Abstract
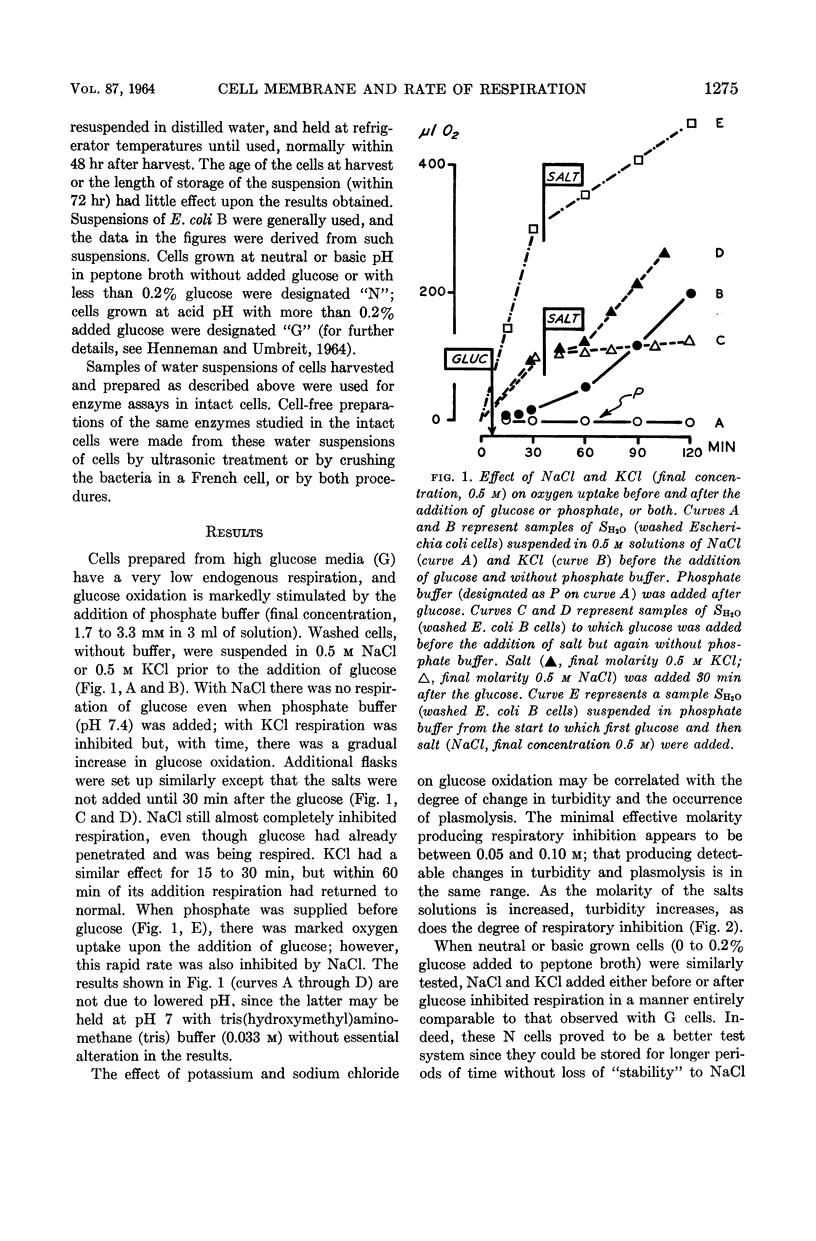
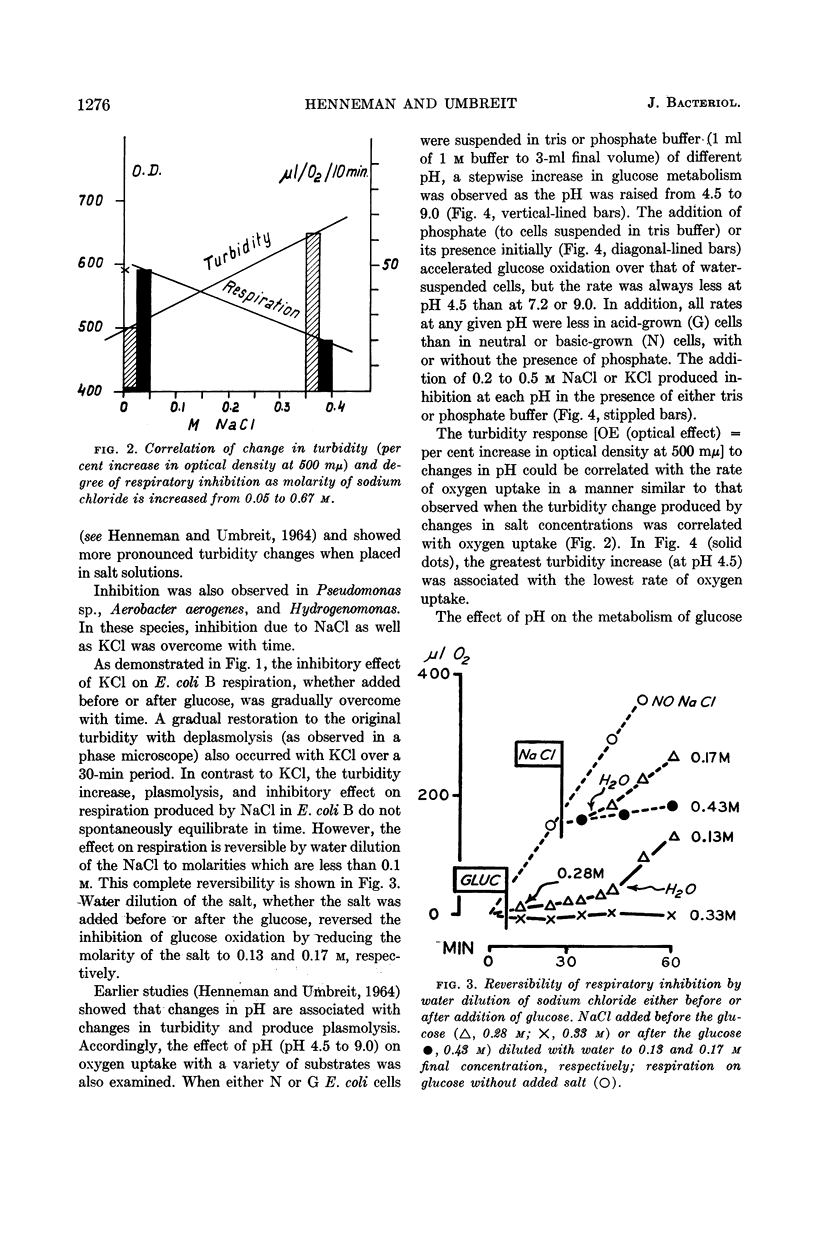
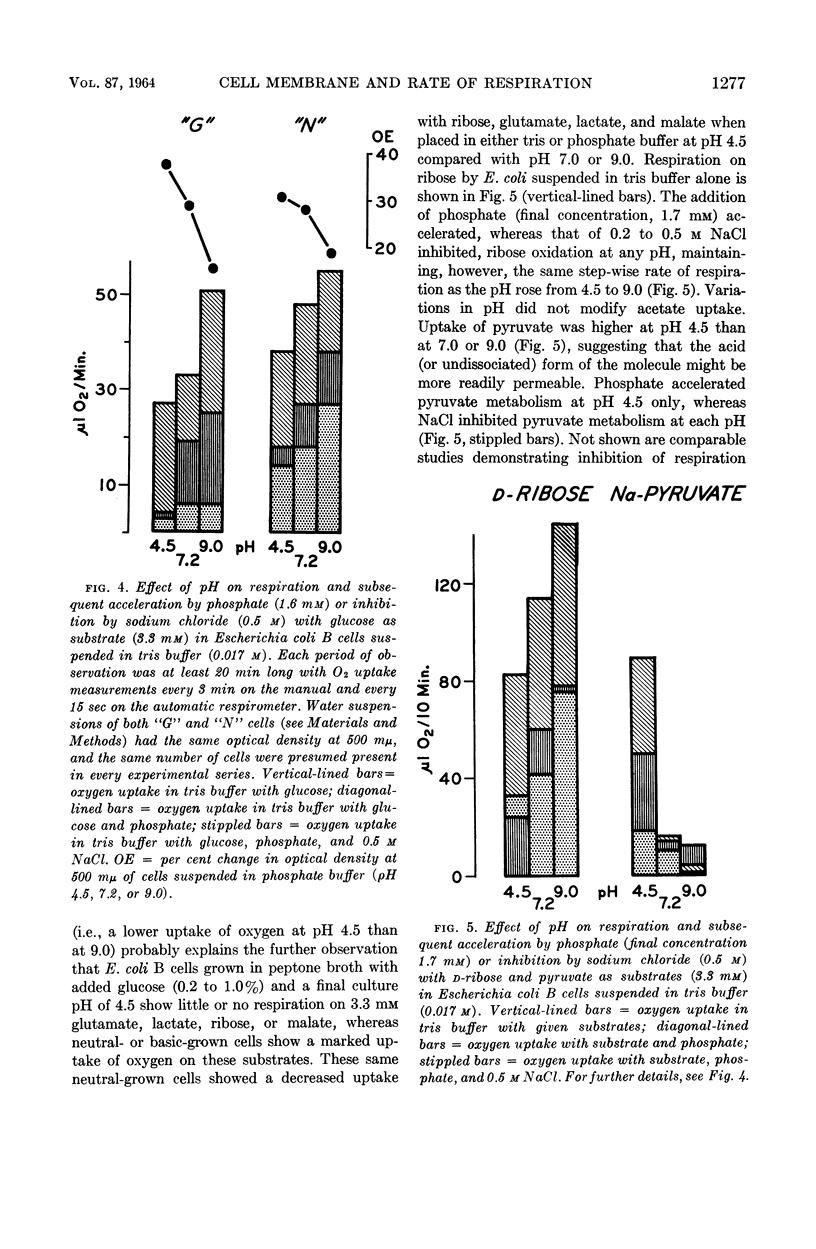
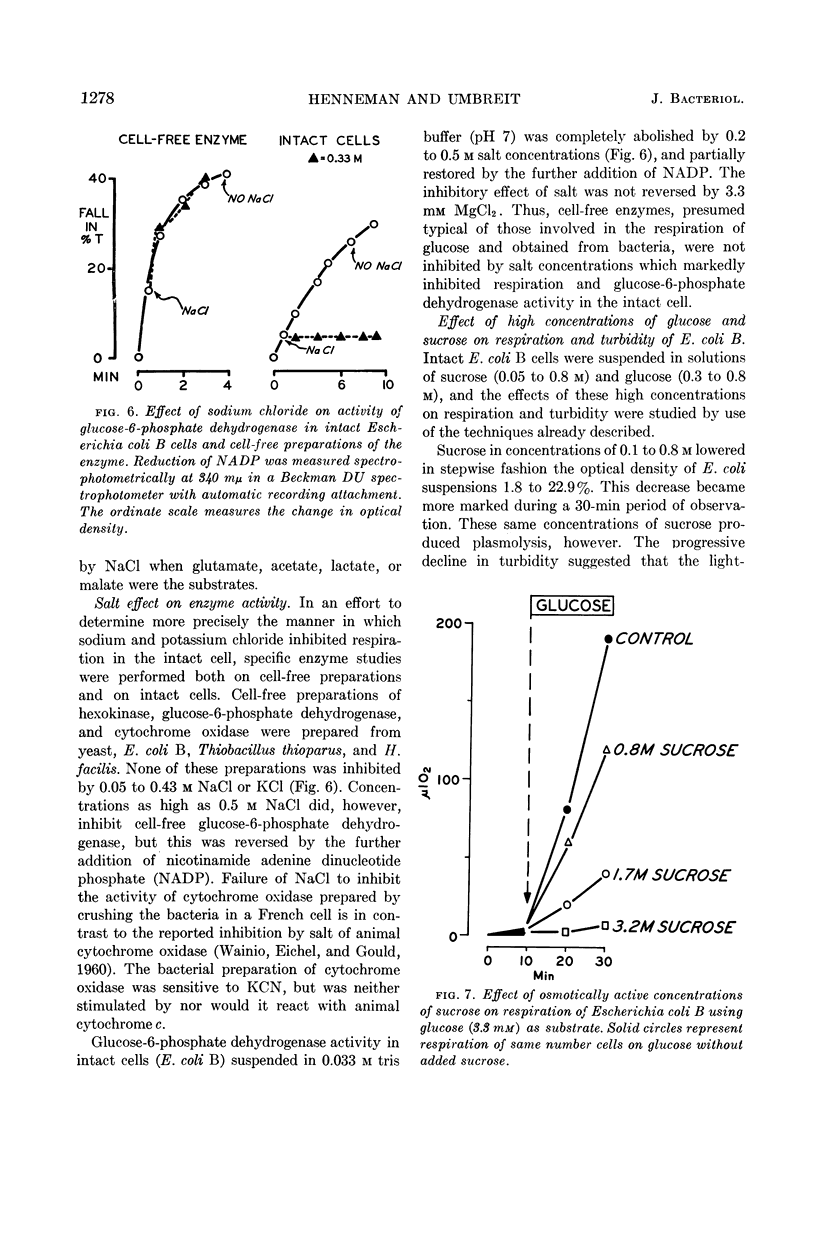
Henneman, Dorothy H. (Rutgers, The State University, New Brunswick, N.J.), and W. W. Umbreit. Influence of the physical state of the bacterial cell membrane upon the rate of respiration. J. Bacteriol. 87:1274–1280. 1964.—NaCl and KCl in concentrations of the order of 0.2 to 0.5 m inhibit the respiration of Escherichia coli B and other gram-negative organisms. Cell-free enzymes concerned in respiration and prepared from the same organisms are not inhibited by these salts, whereas these same enzymes tested in intact cells are. The physical state of the cell membrane appears to be a factor controlling its respiratory activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A. Metabolically dependent penetration of oligosaccharides into bacterial cells and protoplasts. J Biol Chem. 1960 May;235:1281–1285. [PubMed] [Google Scholar]
- AVI-DOR Y., KUCZYNSKI M., SCHATZBERG G., MAGER J. Turbidity changes in bacterial suspensions: kinetics and relation to metabolic state. J Gen Microbiol. 1956 Feb;14(1):76–83. doi: 10.1099/00221287-14-1-76. [DOI] [PubMed] [Google Scholar]
- BERNHEIM F. Factors which affect the size of the organisms and the optical density of suspensions of Pseudomonas aeruginosa and Escherichia coli. J Gen Microbiol. 1963 Jan;30:53–58. doi: 10.1099/00221287-30-1-53. [DOI] [PubMed] [Google Scholar]
- BOVELL C. R., PACKER L., HELGERSON R. PERMEABILITY OF ESCHERICHIA COLI TO ORGANIC COMPOUNDS AND INORGANIC SALTS MEASURED BY LIGHT-SCATTERING. Biochim Biophys Acta. 1963 Sep 24;75:257–266. doi: 10.1016/0006-3002(63)90604-8. [DOI] [PubMed] [Google Scholar]
- HENNEMAN D. H., UMBREIT W. W. FACTORS WHICH MODIFY THE EFFECT OF SODIUM AND POTASSIUM ON BACTERIAL CELL MEMBRANES. J Bacteriol. 1964 Jun;87:1266–1273. doi: 10.1128/jb.87.6.1266-1273.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACKER L., PERRY M. Energy-linked light-scattering changes in Escherichia coli. Arch Biochem Biophys. 1961 Nov;95:379–388. doi: 10.1016/0003-9861(61)90163-1. [DOI] [PubMed] [Google Scholar]
- ROGERS D., YU S. H. TURBIDITY CHANGE DURING GLUCOSE PERMEATION IN ESCHERICHIA COLI. J Bacteriol. 1963 May;85:1141–1149. doi: 10.1128/jb.85.5.1141-1149.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- SMITH L. Structure of the bacterial respiratory-chain system. Respiration of Bacillus subtilis spheroplasts as a function of the osmotic pressure of the medium. Biochim Biophys Acta. 1962 Jul 30;62:145–152. doi: 10.1016/0006-3002(62)90499-7. [DOI] [PubMed] [Google Scholar]


