Abstract
Background
OAT-EP tested the hypothesis that opening a persistently occluded infarct-related artery (IRA) by percutaneous coronary intervention and stenting (PCI) after the acute phase of myocardial infarction (MI) compared to optimal medical therapy alone (MED) reduces markers of vulnerability to ventricular arrhythmias.
Methods and Results
Between April 2003 and December 2005, 300 patients with an occluded native IRA 3 to 28 days after MI (median 12 days) were randomized to PCI or MED. Ten minute digital Holter recordings were obtained prior to randomization, at 30 days, and at one year. The primary endpoint was the change in α1, a nonlinear heart rate variability parameter, between baseline and one year. Major secondary endpoints were the changes in the filtered QRS (fQRS) duration on the signal-averaged ECG (SAECG), and variability in T wave morphology (T wave variability, TWV) between baseline and one year. There were no significant differences in the changes in α1 [-0.04 (-0.12, 0.04)], fQRS [3.0 ms (-4.8 ms, 10.7 ms)], or TWV [2.2 μV (-1.4 μV, 5.9 μV)] between the PCI and MED groups (MED change - PCI change, 95% CI). Multivariable analysis revealed that the results were unchanged after adjustment for baseline clinical variables and medication treatments during the Holter recordings.
Conclusions
PCI with stenting of a persistently occluded IRA during the subacute phase after MI compared to medical therapy alone had no significant effect on changes in heart rate variability, the time-domain SAECG, or TWV during the first year after MI. These findings are consistent with the lack of clinical benefit, including no reduction in sudden death, with PCI for stable patients with persistently occluded IRAs after MI in the main Occluded Artery Trial.
Keywords: myocardial infarction, arrhythmia, artery occlusion, stents, sudden death
Early coronary reperfusion using either thrombolytics or primary percutaneous coronary intervention (PCI) reduces long-term mortality in patients who present with ST-segment elevation myocardial infarction (MI).1 Early coronary reperfusion has been correlated with increased heart rate variability and a decreased incidence of late potentials, suggesting that at least part of the clinical benefit of early reperfusion is explained by a decreased vulnerability to ventricular arrhythmias.2,3 However, up to one third of eligible patients do not receive effective early reperfusion due to such factors as late presentation or lack of efficacy of thrombolytics.3,4
The “late open artery hypothesis” posits that clinical outcomes can be improved by late opening of persistently occluded infarct-related arteries (IRAs) during the subacute phase of MI, when myocardial salvage is not anticipated.5 Several mechanisms have been proposed to account for the putative benefit of late reperfusion, including stabilization of the electrophysiological substrate and downstream prevention of malignant ventricular arrhythmias. Although experimental studies have suggested that late reperfusion decreases arrhythmic events,6 clinical studies that have examined this question have been limited by small sample size, lack of treatment randomization, and technical factors such as lack of stenting that limited sustained vessel patency after PCI.7,8
The Occluded Artery Trial (OAT) was an international randomized trial funded by the National Heart Lung and Blood Institute (NHLBI) that tested the hypothesis that PCI and stenting of occluded IRAs identified on days 3 to 28 after MI compared to medical therapy alone would reduce the combined incidence of death, recurrent MI, and New York Heart Association Class IV heart failure.9 OAT-EP, also funded by the NHLBI, is a mechanistic ancillary study of OAT. The primary aim of OAT-EP was to test the hypothesis that PCI plus optimal medical therapy would result in a greater increase in heart rate variability (HRV) during the first year after MI than optimal medical therapy alone. Major secondary aims were to test the hypotheses that PCI would reduce dynamic changes in T wave morphology, or T wave variability (TWV) and filtered QRS duration (fQRS) on the signal averaged ECG (SAECG) at one year after MI.
Methods
Study Design
OAT-EP enrolled participants between April 2003 and December 2005 at 36 of 216 OAT sites with separate Institutional Review Board approval from OAT (see Appendix for the list of participating centers). OAT-EP patients were OAT participants who provided additional informed consent. Randomization to PCI and stenting plus optimal medical therapy (PCI) or optimal medical therapy alone (MED) was done using the same randomization process as for other OAT participants.
Patient Sample
Eligibility criteria for OAT-EP mirror those of OAT.9 Patients had a documented MI and underwent cardiac catheterization within 3 to 28 days; day 1 was the date of symptom onset, hence the minimum interval between symptom onset and the qualifying cardiac catheterization study was 24 hours. Angiographic criteria included IRA occlusion judged suitable for stenting and at least one high risk criterion: proximal coronary occlusion subtending at least 25% of the left ventricle, or left ventricular ejection fraction < 50%. IRA occlusion was defined as 100% stenosis with Thrombolysis In Myocardial Infarction (TIMI) grade 0 or 1 antegrade flow. Patients with clinical instability, left main or 3-vessel disease, or severe inducible ischemia were excluded. OAT-EP patients were also required to be in sinus rhythm so that HRV and TWV could be measured,10,11 and to have a QRS duration < 120 ms so that the time domain SAECG could be performed.12
Study Procedures
OAT-EP participants gave informed consent for participation prior to OAT randomization to minimize selection bias for participation in the ancillary study. Patients assigned to PCI were to undergo the procedure within 24 hours of randomization whenever possible. Ten- minute digital Holter monitor recordings were performed prior to randomization, at 30 days, and at one year using a Burdick 92510 recorder (Spacelabs-Burdick, Deerfield, WI). The recordings were performed using Frank leads (X, Y, Z) at a sampling rate of 1000 Hz with the patient lying quietly in the supine position. The digital Holter data were stored on PC cards, which were shipped to the Holter Core Laboratory at the University of Maryland for downloading and analysis. The OAT-EP core lab personnel were blinded with respect to the OAT treatment randomization (PCI vs. MED). The Holter data were manually edited to eliminate ectopic beats and signal noise, then analyzed for the fractal scaling exponent (α1), time and frequency domain HRV parameters, the time domain SAECG, and T wave variability (TWV) as previously described.10-15 The SAECGs were acquired in accordance with the published recommendations for recording conditions, lead placement, sampling rate, and signal filtering.16 The analysis of α1 and TWV was conducted at the University of Rochester. Recordings were excluded from HRV analysis if the prevalence of ectopic beats or noisy segments exceeded 25%, from SAECG analysis if the noise level was > 1 μV, and from TWV analysis if the heart rate was unstable or if excessive ectopy or noise was present.10-12
Study Endpoints
The primary study endpoint was the change in α1 at one year. Major secondary endpoints were the changes in fQRS and TWV at one year. These study endpoints were chosen to fully characterize the effects of PCI of occluded IRAs on the major determinants of arrhythmia vulnerability: the autonomic nervous system (HRV), impulse conduction (SAECG), and ventricular repolarization (TWV). The primary endpoint (α1) was chosen because its prognostic value is superior to other HRV variables in post-myocardial infarction patient populations with clinical characteristics similar to those of OAT.13-15 Similarly, fQRS was chosen because it is prognostically the most important SAECG variable,12 while TWV has prognostic value in high-risk patients with coronary artery disease.11
Other secondary variables included standard time domain (RR interval, SDNN, SDANN, rMSSD, pNN50) and frequency domain (total power, VLF, LF, HF, LF/HF ratio) HRV variables, and other time domain SAECG variables (LAS, RMS40).10,12
Statistical Analysis
An intention-to-treat principle was used for the primary treatment-related analyses. The primary endpoint (α1) was evaluated with α=0.05 for a 2-sided t-test comparing change in α1 from baseline to one year between the 2 treatment groups. As in the main OAT trial, a p value of 0.01 for secondary analyses was considered strong evidence of association. Changes in α1 were normally distributed and suitable for the t-test. Changes in TWV and fQRS were not normally distributed, and were winsorized then analyzed with the t-test. Winsorization is a method to control the variability introduced by extreme values. This method typically identifies the top and bottom 5% and reduces them to the next highest (or lowest) value. This method permits the use of procedures that require a normal distribution (i.e. multiple regression), while controlling the influence of these extreme values.17
The planned sample size of 300 subjects was chosen to provide 80% power to detect a clinically relevant difference of 0.1 in the change in α1 from baseline to one year between the 2 treatment groups, based on data from prior studies that indicated that baseline levels of α1 would be 1.0 with a common SD of 0.2.13-15 Because this outcome is a measure of longitudinal change, the correlation between the measurements at baseline and one year must be taken into account. We assumed that the standard deviation in the change in α1 would be between 0.28 (for a correlation (ρ) of 0) and 0.169 (for ρ=0.65) and that the standard deviation of α1 would be the same at baseline and 1 year. Based on these assumptions, the study would have 80% power or more to detect a difference in α1 of 0.14 units (ρ=0) to 0.083 (ρ=0.65) between the PCI and MED patients. This calculation assumed a crossover rate of 25% (PCI in MED patients within 30 days of randomization and no attempt at PCI or failed PCI, which are biological crossovers, in the PCI-assigned patients), and a 15% loss to follow-up.
The analyses were repeated for time domain and frequency domain HRV variables, and for other time domain signal averaged ECG variables. A secondary analysis was conducted using unpaired t tests to determine if the study results were affected by inclusion of additional patients who were excluded due to lack of complete baseline and follow-up information. Secondary analyses of the primary and major secondary endpoints were also performed after excluding crossovers (“as treated” analysis). Other secondary analyses assessed the changes in the primary and major secondary endpoints at 30 days. A sensitivity analysis was conducted because recurrent MI during the year between the Holter measurements could affect changes in arrhythmia marker values. For this analysis, patients with recurrent MI were excluded from the analysis of changes in α1, fQRS, and TWV between baseline and one year.
For multivariable models, we used stepwise linear regression with backward elimination, with a p value of 0.05 required for a variable to be retained in the model. Separate multivariable models were constructed for the change in each arrhythmia marker variable between baseline and one year. The pre-specified variables tested in each stepwise model were treatment group (PCI vs. MED), age, sex, race (white vs non-white), prior MI, interval between qualifying MI and the Holter recording, baseline values of the arrhythmia marker variable, hypertension, diabetes, multivessel disease, collateral arteries, baseline left ventricular ejection fraction, left anterior descending coronary artery culprit, thrombolytics, ST elevation or new Q waves on ECG, beta blocker use at baseline and one year, and angiotensin converting enzyme use at baseline and one year. Since the models of change in α1, fQRS, and TWV had normally distributed residuals that exhibited homoscedasticity, these measures were suitable for analysis with linear regression.
In order to ensure that OAT-EP patients are representative of patients in the community with occluded IRAs post-MI, we compared the clinical characteristics of OAT-EP patients with three relevant patient populations: other randomized OAT patients who did not participate in OAT-EP, the OAT pre-trial Registry18 and the concurrent OAT registry.19 The variables for these comparisons were age, sex, left ventricular ejection fraction < 50%, and culprit IRA. We also compared long-term outcomes in OAT-EP and other OAT patients to ensure that lower risk patients were not inadvertently selected to participate in OAT-EP. These outcomes included the primary OAT endpoint (death, nonfatal reinfarction, and NYHA class IV heart failure) and its individual components. Kaplan-Meier life table analysis was used to calculate estimated event rates and the log-rank test was used for the comparison of outcomes.
The authors had full access to the data and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
The target sample of 300 patients was successfully enrolled in OAT-EP, representing 14% of the OAT study population of 2201 patients. There were an additional 157 patients enrolled in OAT, but not OAT-EP, at participating sites; although the precise reasons for exclusion were not tracked, these patients were typically excluded because they did not meet the additional OAT-EP inclusion criteria, refused to consent for OAT-EP, were randomized before the baseline Holter data could be acquired, or were judged to be unlikely to return for the follow-up one year Holter due to distance from the enrolling site.
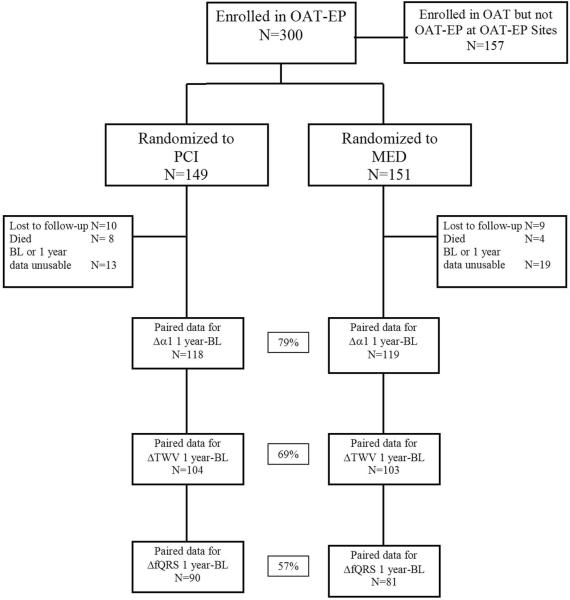
The observed crossovers were 14 failed PCI and 1 PCI not done in the PCI group (10%) and 4 PCI within 30 days in the MED group (2.6%). The loss to follow-up with reasons for patient attrition is shown in Figure 1. Complete baseline and follow-up data for the primary endpoint (α1) was available at baseline and one year for 79% of the study cohort. Complete data for the major secondary endpoints (fQRS and TWV) was available at baseline and one year for 57% and 69% of patients, respectively. This sample size yielded 80% power to detect a change of 0.1 units in α1, 99% power to detect a 10ms change in fQRS, and 91% power to detect a 10 μV change in TWV, each of which represents a clinically relevant change.11-15 More patients were excluded from TWV and fQRS analyses because of excessive signal noise on some recordings, which prohibited performance of these analyses. Data were available at one year for α1, fQRS, and TWV for 86%, 76%, and 80% of patients, respectively.
Figure 1.
OAT-EP study flow. BL=baseline.
Baseline Characteristics
The baseline clinical and angiographic characteristics of the PCI and MED groups were similar in most respects, with the exception of a significant difference in the prevalence of diabetes (Tables 1 and 2). There was no significant difference in the time from the qualifying MI to the Holter recording when the PCI and MED groups were compared (13.3±8.6 days vs. 13.0±7.8 days respectively). OAT-EP patients were representative of the larger OAT study cohort with respect to most clinical and angiographic characteristics. However, OAT-EP patients had a lower incidence of prior MI, were more likely to be hypertensive, were less likely to have received thrombolytic therapy for the index MI, and had a greater prevalence of multivessel disease (Tables 1 and 2). The patients with and without complete data available for the primary endpoint at baseline and one year had similar baseline demographic characteristics, with the exception of median days between MI and randomization (14 days vs 7 days, respectively).
Table 1.
Baseline Clinical Characteristics for OAT-EP and other OAT patients
| OAT-EP PCI Group (N=149) | OAT-EP MED Group (N=151) | OAT-EP Both Groups (N=300) | Other OAT Patients (N=1901) | |
|---|---|---|---|---|
| Age,years,mean±SD | 57.2±10.5 | 57.6±10.5 | 57.4±10.5 | 58.8±11.0 |
| Male,n(%) | 111(74.5) | 127(84.1) | 238(79.3) | 1479(77.8) |
| Race,n(%) | ||||
| White | 115(77.2) | 117(77.5) | 232(77.3) | 1531(80.5) |
| Black | 3(2.0) | 4(2.6) | 7(2.3) | 62(3.3) |
| Hispanic | 26(17.4) | 21(13.9) | 47(15.7) | 230(12.1) |
| Other | 5(3.4) | 9(6.0) | 14(4.7) | 78(4.1) |
| No.(%) with history of: | ||||
| Angina | 28(18.8) | 30(19.9) | 58(19.3) | 437(23.0) |
| Prior MI | 10(6.7) | 10(6.6) | 20(6.7) | 227(11.9) |
| Cerebrovascular disease | 4(2.7) | 6(4.0) | 10(3.3) | 72(3.8) |
| Peripheral vascular disease | 5(3.4) | 6(4.0) | 11(3.7) | 72(3.8) |
| Congestive heart failure | 5(3.4) | 3(2.0) | 8(2.7) | 44(2.3) |
| Prior PCI | 5(3.4) | 10(6.6) | 15(5.0) | 90(4.7) |
| Diabetes | 22(14.8) | 40(26.5) | 62(20.7) | 392(20.6) |
| Hypertension | 81(54.4) | 94(62.3) | 175(58.3) | 896(47.1) |
| Current smoker,n(%) | 55(36.9) | 64(42.4) | 119(39.7) | 740(38.9) |
| NYHA class at randomization,n(%) | ||||
| NYHA class I | 126(84.6) | 122(80.8) | 248(82.7) | 1582(83.4) |
| NYHA class II | 23(15.4) | 29(19.2) | 52(17.3) | 315(16.6) |
| NYHA class III or IV | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) |
| Glomerular filtration rate,ml/min,mean±SD | 83.5±23.6 | 83.5±23.5 | 83.5±23.5 | 80.2±21.2 |
Table 2.
Index MI and Angiographic Characteristics for OAT-EP and other OAT Patients
| OAT-EP PCI Group (N=149) | OAT-EP MED Group (N=151) | OAT-EP Both Groups (N=300) | Other OAT Patients (N=1901) | |
|---|---|---|---|---|
| ST-segment elevation or new Q-waves or loss of R-waves, n(%) | 132(88.6) | 135(89.4) | 267(89.0) | 1638(86.2) |
| Thrombolytic therapy within first 24 hours of Index MI, n(%) | 20(13.4) | 22(14.6) | 42(14.0) | 382(20.1) |
| Interval from MI to randomization, d, Median, (25th percentile, 75th percentile) | 12(6,21) | 11(6,20) | 12(6,21) | 8(5,15) |
| Stress test performed, n(%) | 42(28.2) | 39(25.8) | 81(27.0) | 517(27.2) |
| Ischemia in IRA territory, n(%) | ||||
| Severe | 0(0.0) | 0(0.0) | 0(0.0) | 1(0.2) |
| Moderate | 2(4.8) | 1(2.6) | 3(3.7) | 56(10.8) |
| Mild | 13(30.9) | 10(25.6) | 23(28.4) | 157(30.4) |
| None | 27(64.3) | 28(71.8) | 55(67.9) | 303(58.6) |
| IRA, n(%) | ||||
| Left anterior descending | 52(34.9) | 51(33.8) | 103(34.3) | 690(36.3) |
| Left circumflex | 19(12.8) | 23(15.2) | 42(14.0) | 293(15.4) |
| Right coronary artery | 78(52.3) | 77(51.0) | 155(51.7) | 918(48.3) |
| IRA TIMI flow grade, n(%) | ||||
| Grade 0 | 112(75.2) | 120(79.5) | 232(77.3) | 1573(83.7) |
| Grade 1 | 37(24.8) | 31(20.5) | 68(22.7) | 298(15.8) |
| Grade 2 | 0(0.0) | 0(0.0) | 0(0.0) | 7(0.4) |
| Grade 3 | 0(0.0) | 0(0.0) | 0(0.0) | 2(0.1) |
| Collateral vessels present, n(%) | 131(87.9) | 132(87.4) | 263(87.7) | 1659(88.6) |
| Multivessel disease, n(%) | 27(18.1) | 43(28.5) | 70(23.3) | 309(16.4) |
| Left ventricular ejection fraction, mean±SD | 47.7±10.9 | 47.9±10.3 | 47.8±10.6 | 47.7±11.2 |
| Left ventricular ejection fraction<50%, n(%) | 79(53.0) | 74(49.0) | 153(51.0) | 1017(54.0) |
There were no significant differences between OAT-EP patients and patients in the OAT concurrent Registry with respect to age, sex, and identity of the IRA (data not shown). Patients in the OAT pre-trial Registry were slightly older than OAT-EP patients (62 vs. 57 years) and had fewer men (72% vs. 79%); however, there were no significant differences in the identity of the IRA and left ventricular ejection fraction (data not shown). There were no significant differences in the 3-year OAT primary endpoint event rate (death, nonfatal reinfarction, and NYHA class IV heart failure) or its individual components when OAT-EP and other OAT patients were compared (10.3% vs. 14.3% respectively, p=0.16).
Cardiovascular Medication Use
There were no significant differences between the study groups in cardiovascular medication use during the Holter recordings either at baseline or one year (Table 3). Thienopyridine use was significantly more common in the PCI group at baseline (92% vs 37%).
Table 3.
Medical Therapy During Holter Recording
| Baseline PCI (N=149) | Baseline MED (N=149) | 1 Year PCI (N=131) | 1 Year MED (N=138) | |
|---|---|---|---|---|
| ACE inhibitor, n(%) | 120(80.5) | 121(81.2) | 97(74.0) | 109(79.0) |
| Angiotensin receptor blocker, n(%) | 5(3.4) | 3(2.0) | 9(6.9) | 10(7.2) |
| Beta-blocker, n(%) | 133(89.3) | 138(92.6) | 116(88.5) | 128(92.8) |
| Calcium channel blocker, n(%) | 8(5.4) | 11(7.4) | 7(5.3) | 15(10.9) |
| Digoxin, n(%) | 1(0.7) | 3(2.0) | 0(0.0) | 2(1.4) |
| Diuretic, n(%) | 24(16.1) | 30(20.1) | 25(19.1) | 35(25.4) |
| Spironolactone, n(%) | 6(4.0) | 10(6.7) | 8(6.1) | 10(7.2) |
| Class I or III anti-arrhythmic, n(%) | 2(1.3) | 4(2.7) | 4(3.1) | 2(1.4) |
Primary Endpoint
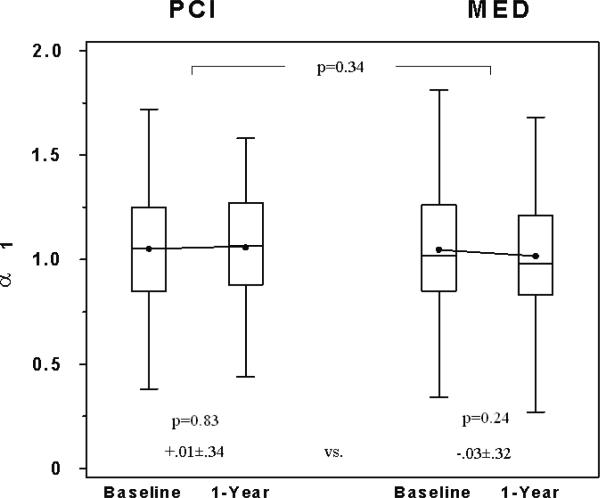
There was no significant difference between the MED and PCI groups in the change in α1 (MED change - PCI change, 95% CI: -0.04 (-0.12, 0.04); Table 4, Figure 2a). On multivariable analysis of the change in α1, the treatment group assignment (PCI vs. MED) was still not a significant predictor (p=0.31) after adjustment for baseline clinical variables and cardiovascular medication use during the Holter recordings. There was no significant change in α1 at one year as compared with baseline within the MED or PCI groups (Table 4, Figure 2a).
Table 4.
Changes in arrhythmia marker values between baseline and one year
| PCI Group | MED Group | MED Change - PCI Change | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline | One Year | Change | N | Baseline | One Year | Change | 95% CI | |||
| Difference | Lower | Upper | |||||||||
| αl | 118 | 1.05±0.30 | 1.06±0.27 | .01±0.34 | 119 | 1.05±0.29 | 1.02±0.29 | -.03±0.32 | -0.04 | -0.12 | 0.04 |
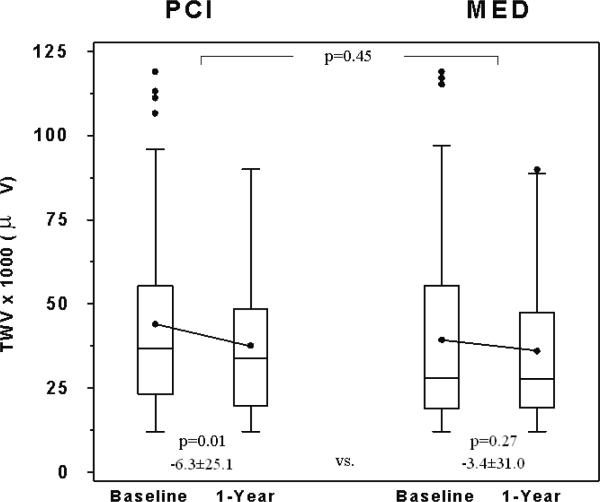
| TWV(μV) | 104 | 44.0±28.4 | 37.7±21.5 * | -6.3±25.1 | 103 | 39.5±27.5 | 36.1±21.3 | -3.4±31.0 | 3.0 | -4.8 | 10.7 |
| Time domain SAECG | |||||||||||
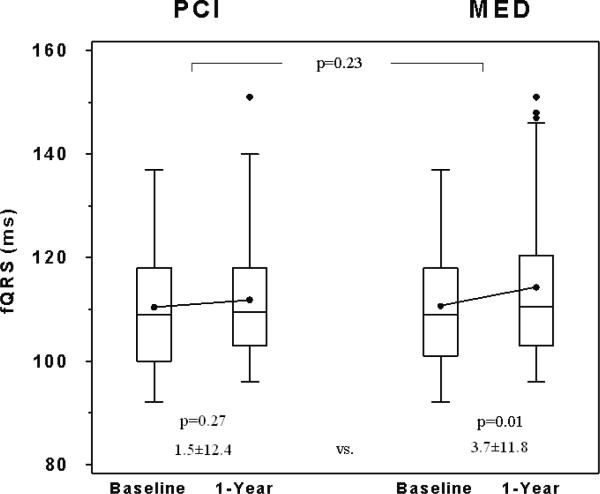
| fQRS (ms) | 90 | 110.4±12.4 | 111.9±13.2 | 1.5±12.4 | 81 | 110.6±12.2 | 114.3±14.9 * | 3.7±11.8 | 2.2 | -1.4 | 5.9 |
| LAS | 94 | 36.5±11.6 | 34.2±11.9 | -2.2±11.6 | 84 | 37.6±12.7 | 36.7±13.3 | -0.9±12.1 | 1.3 | -2.1 | 4.8 |
| RMS | 95 | 27.3±18.1 | 30.4±21.3 | 3.1±19.4 | 84 | 25.8±16.2 | 27.1±18.7 | 1.3±15.1 | -1.8 | -7.0 | 3.4 |
| Time domain HRV | |||||||||||
| Mean RR interval (ms) | 128 | 927.9±162.1 | 980.1±150.9 * | 52.2±191.1 | 122 | 924.4±174.5 | 998.2±184.4 * | 73.8±209.0 | 21.6 | -28.2 | 71.5 |
| SDNN (ms) | 128 | 31.5±14.8 | 38.9±16.4 * | 7.5±18.1 | 122 | 32.2±14.7 | 39.7±17.2 * | 7.5±17.7 | 0.01 | -4.5 | 4.5 |
| SDANN (ms) | 119 | 7.5±6.1 | 9.1±6.9 | 1.6±8.6 | 109 | 8.1±6.3 | 8.6±7.1 | 0.6±9.5 | -1.0 | -3.3 | 1.4 |
| rMSSD (ms) | 128 | 21.2±11.9 | 27.2±14.7 * | 5.9±15.7 | 122 | 21.1±10.7 | 28.1±14.5 * | 7.0±14.6 | 1.1 | -2.7 | 4.9 |
| pNN50 (%) | 75 | 6.5±8.9 | 9.3±10.5 | 2.8±11.5 | 78 | 5.4±7.1 | 10.6±10.7 * | 5.2±10.2 | 2.4 | -1.1 | 5.9 |
| Frequency Domain HRV | |||||||||||
| Total power | 95 | 689.5±685.6 | 947.3±825.8 * | 257.8±897.6 | 81 | 790.4±776.3 | 1154.1±1134.3 * | 363.6±1095.7 | 105.8 | -190.8 | 402.4 |
| VLF | 95 | 282.5±223.4 | 338.2±250.2 | 55.7±347.2 | 81 | 340.5±326.2 | 344.5±286.7 | 4.0±428.4 | -51.6 | -167.0 | 63.8 |
| LF | 95 | 213.3±240.2 | 297.3±303.8 | 83.9±346.6 | 81 | 217.7±257.1 | 346.4±370.9 * | 128.7±334.6 | 44.8 | -57.1 | 146.6 |
| HF | 95 | 111.5±140.7 | 166.4±175.0 * | 54.9±175.3 | 81 | 116.2±147.8 | 200.0±214.8 * | 83.8±208.9 | 28.9 | -28.3 | 86.0 |
| LF/HF ratio | 95 | 3.4±3.1 | 2.7±2.2 | -0.7±3.3 | 81 | 3.2±3.2 | 2.7±2.4 | -0.5±3.5 | 0.13 | -0.89 | 1.15 |
p<0.01 for the change between baseline and one year within the group indicated.
There were no significant differences between the PCI and MED groups in the change of any of the listed variables (p > 0.2 for all comparisons).
Figure 2.
Comparison of baseline and 1-year A) α1, B) fQRS, and C) TWV between the 2 study groups. Box-and-whisker plots indicate medians (lines), means (dots), and interquartile ranges (box). Probability values indicate baseline to 1-year comparisons within each group and between groups as indicated.
Secondary Endpoints
There were no significant differences between the MED and PCI groups in the changes in fQRS or TWV (MED change - PCI change, 95% CI: fQRS [3.0 ms (-4.8 ms, 10.7 ms)], TWV [2.2 μV (-1.4 μV, 5.9 μV)]; Table 4, Figures 2b and 2c). Separate multivariable models were constructed for the change in fQRS and the change in TWV. Treatment group assignment (PCI vs. MED) was still not a significant predictor (p=0.14 for fQRS and p=0.89 for TWV) in either model after adjustment for baseline clinical variables and cardiovascular medication use during the Holter recordings.
There was a small, but significant increase in fQRS within the MED group (Table 4, Figure 2b). No significant changes in fQRS were observed within the PCI group. There was a small, but significant decrease in TWV in the overall study group and within the PCI group (Table 4, Figure 2c). No significant changes in TWV were observed within the MED group.
Secondary analyses
The analysis of all available data at one year revealed no significant differences in α1, fQRS, or TWV (α1: 1.07 vs. 1.02, p=0.19; fQRS: 113.1 vs. 114.3 ms, p=0.55; TWV: 36.4 vs. 36.9 μV, p=0.84; for PCI and MED groups, respectively). Four patients in the PCI group and one patient in the MED group had recurrent MI before the one-year Holter measurements were performed. A sensitivity analysis revealed that excluding patients with recurrent MI did not alter the primary study results (Changes from baseline to one year in α1: 0.00 vs. -0.03, p=0.45; fQRS: +1.6 vs. +3.7 ms, p=0.26; TWV: -6.7 vs. -3.4 μV, p=0.40; for PCI and MED groups, respectively). The primary study results were unchanged when the data were analyzed after excluding crossovers within 30 days (“as treated” analysis, changes from baseline to one year in α1: 0.00 vs. -0.04, p=0.35; fQRS: +2.0 vs. +3.8 ms, p=0.35; TWV: -7.0 vs. -2.7 μV, p=0.29; for PCI and MED groups, respectively). There were no significant differences between the PCI and MED groups in the changes in the primary and major secondary endpoints at 30 days (α1: -0.03 vs. -0.05, p=0.63; fQRS: -1.4 vs. +0.6 ms, p=0.17; TWV: -1.7 vs. -1.0 μV, p=0.88; for PCI and MED groups, respectively).
There were significant increases between baseline and one year in several time domain and frequency domain HRV variables (mean RR, SDNN, pNN50, rMSSD, total power, LF, HF; Table 4). However, there were no significant differences between the PCI and MED groups in the changes in time domain and frequency domain HRV variables, or changes in other time domain signal averaged ECG variables (Table 4).
Discussion
The major findings of this study are that PCI with stenting of a persistently occluded IRA during the subacute phase after MI compared to medical therapy alone had no significant effect on changes in HRV, the time-domain SAECG, or TWV during the first year after MI. Although late IRA patency was achieved in 83% of OAT patients20 and at least moderately retained viability was present at baseline in 69%,9 PCI had no significant effect on changes in the major determinants of arrhythmia vulnerability: the autonomic nervous system (HRV), impulse conduction (SAECG), and ventricular repolarization (TWV).
Several mechanisms have been proposed to explain the presumed benefits of late opening of the IRA, including prevention of ventricular remodeling, improvement of left ventricular function by recovery of hibernating myocardium, and stabilization of the electrophysiological substrate.5 In an animal model of experimental myocardial infarction, late reperfusion reduced the incidence of arrhythmic death as compared to permanent coronary artery occlusion despite identical infarct size in the two groups.6 However, clinical studies that examined the effects of balloon angioplasty without adjunctive stenting on arrhythmia markers have yielded conflicting results.7,8 These studies were limited by small sample sizes, lack of treatment randomization, the use of patients with failed angioplasty as controls, and likely poor long-term vessel patency since stents were not utilized.
The present study is the first large, adequately powered, randomized controlled trial to investigate whether PCI and stenting of occluded coronary arteries late after MI reduces markers of vulnerability to ventricular arrhythmias. We did not observe any significant effect of PCI on changes in HRV (α1), myocardial impulse conduction (fQRS), or ventricular repolarization (TWV) at one year. There was a significant increase in fQRS within the MED group and a significant decrease in TWV within the PCI group. Although these within-group changes could be interpreted as a small benefit of PCI (improvement in TWV within the PCI group, worsening of fQRS within the MED group), these changes were not significantly different when the treatment groups (PCI vs. MED) were compared. Furthermore, the magnitude of these changes was far below the differences that we pre-specified as clinically relevant to detect based on prior outcome data when we calculated the required sample size for the study (10 ms change in fQRS, 10 μV change in TWV).11,12 The results of OAT-EP are concordant with the results of OAT, which did not suggest an effect of PCI on total mortality, cardiac death, death from arrhythmia, sudden unexplained death or development of sustained VT or need for implantable cardioverter-defibrillator placement.9
It is well recognized that traditional time and frequency domain HRV variables change to a greater extent than α1 after MI.21 We observed an identical pattern in our study, as α1 was unchanged but several time and frequency domain HRV variables increased substantially between baseline and one year. Importantly, there were no significant differences between the PCI and MED groups in the changes in any of the study variables. Since we did observe improvement in most HRV variables as well as changes in fQRS and TWV, these data indicate that our study variables were sensitive enough to detect changes in critical electrophysiological characteristics and that PCI had no effect on these variables. Furthermore, since prior studies indicate that HRV is maximally recovered by one year after MI,22 it is unlikely that more extended follow-up would have changed our results.
Study Limitations
Since many OAT-EP centers performed the OAT treatment randomization (PCI vs MED) immediately after the qualifying coronary angiogram, all baseline electrophysiological measures had to be acquired before the angiogram. Long-term Holter recordings and exercise T wave alternans assessment were not feasible since these additional requirements would have delayed the qualifying angiogram and limited enrollment, and because most centers did not have T wave alternans equipment. Although HRV increases with the length of the recording (10 minutes vs 24 hours),10 the results of short-term recordings correlate well with 24-hour recordings and are suitable for measuring changes over time.22 Furthermore, the prognostic value of short-term Holter recordings has been demonstrated for traditional HRV parameters and for α1.15,23,24 There is less experience with TWV than T wave alternans, and the precise relation between the two techniques requires further study. However, since TWV was previously demonstrated to have prognostic value in high-risk patients with coronary artery disease in the landmark MADIT-2 trial,11 it should provide an accurate assessment of ventricular repolarization in OAT-EP patients.
The use of follow-up Holter recordings is subject to bias due to deaths within the first year and difficulty in obtaining follow-up on sicker patients. However, we did not observe any important differences in the baseline clinical characteristics of patients with and without one year data available, which suggests that sicker patients were not excluded. Furthermore, the numbers of patients in the PCI and MED groups who did not have complete data at baseline and one year due to death and other reasons were similar.
While it is possible that OAT-EP patients are not fully representative of patients in the community that meet OAT eligibility criteria, we view this as unlikely given that OAT-EP patients were comparable to patients in the main OAT trial, the OAT pre-trial Registry and the concurrent OAT Registry with respect to most important baseline clinical characteristics. Importantly, long-term outcomes were similar in OAT-EP and other patients in the main OAT trial, suggesting that lower-risk patients were not inadvertently selected to participate in OAT-EP.
Conclusions
PCI with stenting of a persistently occluded IRA during the subacute phase after MI compared to medical therapy alone had no significant effect on changes in HRV, the time-domain SAECG, or TWV during the first year after MI. Given the absence of clinical benefit in OAT,9 routine PCI is not recommended for persistent IRA occlusion in stable patients during the subacute phase after MI.
Acknowledgements
The authors wish to express their gratitude for the excellent work by Traci Brown, Staci Abramsky, Ana Mon, Cory Tschabrunn, Mario Dimitrov, and Betty Mykins.
Sources of Funding The project described was supported by Award Number HL072906 from the National Heart, Lung, And Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. Supplemental grant funds and product donations equivalent to 6% of total study cost from: Eli Lilly, Millenium Pharmaceuticals and Schering Plough, Guidant, Cordis/Johnson and Johnson, Medtronic, Merck and Bristol Myers Squibb Medical Imaging.
Footnotes
Clinical Trial Registration Information: URL:http://www.clinicaltrials.gov. Unique Identifier: NCT00119847
Electrophysiological Effects of Late Percutaneous Coronary Intervention for Infarct-Related Coronary Artery Occlusion. The Occluded Artery Trial - Electrophysiological Mechanisms (OAT-EP)
The “late open artery hypothesis” posits that clinical outcomes can be improved by late opening of persistently occluded infarct-related arteries (IRAs) during the subacute phase of myocardial infarction (MI), when myocardial salvage is not anticipated. Several mechanisms have been proposed for the putative benefit of late reperfusion, including stabilization of the electrophysiological substrate and downstream prevention of malignant ventricular arrhythmias. This ancillary study of the Occluded Artery Trial evaluated the effects of late opening and stenting of an occluded infarct artery on several noninvasive measures of electrophysiologic stability during the first year after MI. Heart rate variability (HRV), assessing the autonomic nervous system, was the primary measure. Major secondary measures evaluated repolarization (dynamic changes in T wave morphology, or T wave variability (TWV)) and delayed myocardial conduction (filtered QRS duration (fQRS) on the signal averaged ECG). Compared to medical therapy alone, late reperfusion and stenting of a persistently occluded IRA did not change HRV, fQRS, or TWV during the first year after MI. These results are consistent with the lack of clinical benefit, including no reduction in sudden death, with PCI for stable patients with persistently occluded IRAs after MI in the main Occluded Artery Trial.
Clinical Trial Registration Information: URL:http://www.clinicaltrials.gov. Unique Identifier: NCT00119847
Conflict of Interest Disclosures Dr. Rashba receives grant support and is a speaker for Medtronic and Boston Scientific. Dr. Hochman received grant support to her institution from Eli Lilly and Bristol Myers Squibb Medical Imaging and product donation from Millennium Pharmaceuticals, Schering-Plough, Guidant, and Merck for OAT. Dr. Hochman receives consultation fees from Eli Lilly, Bristol Myers Squibb, and Sanofi Aventis. Dr. Hochman also receives lecture fees from the Network for Continuing Medical Education (NCME). Dr. Lamas is a consultant to Medtronic, Sanofi Aventis, and CV Therapeutics; he is a speaker for Glaxo Smith Kline, Bristol Myers Squibb, Astra Zeneca, and CV Therapeutics. Dr. Dzavik receives honoraria from Cordis and Johnson and Johnson.
References
- 1.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists' (FTT) Collaborative Group. Lancet. 1994;343:311–22. [PubMed] [Google Scholar]
- 2.Singh N, Mironov D, Armstrong PW, Ross AM, Langer A. Heart rate variability assessment early after acute myocardial infarction. Pathophysiological and prognostic correlates. GUSTO ECG Substudy Investigators. Global Utilization of Streptokinase and TPA for Occluded Arteries. Circulation. 1996;93:1388–95. doi: 10.1161/01.cir.93.7.1388. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg JS, Hochman JS, Morgan CD, Dorian P, Naylor CD, Theroux P, Topol EJ, Armstrong PW. Effects of thrombolytic therapy administered 6 to 24 hours after myocardial infarction on the signal-averaged ECG. Results of a multicenter randomized trial. LATE Ancillary Study Investigators. Late Assessment of Thrombolytic Efficacy. Circulation. 1994;90:746–52. doi: 10.1161/01.cir.90.2.746. [DOI] [PubMed] [Google Scholar]
- 4.Eagle KA, Goodman SG, Avezum A, Budaj A, Sullivan CM, Lopez-Sendon J. Practice variation and missed opportunities for reperfusion in ST-segment-elevation myocardial infarction: findings from the Global Registry of Acute Coronary Events (GRACE) Lancet. 2002;359:373–7. doi: 10.1016/S0140-6736(02)07595-5. [DOI] [PubMed] [Google Scholar]
- 5.Kim CB, Braunwald E. Potential benefits of late reperfusion of infarcted myocardium. The open artery hypothesis. Circulation. 1993;88:2426–36. doi: 10.1161/01.cir.88.5.2426. [DOI] [PubMed] [Google Scholar]
- 6.Opitz CF, Finn PV, Pfeffer MA, Mitchell GF, Pfeffer JM. Effects of reperfusion on arrhythmias and death after coronary artery occlusion in the rat: increased electrical stability independent of myocardial salvage. J Am Coll Cardiol. 1998;32:261–7. doi: 10.1016/s0735-1097(98)00173-9. [DOI] [PubMed] [Google Scholar]
- 7.Dzavik V, Beanlands DS, Leddy D, Davies RF, Kimber S. Does late revascularization alter the evolution of the signal-averaged electrocardiogram in patients with a recent transmural myocardial infarction? Can J Cardiol. 1995;11:378–84. [PubMed] [Google Scholar]
- 8.Lomama E, Helft G, Persoz A, Vacheron A. Late coronary angioplasty and ventricular late potentials. Am J Cardiol. 1998;82:985–7. doi: 10.1016/s0002-9149(98)00520-7. [DOI] [PubMed] [Google Scholar]
- 9.Hochman JS, Lamas GA, Buller CE, Dzavik V, Reynolds HR, Abramsky SJ, Forman S, Ruzyllo W, Maggioni AP, White H, Sadowski Z, Carvalho AC, Rankin JM, Renkin JP, Steg PG, Mascette AM, Sopko G, Pfisterer ME, Leor J, Fridrich V, Mark DB, Knatterud GL. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395–407. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 11.Couderc JP, Zareba W, McNitt S, Maison-Blanche P, Moss AJ. Repolarization variability in the risk stratification of MADIT II patients. Europace. 2007;9:717–23. doi: 10.1093/europace/eum131. [DOI] [PubMed] [Google Scholar]
- 12.Gomes JA, Cain ME, Buxton AE, Josephson ME, Lee KL, Hafley GE. Prediction of long-term outcomes by signal-averaged electrocardiography in patients with unsustained ventricular tachycardia, coronary artery disease, and left ventricular dysfunction. Circulation. 2001;104:436–41. doi: 10.1161/hc2901.093197. [DOI] [PubMed] [Google Scholar]
- 13.Huikuri HV, Poutiainen AM, Makikallio TH, Koistinen MJ, Airaksinen KE, Mitrani RD, Myerburg RJ, Castellanos A. Dynamic behavior and autonomic regulation of ectopic atrial pacemakers. Circulation. 1999;100:1416–22. doi: 10.1161/01.cir.100.13.1416. [DOI] [PubMed] [Google Scholar]
- 14.Makikallio TH, Hoiber S, Kober L, Torp-Pedersen C, Peng CK, Goldberger AL, Huikuri HV. Fractal analysis of heart rate dynamics as a predictor of mortality in patients with depressed left ventricular function after acute myocardial infarction. TRACE Investigators. TRAndolapril Cardiac Evaluation. Am J Cardiol. 1999;83:836–9. doi: 10.1016/s0002-9149(98)01076-5. [DOI] [PubMed] [Google Scholar]
- 15.Perkiomaki JS, Zareba W, Daubert JP, Couderc JP, Corsello A, Kremer K. Fractal correlation properties of heart rate dynamics and adverse events in patients with implantable cardioverter-defibrillators. Am J Cardiol. 2001;88:17–22. doi: 10.1016/s0002-9149(01)01578-8. [DOI] [PubMed] [Google Scholar]
- 16.Breithardt G, Cain ME, el-Sherif N, Flowers NC, Hombach V, Janse M, Simson MB, Steinbeck G. Standards for analysis of ventricular late potentials using high-resolution or signal-averaged electrocardiography. A statement by a Task Force Committee of the European Society of Cardiology, the American Heart Association, and the American College of Cardiology. Circulation. 1991;83:1481–8. doi: 10.1161/01.cir.83.4.1481. [DOI] [PubMed] [Google Scholar]
- 17.Barnett V, Lewis T. Outliers in Statistical Data. Wiley and Sons; New York: 1993. pp. 78–81. [Google Scholar]
- 18.Sadanandan S, Buller C, Menon V, Dzavik V, Terrin M, Thompson B, Lamas G, Hochman JS. The late open artery hypothesis--a decade later. Am Heart J. 2001;142:411–21. doi: 10.1067/mhj.2001.117774. [DOI] [PubMed] [Google Scholar]
- 19.Hochman JS, Lamas GA, Knatterud GL, Buller CE, Dzavik V, Mark DB, Reynolds HR, White HD. Design and methodology of the Occluded Artery Trial (OAT) Am Heart J. 2005;150:627–42. doi: 10.1016/j.ahj.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Dzavik V, Buller CE, Lamas GA, Rankin JM, Mancini GB, Cantor WJ, Carere RJ, Ross JR, Atchison D, Forman S, Thomas B, Buszman P, Vozzi C, Glanz A, Cohen EA, Meciar P, Devlin G, Mascette A, Sopko G, Knatterud GL, Hochman JS. Randomized trial of percutaneous coronary intervention for subacute infarct-related coronary artery occlusion to achieve long-term patency and improve ventricular function: the Total Occlusion Study of Canada (TOSCA)-2 trial. Circulation. 2006;114:2449–57. doi: 10.1161/CIRCULATIONAHA.106.669432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkiomaki JS, Zareba W, Ruta J, Dubner S, Madoery C, Deedwania P, Karcz M, Bayes de Luna A. Fractal and complexity measures of heart rate dynamics after acute myocardial infarction. Am J Cardiol. 2001;88:777–81. doi: 10.1016/s0002-9149(01)01851-3. [DOI] [PubMed] [Google Scholar]
- 22.Bigger JT, Jr., Fleiss JL, Rolnitzky LM, Steinman RC, Schneider WJ. Time course of recovery of heart period variability after myocardial infarction. J Am Coll Cardiol. 1991;18:1643–9. doi: 10.1016/0735-1097(91)90497-w. [DOI] [PubMed] [Google Scholar]
- 23.Perkiomaki JS, Zareba W, Kalaria VG, Couderc J, Huikuri HV, Moss AJ. Comparability of nonlinear measures of heart rate variability between long- and short-term electrocardiographic recordings. Am J Cardiol. 2001;87:905–8. doi: 10.1016/s0002-9149(00)01537-x. [DOI] [PubMed] [Google Scholar]
- 24.Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993;88:927–34. doi: 10.1161/01.cir.88.3.927. [DOI] [PubMed] [Google Scholar]