Abstract
Inhalant abuse in young people is a growing public health concern. We reported previously that acute toluene intoxication in young rats, using a pattern of exposures that approximate abuse patterns of inhalant use in humans, significantly altered neurochemical measures in select brain regions. In this study, adolescent and young adult rats were exposed similarly to an acute (2 × 15 min), high dose (8000 − 12000 ppm) of toluene and high-resolution magic angle spinning proton magnetic resonance spectroscopy (HR-MAS 1H-MRS) was used to assess neurochemical profiles of tissue samples from a number of brain regions collected immediately following solvent exposure. The current investigation focused on N-acetyl-aspartate (NAA), choline-containing compounds, creatine, glutamate, GABA, and glutamine. Contrary to our predictions, no significant alterations were found in levels of NAA, choline, creatine, glutamate, or glutamine in adolescent animals. In contrast to these minimal effects in adolescents, binge toluene exposure altered several neurochemical parameters in young adult rats, including decreased levels of choline and GABA in the frontal cortex and striatum and lowered glutamine and NAA levels in the frontal cortex. One of the more robust findings was a wide-ranging increase in lactate after toluene exposure in adult animals, an effect not observed in adolescents. These age-dependent effects of toluene are distinct from those reported previously in juvenile rats and suggest a developmental difference in vulnerability to the effects of inhalants. Specifically, the results suggest that the neurochemical response to toluene in adolescents is attenuated compared to adults, and imply an association between these neurochemical differences and age-influenced differences in solvent abuse in humans.
Keywords: Toluene, inhalant abuse, magnetic resonance spectroscopy, N-acetyl aspartate, GABA
1. Introduction
Deliberate inhalation of organic compounds with psychotropic properties continues to be a public health concern throughout the world [54]. The popularity of these compounds is due in part to the unrestricted availability of the many industrial and household products containing organic solvents and to the simple methods of administration. Toluene and other abused solvents are ubiquitous in commercial products including paint thinners, cleaning fluids, air fresheners and gasoline [1,17,21]. Acute high-dose inhalation of these vapors intoxicates within seconds and the effects can last for hours with repeated exposure. Inhalant abusers initially feel mild stimulation which progresses – as dose increases – to feelings of disinhibition, euphoria, dizziness and ataxia, slurred speech, and disorientation [1,17].
The easy, legal and inexpensive availability of organic solvents [40] are among the reasons young children and teens are at great risk for their use and abuse [14], especially among lower socioeconomic status (SES) groups [17,35,40,56,79-81]. The most recent National Survey of Drug Use and Health indicates that among the 20.9% of adolescents between the ages of 12 and 17 who reported illicit drug use in the previous year, 45.5% of young adolescents (12−13 years old) report abusing inhalants and 28.4% of older adolescents (14−17 years old) report inhalant abuse, confirming an alarming trend in inhalant use among teenagers. The report further characterized the age-related trends in the type of inhalant abused. Glue, shoe polish and toluene were the most frequently used inhalants among those between 12 and 15 years while among older adolescents, nitrous oxide (or “whippets”) were the most frequently used inhalant [66]. The high prevalence of inhalant use among youth emphasizes the importance of assessing the age- and dose-dependent impact of acute exposures to organic solvents on biobehavioral and neurochemical outcomes in an animal model of binge toluene exposure.
Animal research examining the effects of exposure to abused levels of inhalants has expanded significantly in the last decade (see these reviews: [5,13,15,24]). Animal studies show that, like other drugs of abuse, acute inhalant exposure produces behavioral changes that are concentration-dependent, reversible, and occur at concentrations lower than those necessary to produce explicit toxicity [13,24]. Behavioral effects and the abuse potential of toluene have been characterized in animal models of repeated, brief, high-concentration “binge” exposures (e.g., [5,13,15,24]). For example, toluene produces biphasic concentration-dependent effects on a number of rodent behaviors including locomotor activity [11,33,39], anxiolytic effects [16,43,57,78], and operant performance [30,50-53]. Toluene also supports conditioned place preference [26,28,41,42], enhances the threshold current for self-stimulation [83,84], produces cross-dependence [25], cross-sensitization to cocaine [8], and may be self-administered [9].
The local CNS neurochemical mechanisms underlying these behavioral effects remain to be fully determined. Toluene and related inhalants have effects on various neurotransmitter systems including dopamine, GABA, and glutamate (Glu; reviewed in [5,13,23]). Acute toluene decreases glutamate NMDA currents whereas repeated toluene exposure increases NMDA currents as well as select NMDA receptor subunits [4,20]. Similar NMDA-receptor antagonism has been demonstrated for related solvents such as ethanol, benzene, xylenes, ethylbenzene, propylbenzene, and 1,1,1-trichloroethane [19,62]. Prolonged exposure (10 days) to 8000 ppm toluene increases NMDA receptor subunit levels in the brain [76], similar to reports for prolonged exposure to ethanol in animals and humans. Acute toluene increases extracellular hippocampal glutamate and taurine (but not GABA) levels when measured with microdialysis [77]. Toluene effects on presynaptic GABA transmission are brain region-dependent in that acute toluene increased extracellular GABA in the cerebellum, decreased extracellular GABA in the globus pallidus, and had no effect in the striatum [70,71]. Little is known regarding the effects of acute toluene on neocortical neurochemistry. Toluene, as well as other abused solvents, facilitates GABAA and glycine receptor-mediated neuronal inhibition [4,7]. Finally, like many drugs of abuse with a high potential for dependence, toluene modulates mesolimbic dopamine activity, which may be related to its rewarding effects [27,29,64,65]. A recent review details the findings in this area during the last decade [13].
Despite progress in studies of the neurobiological consequences of toluene exposure and the mechanisms underlying behavioral effects and dependence, the effects of acute toluene exposure on regional changes in neurochemistry remain poorly understood. In our previous investigation, we used high-resolution magic angle spinning proton magnetic resonance spectroscopy (HR-MAS 1H-MRS) (Fig.1) to assess the effects of acute binge toluene inhalation on regional brain concentrations of several MR-visible neurochemicals, including Glu, GABA, and glutamine (Gln), in male and female juvenile rats [55]. High-dose toluene (8,000 ppm and 12,000 ppm), delivered to model patterns of exposure typical of solvent abuse in adolescents, significantly reduced levels of hippocampal GABA and Glu, and the striatal Glu/Gln ratio (an index of glutamatergic tone) that was driven largely by an increase in Gln. We also reported significant high-dose toluene-induced increases in alanine and lactate in several brain regions, indicative of altered oxygen-dependent metabolism which is consistent with what others have reported using MRS following hypoxia-ischemic insult [45,46,63]. Other key metabolites in the MR-visible neurochemical profile, particularly the relatively neuron-specific N-acetyl aspartate (NAA), as well as myo-inositol, creatine (Cre), and various choline (Cho) containing compounds, were unchanged in juvenile rats by acute toluene exposure. These results were consistent with the notion that binge toluene exposure affects juvenile neurochemistry in systems mediating the rewarding and emotional aspects of substance abuse.
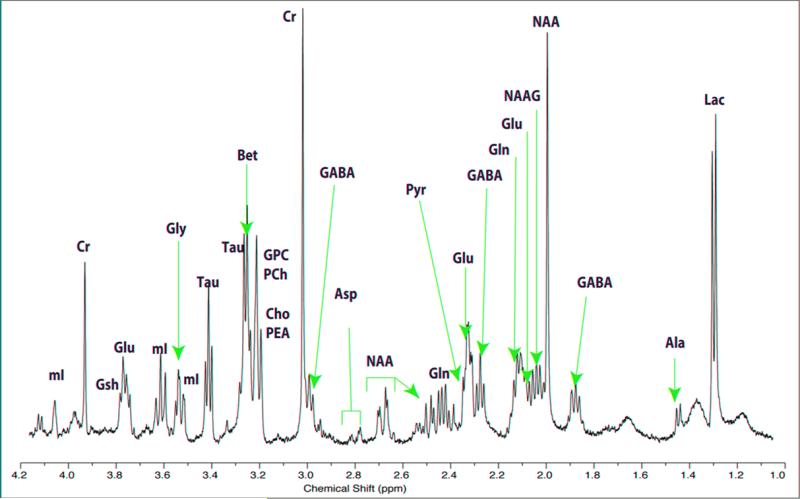
Figure 1.
Representative spectra from an adult male rate striatum. Neurochemicals that can be resolved include (from left to right): mI – myo-inositol, Cre – creatine, GSH – glutathione, Glu – glutamate, Gly – glycine, Tau – Taurine, Bet – betaine, GPC – glycerophosphorylcholine, PCh – phosphorylcholine, Cho – choline, PEA – phosphorylethanolamine, GABA – Gamma-aminobutyric acid, Asp – aspartate, NAA – N-acetyl-aspartate, Gln – glutamine, Pyr – pyruvate, NAAG – N-acetyl-aspartyl glutamate, Ala – alanine, Lac – lactate.
The purpose of the current study was to extend these neurochemical assessments in juveniles to adolescent and adult rats. Characterizing the developmental profile of biobehavioral and neurochemical sensitivity to the effects of toluene is critical to understanding the predominance and, eventually, the mechanism(s) of the long-term consequences of toluene use among youth [44,54]. We hypothesized that acute toluene inhalation using an exposure regime that models patterns of exposure typical of “binge” organic solvent abuse in rats would lead to differential effects on the MR-visible neurochemical profiles assessed at different developmental stages, that is adolescence versus young adulthood [55]. Specifically we hypothesized age- and dose-dependent changes in GABA, Glu, and Gln, consistent with toluene's known or suspected mechanisms of action on the glutamatergic and GABAergic systems [23]. We also hypothesized there would be age-dependent shifts in sensitivity to high-dose toluene-induced alterations in alanine and lactate in select brain regions, indicative of altered oxygen-dependent metabolism. Although unaltered in juveniles, levels of Cre, Cho, NAA, and myo-inositol were also examined in the current study because these compounds are readily visible at clinical field strengths of 1.5 Tesla and have been reported previously to be altered in clinical populations of toluene abusers (e.g., [3,72]).
2. Methods
The Wayne State University Institutional Animal Care and Use Committee approved all animal procedures, which were conducted in accordance with the NIH “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academy Press, NIH Publication No. 85−23, revised 1996). The generation of animals, toluene exposure procedures, brain tissue collection methods, and the HR-MAS 1H MR spectroscopy were detailed previously [55]. The animals tested at PN35 or PN56 in this study were littermates of the rats assessed at PN21 in our previous study.
2.1. Animal generation
Timed-pregnant female Sprague-Dawley rats were acquired on gestation day 4 (GD4; sperm plug positive = GD0) from Charles Rivers Laboratories (Portage, MI, USA). Dams weighed a minimum of 200g on arrival and were housed individually in polypropylene cages (52 cm × 28 cm × 22 cm) with hardwood chip bedding and steel-wire tops. Rodent Lab Diet 5001 (PMI, Nutrition International, Inc., Brentwood, MO) and water were available ad libitum. Animals were kept in a room with controlled temperature (22−24°C) on a 12-h light/12-h dark cycle (lights on 0600 h). Beginning on GD20, dams were inspected between 0800 hrs and 2000 hrs for births. The day of birth was designated as postnatal day 0 (PN0). On PN1, offspring were weighed and examined for obvious physical abnormalities. Litters were culled pseudo-randomly to 10, retaining 5 males and 5 females when possible. Offspring were housed with their dams until weaning on PN21 at which time rats were housed in same-sex groups of 3 until the toluene exposure on PN35 (n=25) or PN56 (n=32).
2.2. Inhalation exposure procedures
Animals within litters were assigned randomly to one of three toluene concentration (dose) exposure groups (0 ppm, 8,000 ppm or 12,000 ppm toluene; gender balanced). On PN35 or PN56 rats were weighed and then exposed via inhalation to 8,000 ppm or 12,000 ppm of toluene (Fisher Scientific Co., Fairlawn, NJ). A separate “air-only” control group received the same handling and placement into exposure chambers without toluene (0 ppm). Rats were administered two 15-min inhalation exposures to air or toluene with a 2-hr interval between exposures. These concentrations and this regime of repeated, brief exposures, in contrast to lower levels of relatively constant environmental or industrial exposures, were used to model the very high levels and patterns of inhalation typical of solvent abusers [33,75]. Exposure to toluene vapor occurred in one of four sealed 36-liter cylindrical glass jars with Plexiglas® lids and located in a chemical fume hood. During toluene exposure, rats were placed one at a time into the chambers onto a grid floor 20 cm from the bottom and 30 cm from the vapor diffuser in the lid. Chambers were sealed and toluene (0.0 mL for 0 ppm air controls, 1.36 mL for 8,000 ppm, 2.05 mL for 12,000 ppm) was injected through a solvent injection port onto a filter paper held in the stainless steel mesh diffuser. The injection port was sealed and a fan in the diffuser volatilized the toluene and spread it throughout the chamber. Following a 15-min exposure, the animals were removed and the chambers evacuated before continuing with the next squad of animals. Within a given squad, the air-only (0 ppm) exposures were restricted to one chamber, but that chamber was counterbalanced across squads. Single wavelength-monitoring infrared spectroscopy (Miran 1-A, Foxboro Analytical, North Haven, CT) was used periodically to ensure consistent ambient toluene concentrations and proper chamber clearance. Actual toluene concentrations in inhalation chambers during the 8,000-ppm and 12,000-ppm exposure conditions were within 2% of nominal within 1 to 2 mins and did not deviate significantly from that asymptote during an entire 15-min session (cf., [10]).
2.3. Tissue collection
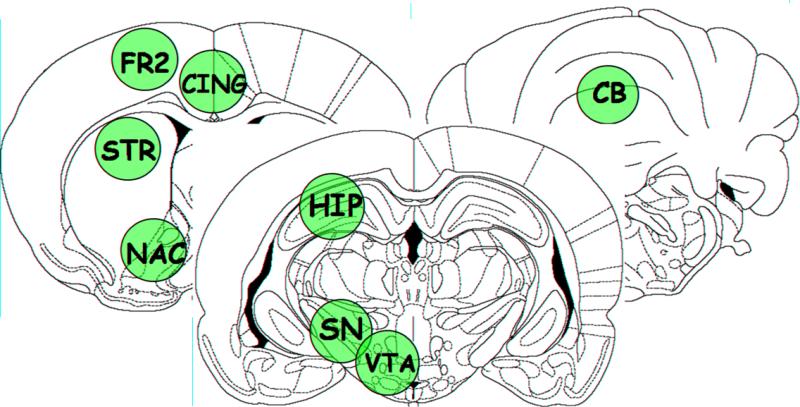
Immediately after completion of the second 15-min inhalation exposure, the subjects were sacrificed, whole brains quickly extracted and placed into an ice-cold matrix, and then sliced into 2-mm thick slices. The left and right frontal neocortex (identified as medial precentral area Fr2 [62]), anterior cingulate cortex, dorsal hippocampus (CA1 region), anterior dorsal striatum, nucleus accumbens, anterior cerebellar vermis, ventral tegmental area (A10) and substantia nigra (A9) were microdissected using a punch technique (2.1-mm dia. punches) from the slice containing the various regions of interests (see Fig. 2) [60]. Some of these brain areas were chosen because of reported clinical effects [2,3,22,37,82] and/or effects at PN21 in rats [55]. (At PN21, the A9 and A10 areas were collected in a single punch [55].) This procedure produced tissue punch samples consistently weighing ∼4 mg. Punches were frozen immediately on solid CO2 and stored at −80°C until the HR-MAS 1H MRS analyses.
Figure 2.
Tissue punches from the regions of interest according to Paxinos and Watson (1997). Fr2 – frontal cortex, STR – anterior striatum, NAC – nucleus accumbens, HIP – hippocampus, SN – substantia nigra, VTA – ventral tegmental area A10, CB – cerebellar vermis.
2.4. HR-MAS 1H MRS methods
For high-resolution magic angle spinning (MAS) proton magnetic resonance spectroscopy (HR-MAS 1H MRS), tissue was rapidly weighed and placed into a Bruker zirconium rotor (2.9-mm dia., 10-μL capacity) containing 5μL of D2O and 3-[(trimethylsilyl)]–1-propane sulfonic acid (TSP) to allow for locking on the center frequency and as a chemical shift reference (0.0 ppm), respectively. The rotor was then inserted immediately into a MAS probe (Bruker) in a 500MHz (11.7-T) Bruker Avance 500 MR spectrometer. The rotor and sample were maintained at 4°C and spun at a MAS rate of 4.2kHz while positioned at 54.7° relative to the magnetic field (B0). A Carr-Purcell-Meiboom-Gill (CPMG) rotor-synchronized pulse sequence [18] (TR = 3500ms, bandwidth 8kHz, 16k complex points, 32 averages) was used to acquire the spectra with a total acquisition time of 3 min 38 sec. The resulting spectra were analyzed using LCModel software that relied on a custom basis set of spectra for all metabolites generated with 27 individual phantom standard solutions as well as unidentified lipid resonances [61]. Cramer-Rao bounds estimated the precision with which LC Model fit the data and were typically below 10% indicating excellent fit. Concentrations were then determined for each visible neurochemical, corrected for tissue sample weight, and expressed as nmol/mg of wet weight and as percent of control values.
2.5. Statistical analysis
Before statistical analyses, data were converted to percent of controls within each Treatment group, Region, Sex and Age. This was necessary because a major upgrade to the Bruker MR spectrometer system was done half way through data collection which prevented direct comparisons of pre- and post-upgrade quantitative values. Converting data to a percent control allowed direct comparisons to assess effects due to toluene treatment within each brain region and age and not an artifact of the system upgrade. Preliminary analyses using MANOVAs within each brain region and age revealed no main effects or interactions with Sex in adolescent animals and just two main effects and one interaction in adult subjects. This, coupled with the relatively small cell sizes, led to collapsing data on Sex for further analyses. In addition, our prior study at PN21 [55] and preliminary analyses here confirmed the presence of significant regional effects for most neurochemicals of interest (all p's< 0.05) so subsequent analyses were conducted for each brain region separately. Neurochemical data were analyzed using separate ANOVAs at each age with acute toluene Treatment as the key between-subjects independent variable. All significant ANOVAs were followed by Dunnett's post hoc comparisons to compare toluene-exposed subjects to controls. A significance level of alpha = 0.05 was maintained for all analyses.
3. Results
Similar to other reports [12], animals exposed to toluene in the present study showed no overt signs of abnormal behavior other than immobility and sedation. There were initial qualitative increases in activity by the rats in the chambers followed by concentration-dependent decreases in activity.
3.1. Glutamate (Glu), glutamine (Gln), GABA & Glycine (Gly)
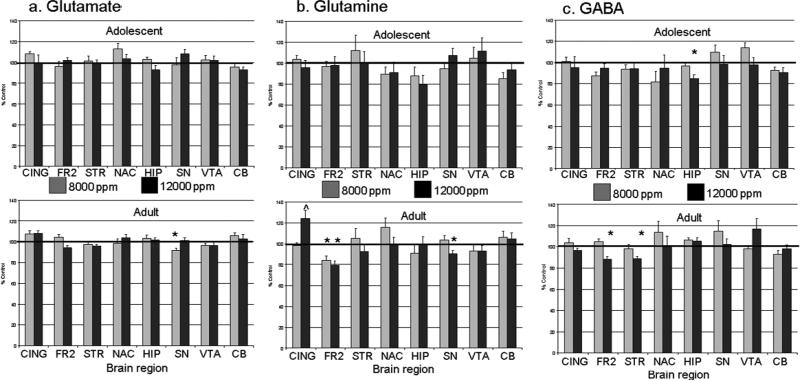
In adolescents, no significant alterations in Glu or Gln were evident in any brain region (all p's>0.05) (Figure 3a & 3b). However, GABA was significantly lower in hippocampus in the 12,000-ppm toluene exposure group compared to controls (F(2,23)=4.75, p<0.05) (Figure 3c). In adults, however, regionally-specific alterations were evident in levels of Gln and GABA (but not Glu) after acute toluene inhalation. (A significant effect on GLU in the SN in adults (F(2,31)=3.36, p<0.05) failed to reach significance in post hoc tests). Specifically, GABA levels in adult animals exposed to 12,000 ppm toluene were reduced in the striatum (F(2,28)=4.57, p<0.05) and the frontal cortex (F(2,29)=12.50, p<0.001) compared to controls (Figure 3c). Compared to controls, Gly levels were significantly reduced after 12,000 ppm toluene in the frontal cortex (F(2, 29)=7.681, p<0.05) and in the anterior dorsal striatum (F(2,28)=3.587, p<0.05). Although Gln was reduced in the 12,000-ppm toluene exposure group compared to controls in the frontal cortex (F(2,29)=5.24, p<0.01) and in the substantia nigra (F(2,29)=3.214, p=0.05), Gln levels were increased significantly compared to controls in the anterior cingulate cortex (F(2,29)=5.24, p<0.01) (Figure 3b).
Figure 3.
Glutamate (a), Glutamine (b), and GABA (c) levels after acute toluene exposure in adolescent (top panels) and adult (bottom panels). Toluene significantly decreased glutamate, glutamine, and GABA in several areas after 8,000 and 12,000 ppm toluene (* p < 0.05).
3.2. N-Acetyl Aspartate (NAA)
In adolescent rats, there was no effect of acute toluene exposure on levels of NAA in any brain region (p's >0.05). However, in adults there was a significant reduction in levels of NAA in the anterior dorsal striatum, after 12,000-ppm toluene, compared to controls (F(2,28)=3.33, p=0.05) (Table 1).
Table 1. Data expressed as a percent of Control. Pink = decreases, Green = increases.
Summary of comparisons among toluene exposure levels groups for each neurochemical and brain region (data presented as percent of control). Brain regions of interest were: ACC – anterior cingulate cortex, Fr2 – frontal neocortex; STR – anterior dorsal striatum; NAC – nucleus accumbens; HIP – hippocampus CA1; VTA – ventral tegmental area A10; SN – substantia nigra; CB – cerebellar vermis. Neurochemicals assessed with HR-MAS 1H-MRS were: Cre – creatine; Cho – choline; NAA – N-acetyl aspartate, ; myo-I – myo-inositol, LAC – lactate; ALA – alanine.
| Age (PN) | Tx | Cing | FR2 | STR | NAC | HIP | SN | VTA | CB | |
|---|---|---|---|---|---|---|---|---|---|---|
| Creatine | 35 | 8000 ppm | 97.9 (4.1) | *89.1 (2.2) | 97.8 (2.7) | 101.1 (1.2) | 94.4 (4.5) | 100.5 (3.7) | 103.6 (3.9) | 92.7 (3.5) |
| 12000 ppm | 107.5 (7.0) | 99.5 (3.2) | 99.3 (3.0) | 101.6 (2.3) | 92.0 (5.2) | 102.7 (1.0) | 99.4 (3.1) | 93.4 (3.2) | ||
| 56 | 8000 ppm | 102.0 (1.9) | 101.0 (1.7) | 98.9 (2.4) | 98.3 (1.4) | 100.0 (2.2) | 102.3 (1.5) | 100.0 (2.2) | 101.7 (3.3 | |
| 12000 ppm | 103.7 (2.2) | 94.9 (2.2) | 96.4 (1.6) | 101.0 (1.3) | 100.9 (1.7) | 99.1 (1.3) | 98.4 (2.2) | 99.5 (3.0) | ||
| Choline | 35 | 8000 ppm | 96.1 (13.5) | 83.8 (11.1) | 126.0 (27 7) | 89 5 (12.1) | 76.3 (9.9) | 97.0 (11.0) | 114.2 (8.9) | 108.9 (7.9) |
| 12000 ppm | 108.8 (14.8) | 103.9 (8.1) | 115.5 (7.2) | 106.7 (9.8) | 79.2 (9.6) | 102.1 (6.5) | 118.9 (9.8) | 107.4 (9.8) | ||
| 56 | 8000 ppm | 89.7 (5.4) | 85.9 (7.5) | 95.7 (5.7) | 114.5 (9.2) | 108.2 (6.7) | 113.1 (2.6) | 85.6 (8.8) | 91.4 (5.2) | |
| 12000 ppm | 105.4 (6.7) | *74.0 (5.0) | *76.8 (5.1) | 87.6 (5.5) | 101.9 (12.8) | 95.1 (7.4) | 83.9 (8.0) | 100.6 (13.3) | ||
| NAA | 35 | 8000 ppm | 101.1 (3.3) | 94.5 (6.4) | 102.4 (4.7) | 103.1 (2.1) | 100.2 (2.8) | 98.5 (4.2) | 108.0 (3.6) | 98.2 (3.3) |
| 12000 ppm | 104.8 (8.8) | 101.5 (2.9) | 102.4 (2.6) | 107.5 (2.2) | 96.1 (4.3) | 102.6 (2.5) | 102.2 (4.2) | 92.6 (3.3) | ||
| 56 | 8000 ppm | 102.7 (2.4) | 104.6 (2.8) | 96.9 (2.0) | 104.9 (1.3) | 99.2 (2.2) | 100.7 (1.9) | 99.9 (2.3) | 101.7 (4.1) | |
| 12000 ppm | 101.6 (2.2) | *91.8 (3.0) | *94.2 (1.5) | 103.3 (1.6) | 100.3 (1.7) | 101.4 (2.1) | 96.9 (1.9) | 99.3 (2.2) | ||
| ml | 35 | 8000 ppm | 85.9 (10.0) | 85.2 (5.0) | 102.4 (6.1) | 92.8 (6.2) | 95.4 (5.7) | 86.5 (3.2) | 99.7 (3.3) | *85.1 (1.2) |
| 12000 ppm | 109.5 (8.3) | 99.8 (7.7) | 103.4 (5.8) | 93.6 (5.4) | 87.1 (3.0) | 97.7 (4.8) | 99.9 (5.2) | #87.1 (5.0) | ||
| 56 | 8000 ppm | 99.1 (2.6) | 95.9 (2.6) | 96.7 (4.7) | 107.4 (4.0) | 101.3 (2.6) | 106.0 (3.0) | 98.1 (4.6) | 95.9 (2.7) | |
| 12000 ppm | 105.7 (2.8) | 92.8 (3.2) | 98.1 (1.9) | 96.1 (3.9) | 103.7 (2.7) | 98.2 (2.6) | 93.2 (3.6) | 98.3 (2.8) | ||
| LAC | 35 | 8000 ppm | 107.1 (6.0) | 100.9 (4.9) | 106.5 (5.0) | 100.8 (1.4) | 96.4 (3.8) | 105.3 (3.9) | 108.6 (3.8) | 89.7 (3.8) |
| 12000 ppm | 112.2 (11.9) | *117.0 (4.1) | 114.0 (5.9) | 108.9 (4.2) | 90.6 (7.9) | 110.7 (5.2) | 102.1 (6.2) | 93.7 (6.1) | ||
| 56 | 8000 ppm | *113.7 (3.4) | *109.4 (2.6) | *112.8 (3.9) | 113.5 (4.5) | 108.3 (4.3) | *115.8 (3.0) | 103.6 (3.7) | 102.3 (2.6) | |
| 12000 ppm | *119.5 (3.5) | 104.0 (2.8) | *110.7 (3.0) | 106.9 (3.0) | 108.8 (2.8) | *117.1 (3.7) | 102.9 (2.9) | 106.1 (3.4) | ||
| ALA | 35 | 8000 ppm | *131.0 (7.4) | 114.6 (12.0) | *126.2 (8.4) | *144.4 (14.0) | 114.3 (11.3) | 94.1 (12.7) | ||
| 12000 ppm | 98.6 (7.5) | 116.9 (9.2) | *122.9 (9.4) | 108.3 (8.7) | 91.64 (8.7) | 88.6 (4.8) | ||||
| 56 | 8000 ppm | 96.9 (9.7) | *124.7 (7.5) | 105.8 (4.9) | 106.5 (10.2) | 116.6 (6.2) | 106.2 (6.9) | |||
| 12000 ppm | 101.8 (14.2) | 108.6 (4.6) | *123.6 (3.6) | 118.76 (6.8) | *124.13 (4.8) | 107.0 (8.3) |
indicates significant difference compared to 0-ppm controls (p < 0.05)
approached significance (p < 0.07)
3.3. Creatine (Cre)
In adolescents, Cre was reduced significantly in the frontal cortex after 8,000 ppm toluene compared to controls (F(2,20)=4.32, p<0.05). In adults, an ANOVA indicated a significant effect of toluene of Cre levels in frontal cortex (F(2,29)=3.41 p<0.05) but post hoc tests failed to reach statistical significance when comparing the 12,000-ppm toluene exposure group and controls (p=0.076).
3.4. Choline-related compounds
Levels of choline (Cho), glycerophosphorylcholine (GPC) and phosphorylcholine (PCh) were unchanged after adolescent toluene exposure in all brain regions (all p's>0.05). However, levels of phosphorylethanolamine (PEA, a precursor for membrane phospholipids such as sphingomyelin) in the nucleus accumbens were significantly lower in the 12,000-ppm group compared to controls (F(2,20)=4.36, p<0.05) and in the anterior cingulate cortex, PEA levels were actually higher in the 8000 ppm group compared to controls (F(2,19)=2.99, p<0.05). In adult animals, Cho was reduced in the 12,000-ppm group compared to control groups in the striatum (F(2,28)=5.79, p<0.01) and the frontal cortex (F(2,27)=3.82, p<0.05) (Table 1). A significant ANOVA indicated a main effect of toluene exposure on levels of GPC in the cingulate, and analysis of means indicated that GPC was reduced in both the 8000 ppm- and 12,000 ppm-groups compared to controls (F(2,27)=3.52, p<0.05), but post hoc tests failed to reach statistical significance. Finally, levels of PEA were increased by 12,000 ppm toluene in adult animals in the cingulate cortex (F(2,29)=3.72, p<0.05) and the nucleus accumbens (F(2,29)=4.34, p<0.05), but decreased in the frontal cortex (F(2,26)=4.01, p<0.05), compared to controls.
3.5. Alanine and lactate
In adolescent animals, alanine levels were increased in the anterior striatum (F(2,19)=6.64, p<0.01), anterior cingulate cortex (F(2,18)=6.71, p<0.01), and the nucleus accumbens (F(2,18)=5.34, p<0.05) after toluene exposure (Table 1). Post hoc tests revealed that toluene significantly increased alanine levels in the striatum after both toluene doses compared to air controls, while in the cingulate and nucleus accumbens, alanine was increased only in the 8,000-ppm treatment group (p's <0.05). In adults, 12,000 ppm of acute toluene exposure significantly increased levels of alanine in the striatum (F(2,28)=6.62, p<0.01) and hippocampus (F(2,28)=4.60, p<0.05) compared to 0-ppm controls. Exposure to 8,000 ppm increased alanine levels in frontal cortex (F(2,28)=6.03, p<0.01). In terms of lactate, toluene significantly increased lactate levels in adolescents in the frontal cortex after 12000 ppm toluene (F(2,20)=6.85, p<0.05). Changes were also evident in lactate in adults; both the 8,000-ppm and 12,000-ppm groups had higher lactate levels than 0-ppm controls in the striatum (F(2,28)=6.01, p<0.01), cingulate (F(2,28)=8.43, p<0.01), and substantia nigra (F(2,29)=10.6, p<0.01). The 8,000-ppm group also had significantly higher lactate levels in frontal cortex (F(2,29)=3.80, p<0.05).
3.6. myo-Inositol and taurine (TAU)
Cerebellar myo-inositol levels were significantly lower in the 8,000-ppm toluene exposure group compared to 0-ppm controls in adolescent animals (F(2,22)=4.05, p<0.05) and a reduction in the 12,000-ppm group approached significance (p=0.053) (Table 1). In adults, taurine levels were lowered significantly by 12,000 ppm toluene compared to controls in frontal cortex (F(2,29)=9.54, p<0.01).
4. Discussion
The major finding from this study was that an acute exposure to toluene, modeled after bingeing patterns in inhalant-abusing humans, produced brain region- and dose-dependent effects on several neurochemicals, and that these effects were substantially different for the adolescent (PN35) and the young adult rats (PN56). Although we had hypothesized that the adolescent animals would be more affected by the toluene exposure, the results suggest that the older animals were more susceptible to toluene-induced alterations in the MR-visible neurochemical profile. Alterations in each neurochemical are discussed below with a particular emphasis on the neurobiology of such changes in the developing animal.
Given toluene's known effects on glutamatergic and GABAergic systems, one of the main objectives of this current study was to characterize the effect of acute toluene exposure on levels of MR-visible Glu and GABA, as well as Gln (see Fig 3). We found no alterations in levels of Glu after toluene exposure at either age, contrary to what we observed previously in juvenile rats [55] as well as the work of others (e.g. [4,20,77]. However, toluene exposure selectively altered Gln levels in adults consistent with disruptions in glutamatergic cycling or flux after acute toluene. After neuronal release of Glu into the synapse, Glu is transported into astroglia and converted to Gln by glutamine synthetase [47]. Gln is then transported out of the glia and back into neurons where it is hydrolyzed into glutamate by phosphate-activated glutaminase. In the current study, adults had reduced Gln levels in frontal cortex and substantia nigra and significantly increased Gln levels in the anterior cingulate cortex. These changes indicate region-specific alterations in Glu/Gln cycling, or an astrocyte response to inflammation and suggest effects of toluene on glial function. However, the direction of change (activation or inhibition) cannot be currently discerned based on changing Gln levels. Nonetheless, toluene does affect astrocytes in that toluene increases glial fibrillary acidic protein (GFAP) [31].
Aside from alterations in Gln, GABA was lower in toluene-exposed adolescents in the hippocampus (consistent with our previous findings in juveniles) and GABA levels were also lower in the frontal cortex and striatum in adults. Changes in GABA could reflect reduced cortical-striatal excitatory drive and a consequent decrement in striatal GABA function. Alternatively, reduced MR-visible GABA in the striatum may be a combined consequence of a toluene-induced increase in extracellular GABA [71] that may alter GABA function directly (by recurrent collateral inhibition) or indirectly (by inhibition of excitatory afferents) at the level of glutamic acid decarboxylase (GAD2) [58] phosphorylation by protein kinase Cε [74]. Finally, cycling of either Gln or GABA is energy demanding [59] and toluene-induced deficits in energy homeostasis would be expected to disrupt the cycle and neurochemical levels. It should be noted that 1H-MRS cannot currently distinguish between intra- and extracellular levels of neurochemicals.
Adult subjects in the current experiment had decreased levels of NAA in the, anterior dorsal striatum. NAA is localized in neuronal mitochondria and synthesized from acetyl-coA and aspartate by aspartate-N-acetyl-transferase. A high rate of NAA turnover and cycling occurs between neurons and oligodendrocytes (reviewed by [6]). Decreased levels of NAA in 1H-MRS studies have been interpreted as neuronal cell loss or damage following a number of chronic conditions ranging from hypoxia-ischemia (e.g., [34,46] to autism [36]. However, a recent review by Moffett and colleagues highlighted NAA's role in bioenergetic metabolism, osmoregulation, and myelin synthesis in the developing brain, and in axon-glial signaling [48]. Reduced striatal NAA in adult animals in this study is probably not reflective of overt neurotoxicity given the acute and relatively short duration of toluene exposure. Rather, the decreased NAA levels in the current experiment (after acute drug challenge) may reflect an alteration in mitochondrial energy status as dictated by demand or availability of acetate for the TCA cycle ([48]). Indeed a recent study in humans suggested that loss of NAA in temporal lobe epilepsy was attributable to altered mitochondrial function rather than neuronal loss [73].
In the current study, Cre was reduced in the frontal cortex by the lower toluene dose in adolescents and by the higher toluene dose in adults. Cre, in equilibrium with phosphocreatine, maintains readily available high-energy phosphate. Reduced striatal NAA combined with decreased cortical Cre may reflect a toluene-dependent disruption in a corticostriatal pathway. However, this interpretation is tentative since medium spiny GABA neurons constitute the majority of striatal neuropil and toluene-induced changes in the striatal neurochemical profile may represent metabolic alterations in these GABA neurons. In fact, the toluene-induced decrease in striatal GABA in the current study is consistent with this latter interpretation. Alternatively, when taken together, decreased NAA and Cre, combined with increased lactate and alanine (see below) are consistent with a shift in the fate of pyruvate (due to energy status and compromised pyruvate transport into mitochondria) followed by a compensatory intra-mitochondrial utilization of creatine and the acetate moiety from NAA for energy demands.
Here we report significant alterations in Cho-containing compounds in adults, especially in cortical areas. Specifically, high-dose toluene exposure reduced free Cho in the striatum and frontal cortex. GPC levels were also significantly reduced in the anterior cingulate cortex after acute binge toluene exposure. In clinical 1H-MRS, the Cho peak is comprised mainly of GPC, a product phospholipase-A2 cleavage of phosphatidylcholine (PtdlCho), and PCh, a further catabolite of GPC and a precursor for PtdlCho synthesis. Although elevated Cho levels might suggest increased turnover of membrane phospholipids (e.g., [49]), the overlapping resonances of PCh and GPC in clinical 1H-MRS profiles render the interpretation of changes in Cho difficult since elevated Cho can reflect opposing processes – membrane precursors and breakdown products, respectively. One major strength of the HR-MAS 1H-MRS method is sufficient resolution to distinguish the resonances of overlapping peaks representing different choline-containing compounds. In our study, the reductions in levels of Cho and GPC may reflect a decrease in membrane turnover or increased conversion of lyso-PtdlCho to the proinflammatory platelet-activating factor, at the expense of GPC production from lyso-PtdlCho. Another precursor for membrane phospholipid synthesis is phosphorylethanolamine (PEA) which was also altered after toluene exposure. Specifically, acute toluene exposure decreased PEA in the nucleus accumbens in adolescents and in the frontal cortex (Fr2 region) in adults. However, PEA was actually increased by toluene in both the anterior cingulate cortex and the nucleus accumbens in adult rats. PEA is a precursor of phosphatidylethanolamine that together with PCh makes up the phosphomonoesters (PME), a pool amenable to measurement with 31P-MRS [69]. PMEs are elevated in tissues where rapid membrane synthesis is taking place [32]. A reduction in PEA could indicate a decrease in membrane synthesis, consistent with our findings with Cho and GPC. However, reductions in PEA, Cho, and GPC were not general across brain regions arguing against a non-specific perturbation of membrane stability. The brain region-specific alterations may represent selective activation of proinflammatory cascades mediated by membrane phospholipid precursors.
These results extend our previous study [56] of the effects of acute, high-dose toluene exposure on regional brain neurochemistry in juvenile rats (i.e., postnatal day 21 [PN21]) to adolescent (PN35) and young adult (PN56) animals, allowing an estimate of a developmental profile. The current findings replicate previous studies [55] in reporting significant regional toluene dose-dependent differences in the neurochemical profile measured by the highly sensitive HR-MAS 1H MRS technique. However, the patterns of significant effects for both the adolescent and adult animals differed from the pattern of effects reported previously for juvenile animals [55]. For example, juveniles showed increases in alanine levels in frontal cortex (like adults) and in striatum and anterior cingulate (like adolescents). Also, there were significant increases in lactate in frontal cortex (Fr2 region) and anterior striatum in the juveniles, consistent with the adults here, while only high-dose toluene elevated lactate in frontal cortex in adolescents. The biphasic age effects for lactate – elevations in juveniles and adults but few changes in adolescents – suggest a lowered general sensitivity to the acute effects of toluene intoxication in adolescence.
These findings of dose-, age- and brain region-dependent patterns of significant effects of acute binge toluene exposure on neurochemistry have implications for the behavioral effects of toluene and perhaps for the potential for abuse, dependence and/or brain damage. The apparent developmental course of toluene's neurochemical effects may underlie maturational changes in toluene's biphasic concentration-dependent effects on locomotor activity [11,33,39], anxiolytic effects [16,43,57,78], and conditioning [30,50-53]. Although the developmental baselines of the various neurotransmitter systems affected by toluene, including GABA and Glu [5,13,23], could not be assessed directly in the current study, the relative differences in the behavioral impact of toluene in adolescence may be related to differences in neurotransmitter development (e.g., [67]). For example, ontogenetic shifts in NMDA currents or receptor sub-unit expression [4,20], extracellular hippocampal Glu levels [77], or presynaptic GABA transmission [70,71], can alter toluene's effects. Adolescent changes in sensitivity of MR-visible neurochemicals may also be secondary to developmental changes in mesolimbic dopamine activity which may be related to toluene's rewarding effects [27,29,38,64,65]. As noted in the introduction, solvent abuse is most likely to occur in youth and adolescents [17,35,40,56,79-81] and the current results offer a possible neurochemical basis for this, in addition to the psychosocial and cultural reasons. The potential influence of toluene's impact on neurochemistry in juveniles or adolescence on lifelong risk for other substance use/abuse is also an important question [68].
In conclusion, the main finding of this study was that the adolescent rat brain appears to be less responsive to high-concentration, binge toluene exposure than brains of either younger juvenile rats [55] or older young-adult rats, as indicated by the fewer number of significant, brain region-dependent alterations in the MR-visible neurochemical profile. The results imply that brain development during the young adult period is more susceptible to toluene-induced brain damage, even after single binge exposures. Because toluene use is usually not a single isolated occurrence but is rather a pattern of abuse over time, future studies should focus on characterizing the effects of long-term or chronic toluene exposure on MR-visible neurochemistry.
Acknowledgments
This work was supported in part by NIDA R01 DA015951 to S.E. Bowen and the Joe Young Sr. Research Fund in Psychiatry. A preliminary report of a portion of this study was presented at the Annual Meeting of the Society for Neuroscience in 2006. The expert technical assistance of Dr. Farhad Ghoddoussi and Dr. Navid Seraji-Bozorgzad is gratefully acknowledged. Dr. O'Leary-Moore is currently at the Fetal Toxicology Division, Bowles Center for Alcohol Studies, 3019 Thurston-Bowles Bldg, CB# 7178, University of North Carolina, Chapel Hill, NC, 27599−7178 (somoore@med.unc.edu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. Literature Cited
- 1.Anderson CE, Loomis GA. Recognition and prevention of inhalant abuse. American family physician. 2003;68:869–74. [PubMed] [Google Scholar]
- 2.Aydin K, Sencer S, Demir T, Ogel K, Tunaci A, Minareci O. Cranial MR findings in chronic toluene abuse by inhalation. Ajnr. 2002;23:1173–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Aydin K, Sencer S, Ogel K, Genchellac H, Demir T, Minareci O. Single-voxel proton MR spectroscopy in toluene abuse. Magn Reson Imaging. 2003;21:777–85. doi: 10.1016/s0730-725x(03)00175-9. [DOI] [PubMed] [Google Scholar]
- 4.Bale AS, Tu Y, Carpenter-Hyland EP, Chandler LJ, Woodward JJ. Alterations in glutamatergic and gabaergic ion channel activity in hippocampal neurons following exposure to the abused inhalant toluene. Neuroscience. 2005;130:197–206. doi: 10.1016/j.neuroscience.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 5.Balster RL. Neural basis of inhalant abuse. Drug and alcohol dependence. 1998;51:207–14. doi: 10.1016/s0376-8716(98)00078-7. [DOI] [PubMed] [Google Scholar]
- 6.Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 2003;28:941–53. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- 7.Beckstead MJ, Weiner JL, Eger EI, 2nd, Gong DH, Mihic SJ. Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Molecular pharmacology. 2000;57:1199–205. [PubMed] [Google Scholar]
- 8.Beyer CE, Stafford D, LeSage MG, Glowa JR, Steketee JD. Repeated exposure to inhaled toluene induces behavioral and neurochemical cross-sensitization to cocaine in rats. Psychopharmacology. 2001;154:198–204. doi: 10.1007/s002130000614. [DOI] [PubMed] [Google Scholar]
- 9.Blokhina EA, Dravolina OA, Bespalov AY, Balster RL, Zvartau EE. Intravenous self-administration of abused solvents and anesthetics in mice. European journal of pharmacology. 2004;485:211–8. doi: 10.1016/j.ejphar.2003.11.068. [DOI] [PubMed] [Google Scholar]
- 10.Bowen SE. Increases in amphetamine-like discriminative stimulus effects of the abused inhalant toluene in mice. Psychopharmacology. 2006;186:517–24. doi: 10.1007/s00213-006-0381-8. [DOI] [PubMed] [Google Scholar]
- 11.Bowen SE, Balster RL. A direct comparison of inhalant effects on locomotor activity and schedule-controlled behavior in mice. Experimental and clinical psychopharmacology. 1998;6:235–47. doi: 10.1037//1064-1297.6.3.235. [DOI] [PubMed] [Google Scholar]
- 12.Bowen SE, Batis JC, Mohammadi MH, Hannigan JH. Abuse pattern of gestational toluene exposure and early postnatal development in rats. Neurotoxicol Teratol. 2005;27:105–16. doi: 10.1016/j.ntt.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Bowen SE, Batis JC, Paez-Martinez N, Cruz SL. The last decade of solvent research in animal models of abuse: mechanistic and behavioral studies. Neurotoxicol Teratol. 2006;28:636–47. doi: 10.1016/j.ntt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Bowen SE, Daniel J, Balster RL. Deaths associated with inhalant abuse in Virginia from 1987 to 1996. Drug and alcohol dependence. 1999;53:239–45. doi: 10.1016/s0376-8716(98)00139-2. [DOI] [PubMed] [Google Scholar]
- 15.Bowen SE, Hannigan JH. Developmental toxicity of prenatal exposure to toluene. The AAPS journal. 2006;8:E419–24. doi: 10.1007/BF02854915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowen SE, Wiley JL, Balster RL. The effects of abused inhalants on mouse behavior in an elevated plus-maze. European journal of pharmacology. 1996;312:131–6. doi: 10.1016/0014-2999(96)00459-1. [DOI] [PubMed] [Google Scholar]
- 17.Brouette T, Anton R. Clinical review of inhalants. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2001;10:79–94. doi: 10.1080/105504901750160529. [DOI] [PubMed] [Google Scholar]
- 18.Cheng LL, Ma MJ, Becerra L, Ptak T, Tracey I, Lackner A, Gonzalez RG. Quantitative neuropathology by high resolution magic angle spinning proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1997;94:6408–13. doi: 10.1073/pnas.94.12.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz SL, Balster RL, Woodward JJ. Effects of volatile solvents on recombinant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Br J Pharmacol. 2000;131:1303–8. doi: 10.1038/sj.bjp.0703666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz SL, Mirshahi T, Thomas B, Balster RL, Woodward JJ. Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. The Journal of pharmacology and experimental therapeutics. 1998;286:334–40. [PubMed] [Google Scholar]
- 21.Cruz SL, Bowen SE. Inhalant Abuse. In: Ubach MM, Mondragon-Ceballos R, editors. Neural Mechanisms of Action of Drugs of Abuse and Natural Reinforcers. Research Signpost; Kerala, India: 2008. pp. 61–87. [Google Scholar]
- 22.Deleu D, Hanssens Y. Cerebellar dysfunction in chronic toluene abuse: beneficial response to amantadine hydrochloride. J Toxicol Clin Toxicol. 2000;38:37–41. doi: 10.1081/clt-100100913. [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg DP. Neurotoxicity and Mechanism of Toluene Abuse. Einstein Quart J Biol Med. 2003;19:150–159. [Google Scholar]
- 24.Evans EB, Balster RL. CNS depressant effects of volatile organic solvents. Neuroscience and biobehavioral reviews. 1991;15:233–41. doi: 10.1016/s0149-7634(05)80003-x. [DOI] [PubMed] [Google Scholar]
- 25.Evans EB, Balster RL. Inhaled 1,1,1-trichloroethane-produced physical dependence in mice: effects of drugs and vapors on withdrawal. The Journal of pharmacology and experimental therapeutics. 1993;264:726–33. [PubMed] [Google Scholar]
- 26.Funada M, Sato M, Makino Y, Wada K. Evaluation of rewarding effect of toluene by the conditioned place preference procedure in mice. Brain Res Brain Res Protoc. 2002;10:47–54. doi: 10.1016/s1385-299x(02)00182-4. [DOI] [PubMed] [Google Scholar]
- 27.Gerasimov MR. Brain uptake and biodistribution of [11C]toluene in nonhuman primates and mice. Methods in enzymology. 2004;385:334–49. doi: 10.1016/S0076-6879(04)85018-3. [DOI] [PubMed] [Google Scholar]
- 28.Gerasimov MR, Collier L, Ferrieri A, Alexoff D, Lee D, Gifford AN, Balster RL. Toluene inhalation produces a conditioned place preference in rats. European journal of pharmacology. 2003;477:45–52. doi: 10.1016/j.ejphar.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Gerasimov MR, Ferrieri RA, Schiffer WK, Logan J, Gatley SJ, Gifford AN, Alexoff DA, Marsteller DA, Shea C, Garza V. Study of brain uptake and biodistribution of [11C]toluene in non-human primates and mice. Life sciences. 2002;70:2811–28. doi: 10.1016/s0024-3205(02)01542-4. others. [DOI] [PubMed] [Google Scholar]
- 30.Glowa JR. Some effects of sub-acute exposure to toluene on schedule-controlled behavior. Neurobehavioral toxicology and teratology. 1981;3:463–5. [PubMed] [Google Scholar]
- 31.Gotohda T, Tokunaga I, Kubo S, Morita K, Kitamura O, Eguchi A. Effect of toluene inhalation on astrocytes and neurotrophic factor in rat brain. Forensic science international. 2000;113:233–8. doi: 10.1016/s0379-0738(00)00215-2. [DOI] [PubMed] [Google Scholar]
- 32.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–53. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 33.Hinman DJ. Biphasic dose-response relationship for effects of toluene inhalation on locomotor activity. Pharmacology, biochemistry, and behavior. 1987;26:65–9. doi: 10.1016/0091-3057(87)90535-1. [DOI] [PubMed] [Google Scholar]
- 34.Hoehn M, Nicolay K, Franke C, van der Sanden B. Application of magnetic resonance to animal models of cerebral ischemia. J Magn Reson Imaging. 2001;14:491–509. doi: 10.1002/jmri.1213. [DOI] [PubMed] [Google Scholar]
- 35.Howard MO, Balster RL, Cottler LB, Wu LT, Vaughn MG. Inhalant use among incarcerated adolescents in the United States: prevalence, characteristics, and correlates of use. Drug and alcohol dependence. 2008;93:197–209. doi: 10.1016/j.drugalcdep.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 36.Kahne D, Tudorica A, Borella A, Shapiro L, Johnstone F, Huang W, Whitaker-Azmitia PM. Behavioral and magnetic resonance spectroscopic studies in the rat hyperserotonemic model of autism. Physiology & behavior. 2002;75:403–10. doi: 10.1016/s0031-9384(01)00673-4. [DOI] [PubMed] [Google Scholar]
- 37.Kamran S, Bakshi R. MRI in chronic toluene abuse: low signal in the cerebral cortex on T2-weighted images. Neuroradiology. 1998;40:519–21. doi: 10.1007/s002340050637. [DOI] [PubMed] [Google Scholar]
- 38.Kasanetz F, Manzoni OJ. Maturation of excitatory synaptic transmission of the rat nucleus accumbens from juvenile to adult. J Neurophysiol. 2009;101:2516–27. doi: 10.1152/jn.91039.2008. [DOI] [PubMed] [Google Scholar]
- 39.Kjellstrand P, Holmquist B, Jonsson I, Romare S, Mansson L. Effects of organic solvents on motor activity in mice. Toxicology. 1985;35:35–46. doi: 10.1016/0300-483x(85)90130-1. [DOI] [PubMed] [Google Scholar]
- 40.Kurtzman TL, Otsuka KN, Wahl RA. Inhalant abuse by adolescents. J Adolesc Health. 2001;28:170–80. doi: 10.1016/s1054-139x(00)00159-2. [DOI] [PubMed] [Google Scholar]
- 41.Lee DE, Gerasimov MR, Schiffer WK, Gifford AN. Concentration-dependent conditioned place preference to inhaled toluene vapors in rats. Drug and alcohol dependence. 2006;85:87–90. doi: 10.1016/j.drugalcdep.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Lee DE, Schiffer WK, Dewey SL. Gamma-vinyl GABA (vigabatrin) blocks the expression of toluene-induced conditioned place preference (CPP) Synapse. 2004;54:183–5. doi: 10.1002/syn.20072. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Rubalcava C, Hen R, Cruz SL. Anxiolytic-like actions of toluene in the burying behavior and plus-maze tests: differences in sensitivity between 5-HT(1B) knockout and wild-type mice. Behavioural brain research. 2000;115:85–94. doi: 10.1016/s0166-4328(00)00241-2. [DOI] [PubMed] [Google Scholar]
- 44.Lubman DI, Yucel M, Lawrence AJ. Inhalant abuse among adolescents: neurobiological considerations. Br J Pharmacol. 2008 doi: 10.1038/bjp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macri MA, D'Alessandro N, Di Giulio C, Di Iorio P, Di Luzio S, Giuliani P, Bianchi G, Esposito E. Regional changes in the metabolite profile after long-term hypoxia-ischemia in brains of young and aged rats: a quantitative proton MRS study. Neurobiol Aging. 2006;27:98–104. doi: 10.1016/j.neurobiolaging.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Malisza KL, Kozlowski P, Ning G, Bascaramurty S, Tuor UI. Metabolite changes in neonatal rat brain during and after cerebral hypoxia-ischemia: a magnetic resonance spectroscopic imaging study. NMR Biomed. 1999;12:31–8. doi: 10.1002/(sici)1099-1492(199902)12:1<31::aid-nbm544>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 47.Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–8. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- 48.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore GJ. Proton magnetic resonance spectroscopy in pediatric neuroradiology. Pediatr Radiol. 1998;28:805–14. doi: 10.1007/s002470050470. [DOI] [PubMed] [Google Scholar]
- 50.Moser VC, Balster RL. The effects of acute and repeated toluene exposure on operant behavior in mice. Neurobehavioral toxicology and teratology. 1981;3:471–5. [PubMed] [Google Scholar]
- 51.Moser VC, Balster RL. The effects of inhaled toluene, halothane, 1,1,1-trichloroethane, and ethanol on fixed-interval responding in mice. Neurobehavioral toxicology and teratology. 1986;8:525–31. [PubMed] [Google Scholar]
- 52.Moser VC, Balster RL. Effects of toluene, halothane and ethanol vapor on fixed-ratio performance in mice. Pharmacology, biochemistry, and behavior. 1985;22:797–802. doi: 10.1016/0091-3057(85)90530-1. [DOI] [PubMed] [Google Scholar]
- 53.Moser VC, Coggeshall EM, Balster RL. Effects of xylene isomers on operant responding and motor performance in mice. Toxicology and applied pharmacology. 1985;80:293–8. doi: 10.1016/0041-008x(85)90086-9. [DOI] [PubMed] [Google Scholar]
- 54.NIDA . Inhalant abuse among children and adolescents: Consultation on building an international research agenda. National Institutes of Health; Washington, D.C.: 2005. Available on http://international.drugabuse.gov/meetings/inhalant_presentations.html, 2005. [Google Scholar]
- 55.O'Leary-Moore SK, Galloway MP, McMechan AP, Hannigan JH, Bowen SE. Region-dependent alterations in glutamate and GABA measured by high-resolution magnetic resonance spectroscopy following acute binge inhalation of toluene in juvenile rats. Neurotoxicol Teratol. 2007;29:466–75. doi: 10.1016/j.ntt.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 56.Oetting ER, Edwards RW, Beauvais F. Social and psychological factors underlying inhalant abuse. NIDA research monograph. 1988;85:172–203. [PubMed] [Google Scholar]
- 57.Paez-Martinez N, Cruz SL, Lopez-Rubalcava C. Comparative study of the effects of toluene, benzene, 1,1,1-trichloroethane, diethyl ether, and flurothyl on anxiety and nociception in mice. Toxicology and applied pharmacology. 2003;193:9–16. doi: 10.1016/s0041-008x(03)00335-1. [DOI] [PubMed] [Google Scholar]
- 58.Patel AB, de Graaf RA, Martin DL, Battaglioli G, Behar KL. Evidence that GAD65 mediates increased GABA synthesis during intense neuronal activity in vivo. J Neurochem. 2006;97:385–96. doi: 10.1111/j.1471-4159.2006.03741.x. [DOI] [PubMed] [Google Scholar]
- 59.Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci U S A. 2005;102:5588–93. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paxinos G, Watson C. The Rat in Stereotaxic Coordinates. 4th ed. Academic Press; San Diego, CA: 1997. Editoin Edition. [Google Scholar]
- 61.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–4. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 62.Raines DE, Gioia F, Claycomb RJ, Stevens RJ. The N-methyl-D-aspartate receptor inhibitory potencies of aromatic inhaled drugs of abuse: evidence for modulation by cation-pi interactions. The Journal of pharmacology and experimental therapeutics. 2004;311:14–21. doi: 10.1124/jpet.104.069930. [DOI] [PubMed] [Google Scholar]
- 63.Raman L, Tkac I, Ennis K, Georgieff MK, Gruetter R, Rao R. In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Brain Res Dev Brain Res. 2005;156:202–9. doi: 10.1016/j.devbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 64.Riegel AC, French ED. An electrophysiological analysis of rat ventral tegmental dopamine neuronal activity during acute toluene exposure. Pharmacology & toxicology. 1999;85:37–43. doi: 10.1111/j.1600-0773.1999.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 65.Riegel AC, Zapata A, Shippenberg TS, French ED. The abused inhalant toluene increases dopamine release in the nucleus accumbens by directly stimulating ventral tegmental area neurons. Neuropsychopharmacology. 2007;32:1558–69. doi: 10.1038/sj.npp.1301273. [DOI] [PubMed] [Google Scholar]
- 66.SAMHSA . The NSDUH Report March 13, 2008 Inhalant Use Across the Adolescent Years. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, MD: 2008. (March 13, 2008) [Google Scholar]
- 67.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 68.Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009;40:31–8. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stanley JA. In vivo magnetic resonance spectroscopy and its application to neuropsychiatric disorders. Canadian journal of psychiatry. 2002;47:315–26. doi: 10.1177/070674370204700402. [DOI] [PubMed] [Google Scholar]
- 70.Stengard K, O'Connor WT. Acute toluene exposure decreases extracellular gamma-aminobutyric acid in the globus pallidus but not in striatum: a microdialysis study in awake, freely moving rats. European journal of pharmacology. 1994;292:43–6. doi: 10.1016/0926-6917(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 71.Stengard K, Tham R, O'Connor WT, Hoglund G, Ungerstedt U. Acute toluene exposure increases extracellular GABA in the cerebellum of rat: a microdialysis study. Pharmacology & toxicology. 1993;73:315–8. doi: 10.1111/j.1600-0773.1993.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 72.Takebayashi K, Sekine Y, Takei N, Minabe Y, Isoda H, Takeda H, Nishimura K, Nakamura K, Suzuki K, Iwata Y. Metabolite alterations in basal ganglia associated with psychiatric symptoms of abstinent toluene users: a proton MRS study. Neuropsychopharmacology. 2004;29:1019–26. doi: 10.1038/sj.npp.1300426. others. [DOI] [PubMed] [Google Scholar]
- 73.Vielhaber S, Niessen HG, Debska-Vielhaber G, Kudin AP, Wellmer J, Kaufmann J, Schonfeld MA, Fendrich R, Willker W, Leibfritz D. Subfield-specific loss of hippocampal N-acetyl aspartate in temporal lobe epilepsy. Epilepsia. 2008;49:40–50. doi: 10.1111/j.1528-1167.2007.01280.x. others. [DOI] [PubMed] [Google Scholar]
- 74.Wei J, Wu JY. Post-translational regulation of L-glutamic acid decarboxylase in the brain. Neurochem Res. 2008;33:1459–65. doi: 10.1007/s11064-008-9600-5. [DOI] [PubMed] [Google Scholar]
- 75.Wilkins-Haug L. Teratogen update: toluene. Teratology. 1997;55:145–51. doi: 10.1002/(SICI)1096-9926(199702)55:2<145::AID-TERA5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 76.Williams JM, Stafford D, Steketee JD. Effects of repeated inhalation of toluene on ionotropic GABA A and glutamate receptor subunit levels in rat brain. Neurochem Int. 2005;46:1–10. doi: 10.1016/j.neuint.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Win-Shwe TT, Mitsushima D, Nakajima D, Ahmed S, Yamamoto S, Tsukahara S, Kakeyama M, Goto S, Fujimaki H. Toluene induces rapid and reversible rise of hippocampal glutamate and taurine neurotransmitter levels in mice. Toxicology letters. 2007;168:75–82. doi: 10.1016/j.toxlet.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 78.Wood RW, Coleman JB, Schuler R, Cox C. Anticonvulsant and antipunishment effects of toluene. The Journal of pharmacology and experimental therapeutics. 1984;230:407–12. [PubMed] [Google Scholar]
- 79.Wu LT, Howard MO. Is inhalant use a risk factor for heroin and injection drug use among adolescents in the United States? Addict Behav. 2007;32:265–81. doi: 10.1016/j.addbeh.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu LT, Howard MO. Psychiatric disorders in inhalant users: results from The National Epidemiologic Survey on Alcohol and Related Conditions. Drug and alcohol dependence. 2007;88:146–55. doi: 10.1016/j.drugalcdep.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu LT, Howard MO, Pilowsky DJ. Substance use disorders among inhalant users: results from the National Epidemiologic Survey on alcohol and related conditions. Addict Behav. 2008;33:968–73. doi: 10.1016/j.addbeh.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamanouchi N, Okada S, Kodama K, Hirai S, Sekine H, Murakami A, Komatsu N, Sakamoto T, Sato T. White matter changes caused by chronic solvent abuse. Ajnr. 1995;16:1643–9. [PMC free article] [PubMed] [Google Scholar]
- 83.Yavich L, Patkina N, Zvartau E. Experimental estimation of addictive potential of a mixture of organic solvents. Eur Neuropsychopharmacol. 1994;4:111–8. doi: 10.1016/0924-977x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 84.Yavich L, Zvartau E. A comparison of the effects of individual organic solvents and their mixture on brain stimulation reward. Pharmacology, biochemistry, and behavior. 1994;48:661–4. doi: 10.1016/0091-3057(94)90328-x. [DOI] [PubMed] [Google Scholar]