Abstract
Estrogens are the best-studied class of drugs for potential use in the prevention of Alzheimer's disease (AD). These steroids have been shown to be potent neuroprotectants both in vitro and in vivo, and to exert effects that are consistent with their potential use in prevention of AD. These include the prevention of the processing of amyloid precursor protein (APP) into beta-amyloid (Aß), the reduction in tau hyperphosphorylation, and the elimination of catastrophic attempts at neuronal mitosis. Further, epidemiological data support the efficacy of early postmenopausal use of estrogens for the delay or prevention of AD. Collectively, this evidence supports the further development of estrogen-like compounds for prevention of AD. Several approaches to enhance brain specificity of estrogen action are now underway in an attempt to reduce the side effects of chronic estrogen therapy in AD.
Keywords: estrogens, estradiol, Alzheimer's disease, neurodegeneration, memory and cognition
Introduction
Estrogens are one of the best-studied classes of molecules for their potential role as a preventative or a disease-modifying therapy for Alzheimer's disease (AD). These polyphenols have been extensively assessed for their capacity to protect neurons from a number of toxic insults, including Aß peptide; in animal models of Alzheimer's neuropathology; and in epidemiological assessments given the hundreds of millions of women years of postmenopausal use of estrogen. Additionally, several placebo controlled clinical trials of estrogen therapy for AD have been conducted. As such, we are now in a position to assess the potential for the use of estrogens as a preventative or a disease-modifying treatment of this neurodegenerative disease.
The neuropathological features of AD for which estrogens have been evaluated are neuronal loss, Aß formation, tau hyperphosphorylation and neurofibrillary tangle formation, oxidative damage, neuroinflammation and the catastrophic attempts of neurons to undergo mitosis. In this treatise, we consider these in vitro and in vivo results as well as the clinical studies conducted to date to determine if estrogen therapy has the potential for preventing AD or modifying its disease course.
We will consider first the in vitro evidence for the potent neuroprotective action of estrogens, then evaluate animal models that demonstrate one or more of the neuropathological features of AD. We will then assess the evidence for and against the clinical use of estrogens for AD prevention or disease modification. Finally, we describe major therapeutic strategies for improving the potential for estrogen use in the prevention of AD.
In vitro evidence supporting estrogen use in preventing Alzheimer's disease
In vitro, 17 ß-estradiol (E2) protects central nervous cells from a variety of oxidative stress-promoting insults. E2 is able to protect neurons from deprivation of serum [Bae et al. 2000; Gollapudi and Oblinger, 1999; Green et al. 1997a, b]; against the toxicity of various Aß peptides [Mook-Jung et al. 1997; Fitzpatrick et al. 2002; Chae et al. 2001; Kim et al. 2001; Hosoda et al. 2001; Bae et al. 2000; Bonnefont et al. 1998; Mattson et al. 1997; Goodman et al. 1996; Green et al. 1996], MPTP [Ba et al. 2004; Gélinas et al. 2004; Gagne et al. 2003; De Girolamo et al. 2001], dopamine [for review see Dluzen and Horstink, 2003], haloperidol [Sagara, 1998], quinolinic acid [Kuroki et al. 2001], hydrogen peroxide (H2O2) [Biewenga et al. 2005; Yu et al. 2004; Brinton et al. 2000; Vedder et al. 2000; Blum-Degen et al. 1998; Bonnefont et al.1998; Sawada et al. 1998; Singer et al., 1998; Behl et al. 1995], SIN-1 [Takao et al. 2004], iodoacetic acid [Perez et al. 2005], paraquat [Gelinas et al. 2004], and hemoglobin [Regan and Guo, 1997]. Further, estrogens ameliorate death from glutathione-depleting agents, such as glutamate [Deecher et al. 2005; Vedder et al. 2000; Behl et al. 1997; Davis and Maher, 1994] and an irreversible blocker of y-glutamyl-cysteine synthase (buthionine sulfoxide) [Mérot et al. 2005; Sawada et al. 2000; Behl et al. 1997], as well as glutamate, NMDA- or kainate-induced excitotoxicity [Kajta et al. 2001; Honda et al. 2000; Singer et al. 1998]. Estrogens also protect against death due to heavy metals (cobalt and mercury) [Olivieri et al. 2002], and iron chloride [Vedder et al. 1999; Mook-Jung et al. 1997].
Estrogens have been shown to reduce inflammatory actions in vitro (for review, see [Brooke and Sapolsky, 2000]. In HIV-related in vitro models, E2 protects against gp120, the coat protein of HIV, HIV regulatory protein (TAT), and exposure to HIV-1 protease [Wallace et al. 2006; Corasaniti et al. 2005; Kendall et al. 2005; Russo et al. 2005; Bruce-Keller et al. 2001; Howard et al. 2001; Turchan et al. 2001; Hawkins et al. 1999]. Estrogens also attenuate gp120-induced cell death in an animal model of HIV [Corasaniti et al. 2005]. In another paradigm, estrogen's ability to inhibit microglial activation by LPS protects dopaminergic neurons by decreasing nitrite production and TNFa expression [Liu et al. 2005]. The protection seen has a greater effect on microglia than neurons [Zemlyak et al. 2005, 2002]. In aged female mice, long-term estrogen treatment elicits a resting pattern in microglia, indicative of an inhibition of astrocytic and microglial activation [Lei et al. 2003]. Estrogen's ability to inhibit microglial superoxide release and phagocytic activity also point to an anti-inflammatory effect [Bruce-Keller et al. 2000]. Further, in AD-models, conditioned media derived from estradiol-treated glia have been shown to protect neuronal cultures from jß-amyloid toxicity [Sortino et al. 2004]. Estrogens have also been shown to protect glial cells directly [Takao et al. 2004; Sur et al. 2003; Vegeto et al. 2001; Bishop and Simpkins, 1994]. These data implicate both neurons and glia as direct cellular targets of estrogen in protecting the brain.
Estrogens have long been recognized as antioxidants in a variety of in vivo and in vitro models. This is important since AD neuropathology has a strong oxidative stress component. This may be due in part to the richness in polyunsaturated fatty acids of neuronal membranes which increases the susceptibility of lipids to oxidative damage. Estrogens have only weak radical scavenging activity [Vegeto et al. 2001; Römer et al. 1997; Sudo et al. 1997] but are able to inhibit oxidative stress markers such as lipid peroxidation [Perez et al. 2005; Bayir et al. 2004; Jung et al. 2004; Vedder et al. 1999; Ruiz-Larrea et al. 1994], protein oxidation [Telci et al. 2002], and DNA damage [Thibodeau et al. 2002; Park, 2001; Sierens et al. 2001; Behl et al. 1995]. In cell-free systems, estrogens inhibit iron-induced lipid peroxidation [Ruiz-Larrea et al. 1995], LDL oxidation, cholesterol oxidation, and conjugated diene formation [Berco and Bhavnani, 2001; Bhavnani et al. 2001; Schwenke et al. 1999; Clemente et al. 1999; Martin et al. 1998; Ayres et al. 1996; McManus et al. 1996; Miller et al. 1996; Tang et al. 1996a; Sack et al. 1994]. This potent anti-oxidant activity is likely due to a novel redox cycling of estrogens [Prokai et al. 2003]. We [Green et al. 1997] and others [Moosmann and Behl, 1999; Behl et al. 1997] have shown that estrogen analogs, with increased capacity to donate a hydrogen radical from the phenolic hydroxyl group on the steroid A ring, are more potent neuroprotectants, suggesting an association between neuroprotection and antioxidant activity.
The lipophilicity of estrogens leads to their accumulation in the hydrophobic plasma membranes and affects membrane fluidity [Liang et al. 2001; Whiting et al. 2000; Dicko et al. 1999; Wiseman, 1994]. With a logP of 4.008, E2 is localized to the lipid environment of membranes, placing it at the site of key peroxidation events, and thereby allowing the prevention of oxidative damage. The lipid membrane is also the site of various signal transduction processes including PI3K/Akt signaling and the translocation of phosphatidylserine form the inner leaflet of the plasma membrane to the outer leaflet during apoptosis.
The redox state of the cell is a key determinant for cell survival and influences parameters such as the ratio of reduced and oxidized glutathione and oxidative state of proteins. Previous work has shown a synergistic interaction between E2 and glutathione (GSH) for neuroprotection [Nakamizo et al. 2000; Gridley et al. 1998; Green et al. 1998] and of the E2 phenoxy radical (E2O∗) with other antioxidants such as a-toco-pherol in chemical systems [Winterle et al. 2001]. Estradiol has also been reported to elicit significant increases in GSH levels in HT-22, primary hippocampal, and primary neocortical cells [Schmidt et al. 2002].
As the major energy source of the cell, mitochondria stand at center stage in aging and neurodegeneration. Mitochondria dysfunction has been implicated in aging and many neurodegenerative diseases such as AD, Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis. Mounting data suggests that estrogens exert neuroprotection through maintaining mitochondrial function. In support of the mitochondria as a relevant target of estrogens, it has been shown that estrogen protects neurons against various mitochondrial toxins such as MPTP [Kenchappa et al. 2004; Shughrue, 2004; Disshon and Dluzen, 1997; Dluzen et al. 1996a] and 3-nitroproprionic acid (3-NPA) [Wang et al. 2001].
Through the mitochondrial electron respiratory chain, mitochondria generate the majority of cellular ATP and reactive oxygen species (ROS). Under oxidative stress, mitochondrial function plays a critical role in cellular life or death decisions. Evidence shows that estrogens may exert direct or indirect effects on mitochondrial function. In cell culture models, E2 preserves mitochondrial function by maintaining the mitochondrial membrane potential [Dykens et al. 2003; Wang et al. 2001], modulating mitochondrial calcium sequestration [Nilsen and Brinton, 2004; Wang et al. 2003, 2001], and attenuating cellular ATP depletion induced by oxidative insults such as 3-NPA [Wang et al. 2001] and H2O2 [Wang et al. 2003; Vedder et al. 2000]. E2 also ameliorates mitochondria-generated ROS [Wang et al. 2001]. Several potential mechanisms that underlie estrogen mitoprotection are described below.
Mitochondrial calcium overloading leads to mitochondrial membrane potential collapse and initiates cell death. Data from our laboratory and others shows that E2 attenuates mitochondrial calcium overloading against oxidative stress [Wang et al. 2003; Nilsen and Brinton, 2003; Wang et al. 2001]. Mitochondrial calcium loading depends on uptake through the uniporter and efflux by Na+/Ca2+ exchanger on mitochondrial membrane [Crompton et al. 1978]. It has been shown that E2 increases Na+-dependent calcium efflux exponentially at concentrations above 10nM in synaptosomal mitochondria [Petrovic et al. 2005; Horvat et al. 2001,2000]. The ability of estrogens to maintain mitochondrial calcium levels may be closely related to their modulatory effect on intracellular calcium homeostasis and mitochondrial membrane potential under oxidative stress.
Long-term supplementation of E2 (80 mg/kg, 16 weeks) in ovariectomy (OVX) rats effectively antagonized the detrimental effects of ovariectomy on brain mitochondrial lipid peroxidation, glutathione loss, and superoxide dismutase (SOD) activity [Feng and Zhang, 2005]. E2 also prevents OVX-induced impairments of mitochondrial complex I and IV activity [Feng and Zhang, 2005]. Furthermore, in OVX rats, E2 treatment increases specific proteins in cerebrovascular mitochondria, such as cytochrome c, subunit IV of electron transport chain (ETC) complex IV, manganese SOD, and subunit I of the ETC complex IV [Stirone et al. 2005]. Incubation of cerebral vessels with 10nM E2 also resulted in elevated levels of mitochondrial cytochrome c [Stirone et al. 2005]. Another important component of ETC, F0F1-ATPase, is also affected by E2. Binding to one subunit of F0F1-ATPase, E2 inhibits its activity and blunt ATP hydrolysis [Zheng and Ramirez, 2000, 1999a, 1999b].
E2 treatment also enhances the ratio of anti-apoptotic proteins to pro-apoptotic proteins, many of which have direct interactions with mitochondria. Physiological levels of E2 and estrogen receptor (ER) agonists increase the levels of an anti-apoptotic protein, bcl-2, in both primary neuronal cultures and ischemic OVX rats [Zhao et al. 2004; Honda et al. 2001; Dubal et al. 1999]. In primary cortical neurons, 10 nM E2 prevented apoptosis, attenuated calpain upregulation, shifted the Bax:Bcl-2 ratio toward survival, and decreased caspase-3 activation [Sribnick et al. 2004]. The PI3-K/Akt signal transduction pathway plays a pivotal role in E2 anti-apoptotic effects [Honda et al. 2001]. In endothelial cells, E2 elicits Akt/PKB localization to mitochondria and phosphorylates Bad protein [Sasaki et al. 2003].
E2 prevents mitochondrial permeability transition pore (MPTP) opening, mitochondrial membrane potential (?m) collapse, and cytochrome c release. Maintaining mitochondrial membrane potential is a critical step in E2 neuroprotection. In SK-N-SH cells, E2 significantly reduced ?m collapse induced by 3-NPA [Wang et al. 2001]. Moreover, in HT-22 cells, 14 estradiol analogs were tested for potency in protection of ?m collapse and increase in neuronal survival. We found a strong correlation between mitoprotection and neuroprotection for these estrogen analogs [Simpkins et al. 2005a]. The preservation of ?m results in prevention of cytochrome c release, which is the crucial point in apoptosis. A plethora of data indicates that E2 reduces cytochrome c release from mitochondria in both in vivo and in vitro models [Zhang and Bhavnani, 2005; Bagetta et al. 2004; Morkuniene et al. 2002; Hosoda et al. 2001].
Although estrogens are known to exert antioxidant effects, they are poor ROS scavengers. In both neuronal and non-neuronal cell cultures, estrogens failed to attenuate H2O2-induced cellular ROS elevation [Simpkins et al. 2005; Wang et al. 2003], but E2 effectively attenuated 3-NPA-induced ROS production from mitochondria [Wang et al. 2001]. We proposed that estrogens reduce mitochondrial free-radical generation by reducing lipid peroxidation, stabilizing ATP production, preserving ?m and mitochondrial ETC efficiency, but not by acting as a direct ROS scavenger. This is supported by evidence that long-term supplementation with E2, ameliorates brain mitochondrial lipid peroxidation and mitochondrial H2O2 production induced by ovariectomy [Feng and Zhang, 2005; Stirone et al. 2005].
Both ERα and ERß localize to mitochondria [Chen et al. 2004a, 2004b; Yang et al. 2004]. This suggests that ERs might play a role in the effects of estrogens on mitochondria function other than through their genomic action. We have addressed this issue by constitutively knocking down ERß in a HT-22 cell line and determining the effects of this manipulation of cell phenotype (Yang et al. unpublished observations). We observed that ERß knockdown had no effect on cell proliferation, but enhanced cell viability in response to a variety of pro-oxidant insults. Additionally, these cells were better able to maintain ?m and cellular ATP levels during oxidative insult. These data suggest that unliganded mitochondrial ERß plays a role in susceptibility of neurons to pro-oxidant stress. By inference, binding of ERß to an estrogen may reduce this ERß involvement in neuronal pro-oxidant susceptibility and provide a novel mechanism for estrogen-induced neuroprotection. This may represent, for the first time, an estrogen ligand-induced loss of function of an ER.
Studies have shown that mitochondrial genes are potential sites of primary action of estrogen [Demonacos et al. 1996]. Mitochondrial proteins are encoded by both mitochondrial and nuclear genes. The 16-kb mitochondrial genome encodes 13 of the more than 100 proteins involved in oxidative phosphorylation and the remainder are encoded by the nuclear genome. Mitochondrial genome contains sequences similar to estrogen response element (ERE). It has been shown that estrogen-specific binding sites were associated with mitochondrial structures, suggesting the mitochondria localization of ERs [Solakidi et al. 2005; Chen and Yager, 2004; Horvat et al. 2001; Noteboom and Gorski, 1965]. In OVX rats, E2 elevated expression of subunit I of complex IV, which is encoded in mtDNA [Stirone et al. 2005].
In cultured female rat hepatocytes, incubation of ethinyl estradiol (EE) for 24 h, increased the transcript levels of the mitochondrial genome-encoded genes cytochrome oxidase subunits I, II and III [Chen et al. 2003]. This effect was accompanied by increased mitochondrial respiratory chain activity, as reflected by increased mitochondrial superoxide generation, and detected by lucigenin-derived chemiluminescence and cellular ATP levels [Chen et al. 2003]. A differential screening of hippocampus cDNA library from estrogen-stimulated OVX rats indicated that complex III mRNA levels significantly increased as early as 3 h following a single dose of E2 treatment [Bettini and Maggi, 1992]. In the pituitary of OVX rats, E2 also remarkably enhanced mitochondria complex II mRNA levels [Van Itallie and Dannies, 1998; Law et al. 1994].
Recently, the Brinton laboratory described the effects of a single dose (30μg/kg) and single time point (24 h post-E2 injection) of E2 on the mitochondrial proteome in 2-week ovariecto-mized rats [Nilsen et al. 2007]. They report that brain mitochondria isolated from E2 treated rats are more efficient in producing ATP, produces less ROS and that 66 proteins isolated by 2D gel electrophoresis were changed more than two-fold by E2 treatment. Of these 66 proteins, 28 showed increased and 38 showed decreased levels. Eighteen spots were selected for identification and these proteins fell into three primary categories: energy-related proteins, oxidative balance proteins and structural and chaperone proteins. Collectively, these data indicate that E2 markedly affects mitochondrial protein expression and thereby can affect mitochondrial structure and function.
Effects of estrogens in models of AD neuropathology
Aß models
In addition to the aforementioned protection provided by estrogens against Aß neurotoxicity and compromised mitochondrial function, estrogens have been demonstrated to affect a variety of processes by which Aß contributes to AD neuropathology. Each of the three major circulating estrogens, estrone, E2 and estriol, dose-depen-dently reduce Aß fibrillation in ex vivo studies [Morinaga et al. 2007]. Also, estrogens enhance the uptake of aggregated Aß into human cortical microglia in vitro [Li et al. 2000]. These actions of estrogens could contribute to their ability to reduce plaque load in animal models (see below).
In cell culture models, E2 has been shown to reduce Aß in HEK293 cells [Chang et al. 1997], to increase soluble APP (a nonamyloido-genic protein) in ZR-75-1 human carcinoma cells [Jaffe et al. 1994], and to accelerate APP trafficking in the trans-Golgi network in vesicles derived from neuroblastoma cells or primary neurons, thereby reducing the production of Aß [Greenfield et al. 2002]. Such effects of estrogens are consistent with its ability to reduce production of neuronal Aß.
Several animal models have been assessed for the effects of estrogens on Aß production and amyloid plaque load. In guinea pigs, which produce the human form of Aß but do not develop plaques, ovariectomy increased, and estrogen treatment reduced, Aβ1-40 and Aβ1-42 concentrations in brain [Petanceska et al. 2000]. Similar results were seen when a double trans-genic (Tg) mouse containing PS-1 and an APP human mutations were subjected to ovariectomy and estrogen replacement [Zheng et al. 2002]. Further, in a mouse Tg model containing the human Swedish APP mutation, a reduction in Aß was seen with treatment with either E2 or its transcriptionally weak diasteriomer, 17 a-estradiol [Levin-Allerhand et al. 2002]. A triple Tg mouse model containing PS-1 and an APP human mutations as well as a mutant human tau construct also showed a reduction in brain Aß with E2 treatment [Carroll et al. 2007]. In contrast to these observations, Green et al. [2005] showed no effect of E2 on Aß in PDAPP mice.
Another means of increasing Aß production in rodents is through induction of a cerebral ischemic event in non-transgenic rats [Shi et al. 1998]. Using this model, we have shown that E2 treatment of ovariectomized rats reduces APP protein and mRNA [Shi et al. 1998] and reduces the expression of BACE-1, the protease responsible for ß-site cleavage of APP [Wen et al. 2004a].
Stroke models
Most cases of dementia are thought to develop from two distinct diseases: Alzheimer's disease or vascular disease. Recent studies suggest that vascular dementia and mixed dementia, in which cerebrovascular and Alzheimer's pathologies coexist, together comprise the majority of dementia cases [Roman, 2002]. Both experimental and clinical investigations have provided evidence that AD and vascular dementia, traditionally considered distinct clinical and pathophysiological entities, could share common features and converging pathogenic mechanisms.
Vascular dementia incorporates cognitive dysfunction with vascular disease. Cerebral ischemia is the major cause of vascular dementia [Roman et al. 2002]. The prevalence of vascular dementia was estimated to be as high as 80% to 90% after stroke according to different criteria [Pohjasvaara et al. 2000]. The traditional concept of vascular dementia postulates that cognitive decline in patients with ischemic stroke can result from the stroke alone when a large volume of brain is affected by infarcts and overcoming the brain's reserve or compensatory mechanisms. However, this traditional concept is not supported by the progressive course of dementia after ischemic stroke demonstrated by epidemiological longitudinal studies. Tatemichi et al. [1990] reported that the incidence of dementia was 6.7% among patients after 1 year of follow-up in a sample of 610 patients who were initially nondemented after stroke. Bornstein et al. [1996] reported that 32% patients who were initially nondemented after stroke developed incident dementia during 5 years of follow-up after first ischemic stroke. Henon et al. [2001] examined a cohort of 169 patients who had been nondemented before stroke onset and reported that the cumulative proportion of patients with incident dementia was 21.3% after 3 years of follow-up. Altieri et al. [2004] assessed 191 non-demented stroke patients for a 4 years follow-up, and found the incidence of dementia increased gradually with 21.5% patients had developed dementia by the end of the follow-up period. In population-based studies of stroke and dementia, Kokmen et al. [1996] reviewed the medical records of a sample of 971 patients who were free of dementia before first stroke. The cumulative incidence of dementia was 7% at 1 year, 10% at 3 years, 15% at 5 years and 23% at 10 years. Desmond et al. [2002] performed functional assessments annually on 334 ischemic stroke patients and 241 stroke-free control subjects, all of whom were nondemented in baseline examinations, and found a progressive course of dementia with the incidence rate of 8.94 per 100 person-years in the stroke cohort and 1.37 cases per 100 person-years in the control cohort. In two studies based on patients presenting with a lacunar infarction as their first stroke, Samuelsson et al. [1996] found that 4.9% and 9.9% of 81 patients developed dementia after 1 and 3 years of follow-up, respectively, and Loeb et al. [1992] found that 23.2% patients developed dementia during an average of 4 years of follow-up.
The progressive course of cognitive decline after ischemic stroke has also been demonstrated in the focal ischemic stroke model. Middle cerebral artery occlusion (MCAO) in rodents is considered to be a convenient, reproducible and reliable model of cerebral ischemia in humans [DeVries et al. 2001; Bederson et al. 1986; Tamura et al. 1981]. MCAO typically results in extensive neuronal death in the cortex and caudate putamen, both centers for sensorimotor function [Bederson et al. 1986]. Sensorimotor behavior impairment has been extensively studied in ischemic stroke models. Spontaneous partial or complete recovery of sensorimotor function has been reported consistently over time after ischemic stroke [Karhunen et al. 2003; DeVries et al. 2001; Roof et al. 2001; Yonemori et al. 1999; Markgraf et al. 1997,1994]. On the other hand, different progression of cognitive impairment has been demonstrated in MCAO models. The Morris water maze has been used to study impairment in spatial learning and memory in experimental stroke studies [Yonemori et al. 1999; Stroemer et al. 1998; Markgraf et al. 1997, 1994, 1992; Smith et al. 1997; Yonemori et al. 1996]. The experimental protocols and indices of performance used in these studies vary considerably; consequently, the conclusions are not always consistent. However, few studies have shown recovery of the cognitive impairment over time after MCAO, and a progressive impairment of cognitive function has been suggested in some of studies [Karhunen et al. 2003; Roof et al. 2001; Stroemer et al. 1998; Markgraf et al. 1992]. A longitudinal behavior study following transient middle cerebral artery occlusion in rats demonstrated sensorimotor and spatial memory impairment for up to 1 year. Interestingly, no progression of sensorimotor dysfunction was found from the repeated test at 7 months and 1 year after transient focal cerebral ischemia [Karhunen et al. 2003]. For the spatial memory test, improvement of performance was found in both stroke and sham animals at 1 year after insult or sham surgery, respectively, as a result of repeated test, compared with the behavior test obtained at 7 months after stroke or sham surgery [Karhunen et al. 2003]. However, the improvement in the stroke animals was profoundly less than that of sham animals, suggesting a progression of spatial memory impairment from 7 month to 1 year after ischemic stroke [Karhunen et al. 2003]. In similar behavior test paradigm, rats with permanent MCAO showed less improvement of performance in repeated spatial memory tests at 1 and 2 weeks after stroke when comparing with shams [Roof et al. 2001].
Taken together, accumulating evidence from both epidemiological studies and basic research has indicated that the progressive cognitive function decline is not solely due to the direct contribution of the primary cerebral ischemic damage [Pasquier and Leys, 1997]. Rather, the progressive course of vascular disease suggests a degenerative disorder [Kokmen et al. 1996; Tatemichi et al. 1994].
Animal research has also demonstrated that Alzheimer's neuropathologies can be induced in the rat MCAO model, suggesting that this model could be useful as a nontransgenic model to discover potential therapeutics for AD. Alz-50-immunoreactive granules are found around the cerebral infarction after an ischemic stroke in gerbil, rats and human patients [Wen et al. 2004b; Ikeda et al. 2000; Dewar et al. 1993]. Ischemic stroke induces abnormal cell cycle molecules, such as cdc2, cyclin B1, together with nonmitotic cdk5 [Kesavapany et al. 2004]. Further, increased cdk5 mRNA and protein in the human brain following acute ischemic stroke is reported [Mitsios et al. 2007]. Cdk5 is a key kinase in tau hyperphosphorylation and AD pathogenesis [Monaco and Vallano, 2005; Cruz and Tsai, 2004]. Ischemic injury induced cdk5 activation is also related to another key pathological feature: amyloid plaque formation [Wen et al. 2008; Vassar, 2007] (see Figure 1). We speculate that estrogens can attenuate cdk5 activations and other cyclin-dependent kinases, in part through activation of protein phospha-tases [Yi et al. 2008, 2005].
Figure 1.
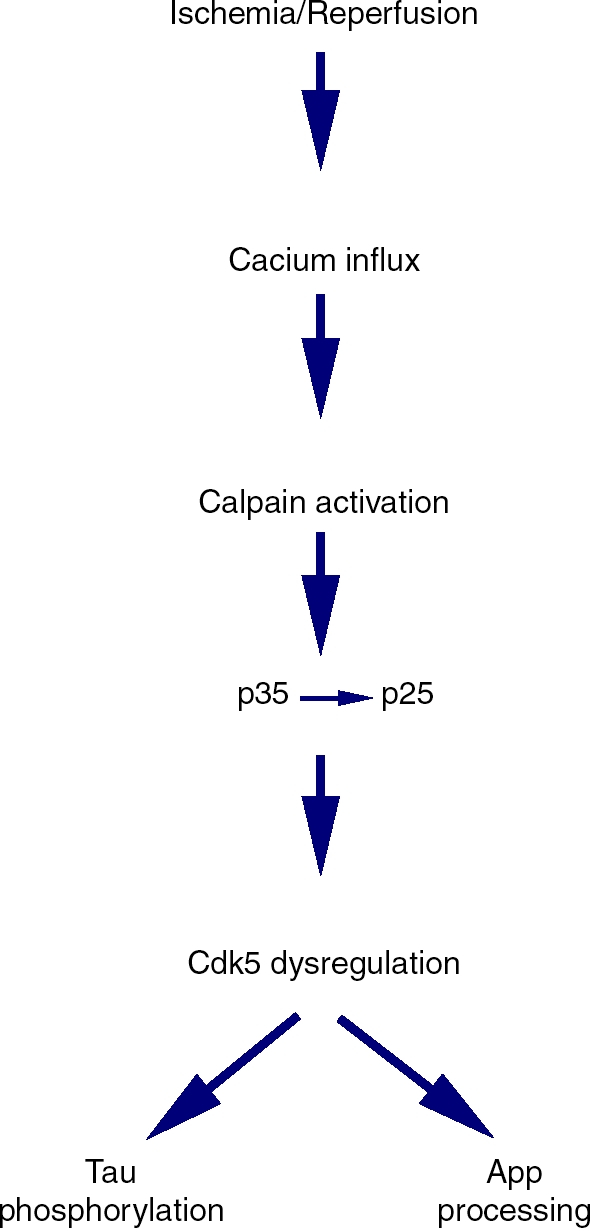
The p25/cdk5 signaling pathway after ischemia/reperfusion injury and its correlation with AD.
Although the effects of estrogens against the progressive decline in cognition have not yet been assessed, the neuroprotective effects of estrogens have been demonstrated in a variety of acute models for acute cerebral ischemia. These include transient and permanent middle cerebral artery occlusion models [Alkayed et al. 1998; Dubal et al. 1998; Simpkins et al. 1997], global forebrain ischemia models [He et al. 2002; Sudo et al. 1997], photothrombotic focal ischemia models [Fukuda et al. 2000], and glutamate-induced focal cerebral ischemia models [Mendelowitsch et al. 2001]. The protective effects of estrogens have been described in rats, mice and gerbils [Culmsee et al. 1999]. Estrogen-induced neuroprotection has been demonstrated in adult female, middle-aged female as well as reproductively senescent female rats [Wise et al. 2001]. Similarly, these effects of estrogens have been shown despite the presence of diabetes and hypertension [Carswell et al. 2000; Toung et al. 2000]. The neuroprotective effects of estrogens have been demonstrated against subarachnoid hemorrhage, a highly prevalent form of stroke in females [Yang et al. 2001]. Finally, the neuro-protective action of estrogen is not limited to the female, inasmuch as estrogen protection is also seen in males [Hawk et al. 1998; Toung et al. 1998]. Collectively, these results indicate that estrogens could be valuable candidates for brain protection during acute stroke. In addition, given the aforementioned in vitro evidence for potent neuroprotection with estrogens, this steroid could have potential in attenuating ischemic-injury-related neurodegeneration and related AD neuropathology. Indeed, the impact of preventive neuroprotective strategies on incidence rates of AD was predictable from epidemiological studies [Shoulson, 1998]. Experimental results have showed that neuropro-tective therapies are expected to slow the rate of neuronal loss, and that even relatively modest neuroprotective effects can lead to dramatic reductions in incidence rates of AD.
Clinical evidence for estrogen prevention of Alzheimer's disease
While the primary clinical indications for estrogen therapy during the perimenopausal and/or postmenopausal period are for its use in reducing hot flushes and the risk of osteoporosis, there have been numerous clinical studies that support the potential role of estrogens in reducing the risk for AD. Support for this hypothesis includes the observation that estrogen therapy can reduce the cognitive decline observed in women that have gone through either natural or surgical menopause [Carlson and Sherwin, 1998; Sherwin, 1997; Phillips and Sherwin, 1992]. Further, there is also evidence that estrogen therapy may affect cognitive function during brain aging as well [Maki et al. 2001; Resnick and Maki, 2001; Resnick et al. 1998]. And with specific regards to AD, starting with the seminal study by Fillit et al. [1986], several epidemiological studies described that postmenopausal estrogen therapy may contribute to the prevention, attenuation, or even delay in the onset of AD [Kawas et al. 1997; Paganini-Hill and Henderson, 1996, 1994; Tang et al. 1996b; Ohkura et al. 1994]. Interestingly, estrogen treatment also improved the efficacy of Tacrine®, an anticholinesterase drug used for the treatment of AD [Schneider et al. 1996], suggesting that estrogen may also facilitate or enhance existing treatments for AD.
However, some recent clinical trials including the Women's Health Initiative (WHI) improved failed to find a positive effect of estrogen on cognitive function and risk for AD patients [Espeland et al. 2004; Shumaker et al. 2004; Rapp et al. 2003; Shumaker et al. 2003; Mulnard et al. 2000]. Further, cardiovascular and thromboembolic risks were also suggested in these studies [Anderson et al. 2004; Manson et al. 2003], although the modest and nonsignificant increase reported was observed only in the conjugated equine estrogen (CEE) plus medrox-yprogesterone acetate (MPA) group [Manson et al. 2003]. In contrast, no increase in the risk for cardiovascular disease in the estrogen alone arm of the WHI was noted, although no beneficial effects were observed either [Anderson et al. 2004]. While these studies were informative, the interpretation of the WHI studies is limited by the hormone preparations used, their route of administration, the regimen of hormone administration (i.e., continuous daily therapy versus cyclic therapy) and the advanced age of the subjects under study [Singh et al. 2008; Simpkins et al. 2005c; Singh and Simpkins 2005]. As such, these results cannot be construed as the ‘final word’ on the role of estrogens in reducing the risk for dementia.
As suggested in the preceding paragraph, an important caveat to the clinical studies described above in which estrogen therapy had negative effects on cognition and/or AD is consideration of the age of the individual. Indeed, the Cache County study [Zandi et al. 2002] revealed that the efficacy of hormone therapy changed not only with increasing age, but also with increasing duration of hormone use. Furthermore, following reanalysis of the WHI Memory Study (WHIMS) data, it has been inferred that women who used hormones at younger ages had lower risks for AD [Henderson et al. 2007].
Collectively, the clinical studies suggest that the beneficial effects of estrogen in AD depend on at least three important factors: the age of the individual (or alternatively, the duration of postmenopausal status prior to initiating hormone therapy), the duration of treatment and, potentially, the formulation of the hormones used in the therapy (i.e., 17yß-estradiol versus CEE). These factors may indeed underlie, at least in part, the apparent discrepancy between the substantial volume of basic science data and the recent results of the WHI. In fact, the reanalyses of the WHI, coupled with new data on the neurobiology of estrogens that address one or more of the three factors listed above, have supported the potential of estrogens in reducing the risk of such neurodegenerative disorders as Alzheimer's disease. Nevertheless, further research is certainly warranted in order to attain a more complete understanding of hormone neurobiology.
Advances in drug discovery of estrogens
One of the major problems faced in applying estrogens to the prevention/therapy of AD is that any successful drug for AD will need to be administered for years to decades. It is now well known that continuous therapy with estrogens leads to an increase in prothrombic events and an increased risk for uterine cancer. As such, estrogen therapy for AD will require types of estrogens that are devoid of these side effects. Two major strategies for AD have developed to address the need for chronic therapy and to eliminate side effects of estrogens.
We have undertaken a planned strategy, using estradiol as a scaffold, to build out of estrogens their ability to interact with ERs, while retaining their potent neuroprotective and antioxidant activities. Two approaches were used to reduce interaction with ERs: the creation of enantiomers and the addition of large bulky groups to the 2-and 4-carbons of the phenolic A ring of the steroid. The former approach capitalized on the stereospecific interactions between ERs and its ligands, while the latter approach utilized the observation that 3/5 of the binding energy of interaction of estrogens with ERs occurs with the insertion of the phenolic A ring into the ER.
We synthesized over 80 compounds that were subjected to screening assays for neuroprotection, antioxidant activity and ER binding [Perez et al. 2006]. Some of the most promising compounds were tested in both in vitro assays and in animal models for neuroprotection. We observed that diasteriomers and enantomers of E2 and estrone were as potent as their parent, naturally occurring estrogen in their antioxidant and neu-roprotective activity, but were much less potent in binding to either ERa or ERß [Perez et al. 2006; Simpkins et al. 2005b; Green et al. 2001; Bishop and Simpkins, 1994]. Additionally, phenolic A ring modification at the 2- and/or 4-carbons produced a number of analogs with neuroprotective potencies 100-fold greater than that of E2 and completely eliminated binding to either ERa or ERß and eliminated in vivo estrogenicity [Jung et al. 2006; Perez et al. 2006; Simpkins et al. 2005b; Perez et al. 2005; Kumar et al. 2005; Simpkins et al. 2004; Liu et al. 2002]. In summary, this strategy promised to produce compounds with enhanced ability to prevent AD neuropathology without stimulating peripheral ERs.
A strategy proposed by the laboratory of Dr Roberta Brinton is to apply the selective estrogen receptor modulator (SERM) approach to brain activity of estrogens. This approach is based on the observations by her laboratory [Zhao et al. 2006] as well as our laboratory [Gridley et al. 1998] that the pan estrogen receptor antagonist, ICI 182 780 is an effective ER antagonist in peripheral tissues, but is an agonist in the brain. She then proposed producing a series of NeuroSERM compounds that have the unique features of ICI 182 780, including a 7a-substitution side chain, but with features which allow for penetrance of the blood-brain barrier [Zhao et al. 2007, 2005]. Poor BBB permeability of ICI 182 780 has limited its use for brain conditions [Zhao et al. 2007, 2005; Brinton, 2004; Howell et al. 1996].
Future clinical research directions
The overall strategy of the future assessment of the potential for the use of estrogens in the treatment/prevention of AD should be three-fold. Over the short-term, studies need to be completed to assess the effects of early estrogen intervention, using estrogen preparations already approved for menopausal syndrome, on the incidence of AD. One of these primary prevention studies, the Kronos Early Estrogen Prevention Study (KEEPS) is ongoing and assesses the effects of initiation at the time of the menopause of a conjugated equine estrogen preparation versus a transdermal estradiol preparation on cognitive decline [Harman, 2006]. While this study is aimed at assessing cognitive decline following the menopause with or without one of two estrogen treatments, its early initiation (at the biologically defined menopause) and proposed duration (4 years) will not allow sampling of women for incident cases of AD. As such, every effort should be made to continue the KEEPS trial for many years after its designed termination to determine if one or the other estrogen treatment delays or prevents AD.
A second approach that we have initiated is to assess estrogen preparations in clinical studies for conditions that predict the later development of AD. Traumatic brain injury (TBI) is known to lead to AD neuropathology [DeKosky et al. 2007; Ikonomovic et al. 2004; Olsson et al. 2004] and is an established risk factor in the development of AD [Van Den Heuvel et al. 2007; Jellinger et al. 2001]. As indicated above, in experimental models estrogens limit brain damage and AD-like neuropathology from trauma. We have received FDA approval to conduct a RESCUE-TBI trial which will assess the effects of a single i.v. dose of Premarin® administered within 2 h of the event on brain damage and functional decline from TBI. While this study will not specifically assess cognitive decline following TBI, protection of brain tissue from damaging effects of trauma will, we believe, predict protection from the eventual development of AD.
A third long-term strategy is to bring other more selective and more potent estrogens to clinical AD trials. As indicated above, NeuroSERMs and/or nonfeminizing estrogens are promising approaches, as both can achieve neuroprotection without stimulation of peripheral estrogen receptors. Such approaches could effectively protect the brain from the neuropathology of AD while avoiding the cancer-promoting and prothrom-botic effects of chronically administered, orally conjugated equine estrogens.
A final issue that affects not only estrogen trials, but also the assessment of all compounds for their potential use in AD prevention is the need for primary prevention trials. The neuropathol-ogy of AD is believed to begin decades before the appearance of clinical symptoms of AD. As such, preventative therapies also must begin early in the disease process and continue into late life when incident cases of AD appear. In the case of estrogen therapy, treatment should begin at the menopause. There are several major obstacles to primary prevention trials that have been recently well described [Aisen et al. 2008]. First, large numbers of subjects are needed due to low incidence of AD prior to age 70 years. Second, treatments initiated at age 50 will then require a 20-year trial. These large subject numbers and trial durations requires that we modify both regulations protecting the patents on tested compounds as well as our mechanism of funding clinical trials. Pharmaceutical companies are not likely to invest in a large 20-year trial for a compound that will be ‘off patent’ at 17 years. Given the current 5-year funding cycle at the NIH, academically conducted trails would need to be refunded three to four times, with little primary outcome data on the effects of the study compound on the incidence of AD. In view of these considerations and the extremely high cost of primary prevention trials, a public–private funding partnership may be needed to conduct these important studies. Nonetheless, primary prevention trials may be the only means of establishing the efficacy and safety of compounds for the treatment of AD.
Acknowledgements
The research described herein was support in part by NIH grants AG10485, AG22550 and AG027956.
Conflict of interests statement
James W. Simpkins holds patents for the use of estrogens to treat neurodegeneration.
Contributor Information
James W. Simpkins, Department of Pharmacology and Neuroscience, Institute for Aging and Alzheimer's Disease Research, Center FOR HER (Focused On Resources for her Health, Education and Research), University of North Texas Health Science Center, Fort Worth, TX, USA jsimpkin@hsc.unt.edu
Evelyn Perez, Department of Pharmacology and Neuroscience, Institute for Aging and Alzheimer's Disease Research, Center FOR HER (Focused On Resources for her Health, Education and Research), University of North Texas Health Science Center, Fort Worth, TX, USA.
Xiaofei Wang, Department of Pharmacology and Neuroscience, Institute for Aging and Alzheimer's Disease Research, Center FOR HER (Focused On Resources for her Health, Education and Research), University of North Texas Health Science Center, Fort Worth, TX, USA.
ShaoHua Yang, Department of Pharmacology and Neuroscience, Institute for Aging and Alzheimer's Disease Research, Center FOR HER (Focused On Resources for her Health, Education and Research), University of North Texas Health Science Center, Fort Worth, TX, USA.
Yi Wen, Department of Pharmacology and Neuroscience, Institute for Aging and Alzheimer's Disease Research, Center FOR HER (Focused On Resources for her Health, Education and Research), University of North Texas Health Science Center, Fort Worth, TX, USA.
Meharvan Singh, Department of Pharmacology and Neuroscience, Institute for Aging and Alzheimer's Disease Research, Center FOR HER (Focused On Resources for her Health, Education and Research), University of North Texas Health Science Center, Fort Worth, TX, USA.
References
- Aisen P., Albert M., Breitner J. C. S., Buckholtz N., Corey-Bloom J.P., Cummings J.L.et al. (2008)Preventing dementia: following in Leon Thal's footsteps,Alzheimer's Dementia 4:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkayed N.J., Harukuni I., Kimes A.S., London E.D., Traystman R.J., Hurn P.D.(1998)Gender-linked brain injury in experimental stroke,Stroke 29:159–165 [DOI] [PubMed] [Google Scholar]
- Altieri M., Di Piero V., Pasquini M., Gasparini M., Vanacore N., Vicenzini E.et al. (2004)Delayed poststroke dementia: a 4-year follow-up study,Neurology 62:2193–2197 [DOI] [PubMed] [Google Scholar]
- Anderson G.L., Limacher M., Assaf A.R., Bassford T., Beresford S.A., Black H.et al. (2004)Effects of conjugated equine estrogen in postmeno-pausal women with hysterectomy: the Women's Health Initiative randomized controlled trial,JAMA 291:1701–1712 [DOI] [PubMed] [Google Scholar]
- Ayres S., Tang M., Subbiah M.T.(1996)Estradiol-17beta as an antioxidant: some distinct features when compared with common fat-soluble antioxidants,J Lab Clin Med 128:367–375 [DOI] [PubMed] [Google Scholar]
- Ba F., Pang P.K., Benishin C.G.(2004)The role of Ca2+ channel modulation in the neuroprotective actions of estrogen in beta-amyloid protein and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) cytotoxic models,Neurochem Int 45:31–38 [DOI] [PubMed] [Google Scholar]
- Bae Y.H., Hwang J.Y., Kim Y.H., Koh J.Y.(2000)Anti-oxidative neuroprotection by estrogens in mouse cortical cultures,J Korean Med Sci 15:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagetta G., Chiappetta O., Amantea D., Iannone M., Rotiroti D., Costa A.et al. (2004)Estradiol reduces cytochrome c translocation and minimizes hippocampal damage caused by transient global ischemia in rat,Neurosci Lett 368:87–91 [DOI] [PubMed] [Google Scholar]
- Bayir H., Marion D.W., Puccio A.M., Wisniewski S.R., Janesko K.L., Clark R.S., Kochanek P.M.(2004)Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients,J Neurotrauma 21:1–8 [DOI] [PubMed] [Google Scholar]
- Bederson J.B., Pitts L.H., Tsuji M., Nishimura M.C., Davis R.L., Bartkowski H.(1986)Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination,Stroke 17:472–476 [DOI] [PubMed] [Google Scholar]
- Behl C., Widmann M., Trapp T., Holsboer F.(1995)17-beta estradiol protects neurons from oxi-dative stress-induced cell death in vitro,Biochem Biophys Res Commun 216:473–482 [DOI] [PubMed] [Google Scholar]
- Behl C., Skutella T., Lezoualc'h F., Post A., Widmann M., Newton C.J.et al. (1997)Neuroprotection against oxidative stress by estrogens: structure-activity relationship,Mol Pharmacol 51:535–541 [PubMed] [Google Scholar]
- Berco M., Bhavnani B.R.(2001)Differential neuroprotective effects of equine estrogens against oxidized low density lipoprotein-induced neuronal cell death,J Soc Gynecol Investig 8:245–254 [DOI] [PubMed] [Google Scholar]
- Bettini E., Maggi A.(1992)Estrogen induction of cytochrome c oxidase subunit III in rat hippocampus,J Neurochem 58:1923–1929 [DOI] [PubMed] [Google Scholar]
- Bhavnani B.R., Cecutti A., Gerulath A., Woolever A.C., Berco M.(2001)Comparison of the antioxidant effects of equine estrogens, red wine components, vitamin E, and probucol on low-density lipoprotein oxidation in postmenopausal women,Menopause 8:408–419 [DOI] [PubMed] [Google Scholar]
- Biewenga E., Cabell L., Audesirk T.(2005)Estradiol and raloxifene protect cultured SN4741 neurons against oxidative stress,Neurosci Lett 373:179–183 [DOI] [PubMed] [Google Scholar]
- Bishop J., Simpkins J.W.(1994)Estradiol treatment increases viability of glioma and neuroblastoma cells in vitro,Mol Cell Neurosci 5:303–308 [DOI] [PubMed] [Google Scholar]
- Blum-Degen D., Haas M., Pohli S., Harth R., Römer W., Oettel M.et al. (1998)Scavestrogens protect IMR 32 cells from oxidative stress-induced cell death,Toxicol Appl Pharmacol 152:49–55 [DOI] [PubMed] [Google Scholar]
- Bonnefont A.B., Munoz F.J., Inestrosa N.C.(1998)Estrogen protects neuronal cells from the cytotoxicity induced by acetylcholinesterase-amyloid complexes,FEBS Lett 441:220–224 [DOI] [PubMed] [Google Scholar]
- Bornstein N.M., Gur A.Y., Treves T.A., Reider-Groswasser I., Aronovich B.D., Klimovitzky S.S.et al. (1996)Do silent brain infarctions predict the development of dementia after first ischemic stroke? Stroke 27:904–905 [DOI] [PubMed] [Google Scholar]
- Brinton R.D., Chen S., Montoya M., Hsieh D., Minaya J.(2000)The estrogen replacement therapy of the Women's Health Initiative promotes the cellular mechanisms of memory and neuronal survival in neurons vulnerable to Alzheimer's disease,Maturitas 34(Suppl. 2):S35–S52 [DOI] [PubMed] [Google Scholar]
- Brinton R.D.(2004)Requirements of a brain selective estrogen: advances and remaining challenges for developing a NeuroSERM,J Alzheimers Dis 6(Suppl. 6):S27–35 [DOI] [PubMed] [Google Scholar]
- Brooke S.M., Sapolsky R.M.(2000)The effects of steroid hormones in HIV-related neurotoxicity: a mini review,Biol Psychiatry 48:881–893 [DOI] [PubMed] [Google Scholar]
- Bruce-Keller A.J., Barger S.W., Moss N., Pham J.T., Keller J.N., Nath A.(2001)Pro-inflammatory and pro-oxidant properties of the HIV protein Tat in a microglial cell line: attenuation by 17 beta-estradiol,J Neurochem 78:1315–1324 [DOI] [PubMed] [Google Scholar]
- Bruce-Keller A.J., Keeling J.L., Keller J.N., Huang F.F., Camondola S., Mattson M.P.(2000)Antiinflammatory effects of estrogen on microglial activation,Endocrinology 141:3646–3656 [DOI] [PubMed] [Google Scholar]
- Carlson L.E., Sherwin B.B.(1998)Steroid hormones, memory and mood in a healthy elderly population [In Process Citation],Psychoneuroendocrinology 23:583–603 [DOI] [PubMed] [Google Scholar]
- Carroll J.C., Rosario E.R., Chang L., Stanczyk F.Z., Oddo S., LaFerla F.M., Pike C.J.(2007)Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice,J Neurosci 27:13357–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell H.V., Dominiczak A.F., Macrae I.M.(2000)Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats,Am J Physiol Heart Circ Physiol 278:H290–H294 [DOI] [PubMed] [Google Scholar]
- Chae H.S., Bach J.H., Lee M.W., Kim H.S., Kim Y.S., Kim K.Y.et al. (2001)Estrogen attenuates cell death induced by carboxy-terminal fragment of amyloid precursorprotein in PC12 through a receptor-dependent pathway,J Neurosci Res 65:403–407 [DOI] [PubMed] [Google Scholar]
- Chang D., Kwan J., Timiras P.S.(1997)Estrogens influence growth, maturation, and amyloid beta-peptide production in neuroblastoma cells and in a beta-APP transfected kidney 293 cell line,Adv Exp Med Biol 429:261–271 [DOI] [PubMed] [Google Scholar]
- Chen J., Delannoy M., Odwin S., He P., Trush M.A., Yager J.D.(2003)Enhanced mitochondrial gene transcript, ATP, bcl-2 protein levels, and altered glutathione distribution in ethinyl estradiol-treated cultured female rat hepatocytes,Toxicol Sci 75:271–278 [DOI] [PubMed] [Google Scholar]
- Chen J.Q., Eshete M., Alworth W.L., Yager J.D.(2004a)Binding of MCF-7 cell mitochondrial proteins and recombinant human estrogen receptors alpha and beta to human mitochondrial DNA estrogen response elements,J Cell Biochem 93:358–373 [DOI] [PubMed] [Google Scholar]
- Chen J.Q., Delannoy M., Cooke C., Yager J.D.(2004b)Mitochondrial localization of ERalpha and ERbeta in human MCF7 cells,Am J Physiol Endocrinol Metab 286:E1011–E1022 [DOI] [PubMed] [Google Scholar]
- Chen J.Q., Yager J.D.(2004)Estrogen's effects on mitochondrial gene expression: mechanisms and potential contributions to estrogen carcinogenesis,Ann N Y Acad Sci 1028:258–272 [DOI] [PubMed] [Google Scholar]
- Clemente C., Caruso M.G., Berloco P., Notarnicola M., D'Attoma B., Osella A.R.et al. (1999)Antioxidant effect of short-term hormonal treatment in postmenopausal women,Maturitas 31:137–142 [DOI] [PubMed] [Google Scholar]
- Crompton M., Moser R., Lüdi H., Carafoli E.(1978)The interrelations between the transport of sodium and calcium in mitochondria of various mammalian tissues,Eur J Biochem 82:25–31 [DOI] [PubMed] [Google Scholar]
- Corasaniti M.T., Amantea D., Russo R., Piccirilli S., Leta A., Corazzari M.et al. (2005)17beta-estradiol reduces neuronal apoptosis induced by HIV-1 gp120 in the neocortex of rat,Neurotoxicology 26:893–903 [DOI] [PubMed] [Google Scholar]
- Cruz J.C., Tsai L.H.(2004)A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease,Curr Opin Neurobiol 14:390–94 [DOI] [PubMed] [Google Scholar]
- Culmsee C., Vedder H., Ravati A., Junker V., Otto D., Ahlemeyer B.et al. (1999)Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: evidence for a receptor-independent antioxidative mechanism,J Cereb Blood Flow Metab 19:1263–1269 [DOI] [PubMed] [Google Scholar]
- Davis J.B., Maher P.(1994)Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line,Brain Res 652:169–173 [DOI] [PubMed] [Google Scholar]
- Deecher D.C., Daoud P., Bhat R.A., O'Connor L.T.(2005)Endogenously expressed estrogen receptors mediate neuroprotection in hippo-campal cells (HT22),J Cell Biochem 95:302–312 [DOI] [PubMed] [Google Scholar]
- De Girolamo L.A., Hargreaves A.J., Billett E.E.(2001)Protection from MPTP-induced neurotoxicity in differentiating mouse N2a neuroblastoma cells,J Neurochem 76:650–660 [DOI] [PubMed] [Google Scholar]
- DeKosky S.T., Abrahamson E.E., Ciallella J.R, Paljug W.R., Wisniewski S.R., Clark R. S. B.et al. (2007)Association of increased cortical soluble A42 levels with diffuse plaques after severe brain injury in humans,Arch Neurol 64:541–544 [DOI] [PubMed] [Google Scholar]
- Demonacos C.V., Karayanni N., Hatzoglou E., Tsiriyiotis C., Spandidos D.A., Sekeris C.E.(1996)Mitochondrial genes as sites of primary action of steroid hormones,Steroids 61:226–232 [DOI] [PubMed] [Google Scholar]
- Desmond D.W., Moroney J.T., Sano M., Stern Y.(2002)Incidence of dementia after ischemic stroke: results of a longitudinal study,Stroke 33:2254–2260 [DOI] [PubMed] [Google Scholar]
- DeVries A.C., Nelson R.J., Traystman R.J., Hurn P.D.(2001)Cognitive and behavioral assessment in experimental stroke research: will it prove useful?,Neurosci Biobehav Rev 25:325–342 [DOI] [PubMed] [Google Scholar]
- Dewar D., Graham D.I., Teasdale G.M., McCulloch J.(1993)Alz-50 and ubiquitin immu-noreactivity is induced by permanent focal cerebral ischaemia in the cat,Acta Neuropathol 86:623–629 [DOI] [PubMed] [Google Scholar]
- Dicko A., Morissette M., Ben Ameur S., Pézolet M., Di Paolo T.(1999)Effect of estradiol and tamoxifen on brain membranes: investigation by infrared and fluorescence spectroscopy,Brain Res Bull 49:401–405 [DOI] [PubMed] [Google Scholar]
- Disshon K.A., Dluzen D.E.(1997)Estrogen as a neuromodulator of MPTP-induced neurotoxicity: effects upon striatal dopamine release,Brain Res 764:9–16 [DOI] [PubMed] [Google Scholar]
- Dluzen D.E., McDermott J.L., Liu B.(1996)Estrogen alters MPTP-induced neurotoxicity in female mice: effects on striatal dopamine concentrations and release,J Neurochem 66:658–666 [DOI] [PubMed] [Google Scholar]
- Dluzen D., Horstink M.(2003)Estrogen as neuroprotectant of nigrostriatal dopaminergic system: laboratory and clinical studies,Endocrine 21:67–75 [DOI] [PubMed] [Google Scholar]
- Dubal D.B., Kashonm M.L., Pettigrewm L. C.J. M., Finklestein S.P., Rau S.W.et al. (1998)Estradiol protects against ischemic injury, J Cereb. Blood Flow Metab 18:1253–1258 [DOI] [PubMed] [Google Scholar]
- Dubal D.B., Shughrue P.J., Wilson M.E., Merchenthaler I., Wise P.M.(1999)Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors,J Neurosci 19:6385–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens J.A., Simpkins J.W., Wang J., Gordon K.(2003)Polycyclic phenols, estrogens and neuroprotection: a proposed mitochondrial mechanism,Exp Gerontol 38:101–107 [DOI] [PubMed] [Google Scholar]
- Espeland M.A., Rapp S.R., Shumaker S.A., Brunner R., Manson J.E., Sherwin B.B.et al. (2004)Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study,J Amer Med Assoc 291:2959–2968 [DOI] [PubMed] [Google Scholar]
- Feng Z., Zhang J.T.(2005)Long-term melatonin or 17beta-estradiol supplementation alleviates oxidative stress in ovariectomized adult rats,Free Radic Biol Med 39:195–204 [DOI] [PubMed] [Google Scholar]
- Fillit H., Weinreb H., Cholst I., Luine V., McEwen B., Amador R.et al. (1986)Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer's type,Psychoneuroendocrinology 11:337–345 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick J.L., Mize A.L., Wade C.B., Harris J.A., Shapiro R.A., Dorsa D.M.(2002)Estrogen-mediated neuroprotection against beta-amyloid toxi-city requires expressionof estrogen receptor alpha or beta and activation of the MAPK pathway,J Neurochem 82:674–682 [DOI] [PubMed] [Google Scholar]
- Fukuda K., Yao H., Ibayashi S., Nakahara T., Uchimura H., Fujishima M.et al. (2000)Ovariectomy exacerbates and estrogen replacement attenuates photothrombotic focal ischemic brain injury in rats,Stroke 31:155–160 [DOI] [PubMed] [Google Scholar]
- Gagné B., Gélinas S., Bureau G., Lagacé B., Ramassamy C., Chiasson K.et al. (2003)Effects of estradiol, phytoestrogens, and Ginkgo biloba extracts against 1-methyl-4-phenylpyridine-induced oxidative stress,Endocrine 21:89–95 [DOI] [PubMed] [Google Scholar]
- Gélinas S., Bureau G., Valastro B., Massicotte G., Cicchetti F., Chiasson K.et al. (2004)Alpha and beta estradiol protect neuronal but not native PC12 cells from paraquat-induced oxidative stress,Neurotox Res 6:141–148 [DOI] [PubMed] [Google Scholar]
- Gollapudi L., Oblinger M.M.(1999)Stable transfection of PC12 cells with estrogen receptor (ERalpha): protective effects of estrogen on cell survival after serum deprivation,J Neurosci Res 56:99–108 [DOI] [PubMed] [Google Scholar]
- Goodman Y., Bruce A.J., Cheng B., Mattson M.P.(1996)Estrogens attenuate and corti-costerone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons,J Neurochem 66:1836–1844 [DOI] [PubMed] [Google Scholar]
- Green P.S., Gridley K.E., Simpkins J.W.(1996)Estradiol protects against beta-amyloid (25-35)-induced toxicity in SK-N-SH human neuroblastoma cells,Neurosci Lett 218:165–168 [DOI] [PubMed] [Google Scholar]
- Green P.S., Bishop J., Simpkins J.W.(1997a)17 alpha-estradiol exerts neuroprotective effects on SK-N-SH cells,J Neurosci 17:511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P.S., Gordon K., Simpkins J.W.(1997b)Phenolic A ring requirement for the neuroprotective effects of steroids,J Steroid Biochem Mol Biol 63:229–235 [DOI] [PubMed] [Google Scholar]
- Green P.S., Gridley K.E., Simpkins J.W.(1998)Nuclear estrogen receptor-independent neuroprotection by estratrienes: a novel interaction with glutathione,Neuroscience 84:7–10 [DOI] [PubMed] [Google Scholar]
- Green P.S., Bales K., Paul S., Bu G.(2005)Estrogen therapy fails to alter amyloid deposition in the PDAPP model of Alzheimer's disease,Endocrinology 146:2774–2781 [DOI] [PubMed] [Google Scholar]
- Green P.S., Yang S.H., Nilsson K.R., Kumar A.S., Covey D.F., Simpkins J.W.(2001)The non-feminizing enantiomer of 17beta-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia,Endocrinology 142:400–6 [DOI] [PubMed] [Google Scholar]
- Greenfield J.P., Leung L.W., Cai D., Kaasik K., Gross R.S., Rodriguez-Boulan E.et al. (2002)Estrogen lowers Alzheimer beta-amyloid generation by stimulating trans-Golgi network vesicle biogenesis,J Biol Chem 277:12128–12136 [DOI] [PubMed] [Google Scholar]
- Gridley K.E., Green P.S., Simpkins J.W.(1998)A novel, synergistic interaction between 17 beta-estradiol and glutathione in the protection of neurons against beta-amyloid 25-35-induced toxicity in vitro,Mol Pharmacol 54:874–880 [DOI] [PubMed] [Google Scholar]
- Harman S.M.(2006)Estrogen replacement in menopausal women: recent and current prospective studies, the WHI and the KEEPS,Gend Med 3:254–269 [DOI] [PubMed] [Google Scholar]
- Hawk T., Zhang Y.Q., Rajakumar G., Day A.L., Simpkins J.W.(1998)Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats,Brain Res 796:296–298 [DOI] [PubMed] [Google Scholar]
- Hawkins V., Shen Q., Chiueh C.C.(1999)Kynostatin and 17beta-estradiol prevent the apoptotic death of human neuroblastoma cells exposed to HIV-1 protease,J Biomed Sci 6:433–438 [DOI] [PubMed] [Google Scholar]
- He Z., He Y.J., Day A.L., Simpkins J.W.(2002)Proestrus levels of estradiol during transient global cerebral ischemia improves the histological outcome of the hippocampal CA1 region: perfusion-dependent and-independent mechanisms,J Neurol Sci 193:79–87 [DOI] [PubMed] [Google Scholar]
- Henderson V.W., Stanford C.A, Espeland M.A., Hogan P.E., Rapp S.R., Stefanick M.L.et al. (2007)Prior use of hormone therapy and incident Alzheimer's disease in the Women's Health Initiative Memory Study. American Academy of Neurology 59th Annual Meeting: Abstract S31.004.
- Henon H., Durieu I., Guerouaou D., Lebert F., Pasquier F., Leys D.(2001)Poststroke dementia: incidence and relationship to prestroke cognitive decline,Neurology 57:1216–1222 [DOI] [PubMed] [Google Scholar]
- Honda K., Sawada H., Kihara T., Urushitani M., Nakamizo T., Akaike A.et al. (2000)Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons,J Neurosci Res 60:321–327 [DOI] [PubMed] [Google Scholar]
- Honda K., Shimohama S., Sawada H., Kihara T., Nakamizo T., Shibasaki H.et al. (2001)Nongenomic antiapoptotic signal transduction by estrogen in cultured cortical neurons,J Neurosci Res 64:466–475 [DOI] [PubMed] [Google Scholar]
- Horvat A., Nikezi G., Petrovi S., Kanazir D.T.(2001)Binding of estradiol to synaptosomal mitochondria: physiological significance,Cell Mol Life Sci 58:636–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat A., Petrovi S., Nedeljkovi N., Martinovi J.V., Nikezi G.(2000)Estradiol affect Na-dependent Ca2+ efflux from synaptosomal mitochondria,Gen Physiol Biophys 19:59–71 [PubMed] [Google Scholar]
- Hosoda T., Nakajima H., Honjo H.(2001)Estrogen protects neuronal cells from amyloid beta-induced apoptotic cell death,Neuroreport 12:1965–1970 [DOI] [PubMed] [Google Scholar]
- Howard S.A., Brooke S.M., Sapolsky R.M.(2001)Mechanisms of estrogenic protection against gp120-induced neurotoxicity,Exp Neurol 168:385–391 [DOI] [PubMed] [Google Scholar]
- Howell A., DeFriend D.J., Robertson J.F., Blamey R.W., Anderson L.et al. (1996)Pharmacokinetics, pharmacological and anti-tumour effects of the specific anti-oestrogen ICI 182780 in women with advanced breast cancer,Br J Cancer 74:300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Akiyama H., Arai T., Kondo H., Haga C., Tsuchiya K.et al. (2000)Neurons containing Alz-50-immunoreactive granules around the cerebral infarction: evidence for the lysosomal degradation of altered tau in human brain?,Neurosci Lett 284:187–189 [DOI] [PubMed] [Google Scholar]
- Ikonomovic M.D., Uryu K., Abrahamson E.E., Ciallella J.R., Trojanowski J.Q., Lee V. M.-Y.et al. (2004)Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury,Exp Neurol 190:192–203 [DOI] [PubMed] [Google Scholar]
- Jaffe A.B., Toran-Allerand C.D., Greengard P., Gandy S.E.(1994)Estrogen regulates metabolism of Alzheimer amyloid beta precursor protein,J Biol Chem 269:13065–13068 [PubMed] [Google Scholar]
- Jellinger K.A., Paulus W., Wrocklage C., Litvan I.(2001)Effects of closed traumatic brain injury and genetic factors on the development of Alzheimer's disease,Eur J Neurol 8:707–710 [DOI] [PubMed] [Google Scholar]
- Jung M.E., Rewal M., Perez E., Wen Y., Simpkins J.W.(2004)Estrogen protects against brain lipid peroxidation in ethanol-withdrawn rats,Pharmacol Biochem Behav 79:573–586 [DOI] [PubMed] [Google Scholar]
- Jung M.E., Wilson A.M., Simpkins J.W.(2006)A nonfeminizing estrogen analog protects against ethanol withdrawal toxicity in immortalized hippo-campal cells,J Pharmacol Exp Ther 319:543–550 [DOI] [PubMed] [Google Scholar]
- Karhunen H., Pitkanen A., Virtanen T., Gureviciene I., Pussinen R., Ylinen A.et al. (2003)Long-term functional consequences of transient occlusion of the middle cerebral artery in rats: a 1-year follow-up of the development of epileptogenesis and memory impairment in relation to sensorimotor deficits,Epilepsy Res 54:1–10 [DOI] [PubMed] [Google Scholar]
- Kajta M., Budziszewska B., Marsza M., Laso W.(2001)Effects of 17-beta estradiol and estriol on NMDA-induced toxicity and apoptosis in primary cultures of rat cortical neurons,J Physiol Pharmacol 52:437–446 [PubMed] [Google Scholar]
- Kawas C., Resnick S., Morrison A., Brookmeyer R., Corrada M., Zonderman A.et al. (1997)A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: the Baltimore Longitudinal Study of Aging,Neurology 48:1517–1521 [DOI] [PubMed] [Google Scholar]
- Kenchappa R.S., Diwakar L., Annepu J., Ravindranath V.(2004)Estrogen and neuroprotection: higher constitutive expression of glutaredoxin in female mice offers protection against MPTP-mediated neurodegeneration,FASEB J 18:1102–1104 [DOI] [PubMed] [Google Scholar]
- Kendall S.L., Anderson C.F., Nath A., Turchan-Cholewo J., Land C.L., Mactutus C.F.et al. (2005)Gonadal steroids differentially modulate neurotoxicity of HIV and cocaine: testosterone and ICI 182,780 sensitive mechanism,BMC Neurosci 6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavapany S., Li B.S., Amin N., Zheng Y.L., Grant P., Pant H.C.(2004)Neuronal cyclin-dependent kinase 5: role in nervous system function and its specific inhibition by the Cdk5 inhibitory peptide,Biochim Biophys Acta 1697:143–153 [DOI] [PubMed] [Google Scholar]
- Kim H., Bang O.Y., Jung M.W., Ha S.D., Hong H.S., Huh K.et al. (2001)Neuroprotective effects of estrogen against beta-amyloid toxicity are mediated by estrogen receptors in cultured neuronal cells,Neurosci Lett 302:58–62 [DOI] [PubMed] [Google Scholar]
- Kokmen E., Whisnant J.P., O'Fallon W.M., Chu C.P., Beard C.M.(1996)Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960-1984),Neurology 46:154–159 [DOI] [PubMed] [Google Scholar]
- Kumar D.M., Perez E., Cai Z.Y., Aoun P., Brun-Zinkernagel A.M., Covey D.F.et al. (2005)Role of nonfeminizing estrogen analogues in neuroprotection of rat retinal ganglion cells against glutamate-induced cytotoxicity,Free Radic Biol Med 38:1152–1163 [DOI] [PubMed] [Google Scholar]
- Kuroki Y., Fukushima K., Kanda Y., Mizuno K., Watanabe Y.(2001)Neuroprotection by estrogen via extracellular signal-regulated kinase against quino-linic acid-induced cell death in the rat hippocampus,Eur J Neurosci 13:472–476 [DOI] [PubMed] [Google Scholar]
- Law S.W., Apostolakis E.M., Samora P.J., O'Malley B.W., Clark J.H.(1994)Hormonal regulation of hypothalamic gene expression: identification of multiple novel estrogen induced genes,J Steroid Biochem Mol Biol 51:131–136 [DOI] [PubMed] [Google Scholar]
- Lei D.L., Long J.M., Hengemihle J., O'Neill J., Manaye K.F., Ingram D.K., Mouton P.R.(2003)Effects of estrogen and raloxifene on neuroglia number and morphology in the hippocampus of aged female mice,Neuroscience 121:659–666 [DOI] [PubMed] [Google Scholar]
- Levin-Allerhand J.A., Lominska C.E., Wang J., Smith J.D.(2002)17Alpha-estradiol and 17beta-estradiol treatments are effective in lowering cerebral amyloid-beta levels in AbetaPPSWE transgenic mice,J Alzheimers Dis 4:449–457 [DOI] [PubMed] [Google Scholar]
- Li R., Shen Y., Yang L.B., Lue L.F., Finch C., Rogers J.(2000)Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex,J Neurochem 75:1447–1454 [DOI] [PubMed] [Google Scholar]
- Liang Y., Belford S., Tang F., Prokai L., Simpkins J.W., Hughes J.A.(2001)Membrane fluidity effects of estratrienes,Brain Res Bull 54:661–668 [DOI] [PubMed] [Google Scholar]
- Liu R., Yang S.H., Perez E., Yi K.D., Wu S.S., Eberst K.et al. (2002)Neuroprotective effects of a novel non-receptor-binding estrogen analogue: in vitro and in vivo analysis,Stroke 33:2485–2491 [DOI] [PubMed] [Google Scholar]
- Liu X., Fan X.L., Zhao Y., Luo G.R., Li X.P., Li R.et al. (2005)Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-alpha and estrogen receptor-beta in microglia,J Neurosci Res 81:653–665 [DOI] [PubMed] [Google Scholar]
- Loeb C., Gandolfo C., Croce R., Conti M.(1992)Dementia associated with lacunar infarction,Stroke 23:1225–1229 [DOI] [PubMed] [Google Scholar]
- Maki P.M., Zonderman A.B., Resnick S.M.(2001)Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy,Am J Psychiatry 158:227–233 [DOI] [PubMed] [Google Scholar]
- Manson J.E., Hsia J., Johnson K.C., Rossouw J.E., Assaf A.R.et al. (2003)Estrogen plus progestin and the risk of coronary heart disease,N Eng J Med 349:523–534 [DOI] [PubMed] [Google Scholar]
- Markgraf C.G., Green E.J., Hurwitz B.E., Morikawa E., Dietrich W.D.et al. (1992)Sensorimotor and cognitive consequences of middle cerebral artery occlusion in rats,Brain Res 575:238–246 [DOI] [PubMed] [Google Scholar]
- Markgraf C.G., Green E.J., Watson B., McCabe P.M., Schneiderman N., Dietrich W.D.et al. (1994)Recovery of sensorimotor function after distal middle cerebral artery photothrombotic occlusion in rats,Stroke 25:153–159 [DOI] [PubMed] [Google Scholar]
- Markgraf C.G., Johnson M.P., Braun D.L., Bickers M.V.(1997)Behavioral recovery patterns in rats receiving the NMDA receptor antagonist MDL 100,453 immediately post-stroke,Pharmacol Biochem Behav 56:391–397 [DOI] [PubMed] [Google Scholar]
- Martín C., Barturen K., Martínez R., Lacort M., Ruiz-Larrea M.B.(1998)In vitro inhibition by estrogens of the oxidative modifications of human lipoproteins,J Physiol Biochem 54:195–202 [PubMed] [Google Scholar]
- Mattson M.P., Robinson N., Guo Q.(1997)Estrogens stabilize mitochondrial function and protect neural cells against the pro-apoptotic action of mutant presenilin-1,Neuroreport 8:3817–3821 [DOI] [PubMed] [Google Scholar]
- McManus J., McEneny J., Young I.S., Thompson W.(1996)The effect of various oestrogens and progestogens on the susceptibility of low density lipoproteins to oxidation in vitro,Maturitas 25:125–131 [DOI] [PubMed] [Google Scholar]
- Mendelowitsch A., Ritz M.F., Ros J., Langemann H., Gratzl O.(2001)17beta-Estradiol reduces cortical lesion size in the glutamate excitotoxicity model by enhancing extracellular lactate: a new neuroprotective pathway,Brain Res 901:230–236 [DOI] [PubMed] [Google Scholar]
- Mérot Y., Ferrière F., Debroas E., Flouriot G., Duval D., Saligaut C.(2005)Estrogen receptor alpha mediates neuronal differentiation and neuroprotection in PC12 cells: critical role of the A/B domain of the receptor,J Mol Endocrinol 35:257–267 [DOI] [PubMed] [Google Scholar]
- Miller C.P., Jirkovsky I., Hayhurst D.A., Adelman S.J.(1996)In vitro antioxidant effects of estrogens with a hindered 3-OH function on the copper induced oxidation of low density lipoprotein,Steroids 61:305–308 [DOI] [PubMed] [Google Scholar]
- Mitsios N., Pennucci R., Krupinski J., Sanfeliu C., Gaffney J., Kumar P.et al. (2007)Expression of cyclin-dependent kinase 5 mRNA and protein in the human brain following acute ischemic stroke,Brain Pathol 17:11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco E.A., 3rd, Vallano M.L.(2005)Role of protein kinases in neurodegenerative disease: cyclin-dependent kinases in Alzheimer's disease,Front Biosci 10:143–159 [DOI] [PubMed] [Google Scholar]
- Mook-Jung I., Joo I., Sohn S., Kwon H.J., Huh K., Jung M.W.(1997)Estrogen blocks neurotoxic effects of beta-amyloid (1-42) and induces neurite extension on B103 cells,Neurosci Lett 235:101–104 [DOI] [PubMed] [Google Scholar]
- Moosmann B., Behl C.(1999)The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties,Proc Natl Acad Sci U S A 96:8867–8872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga A., Hirohata M., Ono K., Yamada M.(2007)Estrogen has anti-amyloidogenic effects on Alzheimer's beta-amyloid fibrils in vitro,Biochem Biophys Res Commun 359:697–702 [DOI] [PubMed] [Google Scholar]
- Morkuniene R., Jekabsone A., Borutaite V.(2002)Estrogens prevent calcium-induced release of cytochrome c from heart mitochondria,FEBS Lett 521:53–56 [DOI] [PubMed] [Google Scholar]
- Mulnard R.A., Cotman C.W., Kawas C., van Dyck C.H., Sano M., Doody R.et al. (2000)Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer's Disease Cooperative Study, J Amer Med Assoc 283:1007–1015 [DOI] [PubMed] [Google Scholar]
- Nakamizo T., Urushitani M., Inoue R., Shinohara A., Sawada H., Honda K.et al. (2000)Protection of cultured spinal motor neurons by estra-diol,Neuroreport 11:3493–3497 [DOI] [PubMed] [Google Scholar]
- Nilsen J., Brinton R.D.(2004)Mitochondria as therapeutic targets of estrogen action in the central nervous system,Curr Drug Targets CNS Neurol Disord 3:297–313 [DOI] [PubMed] [Google Scholar]
- Nilsen J., Brinton R.D.(2003)Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression,Proc Natl Acad Sci USA 100:2842–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J., Irwin R.W., Gallaher T.K., Brinton R.D.(2007)Estradiol in vivo regulation of brain mitochon-drial proteome,J Neurosci. 27:14069–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noteboom W.D., Gorski J.(1965)Stereospecific binding of estrogens in the rat uterus,Arch Biochem Biophys 111:559–568 [DOI] [PubMed] [Google Scholar]
- Ohkura T., Isse K., Akazawa K., Hamamoto M., Yaoi Y., Hagino N.(1994)Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type,Endocr J 41:361–371 [DOI] [PubMed] [Google Scholar]
- Olivieri G., Novakovic M., Savaskan E., Meier F., Baysang G., Brockhaus M.et al. (2002)The effects of beta-estradiol on SHSY5Y neuroblastoma cells during heavy metal induced oxidative stress, neurotoxicity and beta-amyloid secretion,Neuroscience 113:849–855 [DOI] [PubMed] [Google Scholar]
- Olsson A., Csajbok L., Ost M., Höglund K., Nylén K., Rosengren L., Nellga°rd B., Blennow K.(2004)Marked increase of beta-amyloid(1-42) and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injury,J Neurol 251:870–876 [DOI] [PubMed] [Google Scholar]
- Paganini-4/23/2010Hill A., Henderson V.(1994)Estrogen deficiency and risk of Alzheimer's disease in women,Am J Epidemiol 140:256–261 [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A., Henderson V.W.(1996)Estrogen replacement therapy and risk of Alzheimer disease,Arch Intern Med 156:2213–2217 [PubMed] [Google Scholar]
- Park O.J.(2001)Comparison of estrogen and genistein in their antigenotoxic effects, apoptosis and signal transduction protein expression patterns,Biofactors 21:379–382 [DOI] [PubMed] [Google Scholar]
- Pasquier F., Leys D.(1997)Why are stroke patients prone to develop dementia?,J Neurol 244:135–142 [DOI] [PubMed] [Google Scholar]
- Perez E., Liu R., Yang S.H., Cai Z.Y., Covey D.F., Simpkins J.W.(2005)Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo,Brain Res 1038:216–222 [DOI] [PubMed] [Google Scholar]
- Perez E., Cai Z.Y., Covey D.F., Simpkins J.W.(2006)Neuroprotective effects of estratriene analogs: structure-activity relationships and molecular optimization,Drug Development Research 68:78–92 [Google Scholar]
- Petanceska S.S., Nagy V., Frail D., Gandy S.(2000)Ovariectomy and 17beta-estradiol modulate the levels of Alzheimer's amyloid beta peptides in brain,Neurology 54:2212–2217 [DOI] [PubMed] [Google Scholar]
- Petrovic S., Demajo M., Horvat A.(2005)Estradiol affects calcium transport across mitochon-drial membrane in different brain regions,Ann N Y Acad Sci 1048:341–343 [DOI] [PubMed] [Google Scholar]
- Phillips S.M., Sherwin B.B.(1992)Effects of estrogen on memory function in surgically menopausal women,Psychoneuroendocrinology 17:485–495 [DOI] [PubMed] [Google Scholar]
- Pohjasvaara T., Mantyla R., Ylikoski R., Kaste M., Erkinjuntti T.(2000)Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l'Enseignement en Neurosciences, Stroke 31:2952–2957 [DOI] [PubMed] [Google Scholar]
- Prokai L., Prokai-Tatrai K., Perjesi P., Zharikova A.D., Perez E.J., Liu R., Simpkins J.W.(2003)Quinol-based cyclic antioxidant mechanism in estrogen neuroprotection,Proc Natl Acad Sci U S A 100:11741–11746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp S.R., Espeland M.A., Shumaker S.A., Henderson V.W., Brunner R.L., Manson J.E.et al. (2003)Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial,J Amer Med Assoc 289:2663–2672 [DOI] [PubMed] [Google Scholar]
- Regan R.F., Guo Y.(1997)Estrogens attenuate neuronal injury due to hemoglobin, chemical hypoxia, and excitatory amino acids in murine cortical cultures,Brain Res 764:133–140 [DOI] [PubMed] [Google Scholar]
- Resnick S.M., Maki P.M.(2001)Effects of hormone replacement therapy on cognitive and brain aging,Ann N Y Acad Sci 949:203–214 [DOI] [PubMed] [Google Scholar]
- Resnick S.M., Maki P.M., Golski S., Kraut M.A., Zonderman A.B.(1998)Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance,Horm Behav 34:171–182 [DOI] [PubMed] [Google Scholar]
- Roman G.C.(2002)Vascular dementia may be the most common form of dementia in the elderly,J Neurol Sci 203-204:7–10 [DOI] [PubMed] [Google Scholar]
- Roman G.C., Erkinjuntti T., Wallin A., Pantoni L., Chui H.C.(2002)Subcortical ischaemic vascular dementia,Lancet Neurol 1:426–436 [DOI] [PubMed] [Google Scholar]
- Rämer W., Oettel M., Menzenbach B., Droescher P., Schwarz S.(1997)Novel estrogens and their radical scavenging effects, iron-chelating, and total antioxidative activities: 17 alpha-substituted analogs of delta 9(11)-dehydro-17 beta-estradiol,Steroids 62:688–694 [DOI] [PubMed] [Google Scholar]
- Roof R.L., Schielke G.P., Ren X., Hall E.D.(2001)A comparison of long-term functional outcome after 2 middle cerebral artery occlusion models in rats,Stroke 32:2648–2657 [DOI] [PubMed] [Google Scholar]
- Ruiz-Larrea M.B., Leal A.M., Liza M., Lacort M., de Groot H.(1994)Antioxidant effects of estra-diol and 2-hydroxyestradiol on iron-induced lipid per-oxidation of rat liver microsomes,Steroids 59:383–388 [DOI] [PubMed] [Google Scholar]
- Ruiz-Larrea B., Leal A., Martín C., Martínez R., Lacort M.(1995)Effects of estrogens on the redox chemistry of iron: a possible mechanism of the antioxidant action of estrogens,Steroids 60:780–783 [DOI] [PubMed] [Google Scholar]
- Russo R., Navarra M., Maiuolo J., Rotiroti D., Bagetta G., Corasaniti M.T.(2005)17beta-estradiol protects SH-SY5Y Cells against HIV-1 gp120-induced cell death: evidence for a role of estrogen receptors,Neurotoxicology 26:905–13 [DOI] [PubMed] [Google Scholar]
- Sack M.N., Rader D.J., Cannon R.O.(1994)Oestrogen and inhibition of oxidation of low-density lipoproteins in postmenopausal women,Lancet 343:269–270 [DOI] [PubMed] [Google Scholar]
- Sagara Y.(1998)Induction of reactive oxygen species in neurons by haloperidol,J Neurochem 71:1002–1012 [DOI] [PubMed] [Google Scholar]
- Samuelsson M., Soderfeldt B., Olsson G.B.(1996)Functional outcome in patients with lacunar infarction,Stroke 27:842–846 [DOI] [PubMed] [Google Scholar]
- Sasaki K., Sato M., Umezawa Y.(2003)Fluorescent indicators for Akt/protein kinase B and dynamics of Akt activity visualized in living cells,J Biol Chem 278:30945–30951 [DOI] [PubMed] [Google Scholar]
- Sawada H., Ibi M., Kihara T., Urushitani M., Akaike A., Shimohama S.(1998)Estradiol protects mesencephalic dopaminergic neurons from oxidative stress-induced neuronal death,J Neurosci Res 54:707–719 [DOI] [PubMed] [Google Scholar]
- Sawada H., Ibi M., Kihara T., Urushitani M., Honda K., Nakanishi M.et al. (2000)Mechanisms of antiapoptotic effects of estrogens in nigral dopami-nergic neurons,FASEB J 14:1202–1214 [DOI] [PubMed] [Google Scholar]
- Schmidt A.J., Krieg J.C., Vedder H.(2002)Differential effects of glucocorticoids and gonadal steroids on glutathione levels in neuronal and glial cell systems,J Neurosci Res 67:544–550 [DOI] [PubMed] [Google Scholar]
- Schneider L.S., Farlow M.R., Henderson V.W., Pogoda J.M.(1996)Effects of estrogen replacement therapy on response to tacrine in patients with Alzheimer's disease,Neurology 46:1580–1584 [DOI] [PubMed] [Google Scholar]
- Schwenke D.C., Wagner J.D., Adams M.R.(1999)In vitro lipid peroxidation of LDL from post-menopausal cynomolgus macaques treated with female hormones,J Lipid Res 40:235–244 [PubMed] [Google Scholar]
- Sherwin B.B.(1997)Estrogen effects on cognition in menopausal women,Neurology 48:S21–S26 [DOI] [PubMed] [Google Scholar]
- Shi J., Panickar K.S., Yang S.H., Rabbani O., Day A.L., Simpkins J.W.(1998)Estrogen attenuates over-expression of beta-amyloid precursor protein messager RNA in an animal model of focal ischemia,Brain Res 810:87–92 [DOI] [PubMed] [Google Scholar]
- Shoulson I.(1998)Experimental therapeutics of neurodegenerative disorders: unmet needs,Science 282:1072–1074 [DOI] [PubMed] [Google Scholar]
- Shughrue P.J.(2004)Estrogen attenuates the MPTP-induced loss of dopamine neurons from the mouse SNc despite a lack of estrogen receptors (ERalpha and ERbeta),Exp Neurol. 190:468–77 [DOI] [PubMed] [Google Scholar]
- Shumaker S.A., Legault C., Kuller L., Rapp S.R., Thal L., Lane D.S.et al. (2004)Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study,J Amer Med Assoc 291:2947–2958 [DOI] [PubMed] [Google Scholar]
- Shumaker S.A., Legault C., Rapp S.R., Thal L., Wallace R.B., Ockene J.K.et al. (2003)Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial,J Amer Med Assoc 289:2651–2662 [DOI] [PubMed] [Google Scholar]
- Sierens J., Hartley J.A., Campbellm M.J., Leathem A.J., Woodside J.V.(2001)Effect of phytoestrogen and antioxidant supplementation on oxidative DNA damage assessed using the comet assay,Mutat Res 485:169–176 [DOI] [PubMed] [Google Scholar]
- Simpkins J.W., Rajakumar G., Zhang Y.Q., Simpkins C.E., Greenwald D., Yu C.J.et al. (1997)Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat,J Neurosurg 87:724–730 [DOI] [PubMed] [Google Scholar]
- Simpkins J.W., Yang S.H., Liu R., Perez E., Cai Z.Y., Covey D.F.et al. (2004)Estrogen-like compounds for ischemic neuroprotection,Stroke 35(11 Suppl. 1):2648–2651 [DOI] [PubMed] [Google Scholar]
- Simpkins J.W., Wang J., Wang X., Perez E., Prokai L., Dykens J.A.(2005a)Mitochondria play a central role in estrogen-induced neuroprotection,Curr Drug Targets CNS Neurol Disord 4:69–83 [DOI] [PubMed] [Google Scholar]
- Simpkins J.W., Wen Y., Perez E., Yang S., Wang X.(2005b)Role of nonfeminizing estrogens in brain protection from cerebral ischemia: an animal model of Alzheimer's disease neuropathology,Ann N Y Acad Sci 1052:233–242 [DOI] [PubMed] [Google Scholar]
- Simpkins J.W., Yang S.H., Wen Y., Singh M.(2005c)Estrogens, progestins, menopause and neu-rodegeneration: basic and clinical studies,Cell Mol Life Sci 62:271–280 [DOI] [PubMed] [Google Scholar]
- Singer C.A., Rogers K.L., Dorsa D.M.(1998)Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons,Neuroreport 9:2565–2568 [DOI] [PubMed] [Google Scholar]
- Singh M., Simpkins J.W.(2005)The Future of Hormone Therapy: What Basic Science and Clinical Studies Teach Us.New York Academy of Science, New York [Google Scholar]
- Singh M., Sumien N., Kyser C., Simpkins J.W.(2008)Estrogens and progesterone as neuroprotectants: what animal models teach us,Front Biosci 13:1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.E., Hodges H., Sowinski P., Man C.M., Leach M.J., Sinden J.D.et al. (1997)Long-term beneficial effects of BW619C89 on neurological deficit, cognitive deficit and brain damage after middle cerebral artery occlusion in the rat,Neuroscience 77:1123–1135 [DOI] [PubMed] [Google Scholar]
- Solakidi S., Psarra A.M., Nikolaropoulos S., Sekeris C.E.(2005)Estrogen receptors alpha and beta (ERalpha and ERbeta) and androgen receptor (AR) in human sperm: localization of ERbeta and AR in mitochondria of the midpiece,Hum Reprod 20:3481–87 [DOI] [PubMed] [Google Scholar]
- Sortino M.A., Chisari M., Merlo S., Vancheri C., Caruso M., Nicoletti F.(2004)Glia mediates the neuroprotective action of estradiol on beta-amyloid-induced neuronal death,Endocrinology 145:5080–5086 [DOI] [PubMed] [Google Scholar]
- Sribnick E.A., Ray S.K., Nowak M.W., Li L., Banik N.L.(2004)17beta-estradiol attenuates gluta-mate-induced apoptosis and preserves electrophysio-logic function in primary cortical neurons,J Neurosci Res 76:688–696 [DOI] [PubMed] [Google Scholar]
- Stirone C., Duckles S.P., Krausem D.N., Procaccio V.(2005)Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels,Mol Pharmacol 68:959–965 [DOI] [PubMed] [Google Scholar]
- Stroemer R.P., Kent T.A., Hulsebosch C.E.(1998)Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats,Stroke 29:2381–2393 [DOI] [PubMed] [Google Scholar]
- Sudo S., Wen T.C., Desaki J., Matsuda S., Tanaka J., Arai T.et al. (1997)Beta-estradiol protects hippocampal CA1 neurons against transient forebrain ischemia in gerbil,Neurosci Res 29:345–354 [DOI] [PubMed] [Google Scholar]
- Sur P., Sribnick E.A., Wingrave J.M., Nowak M.W., Ray S.K., Banik N.L.(2003)Estrogen attenuates oxidative stress-induced apoptosis in C6 glial cells,Brain Res 971:178–188 [DOI] [PubMed] [Google Scholar]
- Takao T., Flint N., Lee L., Ying X., Merrill J., Chandross K.J.(2004)17beta-estradiol protects oli-godendrocytes from cytotoxicity induced cell death,J Neurochem 89:660–673 [DOI] [PubMed] [Google Scholar]
- Tamura A., Graham D.I., McCulloch J., Teasdale G.M.(1981)Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion, J Cereb Blood Flow Metab 1:53–60 [DOI] [PubMed] [Google Scholar]
- Tang M., Abplanalp W., Ayres S., Subbiah M.T.(1996a)Superior and distinct antioxidant effects of selected estrogen metabolites on lipid peroxidation,Metabolism 45:411–414 [DOI] [PubMed] [Google Scholar]
- Tang M.X., Jacobs D., Stern Y., Marder K., Schofield P., Gurland B.et al. (1996b)Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease,Lancet 348:429–432 [DOI] [PubMed] [Google Scholar]
- Tatemichi T.K., Foulkes M.A., Mohr J.P., Hewitt J.R., Hier D.B., Price T.R.et al. (1990)Dementia in stroke survivors in the Stroke Data Bank cohort. Prevalence, incidence, risk factors, and computed tomographic findings, Stroke 21:858–866 [DOI] [PubMed] [Google Scholar]
- Tatemichi T.K., Paik M., Bagiella E., Desmond D.W., Stern Y., Sano M.et al. (1994)Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study,Neurology 44:1885–1891 [DOI] [PubMed] [Google Scholar]
- Telci A., Cakatay U., Akhan S.E., Bilgin M.E., Turfanda A., Sivas A.(2002)Postmenopausal hormone replacement therapy use decreases oxidative protein damage,Gynecol Obstet Invest 54:88–93 [DOI] [PubMed] [Google Scholar]
- Thibodeau P.A., Kachadourian R., Lemay R., Bisson M., Day B.J., Paquette B.(2002)In vitro pro- and antioxidant properties of estrogens,J Steroid Biochem Mol Biol 81:227–236 [DOI] [PubMed] [Google Scholar]
- Toung T.J., Traystman R.J., Hurn P.D.(1998)Estrogen-mediated neuroprotection after experimental stroke in male rats,Stroke 29:1666–1670 [DOI] [PubMed] [Google Scholar]
- Toung T.K., Hurn P.D., Traystman R.J., Sieber F.E.(2000)Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus,Stroke 31:2701–2706 [DOI] [PubMed] [Google Scholar]
- Turchan J., Anderson C., Hauser K.F., Sun Q., Zhang J., Liu Y., Wise P.M., Kruman I., Maragos W., Mattson M.P., Booze R., Nath A.(2001)Estrogen protects against the synergistic toxi-city by HIV proteins, methamphetamine and cocaine,BMC Neurosci 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel C., Thornton E., Vink R.(2007)Traumatic brain injury and Alzheimer's disease: a review,Prog Brain Res 161:303–316 [DOI] [PubMed] [Google Scholar]
- Van Itallie C.M., Dannies P.S.(1998)Estrogen induces accumulation of the mitochondrial ribonucleic acid for subunit II of cytochrome oxidase in pituitary tumor cells,Mol Endocrinol 2:332–337 [DOI] [PubMed] [Google Scholar]
- Vassar R.(2007)Caspase-3 cleavage of GGA3 stabilizes BACE: implications for Alzheimer's disease,Neuron 54:671–673 [DOI] [PubMed] [Google Scholar]
- Vedder H., Anthes N., Stumm G., Würz C, Behl C, Krieg J.C.(1999)Estrogen hormones reduce lipid peroxidation in cells and tissues of the central nervous system,J Neurochem 72:2531–38 [DOI] [PubMed] [Google Scholar]
- Vedder H., Teepker M., Fischer S., Krieg J.C.(2000)Characterization of the neuroprotective effects of estrogens on hydrogen peroxide-induced cell death in hippocampal HT22 cells: time and dose-dependency,Exp Clin Endocrinol Diabetes 108:120–127 [DOI] [PubMed] [Google Scholar]
- Vegeto E., Bonincontro C., Pollio G., Sala A., Viappiani S., Nardi F.et al. (2001)Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia,J Neurosci 21:1809–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D.R., Dodson S., Nath A., Booze R.M.(2006)Estrogen attenuates gp120- and tat1-72-induced oxidative stress and prevents loss of dopamine transporter function,Synapse 59:51–60 [DOI] [PubMed] [Google Scholar]
- Wang X., Simpkins J.W., Dykens J.A., Cammarata P.R.(2003)Oxidative damage to human lens epithelial cells in culture: estrogen protection of mitochondrial potential, ATP, and cell viability,Invest Ophthalmol Vis Sci 44:2067–2075 [DOI] [PubMed] [Google Scholar]
- Wang J., Green P.S., Simpkins J.W.(2001)Estradiol protects against ATP depletion, mitochon-drial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SKN- SH human neuroblastoma cells,J Neurochem 77:804–811 [DOI] [PubMed] [Google Scholar]
- Wen Y., Onyewuchi O., Yang S., Liu R., Simpkins J.W.(2004a)Increased beta-secretase activity and expression in rats following transient cerebral ischemia,Brain Res 1009:1–8 [DOI] [PubMed] [Google Scholar]
- Wen Y., Yang S., Liu R., Simpkins J.W.(2004b)Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein,Brain Res 1022:30–38 [DOI] [PubMed] [Google Scholar]
- Wen Y., Yu W.H., Maloney B., Bailey J., Ma J., Marié I.et al. (2008)Transcriptional regulation of beta-secretase by p25/cdk5 leads to enhanced amyloi-dogenic processing,Neuron 57:680–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting K.P., Restall C.J., Brain P.F.(2000)Steroid hormone-induced effects on membrane fluidity and their potential roles in non-genomic mechanisms,Life Sci 67:743–757 [DOI] [PubMed] [Google Scholar]
- Winterle J.S., Mill T., Harris T., Goldbeck R.A.(2001)Absolute kinetic characterization of 17-beta-estradiol as a radical-scavenging, antioxidant synergist,Arch Biochem Biophys 392:233–244 [DOI] [PubMed] [Google Scholar]
- Wise P.M., Dubal D.B., Wilson M.E., Rau S.W., Böttner M., Rosewell K.L.(2001)Estradiol is a protective factor in the adult and aging brain: understanding of mechanisms derived from in vivo and in vitro studies,Brain Res Brain Res Rev 37:313–319 [DOI] [PubMed] [Google Scholar]
- Wiseman H.(1994)The antioxidant action of a pure antioestrogen: ability to inhibit lipid peroxidation compared to tamoxifen and 17 beta-oestradiol and relevance to its anticancer potential,Biochem Pharmacol 47:493–498 [DOI] [PubMed] [Google Scholar]
- Yang S.H., He Z., Wu S.S., He Y.J., Cutright J., Millard W.J.et al. (2001)17-beta estradiol can reduce secondary ischemic damage and mortality of subar-achnoid hemorrhage,J Cereb Blood Flow Metab 21:174–181 [DOI] [PubMed] [Google Scholar]
- Yang S.H., Liu R., Perez E.J., Wen Y., Stevens S.M., Jr, Valencia T., Brun-Zinkernagel A.M.et al. (2004)Mitochondrial localization of estrogen receptor beta,Proc Natl Acad Sci U S A 101:4130–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemori F., Yamada H., Yamaguchi T., Uemura A., Tamura A.(1996)Spatial memory disturbance after focal cerebral ischemia in rats,J Cereb Blood Flow Metab 16:973–980 [DOI] [PubMed] [Google Scholar]
- Yonemori F., Yamaguchi T., Yamada H., Tamura A.(1999)Spatial cognitive performance after chronic focal cerebral ischemia in rats,J Cereb Blood Flow Metab 19:483–494 [DOI] [PubMed] [Google Scholar]
- Yi K.D., Chung J., Pang P., Simpkins J.W.(2005)Role of protein phosphatases in estrogen-mediated neuroprotection,J Neurosci 25:7191–7198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K.D., Cai Z.Y., Covey D.F., Simpkins J.W.(2008)Estrogen receptor-independent neuroprotection via protein phosphatase preservation and attenuation of persistent extracellular signal-regulated kinase 1/2 activation,J Pharmacol Exp Ther 324:1188–1195 [DOI] [PubMed] [Google Scholar]
- Yu X., Rajala R.V., McGinnis J.F, Li F., Anderson R.E., Yan X.et al. (2004)Involvement of insulin/phosphoinositide 3-kinase/Akt signal pathway in 17 beta-estradiolmediated neuroprotection,J Biol Chem 279:13086–13094 [DOI] [PubMed] [Google Scholar]
- Zandi P.P., Carlson M.C., Plassman B.L., Welsh-Bohmer K.A., Mayer L.S, Steffens D.C.et al. (2002)Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study,J Amer Med Assoc 288:2123–2129 [DOI] [PubMed] [Google Scholar]
- Zemlyak I., Brooke S.M., Sapolsky R.M.(2002)Protection against gp120-induced neurotoxicity by an array of estrogenic steroids,Brain Res 958:272–276 [DOI] [PubMed] [Google Scholar]
- Zemlyak I., Brooke S., Sapolsky R.(2005)Estrogenic protection against gp120 neurotoxicity: role of microglia,Brain Res 1046:130–136 [DOI] [PubMed] [Google Scholar]
- Zhao L., Wu T.W., Brinton R.D.(2004)Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons,Brain Res 1010:22–34 [DOI] [PubMed] [Google Scholar]
- Zhao L., O'Neill K., Diaz Brinton R.(2005)Selective estrogen receptor modulators (SERMs) for the brain: current status and remaining challenges for developing NeuroSERMs,Brain Res Brain Res Rev 49:472–493 [DOI] [PubMed] [Google Scholar]
- Zhao L., O'Neill K., Brinton R.D.(2006)Estrogenic agonist activity of ICI 182,780 (Faslodex) in hippocampal neurons: implications for basic science understanding of estrogen signaling and development of estrogen modulators with a dual therapeutic profile,J Pharmacol Exp Ther 319:1124–32 [DOI] [PubMed] [Google Scholar]
- Zhao L., Jin C., Mao Z., Gopinathan M.B., Rehder K., Brinton R.D.(2007)Design, synthesis, and estrogenic activity of a novel estrogen receptor modulator - a hybrid structure of 17beta-estradiol and vitamin E in hippocampal neurons,J Med Chem 50:4471–4481 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Bhavnani B.R.(2005)Glutamate-induced apoptosis in primary cortical neurons is inhibited by equine estrogens via down-regulation of caspase-3 and prevention of mitochondrial cytochrome c release,BMC Neurosci 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Ramirez V.D.(2000)Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals,Br J Pharmacol 130:1115–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Ramirez V.D.(1999a)Purification and identification of an estrogen binding protein from rat brain: oligomycin sensitivity-conferring protein (OSCP), a subunit of mitochondrial F0F1-ATP synthase/ATPase,J Steroid Biochem Mol Biol 68:65–75 [DOI] [PubMed] [Google Scholar]
- Zheng J., Ramirez V.D.(1999b)Rapid inhibition of rat brain mitochondrial proton F0F1-ATPase activity by estrogens: comparison with Na+, K+-ATPase of porcine cortex,Eur J Pharmacol 368:95–102 [DOI] [PubMed] [Google Scholar]
- Zheng H., Xu H., Uljon S.N., Gross R., Hardy K., Gaynor J.et al. (2002)Modulation of A(beta) peptides by estrogen in mouse models,J Neurochem 80:191–196 [DOI] [PubMed] [Google Scholar]


