay traditionally has been used to interrogate one SNP in one sample per reaction, novel chip- or bead-based approaches have been tested to make this efficient and accurate assay adaptable to multiplexing and high-throughput SNP genotyping.
Keywords: Cleavase, Biplex invader, SISAR, FRET
1. Introduction
Single nucleotide polymorphisms (SNPs) are the most common form of polymorphisms in the human genome. Commonly, SNPs are substitutions, insertions, or deletions of individual bases in the DNA. Over the past years, numerous large-scale efforts have identified a large number of SNPs, and to date the public repository for these polymorphisms, db-SNP (http://www.ncbi.nlm.nih.gov/SNP), includes information on almost 10 million SNPs (NCBI dbSNP build 121).
As the current issue of this journal shows, a large number of diverse approaches have been developed that allow the efficient and accurate genotyping of SNPs in large numbers of samples. With the intent of using SNPs as markers in genome-wide association studies, cost-efficient methods are essential that allow the quick interrogation of hundreds of thousands of these single base pair sequence changes in thousands of individuals. Here, we discuss one of these methods, the Invader® assay, in detail.
In 1993, Lyamichev et al. [1] described the ability of DNA polymerases to cleave single-stranded DNA not through nucleotide sequence recognition, but in a structure-specific manner. This 5′ exonuclease activity removed excess nucleotides during DNA replication solely based on the bifurcated structure that was formed, not based on any sequence motif. Subsequently, Lyamichev et al. [2] showed that thermostable flap endonucleases (FEN) isolated from the archeae Pyrococcus furiosus and Archaeoglobus fulgidus showed similar properties. Furthermore, their detailed analysis showed that this structure-specific cleavage was highly sensitive to sequence mismatches and thus, would offer the possibility of using the endonuclease activity for sensitive detection of DNA polymorphisms. This initial study suggested that these endonucleases could be used specifically to interrogate SNPs without requiring PCR or thermal cycling of the genotyping reaction.
Below, we describe in detail the different approaches for Invader® assay-based SNP genotyping and their current applications. In addition, we discuss novel platforms that utilize Invader® assays and may offer alternative approaches to the commercial assay formats currently available.
2. Description of the invader reaction
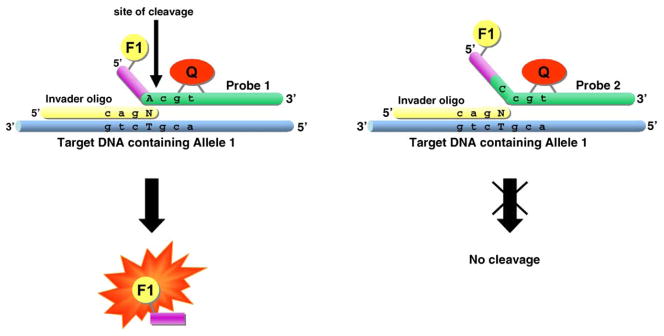
The Invader® assay uses two oligonucleotide probes that are hybridized to target DNA containing a polymorphic site. The two oligonucleotides hybridize to the single-stranded target and form an overlapping invader structure at the site of the SNP. The structure is illustrated in Fig. 1. One oligonucleotide, the Invader® oligo, is complementary to the target sequence 3′ of the polymorphic site and ends with a non-matching base overlapping the SNP nucleotide. The second oligonucleotide, the allele-specific probe, contains the complementary base of the SNP allele and extends to the sequence 5′ of the polymorphic site. This probe can also extend on its 5′ site with additional non-complementary nucleotides. Once the two oligonucleotides anneal to the target DNA, they form a three-dimensional invader structure over the SNP site that can be recognized by cleavase, a FEN enzyme. The enzyme cleaves the probe 3′ of the base complementary to the polymorphic site (i.e. 3′ of the overlapping invader structure). If the probe is designed as a fluorescence resonance energy transfer (FRET) molecule containing a fluorophore at the 5′ end and an internal quencher molecule, the cleavage reaction will separate the fluorophore from the quencher, as shown in Fig. 1, and generates a measurable fluorescent signal. If, in contrast, the probe oligonucleotide does not match the SNP allele present in the target DNA (i.e. the probe is complementary to the alternate SNP allele), then no overlapping invader structure is formed, and the probe is not cleaved. This distinction is highly specific with only minimal unspecific cleavage of the mismatch probe.
Fig. 1.
Allele-specific cleavage in an Invader® reaction by flap endonucleases (FENs).
Commonly, the Invader® oligonucleotide is designed to permanently anneal to the target DNA at the assay temperature. In contrast, the probe oligonucleotide is designed to have a melting temperature close to the assay temperature. As a consequence, the probe constantly anneals and detaches. During the annealing, cleavase can cleave the oligonucleotide, the remnant detaches, and a new uncleaved oligonucleotide probe can re-anneal to the same site. This design ensures a cleavage rate of approximately 3000 probe molecules in 90 min per target molecule [2].
While the described format allows for a highly specific SNP genotyping assay, it requires a significant amount of target molecules to generate a detectable fluorescent signal in a reasonable time. Thus, this assay format would require an initial PCR amplification of the target region, followed by the invader reaction. In addition, the assay requires two separate allele-specific probes (one for each SNP allele) that each needs to be labeled with a fluorophore and a quencher molecule. This significantly increases the cost of the assay, making this format unsuitable for large-scale projects and high-throughput genotyping.
3. Serial invasive signal amplification reaction (SISAR)
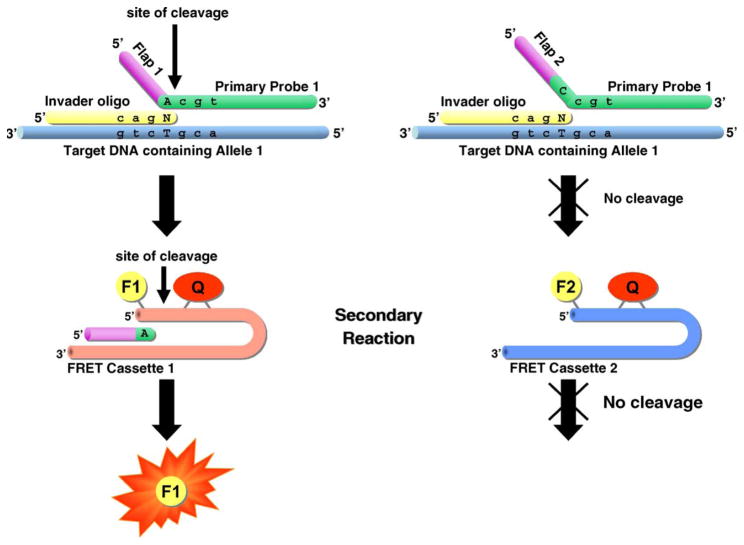
Hall et al. [3] addressed the difficulties of the initial Invader® assay format by developing an assay format that combined two invasive cleavage reactions into a single homogeneous assay. This serial invasive signal amplification reaction (SISAR), which is illustrated in Fig. 2, provides the basis for the majority of Invader® assays used today. The assay was initially applied as a monoplex reaction, requiring the investigation of each SNP allele in a separate reaction [4]. Subsequently, the assay was converted into a single-tube biplex assay using two separate FRET cassette oligonucleotides resulting in distinct fluorescent signals for the two SNP alleles [5].
Fig. 2.
Serial invasive signal amplification reaction (SISAR).
As illustrated in Fig. 2, the initial invasive cleavage reaction is similar to the initial reaction format. Two oligonucleotides complementary to the target sequence anneal to a target molecule and form the three-dimensional invader structure. However, one of the oligonucleotides, the primary probe, is no longer labeled with a fluorophore and a quencher. Instead, the oligonucleotide is extended past the nucleotide complementary to the SNP allele with a 5′ flap sequence which is not complementary to the target sequence at all, and consequently does not anneal to it. This flap sequence is cleaved in the primary reaction, releasing the flap molecule including the base complementary to the SNP allele. Since the flap sequence is independent from the actual target sequence, two generic flap sequences, one for each SNP allele probe, are used in the assay. These flap sequences, once released, serve as Invader® oligonucleotides in a secondary invasive cleavage reaction (see Fig. 2). This time, the flap oligonucleotide anneals to a FRET cassette, an oligonucleotide that partially anneals to itself, thus forming a combined target and probe structure in one molecule. Addition of the flap oligonucleotide as an invader probe forms the required three-dimensional invader structure and cleavase can cleave between the 5′ nucleotide (which is labeled with a fluorophore) and the adjacent quencher molecule. This reaction releases the fluorophore and the signal can be measured. Each flap sequence is specific for one FRET cassette molecule, and thus generates a distinct fluorescent signal.
This assay has several advantages over the previously described format. First, the assay is significantly more sensitive. Hall et al. [3] reported that more than 107 reporter molecules were generated per target molecule in a 4 h reaction. This allowed the successful detection of zeptomol (10−21 mol) levels of target DNA making it possible to use un-amplified genomic DNA as target. Second, the assay no longer requires the synthesis of allele-specific labeled oligonucleotides. Each sequence-specific primary probe is extended with one of two generic flap sequences that react in the secondary reaction with a specific FRET cassette. While this FRET cassette is still expensive, it can now be synthesized in large quantities since it can be used with any Invader® assay regardless of the SNP. Therefore, the cost of synthesis for SNP-specific oligonucleotides is significantly reduced. Finally, the single tube reaction format alleviates concerns about the monoplex assay regarding possible genotyping errors when the reaction in only one tube fails and permits the automated reaction setup and only limited manual steps. This is an essential requirement for use in high-throughput SNP genotyping projects. However, the assay still only interrogates one SNP per reaction and, if run using genomic DNA, requires long incubation times (3–4 h) and large amounts of genomic DNA (20–100 ng) per reaction. Alternatively, the assay can be run using PCR-amplified target in a shorter time (20–40 min).
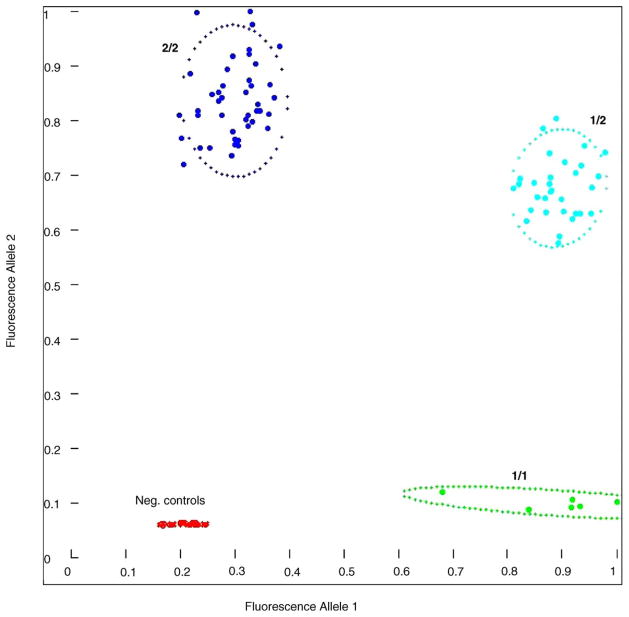
Fig. 3 shows a typical result of a biplex SISAR Invader® assay. The two distinct fluorescent signals are plotted on the two axes and clusters of samples with the different genotypes can be easily identified. Alternatively, data can be analyzed automatically using a clustering algorithm that estimates the probability of each data point belonging to one of the four genotype clusters (homozygous allele 1, heterozygous, homozygous allele 2, negative control cluster). This automated analysis reduces the manual labor involved and provides actual probabilities of genotyping accuracy that can be used for subsequent statistical association analyses [5].
Fig. 3.
Scatter plot of representative genotyping results. Data points for individual samples are plotted based on their relative fluorescence generated by cleavage of each of the two allele-specific FRET cassettes. Dotted lines indicate the confidence interval as determined by clustering for each genotype [5].
Three different studies have assessed the reliability and accuracy of the biplex SISAR Invader® assay. Mein et al. [4] reported a 2.3% failure rate, and a 0.8% error rate, as determined by discrepant calls from duplicate samples. Our own studies found comparable failure rates (1.6–3.5%) but lower error rates (<0.3%), estimated by comparison to sequencing results [5]. Recently, Pati et al. [6] reported on a comparison of commercially available Invader® assays with other genotyping platforms. They estimated a failure rate of 1.04% with an error rate of 0.68% when compared to genotype calls from other platforms and sequencing results. Overall, all three studies attest to the reliable performance of the assay and high genotyping success rates.
4. Other detection approaches
Even though commercially available Invader® assays use FRET detection as the mode of signal generation, other approaches have been used successfully. Alternative detection technologies eliminate the need for the expensive synthesis of two FRET cassettes, and thus reduce the overall cost of the assay.
The first alternative approach uses the detection of alterations in polarized light [7,8]. When a fluorescent molecule is excited by plane polarized light, it emits fluorescence that is also polarized. This fluorescence polarization (FP) is dependent on the molecular weight of the dye molecule. Hsu et al. [7] designed two signal probes with different fluorescent dye labels that react with the two specific flap sequences generated in the primary cleavage of an invader SISAR reaction. The synthesis of these signal probes is significantly simpler than comparable FRET cassettes and the FP probes provide similar sensitivity for the assay. In addition to the reduced assay cost, the use of multiple fluorescent dye molecules on flap-specific signal probes could allow a low degree of multiplexing, which is difficult to achieve when FRET cassettes have to be developed for multiplexed assays.
In a different approach, Berggren et al. [9] used matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) to detect the flap molecules generated in the primary cleavage reaction. While their description focused on the use of the approach for quantifying mRNA molecules using invader technology, the method is clearly adaptable to SNP genotyping since MALDI-TOF is well suited to detect and differentiate oligonucleotide molecules of different sequence and molecular mass. Given the short analysis time of MALDI-TOF MS and the automated systems for high-throughput MS analysis, this approach may offer an alternative detection platform for high-throughput genotyping using invader technology without the requirement of fluorescently labeled oligonucleotide probes.
5. Modified platforms for high-throughput SNP genotyping
In its current commercially available format, the invader platform for SNP genotyping suffers from two limitations. First, it requires relatively large amounts of target DNA per genotype. While genotypes can be obtained reliably directly from genomic DNA, the amount of patient DNA needed for genome-wide association studies would either require the collection of large amounts of sample material from each patient, or initial amplification of the target region by PCR. Second, since only one SNP can be genotyped per reaction, the assay cannot be multiplexed efficiently in its current format. This limits the throughput, despite the development of robotics support systems by Third Wave Technologies [10]. As a consequence, several studies have aimed at either improving the throughput using existing technology or modifying the technology significantly to accommodate multiple SNPs and/or large numbers of samples simultaneously.
Ohnishi et al. [11] developed multiplex PCR reactions to amplify target regions for 100 SNPs in one reaction. They successfully genotyped 98 of 100 SNPs in 10 individuals and confirmed the accuracy of the genotyping results by direct sequencing. The multiplex PCR required 40 ng of genomic DNA. This translates to 0.4 ng of genomic DNA per SNP, an amount that would allow genome-wide SNP association studies using the small samples routinely collected in clinical studies. The method was combined with a novel design for a 384-well card, which drastically reduced the reaction volume for the individual invader reactions, thus further reducing genotyping cost.
Different approaches have aimed at providing a novel platform for Invader® assays that is suitable for high-throughput SNP genotyping. The current restriction of reactions to be run in solution requires reaction volumes that generate sufficient signal in microtiter or PCR plates. Alternatively, allele-specific probes have been successfully attached to solid support material, providing an opportunity for chip- or bead-based assays. Rao et al. [12] generated microspheres with covalently attached fluorescently labeled primary invader probes. After the cleavage reactions, the beads were analyzed using flow cytometry to detect the fluorescent signals on each bead. The method was successfully used to genotype directly from genomic DNA without target amplification. The ability of cleavase to recognize the invader structure was significantly improved when long tethers were used to attach the probes to the surface [13].
Similarly, the probes can be attached directly to activated surfaces [14,15]. The primary probe, the Invader® oligonucleotide, or both can be linked to a surface for successful formation of the invader structure and subsequent cleavage. This approach allows the development of invader SNP chips that would generate genotypes for large numbers of SNPs simultaneously for one DNA sample per chip. It would combine the benefits of the invader technology (accuracy, simplicity of isothermal assay, reaction directly from genomic DNA) with a high-throughput chip platform that would lend itself to automated handling.
Unfortunately, neither method has been successfully applied to large-scale genotyping projects and will require further development before the technology is readily applicable to automated or semi-automated genotyping.
6. Discussion
Invader technology has been used in a number of disease association studies. The method is appealing due to its low failure rate, its isothermal single-tube reaction, and its accuracy. However, the method requires independent reactions per SNP and sample, a restriction that limits the appeal of invader technology for high-throughput operations. Nonetheless, for small- to medium-size genotyping projects, the technology provides a useful and reliable tool that does not require the purchase of expensive equipment. In addition, assays are being provided directly by Third Wave Technologies and can be designed to any SNP of interest.
In addition to SNP genotyping, the method has also been used successfully for accurate detection and quantification of mRNA [16] and most recently, for small inhibitory RNA molecules [17]. This flexibility and wide range of potential applications is an added benefit to a SNP genotyping platform. However, future technology improvements are needed to convert the Invader® assay to a high-throughput platform needed for genome-wide SNP studies.
References
- 1.Lyamichev V, Brow MA, Dahlberg JE. Structure-specific endonucleolytic cleavage of nucleic acids by eubacterial DNA polymerases. Science. 1993;260:778–783. doi: 10.1126/science.7683443. [DOI] [PubMed] [Google Scholar]
- 2.Lyamichev V, Mast AL, Hall JG, Prudent JR, Kaiser MW, Takova T, Kwiatkowski RW, Sander TJ, de Arruda M, Arco DA, Neri BP, Brow MA. Polymorphism identification and quantitative detection of genomic DNA by invasive cleavage of oligonucleotide probes. Nat Biotechnol. 1999;17:292–296. doi: 10.1038/7044. [DOI] [PubMed] [Google Scholar]
- 3.Hall JG, Eis PS, Law SM, Reynaldo LP, Prudent JR, Marshall DJ, Allawi HT, Mast AL, Dahlberg JE, Kwiatkowski RW, de Arruda M, Neri BP, Lyamichev VI. Sensitive detection of DNA polymorphisms by the serial invasive signal amplification reaction. Proc Natl Acad Sci USA. 2000;97:8272–8277. doi: 10.1073/pnas.140225597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mein CA, Barratt BJ, Dunn MG, Siegmund T, Smith AN, Esposito L, Nutland S, Stevens HE, Wilson AJ, Phillips MS, Jarvis N, Law S, de Arruda M, Todd JA. Evaluation of single nucleotide polymorphism typing with invader on PCR amplicons and its automation. Genome Res. 2000;10:330–343. doi: 10.1101/gr.10.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olivier M, Chuang LM, Chang MS, Chen YT, Pei D, Ranade K, de Witte A, Allen J, Tran N, Curb D, Pratt R, Neefs H, de Arruda Indig M, Law S, Neri B, Wang L, Cox DR. High-throughput genotyping of single nucleotide polymorphisms using new biplex invader technology. Nucleic Acids Res. 2002;30:53. doi: 10.1093/nar/gnf052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pati N, Schowinsky V, Kokanovic O, Magnuson V, Ghosh S. A comparison between SNaPshot, pyrosequencing, and biplex invader SNP genotyping methods: accuracy, cost, and throughput. J Biochem Biophys Methods. 2004;60:1–12. doi: 10.1016/j.jbbm.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Hsu TM, Law SM, Duan S, Neri BP, Kwok PY. Genotyping single-nucleotide polymorphisms by the invader assay with dual-color fluorescence polarization detection. Clin Chem. 2001;47:1373–1377. [PubMed] [Google Scholar]
- 8.Kwok PY. SNP genotyping with fluorescence polarization detection. Hum Mutat. 2002;19:315–323. doi: 10.1002/humu.10058. [DOI] [PubMed] [Google Scholar]
- 9.Berggren WT, Takova T, Olson MC, Eis PS, Kwiatkowski RW, Smith LM. Multiplexed gene expression analysis using the invader RNA assay with MALDI-TOF mass spectrometry detection. Anal Chem. 2002;74:1745–1750. doi: 10.1021/ac011167t. [DOI] [PubMed] [Google Scholar]
- 10.de Arruda M, Lyamichev VI, Eis PS, Iszczyszyn W, Kwiatkowski RW, Law SM, Olson MC, Rasmussen EB. Invader technology for DNA and RNA analysis: principles and applications. Expert Rev Mol Diagn. 2002;2:487–496. doi: 10.1586/14737159.2.5.487. [DOI] [PubMed] [Google Scholar]
- 11.Ohnishi Y, Tanaka T, Ozaki K, Yamada R, Suzuki H, Nakamura Y. A high-throughput SNP typing system for genome-wide association studies. J Hum Genet. 2001;46:471–477. doi: 10.1007/s100380170047. [DOI] [PubMed] [Google Scholar]
- 12.Rao KV, Stevens PW, Hall JG, Lyamichev V, Neri BP, Kelso DM. Genotyping single nucleotide polymorphisms directly from genomic DNA by invasive cleavage reaction on microspheres. Nucleic Acids Res. 2003;31:66. doi: 10.1093/nar/gng066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkins Stevens P, Hall JG, Lyamichev V, Neri BP, Lu M, Wang L, Smith LM, Kelso DM. Analysis of single nucleotide polymorphisms with solid phase invasive cleavage reactions. Nucleic Acids Res. 2001;29:77. doi: 10.1093/nar/29.16.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu M, Shortreed MR, Hall JG, Wang L, Berggren T, Stevens PW, Kelso DM, Lyamichev V, Neri B, Smith LM. A surface invasive cleavage assay for highly parallel SNP analysis. Hum Mutat. 2002;19:416–422. doi: 10.1002/humu.10071. [DOI] [PubMed] [Google Scholar]
- 15.Lu M, Hall JG, Shortreed MR, Wang L, Berggren WT, Stevens PW, Kelso DM, Lyamichev V, Neri B, Skinner JL, Smith LM. Structure-specific DNA cleavage on surfaces. J Am Chem Soc. 2002;124:7924–7931. doi: 10.1021/ja012082c. [DOI] [PubMed] [Google Scholar]
- 16.Eis PS, Olson MC, Takova T, Curtis ML, Olson SM, Vener TI, Ip HS, Vedvik KL, Bartholomay CT, Allawi HT, Ma WP, Hall JG, Morin MD, Rushmore TH, Lyamichev VI, Kwiatkowski RW. An invasive cleavage assay for direct quantitation of specific RNAs. Nat Biotechnol. 2001;19:673–676. doi: 10.1038/90290. [DOI] [PubMed] [Google Scholar]
- 17.Allawi HT, Dahlberg JE, Olson S, Lund E, Olson M, Ma WP, Takova T, Neri BP, Lyamichev VI. Quantitation of microRNAs using a modified invader assay. RNA. 2004;10:1153–1161. doi: 10.1261/rna.5250604. [DOI] [PMC free article] [PubMed] [Google Scholar]