Abstract
Vision mediating photoreceptor cells are specialised light-sensitive neurons in the outer layer of the vertebrate retina. The human retina contains approximately 130 million of such photoreceptors which enable images of the external environment to be captured at high-resolution and high–sensitivity (Forrester et al., 2003). Rod and cone photoreceptor subtypes are further specialised for sensing light in low and high illumination, respectively. To enable visual function, these photoreceptors have developed specialised morphological domains for the detection of light (outer segments), for changing cell shape (inner segments) and for synaptic communication with neighbouring retinal neurons (synaptic terminals). Furthermore, rod and cone subtypes have developed unique morphological variations of these specialised features. Here we review the major aspects of vertebrate photoreceptor morphology and key genetic mechanisms that drive their formation. These mechanisms are necessary for cell differentiation as well as function. Their defects lead to cell death.
INTRODUCTION
Sensory neurons are frequently integrated into epithelial structures. While some sensory cells are present on the body surface, others are embedded in epithelia that line internal cavities. This is the case of vertebrate auditory hair cells, for example. Although the lumenal location of vertebrate photoreceptors is less obvious, these cells too differentiate from the epithelial wall of the embryonic optic vesicle. Another common feature of sensory cells is that they detect signals via the apical cell surface domain. To facilitate this function, cells frequently differentiate elaborate apical features. Certain nematode sensory neurons, for example, form apical extensions that resemble tree branches, combs, or spatulas (Ward et al., 1975; Perkins et al., 1986). These structures are supported by a microtubule cytoskeleton. In contrast to that, vertebrate auditory hair cells as well as Drosophila photoreceptors differentiate bundles of finger-like protrusions supported by actin filaments (Hudspeth, 1989; Knust, 2007). In this review, we will focus on the vertebrate photoreceptor, a sophisticated light detector that features equally sophisticated morphology.
MORPHOLOGICAL FEATURES OF THE PHOTORECEPTOR CELL
Vertebrate photoreceptors are tightly aligned parallel to each other in a cell layer that occupies the outer portion of the retina. An isolated photoreceptor has elongated shape and features several morphologically distinct regions. From the apical to the basal terminus, these are: the outer segment, the inner segment, the nuclear region, and the synapse (Fig. 1). Below, we discuss each region in detail.
Fig. 1.
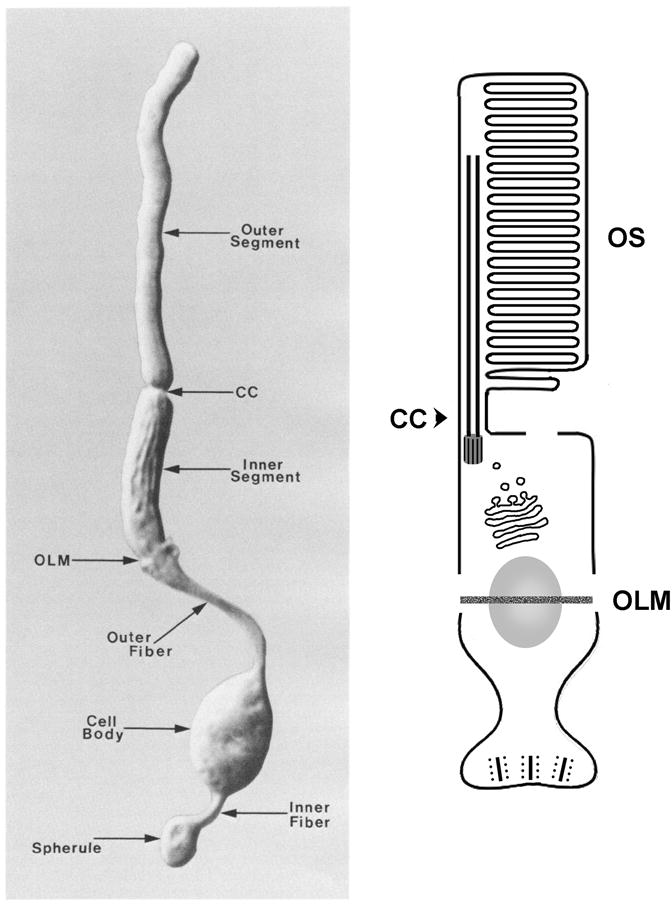
Overview of photoreceptor morphology. Left-hand panel shows a photograph of an isolated rod photoreceptor from the rabbit retina. A schematized view of the same cell is shown to the right. Position of the OLM relative to the nucleus varies for different photoreceptor types. Image to the left reprinted with permission from (Townes-Anderson et al., 1988). Outer segment (OS), connecting cilium (CC), outer limiting membrane (OLM).
The outer segment
A unique characteristic of visual photoreceptor morphology is the photosensitive outer segment. Counter intuitively, rod and cone outer segments are located at the end of photoreceptors furthest away from light entering the pupil, juxtaposed with the retinal pigment epithelium (RPE). Outer segments are enriched in phototransduction proteins including light-sensitive G-protein coupled receptors (GPCR), comprising of a transmembrane opsin protein and the chromophore 11-cis-retinal (a vitamin A metabolite). The compartmentalisation of the phototransduction machinery in the outer segment provides efficiencies in maximising visual sensitivity and in providing localised control over phototransduction. For example, visual sensitivity is regulated by selectively moving downstream components of phototransduction e.g. transducin, recoverin and arrestin between outer and inner segments or inner segments and synaptic regions (Calvert et al., 2006).
The outer segment forms as a protrusion of the apical cell surface which differentiates into an extensive array of photosensitive membranes (discs in rods and lamellae in cones) (Steinberg et al., 1980; Arikawa et al., 1992). Its structure is supported by an array of microtubules that extend from a basal body located at apical-most region of the inner segment (Fig. 2D, E). At the base of the outer segment, a narrow constriction surrounding the ciliary axoneme defines the boundary between the inner and outer segments, and is referred to as the connecting cilium. It is a very active transport route of new outer segment membranes and proteins. A continuous transport of material into the outer segment is necessary to support the renewal of opsin-laden discs/lamellae, a proceess essential for retinal function as shed discs/lamellae are continuously phagocytosed by the RPE apically (Young, 1967; Young, 1971). The disks/lamellae are structurally subdivided into disk surfaces and disk rims. Disk surfaces are the large flat parallel layers of disk membrane, whereas disk rims are the curved membranes that form the edge of the discs.
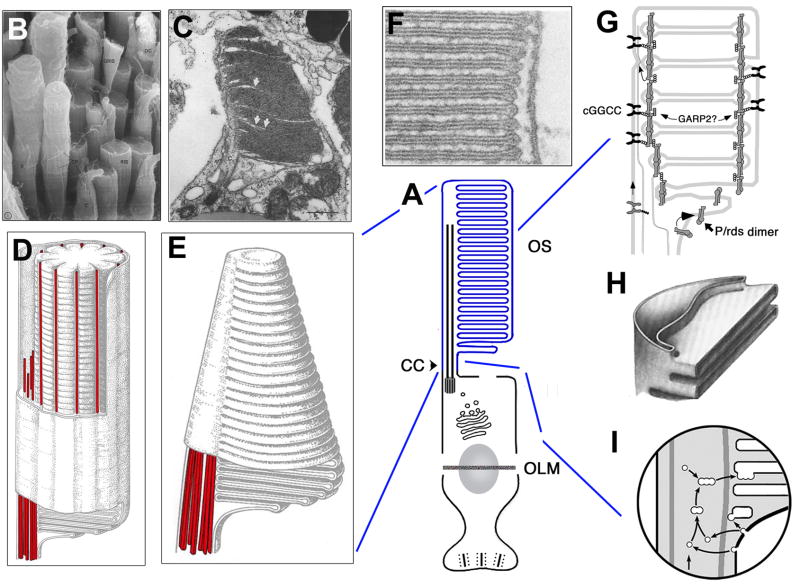
Fig. 2. Photoreceptor Outer Segments.
A: Generalized schematic of photoreceptor morphology; outer segment membranes are coloured in blue.
B: Scanning EM of frog photoreceptors showing intact rods (R) and cones (C), and rod inner segments (RIS) left behind from broken photoreceptors. Calycal processes (CP), intact double cone (DC), green rod inner segment (GRIS). Reprinted with permission (Peters et al., 1983).
C: Transmission EM of a developing Xenopus cone outer segment highlighting distal invaginations occurring between adjacent membranes (white arrows). Reprinted with permission (Eckmiller, 1987).
D. Schematic of a rod outer segment, showing stacks of disk membranes indented by multiple incisures, and a microtubule scaffold highlighted in red. The connecting cilium axoneme contains an array of microtubule doublets that extend from the inner segment into the outer segment, wherein a separate set of longitudinal microtubules run in the indentations of incisures. These microtubules are located in the cytoplasm between the curved rim of the OS disks and the external plasma membrane. Reprinted with permission from (Eckmiller, 2004).
E. Schematic of a cone outer segment showing layers of membranes that are continuous with the rest of the plasma membrane on the ciliary face. Microtubules are highlighted in red. Reprinted with permission from (Eckmiller, 2004).
F: Electron micrograph of a section through the outer segment from a rabbit rod photoreceptor shows membrane folds and closely apposed disks. Reprinted with permission from (Townes-Anderson et al., 1988).
G: A model of protein-protein interactions thought to maintain the architecture of outer segment membranes. This model also shows membrane discs being added via evagination at the base of the outer segment–an alternative to the scenario shown in panel (I). Peripherin/rds (P/rds) tetramers localize to disc rims. cGMP-gated cation channel (cGGCC), which includes a GARP domain, localizes to the plasma membrane and is thought to interact with peripherin. A cytoplasmic GARP protein has been proposed to bridge adjacent discs by interacting with peripherin/rds. Reprinted with permission from (Goldberg, 2006).
H: A detail of disc rim formation according to the open disc “evagination” model. Rim expansion encloses space between adjacent membrane evaginations. Reprinted with permission from Steinberg et al. (Steinberg et al., 1980).
I: A “vesicle fusion” model of rod disk formation. Rod opsin carrying vesicles pinch off at the base of the outer segment, and fuse with rod discs. Reprinted with permission from (Chuang et al., 2007).
Despite gross similarities, rod and cone outer segments differ in shape, structure and renewal process. Indeed, the basis for rod and cone photoreceptor terminology arises from the rod-like or cone-like shape these outer segment stacks adopt, respectively (Fig. 2D, E). In rod outer segments of some higher vertebrates, multiple indentations of the disk membrane and the plasma membrane occur along the length of the outer segment. These specialised indentations are known as incisures and mark the site of a cytoskeletal system containing longitudinal microtubules linked by filaments to the adjacent membranes (Eckmiller, 2000; Eckmiller, 2004) (Fig. 2D). The incisures develop from infolds of the disk rim and surface, and commence formation before rim completion (see below). In rods, the disc membranes are separated from the plasma membrane and stacked internally (Fig. 2B, D, F, G), whereas in cones the layers of lamellae are contiguous with the cell membrane and exposed to the extracellular environment (Fig. 2C, E).
The mechanism by which new disks/lamellae are added to outer segments is not clearly understood, and at least two models have been proposed to explain it. According to the “evagination” model, the morphogenesis of new disk surfaces and rims involves separate steps and regions of the membrane (Steinberg et al., 1980). Basal evaginations from the inner face of the ciliary plasma membrane produce nascent disk membranes that are exposed to the extracellular space, so called “open disks” (Fig. 2G). Then disk rims develop between the lower (basal) membrane of an existing evagination and the upper (apical) membrane of the adjacent, nascent membrane evagination (Fig. 2G). The rims grow around the circumferences of adjacent discs, such that the apical and basal surfaces of a single evagination become surfaces of adjacent discs. In contrast to that, the proponents of the “fusion” model stipulate that rod discs form via regulated fusion of opsin-laden, membranous vesicles (Obata and Usukura, 1992; Chuang et al., 2007) (Fig. 2I). In this scenario, vesicles concentrated at the base of the outer segment assemble into membranous disks. The vesicles may originate via endocytosis of the outer segment basal membrane and/or are trafficked from the inner segment (Chuang et al., 2007) (Fig. 2I). Thus, vesicle fusion mediates the incorporation of opsin into disks and disk formation. One of the key differences between the models is the absence of “open disks” at the basal end of rod outer segments in the “fusion” model. It has been suggested in the literature that the evidence for both the “evagination” and “fusion” model may involve technical artefacts (Chuang et al., 2007; Kleinman and Ambati, 2008; Yang et al., 2008). Intuitively, technical artefacts that disrupt rod outer segment membranes resulting in “open disks” seem more likely. However, nascent outer segment membranes that grow as extracellular evaginations are not simply artefacts, as many vertebrate cones feature extensive membrane lamellae open on the non-ciliary face. It is not clear at present whether only one of these models is correct or both describe mechanisms that function in parallel in the basal region of the photoreceptor cell.
What is the basis for the differences in outer segment shape/structure and are they functionally significant? Differences in the extent of rim formation basally and the ability to produce basal membrane invaginations are proposed to account for the distinct shape and structure of rod and cone outer segments (Eckmiller, 1987). In rod outer segments, disk rim formation initiates at the ciliary side and extends completely around the circumference of the adjacent membranes such that they become pinched off as internal disks separated from the plasma membrane (Fig 2F–H). In cone outer segments, rim development is typically incomplete such that cone membrane lamellae do not “pinch away” to separate from a plasma membrane and as a result the cone membrane is continuous and exposed to the extracellular environment (Eckmiller, 1987).
As mentioned above morphogenesis of outer segments is a dynamic process with disks/lamellae being shed apically and new ones added basally. The ability of rod outer segments to retain their cylindrical shape in this context is believed to relate to complete rim formation such that newly formed disks extend the full width of the outer segment and become isolated stacks that are pushed apically to replace the shed discs. Cone outer segments also add membrane folds basally, but the width of the membranes at the tip of the cone is much shorter than at the base. The generation and retention of cone-shape is proposed to be mediated by unique invaginations (arrows in Fig. 2C) along the outer segment that remodel the membrane lamellae decreasing their width and forming the cone taper (Eckmiller, 1987).
Surrounding photoreceptor inner and outer segments is an extracellular matrix referred to as the retinal interphotoreceptor matrix (IPM) (Mieziewska, 1996). The IPM in mammalian retinas is subdivided into rod and cone-specific compartments, which can be distinguished based on the presence of peanut agglutinin-binding glycoconjugates in cones, and wheat germ agglutinin (WGA)-binding glycoconjugates in rods (Sameshima et al., 1987). Cone matrix sheaths are unique domains of the IPM largely composed of chondroitin 6-sulfate proteoglycan. They are thought to mediate retinal attachment to the RPE (Johnson and Hageman, 1991).
The inner segment
The inner segment corresponds to the photoreceptor domain stretching from the connecting cilium (separating outer and inner segments) to the outer limiting membrane (Fig. 1, OLM). It contains a myoid area with prominent Golgi-apparatus (also rich in contractile microtubules in some lower vertebrates) (Troutt and Burnside, 1988), and an ellipsoid area rich in mitochondria that help fulfil the metabolic requirements of photoreceptors (Fig. 3F). The OLM is itself an important structural feature that divides photoreceptors into apical and basal domains. Although called a “membrane”, it actually consists of junctional complexes between photoreceptors and adjacent Muller glia (Williams et al., 1990). In addition to providing structural support, the OLM has been proposed to act as a semi-permeable barrier, preventing the diffusion of extracellular matrix components surrounding outer and inner segments, as well as a barrier for the diffusion of proteins within the lipid bilayer of the photoreceptor cell membrane itself (Williams et al., 1990).
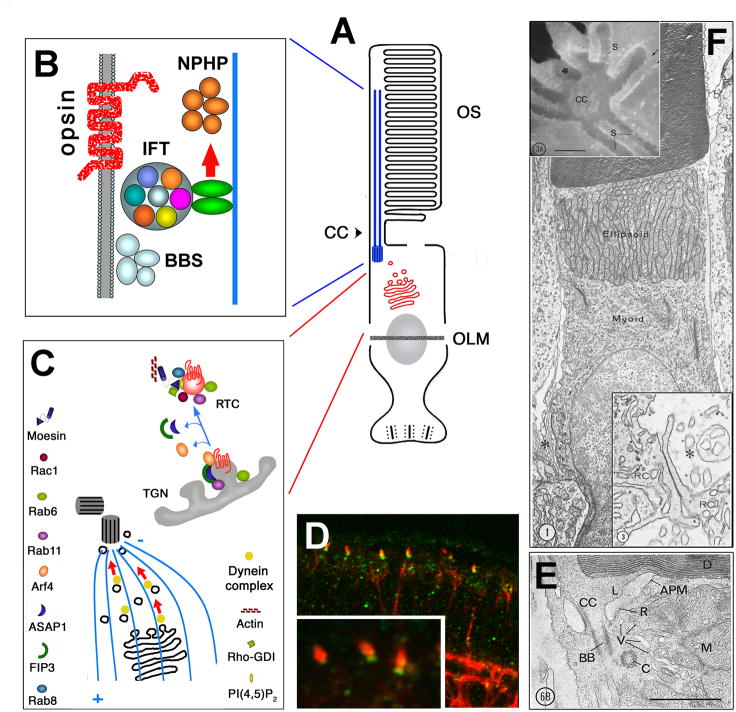
Fig. 3.
Transport into the outer segment. Proteins are synthesized in the cytoplasmic reticulum and from there transported via the Golgi apparatus to the periciliary area at the base of the connecting cilium, and subsequently along ciliary microtubules into the outer segment.
A: A schematic representation of the photoreceptor cell. Ciliary microtubules are highlighted in blue. The Golgi complex and post-Golgi vesicles are in red.
B: Intraflagellar transport (IFT) is thought to translocate proteins into the outer segment along ciliary microtubules. It is mediated via so-called IFT particles, protein complexes that consist of several polypeptides. It is not clear whether IFT is involved in the transport of opsins. In addition to IFT particle components, several other IFT-related proteins, such as BBS gene products, are necessary for photoreceptor survival. Nephrocystins (NPHP) are also required for photoreceptor viability. Their relationship to IFT is not clear.
C: Rod opsin is transported from the Golgi apparatus to the base of the photoreceptor connecting cilium in vesicles. In one proposed scenario, this transport is driven by dynein, a microtubule-dependant motor. The budding of RTCs from the trans Golgi network and subsequently their fusion at the base of the connecting cilium appear to require a number of proteins, including a small GTPases Arf4, Rab8, and Rab11, a GTPase effector FIP3, and a GAP factor, ASAP1. Several other proteins are also proposed to participate in these processes (listed in the figure). The representation of RTC budding provided courtesy of D. Deretic.
D: Immunolocalization of IFT88 (green) to the base of the connecting cilium in photoreceptors of larval zebrafish (Malicki lab).
E: Longitudinal section of a frog rod cut along the axis of the connecting cilium reveals structural details in the periciliary ridge complex. Apical plasmalemma (APM) of the inner segment. Basal bodies (BB), Centriole (C), Connecting cilium (CC), Disks (D) in the outer segment, Lip (L), Mitochondria (M), Ridge (R), Carrier vesicles (V), Reprinted with permission from (Peters et al., 1983).
F: Ultrastructure of a salamander rod photoreceptor. The outer segment contains stacks of membranous disks. The inner segment consists of a mitochondria-rich ellipsoid and a myoid region containing a Golgi apparatus. The inset in the bottom right corner (magnified at location of asterisk in main panel) shows photoreceptor fins, that interdigitate with microvilli of Muller cells above the external limiting membrane. Reprinted with permission (Townes-Anderson et al., 1985). Inset in top left corner shows scanning electron micrograph of the periciliary ridge complex. Reprinted with permission from (Peters et al., 1983).
Some aspect of the models presented in this figure should be considered hypothetical.
In lower vertebrates, photoreceptor inner segment morphology undergoes extensive length remodelling in response to changes in ambient lighting and circadian rhythms. In darkness, the myoid regions of cones and rods elongate and contract, respectively. This positions rod outer segments close to the outer limiting membrane and cone outer segments closer to the RPE pigment granules (Ali, 1971). The reverse “retinomotor movements” occur in light. In addition to diurnal light regulation, retinomotor movements exhibit circadian regulation. When placed in constant darkness photoreceptor elongation and retraction occur at the “expected” light on and off phases (Pierce and Besharse, 1985; Pierce and Besharse, 1988; Menger et al., 2005). Melatonin is a neuromodulator secreted from the pineal under circadian regulation, with highest expression during the dark phase. In agreement with that, melatonin induces cone elongation (Pierce and Besharse, 1985). Other neuromodulators include adenosine and GABA which induce cone elongation (Pierce and Besharse, 1988; Rey and Burnside, 1999) and dopamine which induces cone contraction (Pierce and Besharse, 1985; Dearry et al., 1990). Cone myoid elongation is microtubule-dependent and contraction is actin-dependent, whereas rod elongation and contraction are actin-based (Warren and Brunside, 1978; O’Connor and Burnside, 1981; O’Connor and Burnside, 1982; Burnside et al., 1983). Interestingly, retinomotor movements appear to require functionally mature photoreceptors (Hodel et al., 2006). They are proposed to optimally position rod and cone photoreceptor outer segments to maximise light capture in scotopic and photopic environments.
The sequestration of mitochondria to the inner segment ellipsoid is a morphological adaptation proposed to bring them closer to the choroidal blood supply for more efficient sourcing of oxygen (Stone et al., 2008). Mitochondria are also clustered in the axon terminals of species with an intraretinal vasculature, which brings them closer to this inner retinal blood supply (Stone et al., 2008).
The nuclear region
The nucleus is positioned between the synaptic terminus and the inner segment (Figs. 1, and 4). It is a bulky organelle, which occupies a substantial portion of cell volume. In many species, cone and rod nuclei have somewhat different shapes, stain differently in histological preparations, and segregate to different sublaminae of the outer nuclear layer. In the mouse and zebrafish, rod nuclei tend to be located basally (vitreally) relative to these of cones (Carter-Dawson and LaVail, 1979; Branchek and Bremiller, 1984). It has also been noted that mouse nuclei tend to form apico-basal columns (Carter-Dawson and LaVail, 1979). The Golgi apparatus localizes apically to the nucleus while endoplasmic reticulum is found both apically and basally (Mercurio and Holtzman, 1982).
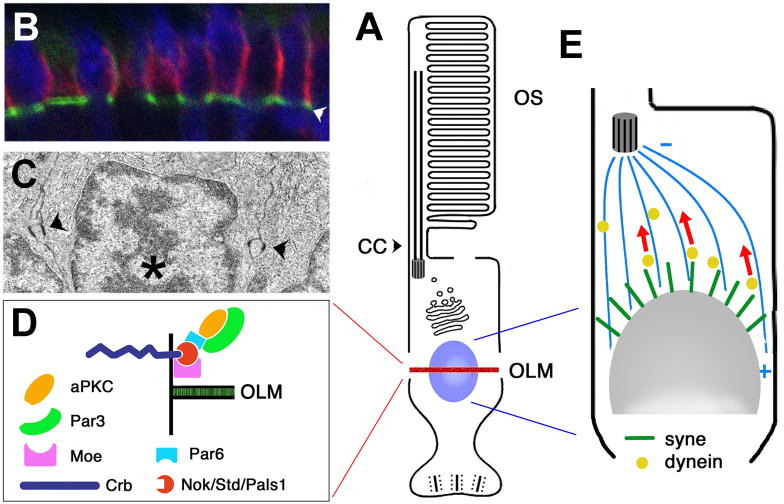
Fig. 4. Photoreceptor cell body.
A: A schematic representation of the photoreceptor cell. The nucleus is highlighted in blue. A belt of cell junctions, erroneously called the outer limiting membrane (OLM), subdivides the photoreceptor cell surface into the apical and baso-lateral domains (highlighted in red).
B: Confocal image of the outer limiting membrane in the retina of larval zebrafish. Cell junctions are visualized by phalloidin staining (in green, indicated with an arrowhead). The Crumbs polypeptide is detected via antibody staining (in red). It localizes apical to cell junctions. (Malicki lab)
C: Electron micrograph of a section through the photoreceptor cell layer in larval zebrafish. Arrowheads indicate cell junctions, the nucleus is indicated with an asterisk. (Malicki lab)
D: A schematic representation of the protein complex that regulates the formation of cell junctions in the outer limiting membrane. The formation of the apical cell membrane domain of the photoreceptor cell, and the integrity of the junctional complexes in the OLM require the function of Crumbs, a transmembrne protein that features a large extracellular domain and a short cytoplasmic tail. Crumbs cytoplasmic moiety binds a MAGUK protein Stardust/Nagie oko and a FERM-domain protein Mosaic eyes (Moe), which also bind each other. Par6, Par3, and aPKC are also thought to contribute to this protein complex. Crb, Crumbs; Moe, Mosaic eyes; Nok, Nagie oko; Std, Stardust.
E: The nucleus is by far the most voluminous organelle in the cytoplasm of the photoreceptor cell. Its position is affected by the activity of a microtubule dependant motor, dynein, and nuclear envelope components that feature a C-terminal KASH domain (Syne family proteins in vertebrates). The KASH domain contains a lipophilic segment thought to span the outer membrane of the nuclear envelope. The cytoplasmic portion of many KASH-domain proteins is exceptionally long (close to 10,000 amino acids in some cases). It is not clear how they interact with the dynein complex.
The synapse
Photoreceptors synapse with bipolar and horizontal cells at the outer plexiform layer of the retina. Similar to mechanosensory hair cells, photoreceptors utilise the ribbon synapse (Fig. 5), a specialised morphological adaptation of conventional chemical synapses, to communicate with downstream targets (tom Dieck and Brandstatter, 2006). The ribbon synapse is a presynaptic electron-dense “plate”, perpendicular to the plasma membrane, and surrounded by a large pool of synaptic vesicles (Fig 5B). It extends from the synaptic active zone into the presynaptic cytoplasm (Lenzi and von Gersdorff, 2001; Sterling and Matthews, 2005; tom Dieck and Brandstatter, 2006).
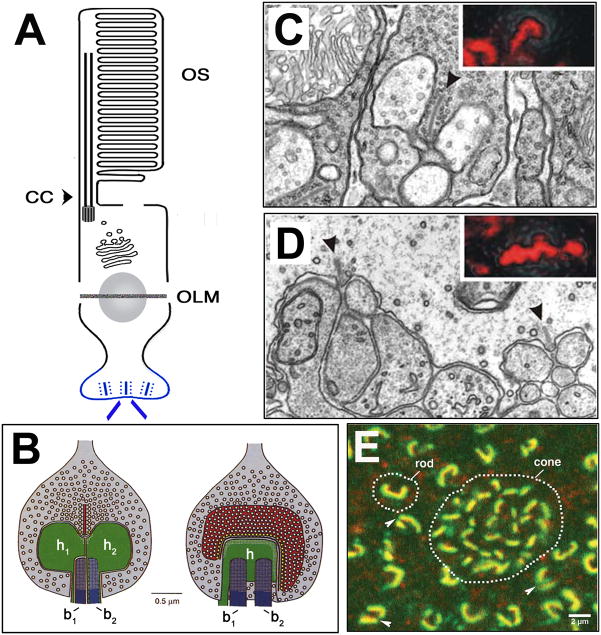
Fig. 5. The Synaptic Terminal.
A: Schematic of photoreceptor morphology; synaptic terminal is labeled in blue.
B: Schematic of a rod photoreceptor synapse. Views of the rod terminal perpendicular (left) and parallel (right) to the face of ribbon. Presynaptically, the ribbon tethers several hundred vesicles (white circles in red ribbon) and the active zone docks ca. 100 vesicles (yellow circles) and tethers ca. 770 vesicles. Postsynaptically, processes of 2 horizontal (h1, h2) and 2 bipolar (b1, b2) cells occupy the invagination. Reprinted with permission from (Rao-Mirotznik et al., 1995).
C. EM images of the “tetrad” ribbon synapse of a mammalian rod photoreceptor. Ribbon is indicated by an arrowhead and the inset is a confocal image of RIBEYE antibody staining. Reprinted with permission from (tom Dieck et al., 2005).
D. EM images of the “triad” ribbon synapse of a mammalian cone photoreceptor. Ribbons are indicated by arrowheads and the inset is a confocal image of RIBEYE antibody staining. Reprinted with permission from (tom Dieck et al., 2005).
E. Confocal image of a tangential section through macaque photoreceptor terminals. Dotted lines outline rod and cone presynaptic terminals immunostained for Bassoon (green), and the 1F subunit of an L-type Ca2C channel (red). The rod terminal typically comprises of a single, crescent-shaped ribbon, but can appear as two separate but linked ribbons (arrowheads). The cone terminal contains an array of smaller ribbons that serve separate invaginations. Reprinted with permission (Wassle, 2003).
Again, cone and rod photoreceptors have developed distinct structural variations of the ribbon synapse (Sterling and Matthews, 2005; tom Dieck and Brandstatter, 2006). The synaptic end is called a pedicle in cones and a spherule in rods. Typically, rod basal ends branch into many small terminals, each containing a single ribbon enveloping a “tetrad” of two lateral AMPA receptor-regulated horizontal cell processes and two or more central mGluR6 receptor-regulated bipolar cell dendrites (Fig. 5C) (Sterling and Matthews, 2005; tom Dieck and Brandstatter, 2006). Cones, in general, have larger terminals containing multiple ribbons and more vesicle docking sites than rod ribbon synapses (Fig 5E). The cone ribbons synapses are associated with “triads” consisting of two lateral AMPA-positive horizontal cell dendrites and one or more central, mGluR6-positive, bipolar cell dendrites (Fig. 5D). Cone but not rod terminals also synapse with tips of additional mGluR1/5/6/7-regulated bipolar cells via distinct basal contacts at characteristic distances from the triad. Further away from the ribbon synapse, even more glutamate receptor-positive clusters which arise from horizontal cell processes are observed. It is proposed that the ability of glutamate released from cone ribbon synapses to couple with these additional cell types enables higher order signalling (Sterling and Matthews, 2005). Functionally the ribbon synapse structure enables much higher rates of exocytosis of glutaminergic vesicles and more tightly controlled release of glutamate, than conventional synapses (Sterling and Matthews, 2005; tom Dieck and Brandstatter, 2006). Thus, ribbon synapses enable photoreceptors (especially cones) to transmit graded signals, fine-tuning information relating to a wide range of light intensities in “real time”.
In teleost cones, but not rods, the number of synaptic ribbons decreases at night. The decrease is circadian, regulated by intracellular calcium, and coincides with enhanced transmitter release (Vollrath and Spiwoks-Becker, 1996). Recent findings indicate that ribbons of mammalian rods also undergo significant morphological transformations in response to diurnal changes in light, although these alterations are not circadian in nature (Spiwoks-Becker et al., 2004). In the dark, rod ribbons are large and smooth, but small and “club-shaped” during light. These differences have been proposed to arise from phototransduction-mediated changes in intracellular calcium. Thus, rod ribbons may traffic fewer vesicles in light than in dark as a means of adaptation.
Morphogenesis from Progenitor Cells
The striking morphological features of vertebrate photoreceptors described above develop from differentiating retinal progenitor cells, which are characterized by a very different epithelilal morphology. An important attribute of a differentiating photoreceptor is that it remains connected via cell junctions to the surrounding cells (Hinds and Hinds, 1979; Schmitt and Dowling, 1999). These cell junctions appear to persist throughout development and adulthood as the outer limiting membrane. Morphogenetic changes to photoreceptor progenitors have been described in several species (Hinds and Hinds, 1979; Schmitt and Dowling, 1999; Martinez-Navarrete et al., 2008; Stone et al., 2008). In zebrafish, for example, initially short inner segments containing connecting cilia at the apical end are observed to extend towards the ventricular surface. These inner segments also contain rough endoplasmic reticulum, ribosomes and mitochondria. Subsequently, outer segments and synaptic termini can be distinguished, although synaptic ribbons have not yet formed (Schmitt and Dowling, 1999). The inner segments enlarge and the ellipsoid and myoid are distinguishable. Thereafter, unanchored ribbons are observed in the synaptic terminals. Finally, expanding outer segments are observed as are enlarged synaptic terminals with anchored ribbons and invaginating processes.
Neurogenesis of photoreceptors from progenitor cell niches can also occur in adults. In fish and amphibians, a ciliary marginal zone (CMZ) niche adds photoreceptors as part of the growth of the eye throughout adulthood, and postnatal chicks retinas also contain multipotent progenitors in the retinal margin (Wetts et al., 1989; Harris and Perron, 1998; Fischer and Reh, 2000). As recently reported, neurogenesis of rod and cone photoreceptors from retinal progenitors may also occur at the margin of monkey and human retinas (Martinez-Navarrete et al., 2008).
MOLECULAR MECHANISMS OF PHOTORECEPTOR MORPHOGENESIS
The sophisticated morphology that characterizes the vertebrate photoreceptor cell requires sophisticated mechanisms to drive its formation. Molecular motors play a major role in photoreceptor morphogenesis and so do transmembrane proteins that confer distinct identity on cell membrane domains, and mediate their intricate folding. Below, we discuss the major molecular mechanisms that contribute to photoreceptor morphogenesis. Most, if not all, of them are also necessary for long-term viability of the cell.
Membrane compartmentalization
Compartmentalization of the cell membrane is an essential feature of many cells (for a recent review see Caudron and Barral, 2009). The best studied example is the subdivision of epithelial cells into apical and basolateral cell membrane domains. In epithelia, the need for membrane subdivision is obvious, as one area of the cell surface faces the outside environment while another one the extracellular environment of the tissue. Clearly, the different characteristics of the external and internal environments dictate differences in protein composition. This is particularly obvious in the case of transporters that facilitate directional movement of ions, small molecules, and proteins into or out of the cell. A diffusion barrier between these two compartments is set by cell junctions and associated structures. It prevents the mixing of the apical and the baso-lateral proteins.
The subdivision of the cell membrane into the apical and basolateral domains is also present in many sensory cells, including hair cells, olfactory sensory neurons, and vertebrate and insect photoreceptors (Hirokawa and Tilney, 1982; Hansen and Zeiske, 1993; Longley and Ready, 1995) (Fig. 4B, C). A substantial body of evidence indicates that the genetic underpinnings of apicobasal polarity in epithelial sheets and photoreceptors are closely related.
One of the key regulators of apicobasal polarity is the crumbs gene. Initially discovered during saturation mutagenesis screens in the fly (Jurgens et al., 1984), crumbs genes encode transmembrane proteins which feature a short C-terminal region of ca. 40 amino acids and extracellular domain of varying length. crumbs genes are necessary for the proper formation of cell junctions which define the boundary between the apical and basolateral domains of the cell membrane (Knust and Bossinger, 2002). While crumbs loss-of-function causes a disintegration of cell junctions (Grawe et al., 1996), its overexpression results in a massive expansion of the apical cell surface domain in fly embryonic epithelia (Wodarz et al., 1995). Similar phenotypes are seen in fly photoreceptor cells (Pellikka et al., 2002). Similar to Drosophila, vertebrate crumbs mutations also affect cell membrane polarity. In zebrafish, loss of crumbs activity results in a dramatic decrease of the apical membrane size in photoreceptor cells as measured by the distance between the outer limiting membrane and the outer segment (Omori and Malicki, 2006). In the mouse, crumbs mutants are characterized by the loss of cell junctions of the outer limiting membrane (Mehalow et al., 2003; van de Pavert et al., 2004). In tests performed so far, crumbs overexpression does not, however, cause an expansion of the apical membrane in zebrafish photoreceptors (Omori and Malicki, 2006).
The crumbs gene product functions as component of a larger protein complex. In zebrafish, its C-terminus binds a FERM (Four point one, Ezrin, Radixin, Moesin) domain protein, encoded by the mosaic eyes (moe) locus (Hsu et al., 2006). The Moe protein is expressed throughout the photoreceptor cell surface and in contrast to crumbs, its loss of function is thought to result in an expansion of the apical cell surface domain, the outer segment in particular (Hsu et al., 2006). Given their contrasting mutant phenotypes, Moe has been proposed to function as a negative regulator of Crumbs. Other than phenotypic observations, direct evidence to support this model is not, however, available so far. In addition to Moe, the C-termini of Crumbs proteins also interact with a MAGUK factor encoded by the nagie oko/stardust (pals1 in the mouse) locus and with a PDZ domain protein, Par6 (Hong et al., 2001; Lemmers et al., 2004). Par6, in turn, binds Par3 and aPKC, two proteins known to function as regulators of apico-basal polarity in fly embryonic epithelia (reviewed in Shin et al., 2006) (Fig. 4D). In the zebrafish retina, Nok (Nagie oko), Par3, and Has/aPKC colocalize with Crumbs apical to the cell junctions of the outer limiting membrane (Fig. 4B) (Horne-Badovinac et al., 2001; Wei and Malicki, 2002; Wei et al., 2004). It is not known, however, whether they also function as regulators of the apical cell surface size in vertebrate photoreceptors. In the case of nok, for example, although mutant strains are available, this question is difficult to address because the retinae of nok mutant animals are disorganized, due to earlier function of this gene in the retinal neroepithelium and in the RPE (Zou et al., 2008).
Another important subdivision of the cell membrane, which most likely exists in all ciliated cells, is that which defines the ciliary compartment at the apical surface, and is termed the “ciliary pore” (Rosenbaum and Witman, 2002). As photoreceptor outer segments are among the most prominent cilia-derived structures, this subdivision plays a critical role in photoreceptor function. It assures, for example, that opsins do not diffuse from the outer segment into the rest of the photoreceptor cell membrane. In the absence of the outer segment, opsins are present throughout the cell membrane and contribute to the degeneration of photoreceptors (see for example, Tsujikawa and Malicki, 2004). The nature of the diffusion barrier at the base of the outer segment or other types of cilia is not clear, as no distinct structural analogs to the belt of cell junctions that separate the apical and basolateral surface domains are seen in ultrastructural or histochemical analyses. The most plausible candidates for structural components of this diffusion barrier are so-called transitional fibers seen in some types of cilia (Dute and Kung, 1978; Perkins et al., 1986; Deane et al., 2001), or elements of the ciliary necklace (Gilula and Satir, 1972). The molecular components of these ciliary structures are, however, nearly unknown.
Transport Mechanisms
The outer segment is one of the most voluminous parts of the vertebrate photoreceptor, but it lacks ribosomes, and thus does not synthesize its own proteins. Consequently, all outer segment proteins are delivered from the cell body. This is clearly a challenging task, given that opsin alone is present in ca. 1 billion copies in the outer segment and is constantly being replenished (Pugh and Lamb, 2000). As its absence results in the lack of outer segment formation, rod opsin is not only a transmembrane receptor but also an important structural component (Lem et al., 1999). This may be one reason why the amount of opsin in the outer segment has to be carefully regulated: reducing opsin content by half results in photoreceptor degeneration and so does opsin overexpression (Olsson et al., 1992; Lem et al., 1999). Opsin is the most abundant protein of the outer segment, and consequently it has received by far the most attention in the analysis of photoreceptor protein transport.
The journey of proteins from the Golgi to outer segment membranes consists of two parts that are related to the two apical membrane compartments. The first section of the transport route carries proteins from the Golgi to base of the connecting cilium (Fig. 3C). Transport proceeds towards the minus ends of microtubules, and appears to be mediated, at least in part, by dynein, a minus-end directed microtubule dependent motor (Tai et al., 1999). The second segment of transport occurs within the ciliary compartment (Fig. 3B), which, as discussed above, is delimited by a specialized region of the apical cell membrane. There transport proceeds toward the plus ends of microtubules and is driven, at least in part, by kinesins.
Transport from the Golgi to the Apical Membrane
Rod opsin, and presumably many other outer segment proteins, are transported from the endoplasmic reticulum into the Golgi, and from there to the base of the photoreceptor outer segment. This process can be subdivided into 3 stages: budding of opsin carrier vesicles, translocation of vesicles, and finally their fusion with the cell membrane in the vicinity of the connecting cilium. Undoubtedly, each of these steps involves complex molecular machinery and our understanding of its intricacies appears rudimentary at best. Some of the players begin to emerge, however, and are discussed below.
Budding of opsin carrier vesicles
The 44 C-terminal amino acids of rod opsin are both necessary and sufficient for its correct transport to the outer segment (Tam et al., 2000; Perkins et al., 2002). Given this function, it is not surprising that this region of the rod opsin peptide appears to interact with a number of proteins (Tai et al., 1999; Deretic et al., 2005; Chuang et al., 2007; Mazelova et al., 2009). One of its interacting partners is the small GTPase, Arf4, which localizes to the basal part of the photoreceptor inner segment, an area also occupied by the Golgi apparatus (Deretic et al., 2005). GTP-bound Arfs are thought to stimulate vesicle budding via the recruitment of coat proteins (Nie et al., 2003; Nie and Randazzo, 2006). Consistently, antibody blocking of Arf4 function inhibits the formation of rhodopsin carrier vesicles in a retinal extract assay (Deretic et al., 2005). In the same assay, interference with the function of the opsin terminus itself produces a similar phenotype (Deretic et al., 1998). These results suggest that the interaction of Arf4 with the opsin C-terminal sequence is necessary for the budding of opsin transport carrier vesicles. Clearly, Arf4 cannot be the only element of the molecular cascade that regulates vesicle budding. Indeed, recent studies suggest that Arf4 forms a complex with an Arf GAP, ASAP1; another small GTPase, Rab11; and an Arf and Rab11 effector, FIP3 (Mazelova et al., 2009) (Fig. 3C).
Vesicle transport
Once formed, opsin-containing vesicles translocate towards the base of the outer segment. This process was initially inferred based on microscopy and radiolabelling experiments (Young and Droz, 1968; Hall et al., 1969; Papermaster et al., 1985; Papermaster et al., 1986). As photoreceptor inner segment microtubules are directed towards the apical surface (Troutt and Burnside, 1988), dynein is a good candidate for a motor that drives this process. Indeed, this idea is supported by the observation that opsin-bearing vesicles translocate along microtubules in an in vitro assay. Interestingly, this process appears to be mediated by a direct binding interaction between the C-terminus of the opsin polypeptide and one of the dynein light chains (Tai et al., 1999). Given the huge amount of opsin to be transported to the outer segment, and the dire consequences of opsin mistargeting (see “morphogenesis and disease” section below), one would expect a redundancy in this transport mechanism. Alternative mechanisms for the interaction of opsin-bearing vesicles with motor complexes have not been documented however.
Apart from opsins, the targeting of proteins to the outer segment region is poorly investigated. One would expect that all outer segment-bound proteins may share a common targeting signal. This does not, however, appear to be the case. The C-terminal peptide that targets Peripherin/Rds to outer segment membranes does not appear to be related to the opsin targeting sequence (Tam et al., 2004). This lack of obvious targeting motifs is consistent with a recent idea that for many proteins the outer segment is a “default” destination in photoreceptor trafficking, and so it is the proteins that reside outside of the outer segment that require appropriate targeting mechanisms to redirect them elsewhere (Baker et al., 2008).
Fusion of vesicles with the target membrane
Once at the base of the connecting cilium, opsin carrier vesicles appear to merge with the cell membrane in a specialized apical area that consists of several membrane folds (ridges) extending radially from the apical cilium, and referred to as the periciliary ridge complex (Peters et al., 1983) (Fig 3F, inset). It is noteworthy that at least in amphibians, nine membrane folds surround the connecting cilium, suggesting that this structure is related to ciliary architecture, which features nine pairs of microtubules. Several microscopic techniques have been used to demonstrate that opsin carrier vesicles fuse with the membranes of the periciliary ridge complex (Papermaster et al., 1985; Papermaster et al., 1986). On the molecular level, one of the best characterized genetic regulators of this process is Rab8. It localizes to the vicinity of the connecting cilium and its basal body (Deretic et al., 1995), and the overexpression of its dominant negative form results in the accumulation of vesicles at the base of the connecting cilium and a rapid photoreceptor degeneration. This striking phenotype suggests that Rab8 plays a key role in the fusion of opsin carrier vesicles with the cell membrane (Moritz et al., 2001).
The role of Rab8 in outer segment formation is consistent with recent findings that this small GTPase is an important regulator of ciliogenesis. Rab8 localizes to the ciliary membrane in cultured RPE cells, and interacts both with the BBS (Bardet-Biedl syndrome) protein complex and the IFT (intraflagellar transport, see detailed discussion below) particle (Nachury et al., 2007; Omori et al., 2008). These interactions are mediated via its putative effectors, Rabin8 and Rabaptin5. While Rabin8 binds BBS1 (Nachury et al., 2007), Rabaptin5 binds to the Elipsa protein, which in turn strongly associates with IFT20 (Omori et al., 2008; Follit et al., 2009). The Rab8 effector pathways appear to contribute to outer segment morphogenesis as mutations of both BBS1 and Elipsa result in photoreceptor loss (Doerre and Malicki, 2002; Mykytyn et al., 2002). In fact, in elipsa mutants, photoreceptor outer segments are entirely absent. This may be related, however, to a putative function of Elipsa in IFT particle assembly and not to its binding interactions with the Rab8 complex (see below).
Given the involvement of Rab8 in vesicle fusion at the base of the photoreceptor connecting cilium, it is tempting to speculate that perhaps this process could be modeled after yeast exocytosis, which is mediated by the so-called exocyst complex (Jahn et al., 2003). The exocyst does, in fact, localize to cilia in MDCK cells (Rogers et al., 2004), and one of the key initial steps in its assembly in yeast involves a small GTPase, Sec4p, a Rab8 homolog (Ang et al., 2003). Similar to Sec4p, GTP-bound Rab8 could thus initiate vesicle fusion at the base of the connecting cilium. Another small GTPase, which has been proposed to fulfill Sec4p function in multicellular eukaryotes is Rab11 (Beronja et al., 2005). Similar to Rab8 in the vertebrate eye, a reduction of Rab11 activity in fly photoreceptors leads to the accumulation of opsin vesicles in the cytoplasm (Satoh et al., 2005). Rab11 associates with post Golgi vesicles in frog retinae, but its function has been mostly associated with vesicle budding and not fusion so far (Deretic et al., 1996).
The similarity between yeast exocytosis and the targeting of vesicles in photoreceptor cells can be extended further in the fly. In the yeast exocyst formation pathway, GTP-bound Sec4p associates with Sec15p (Guo et al., 1999). A related binding interaction may take place between Rab11 and Sec15 in fly photoreceptors, as Sec15 mutant photoreceptor cells display abnormal distribution of Rab11 and differentiate shorter rhabdomeres (Wu et al., 2005). To our knowledge, a role for Sec15 in vertebrate photoreceptors has not been proposed so far. In addition to homologs of exocyst complex components, Ezrin, Moesin, and Rac1 are candidate regulators of opsin vesicle fusion with the photoreceptor periciliary membrane. They co-localize with Rab8 in the vicinity of the connecting cilium, and are displaced from this area coincident with the blockage of opsin vesicle fusion (Deretic et al., 2004).
Transport within the ciliary compartment
Once at the base of the connecting cilium, outer segment proteins are transported along the ciliary axoneme into the photoreceptor outer segment (Fig. 3B). Although nomenclature suggests that the outer segment and the connecting cilium are distinct entities, the entire outer segment can be thought of as a cilium characterized by a highly expanded and folded membrane.
The motors
Compared to the inner segment, the direction of outer segment microtubules is reversed (Troutt and Burnside, 1988), and accordingly apically-directed microtubule-dependent transport of opsins within the outer segment requires plus end-directed motors. Indeed, kinesins play a crucial role in the transport of opsins and other proteins into the outer segment. In mouse conditional Kif3a knock-out animals, Opsin, Arestin, and Peripherin mislocalize to the inner segment (Marszalek et al., 2000). In contrast to that, the localization of α-transducin is not affected.
The normal localization of transducin in Kif3a mutants is a curious observation, which could be explained by the presence of another parallel transport mechanism, perhaps driven by a different kinesin. Multiple kinesins do, in fact, co-operate in nematode cilia formation (reviewed in Blacque et al., 2008). In the so-called amphidial channels of C. elegans, two kinesins drive cilia formation: a heterotrimeric kinesin, Kif3; and a homodimeric one, OSM-3. The function of these two motors is partially redundant: while the loss of Kif3 function alone does not produce a phenotype, and the absence of OSM-3 affects the distal ciliary segment only, a deficiency of both motors causes a nearly complete loss of the ciliary axoneme (Snow et al., 2004; Ou et al., 2005; Pan et al., 2006). Thus while OSM-3 alone is necessary for the formation of the distal part of amphidial cilia, both motors function redundantly in the proximal part. Interestingly, these genetically distinct mechanisms correlate with structural features of amphidial cilia: while the proximal part of these cilia contains microtubule doublets, the OSM-3 dependant distal part features single microtubules (Ward et al., 1975; Snow et al., 2004).
The nematode studies prompted tests of Kif17, a vertebrate homolog of the nematode OSM-3 kinesin, for a role in photoreceptor outer segment formation. Indeed, an antisense knockdown of Kif17 in zebrafish produces outer segment loss (Insinna et al., 2008). This phenotype appears to be more severe, compared to that associated with Kif3a defect in the mouse: outer segments are largely absent in larval zebrafish following morpholino knockdown (Insinna et al., 2008). Thus two kinesins function in nematode amphidial cilia and vertebrate photoreceptor cells alike. Moreover, similar to nematode cilia, the microtubules of vertebrate photoreceptor cells form doublets at the base of the outer segment and singlets in its distal region (Cohen, 1965; Yacob et al., 1977). Despite these genetic and structural similarities, it is unlikely that the nematode model is applicable to vertebrate photoreceptor cells. In contrast to nematode amphidial cilia, kinesins do not appear to function redundantly in the basal part of the outer segment (Insinna et al., 2008). This is not surprising as even in C. elegans different classes of cilia require differing contributions of homodimeric and heterotrimeric kinesins: these motors are fully redundant in building only a subset of amphidial channel cilia (Evans et al., 2006; Mukhopadhyay et al., 2007). The relative contributions of homodimeric and heterotrimeric kinesins to outer segment formation need to be explored further.
The intraflagellar particle
Ciliary kinesins translocate a protein complex, known as the intraflagellar transport (IFT) particle, which is currently thought to consist of ca. 15–20 polypeptides (for a recent review see Pedersen and Rosenbaum, 2008). IFT proteins are of paramount importance for cilia formation, including the formation of the photoreceptor outer segment. In hypomorphic mutants of the mouse Ift88/polaris gene, photoreceptor outer segments are disorganized and in the mutants of the zebrafish ift88/oval locus they are entirely absent, although some outer segment-like membrane stacks form on the lateral surfaces of the cell (Tsujikawa and Malicki, 2004). Similarly, in zebrafish mutants of elipsa and fleer loci, both encoding IFT particle-associated proteins, outer segments are entirely missing (Doerre and Malicki, 2002). Although one might be tempted to assume that all IFT particle components contribute equally to the particle assembly and function, this does not seem to be the case. In contrast to oval, elipsa, and fleer, the zebrafish curly mutant, a null for ift57 function, does differentiate a short outer segment (Krock and Perkins, 2008). Thus oval and curly loci appear to play somewhat different roles at least in the photoreceptor outer segment, but perhaps also in other cilia. These differences may have to do with the IFT particle structure, a poorly investigated topic at this time.
How does the IFT particle interact with kinesin motors? As Kif3b binds Ift20 in a yeast two-hybrid assay (Baker et al., 2003), it would appear likely that the heterotrimeric kinesin interacts with the IFT particle by binding to its Ift20 component. The Ift20 role does not, however, appear to be that simple. In zebrafish ift57/curly mutants, Ift20 no longer co-precipitates with other IFT proteins and, interestingly, the heterotrimetic kinesin appears to bind the IFT particle stronger (Krock and Perkins, 2008). These experiments suggest that Ift20 is not necessary for kinesin binding, and, to the contrary, may function as a negative regulator of this process. Thus it remains unknown which IFT protein mediates the binding of the particle to Kif3. IFT particle interactions with Kif17 are even less clear.
A good understanding of binding relationships within the IFT particle would help to study its interactions with other protein complexes. Several studies have attempted to determine how IFT proteins bind each other (Baker et al., 2003; Lucker et al., 2005; Omori et al., 2008). For example, IFT57 and IFT20 appear to bind each other, and so do IFT74 and IFT81. As the binding partners of many other IFT proteins remain unknown, it is safe to say that a lot remains to be done before this area is fully understood.
The cargo
It is usually assumed that the main function of the IFT particle is to mediate the transport of polypeptides within the cilium. Perhaps the best documented example of that is the transport of radial spokes to the tips of Chlamydomonas flagella (Qin et al., 2004; Pan and Snell, 2005). In addition to cytoskeletal proteins, IFT is also thought to support the transport of membrane-associated proteins, such as TRP channels (Qin et al., 2005). Naturally, in the context of the photoreceptor one would like to know whether opsin transport requires IFT. Although it is tempting to assume that membrane-embedded opsins interact with IFT particle proteins, biochemical evidence to support this model has not been generated so far. The C-terminal 40 amino acids of opsin do interact, however, with SARA (Smad anchor for receptor activation), a protein initially identified as a component of the TGFβ signaling pathway (Tsukazaki et al., 1998). Interestingly, in photoreceptors SARA localizes to vesicular structures found along the ciliary axoneme, predominantly at the base of the outer segment (Chuang et al., 2007). Interference with SARA function leads to the accumulation of vesicles in this area, a striking phenotype which sometimes appears to result in the rupture of the entire outer segment (Chuang et al., 2007). SARA binds Syntaxin 3 and is thought to mediate a fusion of opsin containing vesicles at the base of the outer segment, a process hypothesized to mediate outer segment disc formation (Fig. 2B). As we pointed out previously, this view contrasts with previous models, suggesting that rod discs form via membrane evagination, followed by enclosure of extracellular space between adjacent evaginations by bilaterally outgrowing disc rim membrane (Tokuyasu and Yamada, 1959; Steinberg et al., 1980). How SARA-positive vesicles form and whether IFT plays a role in their movement remains unknown.
Other ciliary proteins
Many other genes are likely to be involved in the transport of proteins through the photoreceptor connecting cilium. Some of them are clearly required for outer segment formation so that in their absence the outer segment does not form at all. In the case of others, mutant phenotypes involve disorganized outer segments, or photoreceptor degeneration. Retinitis Pigmentosa GTPase Regulator (RPGR) and its binding partner RPGR-interacting protein (RPGRIP), for example, are a pair of proteins that may be involved in photoreceptor ciliary transport (Meindl et al., 1996; Roepman et al., 2000). The evidence for this is based on the observation that the RPGR gene product co-immunoprecipitates with IFT88 (Khanna et al., 2005). The two proteins appear to be functionally related as the localization of RPGR to the connecting cilium requires the presence of RPGRIP, and knockouts of either protein cause outer segment disorganization (Zhao et al., 2003). Another group of proteins that may be involved in photoreceptor ciliary transport are these encoded by Bardet-Biedl syndrome (BBS) loci. In nematode cilia, BBS proteins translocate with the speed characteristic of the IFT particle, and the cilia of nematode BBS mutants are shorter, compared to the wild type (Blacque et al., 2004). In mouse mutants of BBS genes, opsins are mislocalized, a phenotype which may be associated with ciliary transport defect (Nishimura et al., 2004; Abd-El-Barr et al., 2007; Davis et al., 2007).
Nephrocystins are yet another group of proteins that may be involved in ciliary transport. The two nephrocystins that have been investigated in the nematode model so far localize to the base of cilia and function redundantly with B9 domain proteins: double mutants of nephrocystins and B9 proteins display a shortening of cilia (Williams et al., 2008). In addition, the levels of certain IFT machinery components appear reduced in C. elegans nephrocystin mutant cilia, and the speed of IFT52 translocation is also altered (Jauregui et al., 2008). These observations raise the possibility that NPHP genes regulate IFT in at least some types of cilia. In vertebrate photoreceptors, nephrocystin 5 is strongly expressed in the entire outer segment, while nephrocystin 1 localizes to the vicinity of the basal body (Otto et al., 2005; Fliegauf et al., 2006). Interestingly, NPHP6, known also as CEP290, is required for the localization of Rab8 to the primary cilium (Kim et al., 2008; Tsang et al., 2008). Given that Rab8 is thought to function in the delivery of opsin carrier vesicles to the base of the outer segment (see above), it appears that Nephrocystins may participate in multiple molecular events to form and maintain photoreceptor structure.
Finally, it is worth noting that an excessive amount of opsin accumulates near the basal body and in the connecting cilium of mouse strains that carry mutations in the myosin VIIa (Myo7a) gene (Liu et al., 1999). This led to the idea that Myo7a may also contribute to opsin transport. Although this may be the case, this contribution is most likely relatively small as photoreceptors of Myo7a mutant mice display normal or near-normal survival rates (Liu et al., 1999).
Outer Segment Architecture
It is difficult not to be impressed by the regular parallel arrangement of hundreds of membrane folds in the photoreceptor outer segment, and wonder what mechanisms mould them into such an exquisite piece of cellular machinery. Below we review genes currently known to function in the development of outer segment morphology. Most studies have incorporated rodent models and therefore the phenotypes described predominantly relate to rods.
Rhodopsin and disk biogenesis
Although usually thought of as solely a phototransduction protein, rhodopsin is also an indispensable structural protein, as outer segments completely fail to develop in rhodopsin knockout mice (Humphries et al., 1997; Lem et al., 1999). Rhodopsin is expressed in mature disks and the surrounding plasma membrane and thus could be envisaged to have a simple structural role in supporting outer segments. However, rhodopsin is also expressed in nascent disks, and the failure of outer segments to form in rhodopsin knockout mice implicates an unexpected role for rhodopsin in outer segment biogenesis. These findings are cited as supporting evidence for the “fusion” model of outer segment formation mediated by opsin-laden vesicles (Chuang et al., 2007).
Rim proteins
Peripherin/rds and rom-1 are tetraspanin or transmembrane 4 superfamily proteins required for normal disk morphogenesis (reviewed by Goldberg, 2006). In contrast to Opsin, Periperin/rds and Rom-1 specifically localise throughout the disk rims of mature rod disks (Fig. 2G) (Arikawa et al., 1992; Bascom et al., 1992). Periperin/rds is also expressed adjacent to the cilium on the edges of nascent rod disks (Arikawa et al., 1992). Knockout of periperin reveals a differential requirement in rods and cones. Taking advantage of the enrichment of cones in Nrl knockout mice, cone photoreceptor differentiation was studied in peripherin/rds:nrl double knockout mice (Farjo et al., 2006). In these animals, cone outer segments do develop and do retain visual function (Farjo et al., 2006). They are, however, enlarged and morphologically disorganised. These structural abnormalities were proposed to result from impaired folding of evaginating lamellae (Farjo et al., 2006). By contrast, rod outer segments fail to form entirely in periperin/rds mutants (Sanyal and Jansen, 1981; Jansen and Sanyal, 1984). These phenotypic differences between rods and cones may reflect different localization patterns: in cones, peripherin/rds localises to outer segment edges in the areas adjacent the connecting cilium, while in rods it is present around the entire rim of rod discs. The loss of peripherin/rds also prevents the extracellular cone matrix sheaths from enveloping the cone outer segment (Farjo et al., 2006). This suggests that Peripherin/rds anchors both cone outer segments and their extracellular environment. Thus, these studies reveal an essential requirement for the periperin/rds gene in rod outer segment biogenesis, and a more moderate role in the structural organisation of cones.
Rom-1 and peripherin/rds are homologous proteins that form heteroligomers (Goldberg and Molday, 1996; Loewen and Molday, 2000; Loewen et al., 2001). Knockout of Rom-1 results in enlarged disks and disorganised rod outer segments, reminiscent of the phenotype described for the loss of Peripherin/rds in cone outer segments (Clarke et al., 2000). The less severe phenotype seen in the rods of Rom-1 versus Peripherin/rds null mutants may arise from the ability of peripherin to form homotetramers, which presumably can compensate for the absence of Rom-1. The aforementioned complexes of Rom-1 and peripherin/rds have also been proposed to form the curved disk rims by oligomerisation around disk edges or across adjacent membranes (Loewen and Molday, 2000; Boesze-Battaglia et al., 2002).
Base outer segment proteins
Prominin 1 and Protocadherin 21 display another intriguing subcellular distribution: they both localise to the base of the outer segment. They also present with similar mutant phenotypes, suggesting related functions in outer segment morphogenesis. Prominin 1 is a transmembrane glycoprotein, and ultastructural analysis of transgenic mice expressing a mutant form of prominin-1 reveals overgrown and misoriented disk membranes (Yang et al., 2008). Protocadherin 21 is a photoreceptor-specific cadherin localised to nascent outer segment discs on the edges opposite to the connecting cilium. Its localization is complementary to that of peripherin/rds (Rattner et al., 2001). Knockout of the protocadherin 21 gene results in grossly oversized outer segment disks (Rattner et al., 2001). Similar disk overgrowth phenotypes occur following actin filament depolymerisation with cytochalasin D (Williams et al., 1988; Vaughan and Fisher, 1989). This phenotype involves excessive growth of a few nascent disks rather than normal growth of many.
Several observations suggest that Prominin 1 and Protocadherin 21 form a functional complex. First, consistent with their similar expression and disk morphogenesis phenotypes, Prominin 1 has been shown via binding studies to physically interact with Protocadherin 21 and Actin (Yang et al., 2008). Furthermore, the expression of mutant Prominin 1 reduces the levels of cleaved Protocadherin 21 normally observed during disk morphogenesis and reduces interaction with actin. Finally, mutant Prominin 1 expression also results in the mislocalisation of both wild-type Prominin 1 and Protocadherin throughout the photoreceptor, while Rom-1 and a component of the cGMP-gated channel exhibit normal localisation (Yang et al., 2008). What then is the function of the Prominin/Protocadherin complex? As Prominin 1 is associated with a variety of membrane protrusions, it has been proposed that it provides curvature to forming disks or link adjacent disks during outer segment morphogenesis, by cis- and trans- dimerisation of its leucine zipper domains, respectively (Jaszai et al., 2007). The recent identification of physical interactions of Prominin 1 and Protocadherin 21 suggest that these heterodimeric complexes may also regulate the proper alignment of nascent disks (Yang et al., 2008). Finally, Prominin 1 may also function in cooperation with an extracellular matrix protein Spacemaker. Drosophila Prominin and Spacemaker interact at the tips of rhabdomeral microvilli to keep rhabdomeres of neighouring photoreceptors apart from each other (Zelhof et al., 2006).
Plasma membrane proteins
In contrast to Peripherin/rds and Rom-1, which localise to disk rims, the cGMP-gated channel and the Na/Ca-K exchanger localise to the outer segment plasma membrane (Fig. 2G). The β-subunit of the cGMP-gated channel features an N-terminal glutamic acid- and proline-rich region, referred to as GARP (glutamic acid rich protein). In addition, two cytoplasmic GARPs are found in the outer segment. All three proteins appear to be alternative splicing products of a single gene (Colville and Molday, 1996). GARPs localise to the disc rims and incisures of rods but are not found in cone outer segments (Colville and Molday, 1996). As photoreceptor GARPs bind Peripherin/Rds (Poetsch et al., 2001), it has been proposed that their soluble forms bridge Peripherin/rds molecules found on adjacent outer segment discs (Fig. 2G). In addition, the cGMP-gated channel GARP may bridge the plasma membrane with peripherin-2 oligomers in the rim of disk membranes (Poetsch et al., 2001). These results suggest that GARPs mediate photoreceptor morphogenesis by providing adhesive interactions both between outer segment discs and between disc rims and the plasma membrane.
Organelle Distribution
In many cells, organelles distribute in a non-random manner in the cytoplasm (reviewed in Chen and Chan, 2006; Bornens, 2008). In skeletal muscle cells, for example, a cluster of several mitochondria accumulates beneath the neuromuscular junction (Grady et al., 2005). Similarly in neurons, mitochondria are found more frequently at the nerve terminal (Palay, 1956). Photoreceptor cell organelles also clearly distribute in defined fashion: basal to the connecting cilium, the photoreceptor cytoplasm is occupied by a dense cluster of mitochondria (Fig. 3F). This part of the cell is referred to as the ellipsoid (Fig 3F). The Golgi apparatus localizes basal to mitochondria and apical to the cell nucleus (Fig 1), and finally the synaptic apparatus invariably occupies the basal-most portion of the photoreceptor cell (Rodieck, 1973).
The cell nucleus is by far the most bulky cytoplasmic organelle of the photoreceptor and so its localization is critical for the morphology of the entire cell as well as the correct organization of the photoreceptor cell layer (Fig. 4). The mechanism of nuclear positioning was analyzed extensively in Drosophila photoreceptor cells. Studies in this model uncovered that dynein and kinesin motor complexes regulate nuclear position by pulling the nucleus in opposite directions, towards the apical terminus of the cell, and the axon, respectively (Fan and Ready, 1997; Whited et al., 2004). Consistent with these observations, mutations in a dynein cofactor, the DLis-1 polypeptide, also cause a severe basal displacement of photoreceptor nuclei in the fly (Swan et al., 1999). Besides genes that encode motor complex components, the activity of several other loci, including Bic-D, klarsicht, misshapen, disabled, and lamin and are known to influence nuclear position in fly photoreceptor cells (Mosley-Bishop et al., 1999; Swan et al., 1999; Patterson et al., 2004; Houalla et al., 2005; Pramatarova et al., 2006). Two of these genes, klarsicht and laminin, encode nuclear envelope-associated proteins. The Klarsicht protein features a KASH domain: a C-terminal region containing a single transmembrane hydrophobic sequence and a short cytoplasmic tail of ca. 10–35 amino acids (Starr and Fischer, 2005). It localizes to the nuclear envelope and contains an extensive extracellular domain of ca. 2000 amino acids. KASH domain proteins in general are involved in nuclear positioning in several model systems (Starr and Han, 2002; Grady et al., 2005).
Recent experiments reveal that mechanisms regulating nuclear position are very similar in insect and vertebrate photoreceptors. Insights into this area came from the analysis of zebrafish mutant mikre oko (mok). As mokm632 mutant photoreceptors degenerate rapidly (Doerre and Malicki, 2001), the analysis of nuclear position in mutant cells was performed in genetically mosaic retinae. It revealed that the nucleus in mok mutant photoreceptors is strongly displaced towards the synaptic terminus, so that in the most extreme cases it localizes to the outer plexiform layer, and appears to interfere with synaptic differentiation (Tsujikawa et al., 2007). The mikre oko locus encodes the p150 (Dynactin 1) subunit of the dynactin complex and is homologous of the fly glued gene, also involved in the localization of photoreceptor nuclei (Fan and Ready, 1997). Interestingly, both zebrafish and fly p150 nuclear positioning mutants involve similar C-terminal truncations of the polypeptide (Tsujikawa et al., 2007).
Similarities between fly and fish photoreceptors extend further. The interference with another dynactin complex component, p50 (dynactin 2), as well as with the lis1 protein cause a basal mislocalization of nuclei also in zebrafish (Tsujikawa et al., 2007). Furthermore, both in fly and in zebrafish photoreceptors, nuclear positioning is mediated by a KASH-domain proteins, thought to localize to the nuclear envelope (Fig. 4E). These studies support the presence of a mechanism that involves a microtubule-dependent motor(s) which interact with proteins anchored in the nuclear envelope (Tsujikawa et al., 2007).
The current model of nuclear positioning is not without shortcomings. As we already mentioned, KASH proteins feature a short C-terminus and an extremely large N-terminal region, in some cases many thousands of amino acids in length (reviewed in Wilhelmsen et al., 2006). While the C-terminal region is known to interact with Sun proteins (reviewed in Worman and Gundersen, 2006), the N-terminus presumably interacts directly or indirectly with motor complexes. The lack of a molecular mechanisms that would mediate this interaction remains the most significant deficiency of this model.
In contrast to the nucleus, the mechanisms that regulate the distribution of other photoreceptor organelles, the mitochondria and the Golgi apparatus in particular, are nearly unknown. In tissue culture cells, the positioning of the Golgi is also regulated by microtubule dependent motors, dynein in particular (Harada et al., 1998; Smith et al., 2000). While the positioning of the photoreceptor Golgi has not been investigated in mok mutant zebrafish, Kif3a mutant mouse displays a normal localization of the Golgi apparatus, arguing against a role for this particular motor in Golgi positioning (Marszalek et al., 2000). Similarly, so far there is no evidence for the involvement of microtubule- dependent motors in the positioning of mitochondria, although some of these organelles may be mislocalized in the mok mutant as well (Marszalek et al., 2000; Tsujikawa et al., 2007). In many species, mitochondria form a tight cluster in the very apical region of the inner segment (Fig. 3F). It remains to be investigated what mediates their adhesion to each other and what mechanisms position the mitochondrial cluster in a specific area of the cell.
Synapse formation
The synaptic apparatus is the basal-most feature of the vertebrate photoreceptor cell (Fig. 1, 5). Developmentally, synaptic ribbons arise from electron-dense precursor spheres present in photoreceptor terminals, which change shape to form unanchored plate-like ribbons, and finally mature ribbon synapses (Fig. 5) (Regus-Leidig et al., 2009). The precursor spheres transport cytomatrix proteins including Bassoon, Piccolo, RIBEYE, and RIM1 to the location where the mature ribbon synapses settle (Regus-Leidig et al., 2009).
The molecular constituents defining ribbon synapses are becoming clearer. As ribbon synapses are morphological adaptations of conventional chemical synapses, these include standard components of conventional synapses and specialised ribbon synapse proteins. In addition, genetic and biochemical studies have characterised several factors involved in cell-cell/ECM contact, Ca2+-mediated exocytosis and intracellular transport that are required for proper morphogenesis/function of photoreceptor terminals/ribbon synapses (see below).
Ribbon Proteins
Components of the ribbon itself include RIBEYE, Kif3a, RIM, Piccolo, Bassoon and ERC/CAST (Lenzi and von Gersdorff, 2001; Sterling and Matthews, 2005; tom Dieck et al., 2005; tom Dieck and Brandstatter, 2006). RIBEYE is a multidomain protein alternatively transcribed from the Ctbp2 locus (Schmitz et al., 2000). Knockout of RIBEYE by the elimination of the entire Ctbp2 locus was not informative due to early lethality (Hildebrand and Soriano, 2002). However, knockdown of ribeye a in zebrafish results in visual behaviour defects and shortened photoreceptor ribbons (Wan et al., 2005). Consistent with this evidence of a structural role, binding interactions of RIBEYE with Munc119 and Basoon suggest a scaffolding function (tom Dieck et al., 2005; Alpadi et al., 2008). Munc119 is a ribbon-associated protein essential for chemical transmission at the synapse. A nonsense mutation in Munc119 that removes its prenyl-binding domain produces ERG changes and photoreceptor degeneration in the mouse (Kobayashi et al., 2000). The presence of this domain suggests that Munc119 is involved in intracellular membrane and protein trafficking. Munc119 has recently been shown to also physically interact with a calmodulin-like calcium-binding protein, CaBP4 (Alpadi et al., 2008; Haeseleer, 2008). Thus, RIBEYE-Munc119-CaBP4 interactions provide a putative signaling complex that links intracellular calcium levels and the ribbon-associated synaptic vesicles. As pointed out above, RIBEYE also binds Bassoon, a cytomatrix protein localised to the base of the photoreceptor synaptic ribbon (tom Dieck et al., 2005). In Bassoon null mutants, most ribbons float free in the cytoplasm and synaptic transmission is attenuated (Dick et al., 2003). Basoon, also binds ERC2/CAST1 at the ribbon synapse, which suggests that it functions as an intermediary protein anchoring the ribbon to the presynaptic membrane (tom Dieck et al., 2005).
Vesicle and SNARE Proteins
Most proteins localised to vesicles of conventional synapses (e.g. synaptotagmin, synaptophysin, SV2 and Rab3a) are also present in vesicles of ribbon synapses (Lenzi and von Gersdorff, 2001). The exceptions are the synapsins which tether vesicles to the cytoskeleton in conventional synaptic apparatus but are absent from ribbon synapses. This difference may simply reflect the ribbon structure, which provides the same tethering function as the synapsins, or alternatively the unique need of ribbon synapses to accumulate large pools of readily releasable synaptic vesicles, which in turn requires different tethering mechanisms (tom Dieck and Brandstatter, 2006). The core SNARE-complex proteins synaptobrevin, syntaxin and SNAP-25, are also present at ribbon synapses, mediating the fusion of synaptic vesicles with the plasma membrane.
Regulators of calcium-dependent neurotransmitter release
At synaptic termini, glutamate is released as a neurotransmitter by calcium-dependent exocytosis. Elevated intracellular calcium triggers glutamate-laden vesicles to fuse with the presynaptic terminal and release their neurotransmitter into the synaptic cleft. Mutations in molecular components mediating calcium-dependent exocytosis of glutamate are associated with structure-function defects at ribbon synapses. L-type voltage-dependent calcium channels (VDCC) and their regulator, CaBP4, both localize to ribbon synapses. Mice carrying null mutations in an α1F-subunit of VDCC display disorganized synaptic structures, and ectopic horizontal and bipolar cell extensions synapsing in the outer nuclear layer (Mansergh et al., 2005; Chang et al., 2006a; Bayley and Morgans, 2007). Consistently, knockout of a VDCC β2 subunit also results in thinning of the OPL, a significant loss of ribbon synapses, and abnormal ERG b-waves (Ball et al., 2002). Calcium channel activity is regulated by CaBP4, a photoreceptor-specific member of the calmodulin-like calcium-binding protein family (Haeseleer et al., 2004). Cabp4 null mutants also display a thin OPL, reduced numbers of photoreceptor terminals and synaptic ribbons, and reduced ERG a- and b-waves (Haeseleer et al., 2004). As mentioned above, CaBP4 also binds Munc119, forming a potential link between intracellular calcium regulation and ribbon synapse exocytosis. These findings demonstrate that VDCC function is required for the proper establishment and/or maintenance of photoreceptor ribbon synapses.
RIM1 is a Rab3-interacting protein proposed to function in late steps of exocytosis. It interacts with Munc13-1, ERC2/CAST1 and VDCC, all of which regulate synaptic vesicle priming and neurotransmitter release at the presynaptic active zone (tom Dieck and Brandstatter, 2006). Munc13-1 is present only at the active zone compartment of the photoreceptor ribbon synapse wherein it likely primes vesicles for release in conjunction with the VDCC and ERC2/CAST1 (tom Dieck et al., 2005; tom Dieck and Brandstatter, 2006).
Downstream of neurotransmitter release there appears to be a unique requirement for synaptophysin, an integral synaptic vesicle protein, in the formation and recycling of synaptic vesicles at photoreceptor synapses. In most synapses, knockout of synaptophysin does not cause structural defects. In photoreceptors, however, which appear to lack a compensatory protein, knockout of synaptophysin results in a significant reduction of synaptic vesicle number (Spiwoks-Becker et al., 2001).
As signalling mechanisms that connect VDCCs to the release of synaptic vesicles are progressively better understood, it is becoming clear that components of calcium-dependent neurotransmitter release are frequently associated with the development of ribbon synapse morphology, and their defects cause human disease (see below).
Molecular Motors
myosin Va is an actin-based molecular motor involved in organelle and vesicle transport (Tyska and Mooseker, 2003). In the retina, Myosin Va localises predominantly to the synaptic terminals of photoreceptors. Disruption of mouse Myosin Va in the dilute lethal mutant results in abnormal ERGs, with particularly reduced b-wave amplitudes (Libby et al., 2004). Although the ribbons of mutant photoreceptors are properly localized, they often present with an abnormal “club-like” shape and lack synaptic vesicles, reminiscent of the changes in ribbon structure observed in light (Libby et al., 2004). These data suggest that myosin Va functions to establish or maintain the ribbon synapse structure.
As mentioned above, the microtubule-dependent motor, Kif3a, is also a component of the ribbon. It has been proposed that Kif3a moves synaptic vesicles along the ribbon to the release site. Conditional knockout of kif3a in mouse photoreceptors does not, however, result in obvious synaptic abnormalites, although transport to the outer segment is defective (Marszalek et al., 2000). The ultrastructure of the ribbon synapse in kif3a mutants has not been investigated so far.
Extracellular Matrix
The extracellular matrix (ECM) surrounding photoreceptor termini provides a structure for the formation and function of ribbon synapses. A critical component of the photoreceptor synaptic ECM is Pikachurin, a dystroglycan binding-protein that localises to the synaptic cleft of ribbon synapses (Sato et al., 2008). In pikachurin null mice, bipolar cell dendrites fail to invaginate at ribbon synpases, whereas horizontal cell processes are unaffected (Sato et al., 2008). The abnormal morphology of ribbon synapses is associated with defects in retinal physiology. Pikachurin binds Dystroglycan, a component of the Dystrophin-glycoprotein complex (DGC) that links the extracellular matrix to the intracellular cytoskeleton (Drenckhahn et al., 1996). Consistent with that, Dystrophin also localises to rod and cone terminals (Drenckhahn et al., 1996). Moreover, knockouts of laminin β2, yet another extracellular matrix component, cause ultrastructural and physiological defects similar to those observed in pikachurin and dystrophin mutants (Libby et al., 1999). This is in agreement with observations that Dystrophins interact with Laminins via the Dystroglycan complex (Talts et al., 1999). Taken together, these observations suggest that Dystrophin, Dystroglycan, Pikachurin and Laminin function in a network that controls the development and maintenance of photoreceptor synapses.
Endocytosis
As ribbon synapses have significantly higher rates of synaptic vesicle exocytosis than conventional synapses, it is anticipated that they feature compensatory endocytotic mechanisms. Recently, mutations in the endocytic protein, Synaptojanin, have been associated with disrupted ribbon architecture in the zebrafish nrc mutant (Van Epps et al., 2004). Similar to basoon null mutants, nrc animals display an abnormal ERG and floating ribbons (Van Epps et al., 2004). In addition, synaptic vesicles are reduced in number and abnormally distributed within a dense cytoskeletal matrix at the synaptic terminus. nrc synaptojanin 1 is a polyphosphoinositide phosphatase implicated in endocytosis (Van Epps et al., 2004). The increased number of synaptic vesicles and endosomes most likely reflects enhanced actin polymerization and defects in the endocytotic release of clathrin-coated vesicles, associated with impaired PI(4,5)P2 hydrolysis. The function of synaptojanin in anchoring ribbon synapses may reflect a direct requirement for phosphoinositide metabolism or an indirect effect, secondary to defects in actin polymerisation or endocytosis. Overall, these findings reveal a novel link between phosphoinositide metabolism and ribbon morphology.
Photoreceptor Morphogenesis and Disease
One has to wonder how much effort should be invested into the studies of the intricate features that characterize photoreceptor morphology. Is it worthwhile to analyze molecular mechanisms that shape such esoteric structures as the periciliary ridge complex or rod disc incisures? The answer to this question becomes clear when one realizes that many of the mechanisms that we discussed above are involved in defects that lead to photoreceptor degeneration and consequently human blindness. As numerous reviews have been written on the subject of photoreceptor degeneration and the underlying causes (see for example Hartong et al., 2006; Adams et al., 2007; den Hollander et al., 2008; Hildebrandt et al., 2009; Saihan et al., 2009), here we will outline only the most obvious relationships between specific morphogenetic mechanisms and photoreceptor loss. These are also listed in Table 1.
TABLE 1.
Photoreceptor Morphogenesis and Human Disease
| Process | Gene Affected | Disease | References |
|---|---|---|---|
| Membrane Polarity | CRB1 | Leber Congenital Amurosis | (den Hollander et al., 2001) |
| Retinitis Pigmentosa | (den Hollander et al., 1999; den Hollander et al., 2004) | ||
| Transport in the IS | Rod Opsin* | Autosomal Dominant Retinitis Pigmentosa | (Bessant et al., 1999) |
| Peripherin/Rds* | Macular Distrophy | (Nichols et al., 1993) | |
| Transport in the | RP1 | Retinitis pigmentosa | (Pierce et al., 1999) |
| OS/Ciliogenesis | RPGR | Retinitis pigmentosa | (Meindl et al., 1996) |
| RPGRIP | Leber Congenital Amurosis | (reviewed in Koenekoop, 2005) | |
| BBS loci | Bardet Biedl Syndrome | (reviewed in Katsanis, Katsanis)] | |
| NPHP loci | Senior Locken Syndrome | (O’Toole et al., 2006; reviewed in Hildebrandt and Zhou, 2007) | |
| Membrane architecture in the OS | Rod opsin | Retinitis Pigmentosa | (Rosenfeld et al., 1992; Kumaramanickavel et al., 1994) |
| Peripherin/rds | Retinitis Pigmentosa | (Farrar et al., 1991; Kajiwara et al., 1991; reviewed in Goldberg, 2006) | |
| ROM-1 | Retinitis Pigmentosa | (Kajiwara et al., 1994) | |
| Prominin | Macular Degeneration | (Yang et al., 2008) | |
| Spacemaker | Retinitis Pigmentosa | (Collin et al., 2008) | |
| Synapse Fomation and Maintenance | Cacna1f | Congenital Stationary Night Blingness | (Strom et al., 1998) |
| Munc119 | Cone rod dystrophy | (Kobayashi et al., 2000) | |
| RIM1 | Cone rod dystrophy | (Johnson et al., 2003; Michaelides et al., 2005) | |
| Dystrophin | Visual dysfunction associated with Duchenne muscular dustrophy. | (Lenk et al., 1996) |
These defects involve amino acid sequences that mediate transport to the outer segment.
As it is discussed above, when studied in animal models, crumbs genes regulate the size of the apical surface in photoreceptors and epithelial cells alike (Wodarz et al., 1995; Pellikka et al., 2002; Omori and Malicki, 2006). Consistent with these analyses, mutations in one of the three human crumbs paralogs, CRB1, also cause photoreceptor abnormalities: carriers of CRB1 defects display several forms or retinitis pigmentosa as well as Leber Congenital Amaurosis (LCA) (den Hollander et al., 1999; den Hollander et al., 2001; den Hollander et al., 2004). One has to note, however, that vertebrate crumbs genes carry out several functions in the retina, not all confined to the photoreceptor cell. In the zebrafish oko meduzy (ome) mutant, which affects the crb2a gene, the entire retina is disorganized, most likely as the result of a neuroepithelial polarity defect (Omori and Malicki, 2006). Also in the zebrafish, a reduction of apical membrane size in photoreceptors is caused by a knockdown of the crb2b, and not crb1 (Omori and Malicki, 2006). A crb1 phenotype has not been identified in zebrafish so far, and so it remains possible that human CRB1 defects are not related to the regulation of apical cell membrane size, but to CRB1 function in Muller glia or in the eye neuroepithelium. Consistent with the latter possibility, the retinae of at least some human carriers of CRB1 mutations display abnormal layering (Jacobson et al., 2003).
Defects in intracellular transport are a frequent cause of photoreceptor loss as well. The cytoplasmic tail of rod opsin, which targets it to the outer segment, is frequently mutated in autosomal dominant retinitis pigmentosa. Interestingly, most of these mutations affect 5 amino acids located at the very C-terminus of the polypeptide. This mutational hot spot correlates with the VXPX motif, essential for opsin trafficking (reviewed in Deretic, 2006). The importance of the C-terminal amino acids is further underscored by the observation that mutations in this area lead to more severe disease symptoms, compared to defects in other regions of the rod opsin polypeptide (Bessant et al., 1999; Berson et al., 2002).
Cilia-related genes are one of the major causes of hereditary photoreceptor loss. Photoreceptor defects found in ciliary disorders may be due to aberrant transport through the connecting cilium or to structural abnormalities of the outer segment. These two possibilities can be distinguished to some extent using animal models (Gao et al., 2002; Chang et al., 2006b; Abd-El-Barr et al., 2007; Davis et al., 2007). As cilia are ubiquitously present in vertebrate tissues, many cilia-related photoreceptor defects are syndromic, i.e. involve abnormalities of other organs. Photoreceptor loss is thus found in association with kidney malfunction, brain defects, and less frequently with obesity, polydactyly, and several other pathologies. The most common forms of syndromic cilia-related photoreceptor loss are Bardet-Biedl Syndrome (BBS) and nephronoptisis (NPHP). For example, mutations in NPHP6/CEP290 are the most frequent cause of LCA (den Hollander et al., 2007). Defects in this gene are also associated with Joubert, Senior-Loken, and Bardet-Biedl syndromes. Jeune, Mekel-Grueber, Alstrom as well as several other syndromes are less frequent cilia-related disorders (reviewed in Adams et al., 2007). Not all forms of cilia-related photoreceptor loss are syndromic. RP1 and RPGR mutations, for example, produce mostly non-syndromic pathologies (Vervoort and Wright, 2002; Schwartz et al., 2003).
The many protrusions and folds of the photoreceptor cell membrane require specialized molecular mechanisms for both formation and maintenance. Two tetraspanins, peripherin and ROM1, are thought to facilitate the folding of the outer segment membrane (reviewed in Goldberg, 2006). Both were found responsible for retinitis pigmentosa (Farrar et al., 1991; Kajiwara et al., 1991; Kajiwara et al., 1994). Recently, mutations in prominin 1 and a human ortholog of the Drosophila spacemaker gene have also been linked to inherited macular degeneration (Collin et al., 2008; Yang et al., 2008). Another group of proteins that may be associated with the folding of the photoreceptor cell membrane are these found defective in the Usher syndrome. Many of them feature transmembrane domains and several other are likely to function as scaffolding factors (Williams, 2008; Saihan et al., 2009). The USH2A protein localizes to the periciliary but not the ciliary membrane, suggesting a role in the formation of the periciliary ridge complex or related structures (Liu et al., 2007).
Finally, as the photoreceptor synapse is characterized by many intricate morphological features, it is not surprising that defects in several aspects of synaptic architecture lead to cell loss and blindness. A missense mutation in the dystroglycan binding-domain of dystrophin, an extracellular matrix compenent, has been reported to associate with attenuated visual function (Lenk et al., 1996; Schmitz and Drenckhahn, 1997). This pathology may involve a breakdown of pikachurin-dystroglycan interactions at ribbon synapse (Sato et al., 2008). Likewise, mutations in two proteins thought to be involved in synaptic vesicle release are found in cone-rod dystrophies: a nonsense mutation in Munc119 that removes its prenyl-binding protein domain, and a point mutation in the RIM1 gene (Kobayashi et al., 2000; Johnson et al., 2003; Michaelides et al., 2005). Finally, mutations in Cacna1f, the gene encoding the α1F-subunit of Cav1.4 calcium channes result in inherited human congenital stationary night blindness (Bech-Hansen et al., 1998; Strom et al., 1998).
It is also worth noting that human genetic defects have not been found in several mechanisms known to be involved in photoreceptor loss in animal models. Mutations in IFT genes, for example, cause photoreceptor loss both in mouse and zebrafish models (Pazour et al., 2002; Tsujikawa and Malicki, 2004; Sukumaran and Perkins, 2009). In contrast to that, human defects in these genes have not been reported. Although several Jeune syndrome patients were found to carry a mutation in the IFT80 gene, retinal defects appeared to be absent in these individuals (Beales et al., 2007). Similarly, the absence of dynactin mutants in the human patient population is somewhat puzzling, given that a group of hypomorphic p150 mutations result in phenotypes largely confined to the retina both in the fly and in zebrafish. The opposite has also been observed: mouse mutants of human Usher genes do not develop photoreceptor degeneration (Saihan et al., 2009). These observations indicate that significant differences exist between human and animal models in mechanisms that regulate photoreceptor morphogenesis.
CLOSING REMARKS
As the preceding sections of this article reveal, a wealth of information is available about quite a few aspects of photoreceptor morphogenesis. In spite of that, unanswered questions abound in almost every area of photoreceptor differentiation. Some of these are quite fundamental. For example, how are opsins transported along the connecting cilium and into the outer segment? Does this process involve the IFT particle and if so does opsin bind any of the IFT proteins directly? We seem to have some information about how opsin may be added to outer segment discs, but what regulates the size of discs so that they stack parallel to each other in such a precise way is not clear. Several protein-protein interactions have been proposed to mediate adhesion between outer segment membranes but it is not obvious whether these account for all features of outer segment architecture.
The mechanism that mediates the delivery of opsins and other transmembrane proteins to the periciliary membrane is also far from being fully understood. As Rab8 alone is unlikely to mediate the fusion of opsin carrier vesicles with the target membrane, one would like to know what other proteins facilitate this event. Remarkably, once proteins are delivered to the basal body area, they do not diffuse to the rest of the inner segment membrane. The nature of the barrier that accounts for the absence of such diffusion is not known. The mechanisms that mediate oraganelle distribution in cell’s cytoplasm are also incompletely understood. The nature of the interaction between nuclear envelope proteins and microtubule-dependent motors remains an obvious missing piece of the nuclear positioning mechanism. Finally, it is not clear what molecular processes mediate the formation of complex membrane incavitations in the photoreceptor synapse.
Answers to these questions will have to be generated through multiple parallel approaches, including biochemical tests, analysis of cell-free tissue extracts, tissue culture studies, and finally animal models. These approaches are not without challenges as photoreceptors cannot be maintained in tissue culture for prolonged periods of time, tissue extracts do not necessarily reflect in vivo conditions, and genetic studies in animal models are limited by the redundancy and pleiotropy of gene function. The latter problem is particularly acute when studying the molecular bases of ubiquitous processes, intracellular transport or exocytosis for example, in a highly differentiated cell such as the photoreceptor. These obstacles did not, however, stop progress in the past and will not do so in the future.
Human genetic studies have been one of the most productive routes of discovery for loci involved in photoreceptor differentiation. Progress in this area has been remarkably accelerated by advances in mapping and sequencing technologies. As sequencing costs are decreasing at a breathtaking rate, human genetics will continue to be a very exciting field. Unfortunately, discovery of a disease gene does not usually contribute a lot to the understanding of its function on a cellular or molecular level, and hence the uses of animal models and biochemical approaches are of paramount importance. Human genetic studies are also biased towards loci that do not affect embryogenesis, a possible reason why IFT or dynein mutations have not been reported to cause blindness in the human population. In this regard, animal models complement human genetics very well. The mouse, Xenopus and zebrafish are the animal models that have contributed the most to the studies of the photoreceptor cell. Each of these has unique advantages, such as the ability to generate conditional knockouts in the mouse or perform genetic screens in zebrafish. It is thus to be expected that they will be fruitfully used in parallel to each other in future studies. As problems to be solved abound, we expected many exciting advances in the future.
Acknowledgments
The authors wish to thank the following scientists for commenting on earlier versions of this manuscript: Dr. Anneke den Hollander; Dr. Oliver Blacque; Dr. Ching-Hwa Sung; Dr. Tiffany Cook, and Dr. Dusanka Deretic. This work was supported by an NIH RO1 awards EY016859 and EY018176 (to JM), and Science Foundation Ireland and Health Research Board awards (BK).
References
- Abd-El-Barr MM, Sykoudis K, Andrabi S, Eichers ER, Pennesi ME, Tan PL, Wilson JH, Katsanis N, Lupski JR, Wu SM. Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet-Biedl syndrome. Vision Res. 2007;47:3394–3407. doi: 10.1016/j.visres.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams NA, Awadein A, Toma HS. The retinal ciliopathies. Ophthalmic Genet. 2007;28:113–125. doi: 10.1080/13816810701537424. [DOI] [PubMed] [Google Scholar]
- Ali MA. [Retinomotor response: characteristics and mechanisms] Vision Res. 1971;11:1225–1288. doi: 10.1016/0042-6989(71)90010-1. [DOI] [PubMed] [Google Scholar]
- Alpadi K, Magupalli VG, Kappel S, Koblitz L, Schwarz K, Seigel GM, Sung CH, Schmitz F. RIBEYE recruits Munc119, a mammalian ortholog of the Caenorhabditis elegans protein unc119, to synaptic ribbons of photoreceptor synapses. J Biol Chem. 2008;283:26461–26467. doi: 10.1074/jbc.M801625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang AL, Folsch H, Koivisto UM, Pypaert M, Mellman I. The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J Cell Biol. 2003;163:339–350. doi: 10.1083/jcb.200307046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa K, Molday LL, Molday RS, Williams DS. Localization of peripherin/rds in the disk membranes of cone and rod photoreceptors: relationship to disk membrane morphogenesis and retinal degeneration. J Cell Biol. 1992;116:659–667. doi: 10.1083/jcb.116.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Freeman K, Luby-Phelps K, Pazour GJ, Besharse JC. IFT20 links kinesin II with a mammalian intraflagellar transport complex that is conserved in motile flagella and sensory cilia. J Biol Chem. 2003;278:34211–34218. doi: 10.1074/jbc.M300156200. [DOI] [PubMed] [Google Scholar]
- Baker SA, Haeri M, Yoo P, Gospe SM, 3rd, Skiba NP, Knox BE, Arshavsky VY. The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J Cell Biol. 2008;183:485–498. doi: 10.1083/jcb.200806009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, Gregg RG. Role of the beta(2) subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci. 2002;43:1595–1603. [PubMed] [Google Scholar]
- Bascom RA, Manara S, Collins L, Molday RS, Kalnins VI, McInnes RR. Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron. 1992;8:1171–1184. doi: 10.1016/0896-6273(92)90137-3. [DOI] [PubMed] [Google Scholar]
- Bayley PR, Morgans CW. Rod bipolar cells and horizontal cells form displaced synaptic contacts with rods in the outer nuclear layer of the nob2 retina. J Comp Neurol. 2007;500:286–298. doi: 10.1002/cne.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, Johnson C, Irving M, Elcioglu N, Winey M, Tada M, Scambler PJ. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–729. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:264–267. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- Beronja S, Laprise P, Papoulas O, Pellikka M, Sisson J, Tepass U. Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J Cell Biol. 2005;169:635–646. doi: 10.1083/jcb.200410081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson EL, Rosner B, Weigel-DiFranco C, Dryja TP, Sandberg MA. Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations. Invest Ophthalmol Vis Sci. 2002;43:3027–3036. [PubMed] [Google Scholar]
- Bessant DA, Khaliq S, Hameed A, Anwar K, Payne AM, Mehdi SQ, Bhattacharya SS. Severe autosomal dominant retinitis pigmentosa caused by a novel rhodopsin mutation (Ter349Glu). Mutations in brief no. 208. Online. Hum Mutat. 1999;13:83. doi: 10.1002/(SICI)1098-1004(1999)13:1<83::AID-HUMU12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Blacque OE, Cevik S, Kaplan OI. Intraflagellar transport: from molecular characterisation to mechanism. Front Biosci. 2008;13:2633–2652. doi: 10.2741/2871. [DOI] [PubMed] [Google Scholar]
- Blacque OE, Reardon MJ, Li C, McCarthy J, Mahjoub MR, Ansley SJ, Badano JL, Mah AK, Beales PL, Davidson WS, Johnsen RC, Audeh M, Plasterk RH, Baillie DL, Katsanis N, Quarmby LM, Wicks SR, Leroux MR. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 2004;18:1630–1642. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Dispoto J, Kahoe MA. Association of a photoreceptor-specific tetraspanin protein, ROM-1, with triton X-100-resistant membrane rafts from rod outer segment disk membranes. J Biol Chem. 2002;277:41843–41849. doi: 10.1074/jbc.M207111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornens M. Organelle positioning and cell polarity. Nat Rev Mol Cell Biol. 2008;9:874–886. doi: 10.1038/nrm2524. [DOI] [PubMed] [Google Scholar]
- Branchek T, Bremiller R. The Development of Photoreceptors in the Zebrafish, Brachydanio rerio. I. Structure. J Comp Neurol. 1984;224:107–115. doi: 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- Burnside B, Adler R, O’Connor P. Retinomotor pigment migration in the teleost retinal pigment epithelium. I. Roles for actin and microtubules in pigment granule transport and cone movement. Invest Ophthalmol Vis Sci. 1983;24:1–15. [PubMed] [Google Scholar]
- Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Caudron F, Barral Y. Septins and the lateral compartmentalization of eukaryotic membranes. Dev Cell. 2009;16:493–506. doi: 10.1016/j.devcel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Chang B, Heckenlively JR, Bayley PR, Brecha NC, Davisson MT, Hawes NL, Hirano AA, Hurd RE, Ikeda A, Johnson BA, McCall MA, Morgans CW, Nusinowitz S, Peachey NS, Rice DS, Vessey KA, Gregg RG. The nob2 mouse, a null mutation in Cacna1f: anatomical and functional abnormalities in the outer retina and their consequences on ganglion cell visual responses. Vis Neurosci. 2006a;23:11–24. doi: 10.1017/S095252380623102X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, Sayer JA, Otto EA, Attanasio M, O’Toole JF, Jin G, Shou C, Hildebrandt F, Williams DS, Heckenlively JR, Swaroop A. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006b;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chan DC. Critical dependence of neurons on mitochondrial dynamics. Curr Opin Cell Biol. 2006;18:453–459. doi: 10.1016/j.ceb.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Chuang JZ, Zhao Y, Sung CH. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell. 2007;130:535–547. doi: 10.1016/j.cell.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Goldberg AF, Vidgen D, Collins L, Ploder L, Schwarz L, Molday LL, Rossant J, Szel A, Molday RS, Birch DG, McInnes RR. Rom-1 is required for rod photoreceptor viability and the regulation of disk morphogenesis. Nat Genet. 2000;25:67–73. doi: 10.1038/75621. [DOI] [PubMed] [Google Scholar]
- Cohen AI. New Details of the Ultrastructure of the Outer Segments and Ciliary Connectives of the Rods of Human and Macaque Retinas. Anat Rec. 1965;152:63–79. doi: 10.1002/ar.1091520108. [DOI] [PubMed] [Google Scholar]
- Collin RW, Littink KW, Klevering BJ, van den Born LI, Koenekoop RK, Zonneveld MN, Blokland EA, Strom TM, Hoyng CB, den Hollander AI, Cremers FP. Identification of a 2 Mb human ortholog of Drosophila eyes shut/spacemaker that is mutated in patients with retinitis pigmentosa. Am J Hum Genet. 2008;83:594–603. doi: 10.1016/j.ajhg.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville CA, Molday RS. Primary structure and expression of the human beta-subunit and related proteins of the rod photoreceptor cGMP-gated channel. J Biol Chem. 1996;271:32968–32974. doi: 10.1074/jbc.271.51.32968. [DOI] [PubMed] [Google Scholar]
- Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, Agassandian K, Philp AR, Searby CC, Andrews MP, Thompson S, Berry CJ, Thedens DR, Yang B, Weiss RM, Cassell MD, Stone EM, Sheffield VC. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci U S A. 2007;104:19422–19427. doi: 10.1073/pnas.0708571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Dearry A, Edelman JL, Miller S, Burnside B. Dopamine induces light-adaptive retinomotor movements in bullfrog cones via D2 receptors and in retinal pigment epithelium via D1 receptors. J Neurochem. 1990;54:1367–1378. doi: 10.1111/j.1471-4159.1990.tb01971.x. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Davis J, van der Velde-Visser SD, Zonneveld MN, Pierrottet CO, Koenekoop RK, Kellner U, van den Born LI, Heckenlively JR, Hoyng CB, Handford PA, Roepman R, Cremers FP. CRB1 mutation spectrum in inherited retinal dystrophies. Hum Mutat. 2004;24:355–369. doi: 10.1002/humu.20093. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Heckenlively JR, van den Born LI, de Kok YJ, van der Velde-Visser SD, Kellner U, Jurklies B, van Schooneveld MJ, Blankenagel A, Rohrschneider K, Wissinger B, Cruysberg JR, Deutman AF, Brunner HG, Apfelstedt-Sylla E, Hoyng CB, Cremers FP. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am J Hum Genet. 2001;69:198–203. doi: 10.1086/321263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, Koenekoop RK, Mohamed MD, Arts HH, Boldt K, Towns KV, Sedmak T, Beer M, Nagel-Wolfrum K, McKibbin M, Dharmaraj S, Lopez I, Ivings L, Williams GA, Springell K, Woods CG, Jafri H, Rashid Y, Strom TM, van der Zwaag B, Gosens I, Kersten FF, van Wijk E, Veltman JA, Zonneveld MN, van Beersum SE, Maumenee IH, Wolfrum U, Cheetham ME, Ueffing M, Cremers FP, Inglehearn CF, Roepman R. Mutations in LCA5, encoding the ciliary protein lebercilin, cause Leber congenital amaurosis. Nat Genet. 2007;39:889–895. doi: 10.1038/ng2066. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, ten Brink JB, de Kok YJ, van Soest S, van den Born LI, van Driel MA, van de Pol DJ, Payne AM, Bhattacharya SS, Kellner U, Hoyng CB, Westerveld A, Brunner HG, Bleeker-Wagemakers EM, Deutman AF, Heckenlively JR, Cremers FP, Bergen AA. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12) Nat Genet. 1999;23:217–221. doi: 10.1038/13848. [DOI] [PubMed] [Google Scholar]
- Deretic D. A role for rhodopsin in a signal transduction cascade that regulates membrane trafficking and photoreceptor polarity. Vision Res. 2006;46:4427–4433. doi: 10.1016/j.visres.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS. rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995;108 ( Pt 1):215–224. doi: 10.1242/jcs.108.1.215. [DOI] [PubMed] [Google Scholar]
- Deretic D, Puleo-Scheppke B, Trippe C. Cytoplasmic domain of rhodopsin is essential for post-Golgi vesicle formation in a retinal cell-free system. J Biol Chem. 1996;271:2279–2286. doi: 10.1074/jbc.271.4.2279. [DOI] [PubMed] [Google Scholar]
- Deretic D, Schmerl S, Hargrave PA, Arendt A, McDowell JH. Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proc Natl Acad Sci U S A. 1998;95:10620–10625. doi: 10.1073/pnas.95.18.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D, Traverso V, Parkins N, Jackson F, Rodriguez de Turco EB, Ransom N. Phosphoinositides, ezrin/moesin, and rac1 regulate fusion of rhodopsin transport carriers in retinal photoreceptors. Mol Biol Cell. 2004;15:359–370. doi: 10.1091/mbc.E03-04-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4) Proc Natl Acad Sci U S A. 2005;102:3301–3306. doi: 10.1073/pnas.0500095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, Gundelfinger ED, Brandstatter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- Doerre G, Malicki J. A Mutation of Early Photoreceptor Development, mikre oko, Reveals Cell- Cell Interactions Involved in the Survival and Differentiation of Zebrafish Photoreceptors. Journal of Neuroscience. 2001;21:6745–6757. doi: 10.1523/JNEUROSCI.21-17-06745.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerre G, Malicki J. Genetic analysis of photoreceptor cell development in the zebrafish retina. Mechanisms of Development. 2002;110:125–138. doi: 10.1016/s0925-4773(01)00571-8. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D, Holbach M, Ness W, Schmitz F, Anderson LV. Dystrophin and the dystrophin-associated glycoprotein, beta-dystroglycan, co-localize in photoreceptor synaptic complexes of the human retina. Neuroscience. 1996;73:605–612. doi: 10.1016/0306-4522(96)00069-3. [DOI] [PubMed] [Google Scholar]
- Dute R, Kung C. Ultrastructure of the proximal region of somatic cilia in Paramecium tetraurelia. J Cell Biol. 1978;78:451–464. doi: 10.1083/jcb.78.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmiller MS. Cone outer segment morphogenesis: taper change and distal invaginations. J Cell Biol. 1987;105:2267–2277. doi: 10.1083/jcb.105.5.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmiller MS. Microtubules in a rod-specific cytoskeleton associated with outer segment incisures. Vis Neurosci. 2000;17:711–722. doi: 10.1017/s0952523800175054. [DOI] [PubMed] [Google Scholar]
- Eckmiller MS. Defective cone photoreceptor cytoskeleton, alignment, feedback, and energetics can lead to energy depletion in macular degeneration. Prog Retin Eye Res. 2004;23:495–522. doi: 10.1016/j.preteyeres.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Evans JE, Snow JJ, Gunnarson AL, Ou G, Stahlberg H, McDonald KL, Scholey JM. Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J Cell Biol. 2006;172:663–669. doi: 10.1083/jcb.200509115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan SS, Ready DF. Glued participates in distinct microtubule-based activities in Drosophila eye development. Development. 1997;124:1497–1507. doi: 10.1242/dev.124.8.1497. [DOI] [PubMed] [Google Scholar]
- Farjo R, Skaggs JS, Nagel BA, Quiambao AB, Nash ZA, Fliesler SJ, Naash MI. Retention of function without normal disc morphogenesis occurs in cone but not rod photoreceptors. J Cell Biol. 2006;173:59–68. doi: 10.1083/jcb.200509036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar GJ, Kenna P, Jordan SA, Kumar-Singh R, Humphries MM, Sharp EM, Sheils DM, Humphries P. A three-base-pair deletion in the peripherin-RDS gene in one form of retinitis pigmentosa. Nature. 1991;354:478–480. doi: 10.1038/354478a0. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220:197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fliegauf M, Horvath J, von Schnakenburg C, Olbrich H, Muller D, Thumfart J, Schermer B, Pazour GJ, Neumann HP, Zentgraf H, Benzing T, Omran H. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J Am Soc Nephrol. 2006;17:2424–2433. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- Follit JA, Xu F, Keady BT, Pazour GJ. Characterization of mouse IFT complex B. Cell Motil Cytoskeleton. 2009 doi: 10.1002/cm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester Dick, McMenamin Lee. The Eye, Basic Sciences in Practice. Saunders Press; 2003. [Google Scholar]
- Gao J, Cheon K, Nusinowitz S, Liu Q, Bei D, Atkins K, Azimi A, Daiger SP, Farber DB, Heckenlively JR, Pierce EA, Sullivan LS, Zuo J. Progressive photoreceptor degeneration, outer segment dysplasia, and rhodopsin mislocalization in mice with targeted disruption of the retinitis pigmentosa-1 (Rp1) gene. Proc Natl Acad Sci U S A. 2002;99:5698–5703. doi: 10.1073/pnas.042122399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972;53:494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AF. Role of peripherin/rds in vertebrate photoreceptor architecture and inherited retinal degenerations. Int Rev Cytol. 2006;253:131–175. doi: 10.1016/S0074-7696(06)53004-9. [DOI] [PubMed] [Google Scholar]
- Goldberg AF, Molday RS. Subunit composition of the peripherin/rds-rom-1 disk rim complex from rod photoreceptors: hydrodynamic evidence for a tetrameric quaternary structure. Biochemistry. 1996;35:6144–6149. doi: 10.1021/bi960259n. [DOI] [PubMed] [Google Scholar]
- Grady RM, Starr DA, Ackerman GL, Sanes JR, Han M. Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc Natl Acad Sci U S A. 2005;102:4359–4364. doi: 10.1073/pnas.0500711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grawe F, Wodarz A, Lee B, Knust E, Skaer H. The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development. 1996;122:951–959. doi: 10.1242/dev.122.3.951. [DOI] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. Embo J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F. Interaction and colocalization of CaBP4 and Unc119 (MRG4) in photoreceptors. Invest Ophthalmol Vis Sci. 2008;49:2366–2375. doi: 10.1167/iovs.07-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Imanishi Y, Maeda T, Possin DE, Maeda A, Lee A, Rieke F, Palczewski K. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nat Neurosci. 2004;7:1079–1087. doi: 10.1038/nn1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MO, Bok D, Bacharach AD. Biosynthesis and assembly of the rod outer segment membrane system. Formation and fate of visual pigment in the frog retina. J Mol Biol. 1969;45:397–406. doi: 10.1016/0022-2836(69)90114-4. [DOI] [PubMed] [Google Scholar]
- Hansen A, Zeiske E. Development of the olfactory organ in the zebrafish, Brachydanio rerio. J Comp Neurol. 1993;333:289–300. doi: 10.1002/cne.903330213. [DOI] [PubMed] [Google Scholar]
- Harada A, Takei Y, Kanai Y, Tanaka Y, Nonaka S, Hirokawa N. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol. 1998;141:51–59. doi: 10.1083/jcb.141.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WA, Perron M. Molecular recapitulation: the growth of the vertebrate retina. Int J Dev Biol. 1998;42:299–304. [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Soriano P. Overlapping and unique roles for C-terminal binding protein 1 (CtBP1) and CtBP2 during mouse development. Mol Cell Biol. 2002;22:5296–5307. doi: 10.1128/MCB.22.15.5296-5307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. J Am Soc Nephrol. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- Hinds J, Hinds P. Differentiation of photoreceptors and horizontal cells in the embryonic mouse retina: an electron microscopic, serial section analysis. J Comp Neur. 1979;187:495–512. doi: 10.1002/cne.901870303. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Tilney LG. Interactions between actin filaments and between actin filaments and membranes in quick-frozen and deeply etched hair cells of the chick ear. J Cell Biol. 1982;95:249–261. doi: 10.1083/jcb.95.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel C, Neuhauss SC, Biehlmaier O. Time course and development of light adaptation processes in the outer zebrafish retina. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:653–662. doi: 10.1002/ar.a.20329. [DOI] [PubMed] [Google Scholar]
- Hong Y, Stronach B, Perrimon N, Jan LY, Jan YN. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 2001;414:634–638. doi: 10.1038/414634a. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Lin D, Waldron S, Schwarz M, Mbamalu G, Pawson T, Jan Y, Stainier DY, Abdelilah-Seyfried S. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr Biol. 2001;11:1492–1502. doi: 10.1016/s0960-9822(01)00458-4. [DOI] [PubMed] [Google Scholar]
- Houalla T, Hien Vuong D, Ruan W, Suter B, Rao Y. The Ste20-like kinase misshapen functions together with Bicaudal-D and dynein in driving nuclear migration in the developing drosophila eye. Mech Dev. 2005;122:97–108. doi: 10.1016/j.mod.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Willoughby JJ, Christensen AK, Jensen AM. Mosaic Eyes is a novel component of the Crumbs complex and negatively regulates photoreceptor apical size. Development. 2006;133:4849–4859. doi: 10.1242/dev.02685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. How the ear’s works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev Biol. 2008;316:160–170. doi: 10.1016/j.ydbio.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Aleman TS, Pianta MJ, Sumaroka A, Schwartz SB, Smilko EE, Milam AH, Sheffield VC, Stone EM. Crumbs homolog 1 (CRB1) mutations result in a thick human retina with abnormal lamination. Hum Mol Genet. 2003;12:1073–1078. doi: 10.1093/hmg/ddg117. [DOI] [PubMed] [Google Scholar]
- Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Jansen HG, Sanyal S. Development and degeneration of retina in rds mutant mice: electron microscopy. J Comp Neurol. 1984;224:71–84. doi: 10.1002/cne.902240107. [DOI] [PubMed] [Google Scholar]
- Jaszai J, Fargeas CA, Florek M, Huttner WB, Corbeil D. Focus on molecules: prominin-1 (CD133) Exp Eye Res. 2007;85:585–586. doi: 10.1016/j.exer.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Jauregui AR, Nguyen KC, Hall DH, Barr MM. The Caenorhabditis elegans nephrocystins act as global modifiers of cilium structure. J Cell Biol. 2008;180:973–988. doi: 10.1083/jcb.200707090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Hageman GS. Structural and compositional analyses of isolated cone matrix sheaths. Invest Ophthalmol Vis Sci. 1991;32:1951–1957. [PubMed] [Google Scholar]
- Johnson S, Halford S, Morris AG, Patel RJ, Wilkie SE, Hardcastle AJ, Moore AT, Zhang K, Hunt DM. Genomic organisation and alternative splicing of human RIM1, a gene implicated in autosomal dominant cone-rod dystrophy (CORD7) Genomics. 2003;81:304–314. doi: 10.1016/s0888-7543(03)00010-7. [DOI] [PubMed] [Google Scholar]
- Jurgens G, Wieschaus E, Nusslein-Volhard C, Kluding C. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Roux Archiv of Dev Bio. 1984;193:283–295. doi: 10.1007/BF00848157. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Berson EL, Dryja TP. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science. 1994;264:1604–1608. doi: 10.1126/science.8202715. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Hahn LB, Mukai S, Travis GH, Berson EL, Dryja TP. Mutations in the human retinal degeneration slow gene in autosomal dominant retinitis pigmentosa. Nature. 1991;354:480–483. doi: 10.1038/354480a0. [DOI] [PubMed] [Google Scholar]
- Katsanis N. The oligogenic properties of Bardet-Biedl syndrome. Hum Mol Genet. 2004;13(Spec No 1):R65–71. doi: 10.1093/hmg/ddh092. [DOI] [PubMed] [Google Scholar]
- Khanna H, Hurd TW, Lillo C, Shu X, Parapuram SK, He S, Akimoto M, Wright AF, Margolis B, Williams DS, Swaroop A. RPGR-ORF15, which is mutated in retinitis pigmentosa, associates with SMC1, SMC3, and microtubule transport proteins. J Biol Chem. 2005;280:33580–33587. doi: 10.1074/jbc.M505827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet. 2008;17:3796–3805. doi: 10.1093/hmg/ddn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman ME, Ambati J. Fifty years later: the disk goes to the prom. J Clin Invest. 2008;118:2681–2684. doi: 10.1172/JCI36515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E. Photoreceptor morphogenesis and retinal degeneration: lessons from Drosophila. Curr Opin Neurobiol. 2007;17:541–547. doi: 10.1016/j.conb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Higashide T, Hamasaki D, Kubota S, Sakuma H, An W, Fujimaki T, McLaren MJ, Weleber RG, Inana G. HRG4 (UNC119) mutation found in cone-rod dystrophy causes retinal degeneration in a transgenic model. Invest Ophthalmol Vis Sci. 2000;41:3268–3277. [PubMed] [Google Scholar]
- Koenekoop RK. RPGRIP1 is mutated in Leber congenital amaurosis: a mini-review. Ophthalmic Genet. 2005;26:175–179. doi: 10.1080/13816810500374441. [DOI] [PubMed] [Google Scholar]
- Krock BL, Perkins BD. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci. 2008;121:1907–1915. doi: 10.1242/jcs.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaramanickavel G, Maw M, Denton MJ, John S, Srikumari CR, Orth U, Oehlmann R, Gal A. Missense rhodopsin mutation in a family with recessive RP. Nat Genet. 1994;8:10–11. doi: 10.1038/ng0994-10. [DOI] [PubMed] [Google Scholar]
- Lem J, Krasnoperova NV, Calvert PD, Kosaras B, Cameron DA, Nicolo M, Makino CL, Sidman RL. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci U S A. 1999;96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi MH, Medina E, Arsanto JP, Le Bivic A. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell. 2004;15:1324–1333. doi: 10.1091/mbc.E03-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk U, Oexle K, Voit T, Ancker U, Hellner KA, Speer A, Hubner C. A cysteine 3340 substitution in the dystroglycan-binding domain of dystrophin associated with Duchenne muscular dystrophy, mental retardation and absence of the ERG b-wave. Hum Mol Genet. 1996;5:973–975. doi: 10.1093/hmg/5.7.973. [DOI] [PubMed] [Google Scholar]
- Lenzi D, von Gersdorff H. Structure suggests function: the case for synaptic ribbons as exocytotic nanomachines. Bioessays. 2001;23:831–840. doi: 10.1002/bies.1118. [DOI] [PubMed] [Google Scholar]
- Libby RT, Lavallee CR, Balkema GW, Brunken WJ, Hunter DD. Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS. J Neurosci. 1999;19:9399–9411. doi: 10.1523/JNEUROSCI.19-21-09399.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Lillo C, Kitamoto J, Williams DS, Steel KP. Myosin Va is required for normal photoreceptor synaptic activity. J Cell Sci. 2004;117:4509–4515. doi: 10.1242/jcs.01316. [DOI] [PubMed] [Google Scholar]
- Liu X, Bulgakov OV, Darrow KN, Pawlyk B, Adamian M, Liberman MC, Li T. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc Natl Acad Sci U S A. 2007;104:4413–4418. doi: 10.1073/pnas.0610950104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Udovichenko IP, Brown SD, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J Neurosci. 1999;19:6267–6274. doi: 10.1523/JNEUROSCI.19-15-06267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen CJ, Molday RS. Disulfide-mediated oligomerization of Peripherin/Rds and Rom-1 in photoreceptor disk membranes. Implications for photoreceptor outer segment morphogenesis and degeneration. J Biol Chem. 2000;275:5370–5378. doi: 10.1074/jbc.275.8.5370. [DOI] [PubMed] [Google Scholar]
- Loewen CJ, Moritz OL, Molday RS. Molecular characterization of peripherin-2 and rom-1 mutants responsible for digenic retinitis pigmentosa. J Biol Chem. 2001;276:22388–22396. doi: 10.1074/jbc.M011710200. [DOI] [PubMed] [Google Scholar]
- Longley RL, Jr, Ready DF. Integrins and the development of three-dimensional structure in the Drosophila compound eye. Dev Biol. 1995;171:415–433. doi: 10.1006/dbio.1995.1292. [DOI] [PubMed] [Google Scholar]
- Lucker BF, Behal RH, Qin H, Siron LC, Taggart WD, Rosenbaum JL, Cole DG. Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. J Biol Chem. 2005;280:27688–27696. doi: 10.1074/jbc.M505062200. [DOI] [PubMed] [Google Scholar]
- Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- Martinez-Navarrete GC, Angulo A, Martin-Nieto J, Cuenca N. Gradual morphogenesis of retinal neurons in the peripheral retinal margin of adult monkeys and humans. J Comp Neurol. 2008;511:557–580. doi: 10.1002/cne.21860. [DOI] [PubMed] [Google Scholar]
- Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehalow AK, Kameya S, Smith RS, Hawes NL, Denegre JM, Young JA, Bechtold L, Haider NB, Tepass U, Heckenlively JR, Chang B, Naggert JK, Nishina PM. CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Hum Mol Genet. 2003;12:2179–2189. doi: 10.1093/hmg/ddg232. [DOI] [PubMed] [Google Scholar]
- Meindl A, Dry K, Herrmann K, Manson F, Ciccodicola A, Edgar A, Carvalho MR, Achatz H, Hellebrand H, Lennon A, Migliaccio C, Porter K, Zrenner E, Bird A, Jay M, Lorenz B, Wittwer B, D’Urso M, Meitinger T, Wright A. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3) Nat Genet. 1996;13:35–42. doi: 10.1038/ng0596-35. [DOI] [PubMed] [Google Scholar]
- Menger GJ, Koke JR, Cahill GM. Diurnal and circadian retinomotor movements in zebrafish. Vis Neurosci. 2005;22:203–209. doi: 10.1017/S0952523805222083. [DOI] [PubMed] [Google Scholar]
- Mercurio AM, Holtzman E. Smooth endoplasmic reticulum and other agranular reticulum in frog retinal photoreceptors. J Neurocytol. 1982;11:263–293. doi: 10.1007/BF01258247. [DOI] [PubMed] [Google Scholar]
- Michaelides M, Holder GE, Hunt DM, Fitzke FW, Bird AC, Moore AT. A detailed study of the phenotype of an autosomal dominant cone-rod dystrophy (CORD7) associated with mutation in the gene for RIM1. Br J Ophthalmol. 2005;89:198–206. doi: 10.1136/bjo.2004.050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieziewska K. The interphotoreceptor matrix, a space in sight. Microsc Res Tech. 1996;35:463–471. doi: 10.1002/(SICI)1097-0029(19961215)35:6<463::AID-JEMT5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12:2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley-Bishop KL, Li Q, Patterson L, Fischer JA. Molecular analysis of the klarsicht gene and its role in nuclear migration within differentiating cells of the Drosophila eye. Curr Biol. 1999;9:1211–1220. doi: 10.1016/s0960-9822(99)80501-6. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Lu Y, Qin H, Lanjuin A, Shaham S, Sengupta P. Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in C. elegans. Embo J. 2007;26:2966–2980. doi: 10.1038/sj.emboj.7601717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytyn K, Nishimura DY, Searby CC, Shastri M, Yen HJ, Beck JS, Braun T, Streb LM, Cornier AS, Cox GF, Fulton AB, Carmi R, Luleci G, Chandrasekharappa SC, Collins FS, Jacobson SG, Heckenlively JR, Weleber RG, Stone EM, Sheffield VC. Identification of the gene (BBS1) most commonly involved in Bardet-Biedl syndrome, a complex human obesity syndrome. Nat Genet. 2002;31:435–438. doi: 10.1038/ng935. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nichols BE, Drack AV, Vandenburgh K, Kimura AE, Sheffield VC, Stone EM. A 2 base pair deletion in the RDS gene associated with butterfly-shaped pigment dystrophy of the fovea. Hum Mol Genet. 1993;2:1347. doi: 10.1093/hmg/2.8.1347-a. [DOI] [PubMed] [Google Scholar]
- Nie Z, Hirsch DS, Randazzo PA. Arf and its many interactors. Curr Opin Cell Biol. 2003;15:396–404. doi: 10.1016/s0955-0674(03)00071-1. [DOI] [PubMed] [Google Scholar]
- Nie Z, Randazzo PA. Arf GAPs and membrane traffic. J Cell Sci. 2006;119:1203–1211. doi: 10.1242/jcs.02924. [DOI] [PubMed] [Google Scholar]
- Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang B, Carmi R, Stone EM, Sheffield VC. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci U S A. 2004;101:16588–16593. doi: 10.1073/pnas.0405496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor P, Burnside B. Actin-dependent cell elongation in teleost retinal rods: requirement for actin filament assembly. J Cell Biol. 1981;89:517–524. doi: 10.1083/jcb.89.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor P, Burnside B. Elevation of cyclic AMP activates an actin-dependent contraction in teleost retinal rods. J Cell Biol. 1982;95:445–452. doi: 10.1083/jcb.95.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole JF, Otto EA, Frishberg Y, Hildebrandt F. Retinitis pigmentosa and renal failure in a patient with mutations in INVS. Nephrol Dial Transplant. 2006;21:1989–1991. doi: 10.1093/ndt/gfl088. [DOI] [PubMed] [Google Scholar]
- Obata S, Usukura J. Morphogenesis of the photoreceptor outer segment during postnatal development in the mouse (BALB/c) retina. Cell Tissue Res. 1992;269:39–48. doi: 10.1007/BF00384724. [DOI] [PubMed] [Google Scholar]
- Olsson JE, Gordon JW, Pawlyk BS, Roof D, Hayes A, Molday RS, Mukai S, Cowley GS, Berson EL, Dryja TP. Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa. Neuron. 1992;9:815–830. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- Omori Y, Malicki J. oko meduzy and Related crumbs Genes Are Determinants of Apical Cell Features in the Vertebrate Embryo. Current Biology. 2006;16:945–957. doi: 10.1016/j.cub.2006.03.058. [DOI] [PubMed] [Google Scholar]
- Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, Furukawa T, Sengupta P, Veraksa A, Malicki J. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nature Cell Bio. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O’Toole JF, Helou J, Attanasio M, Utsch B, Sayer JA, Lillo C, Jimeno D, Coucke P, De Paepe A, Reinhardt R, Klages S, Tsuda M, Kawakami I, Kusakabe T, Omran H, Imm A, Tippens M, Raymond PA, Hill J, Beales P, He S, Kispert A, Margolis B, Williams DS, Swaroop A, Hildebrandt F. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005;37:282–288. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–587. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- Palay SL. Synapses in the central nervous system. J Biophys Biochem Cytol. 1956;2:193–202. doi: 10.1083/jcb.2.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Snell WJ. Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking, and blocking anterograde cargo loading. Dev Cell. 2005;9:431–438. doi: 10.1016/j.devcel.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Pan X, Ou G, Civelekoglu-Scholey G, Blacque OE, Endres NF, Tao L, Mogilner A, Leroux MR, Vale RD, Scholey JM. Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J Cell Biol. 2006;174:1035–1045. doi: 10.1083/jcb.200606003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, Besharse JC. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest Ophthalmol Vis Sci. 1985;26:1386–1404. [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, DeFoe D, Besharse JC. Biosynthesis and vectorial transport of opsin on vesicles in retinal rod photoreceptors. J Histochem Cytochem. 1986;34:5–16. doi: 10.1177/34.1.2934469. [DOI] [PubMed] [Google Scholar]
- Patterson K, Molofsky AB, Robinson C, Acosta S, Cater C, Fischer JA. The functions of Klarsicht and nuclear lamin in developmentally regulated nuclear migrations of photoreceptor cells in the Drosophila eye. Mol Biol Cell. 2004;15:600–610. doi: 10.1091/mbc.E03-06-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Rosenbaum JL. Chapter Two Intraflagellar Transport (IFT) Role in Ciliary Assembly, Resorption and Signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, Ready DF, Tepass U. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–149. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- Perkins BD, Kainz PM, O’Malley DM, Dowling JE. Transgenic expression of a GFP-rhodopsin COOH-terminal fusion protein in zebrafish rod photoreceptors. Vis Neurosci. 2002;19:257–264. doi: 10.1017/s0952523802192030. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Peters KR, Palade GE, Schneider BG, Papermaster DS. Fine structure of a periciliary ridge complex of frog retinal rod cells revealed by ultrahigh resolution scanning electron microscopy. J Cell Biol. 1983;96:265–276. doi: 10.1083/jcb.96.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce EA, Quinn T, Meehan T, McGee TL, Berson EL, Dryja TP. Mutations in a gene encoding a new oxygen-regulated photoreceptor protein cause dominant retinitis pigmentosa. Nat Genet. 1999;22:248–254. doi: 10.1038/10305. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Besharse JC. Circadian regulation of retinomotor movements. I. Interaction of melatonin and dopamine in the control of cone length. J Gen Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce ME, Besharse JC. Circadian regulation of retinomotor movements: II. The role of GABA in the regulation of cone position. J Comp Neurol. 1988;270:279–287. doi: 10.1002/cne.902700208. [DOI] [PubMed] [Google Scholar]
- Poetsch A, Molday LL, Molday RS. The cGMP-gated channel and related glutamic acid-rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J Biol Chem. 2001;276:48009–48016. doi: 10.1074/jbc.M108941200. [DOI] [PubMed] [Google Scholar]
- Pramatarova A, Ochalski PG, Lee CH, Howell BW. Mouse disabled 1 regulates the nuclear position of neurons in a Drosophila eye model. Mol Cell Biol. 2006;26:1510–1517. doi: 10.1128/MCB.26.4.1510-1517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E, Lamb T. Phototransduction in Vertebrate Rods and Cones. Handbook of Biological Physics. 2000:183–255. [Google Scholar]
- Qin H, Burnette DT, Bae YK, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr Biol. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao-Mirotznik R, Harkins AB, Buchsbaum G, Sterling P. Mammalian rod terminal: architecture of a binary synapse. Neuron. 1995;14:561–569. doi: 10.1016/0896-6273(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Rattner A, Smallwood PM, Williams J, Cooke C, Savchenko A, Lyubarsky A, Pugh EN, Nathans J. A photoreceptor-specific cadherin is essential for the structural integrity of the outer segment and for photoreceptor survival. Neuron. 2001;32:775–786. doi: 10.1016/s0896-6273(01)00531-1. [DOI] [PubMed] [Google Scholar]
- Regus-Leidig H, Tom Dieck S, Specht D, Meyer L, Brandstatter JH. Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: the involvement of precursor spheres. J Comp Neurol. 2009;512:814–824. doi: 10.1002/cne.21915. [DOI] [PubMed] [Google Scholar]
- Rey HL, Burnside B. Adenosine stimulates cone photoreceptor myoid elongation via an adenosine A2-like receptor. J Neurochem. 1999;72:2345–2355. doi: 10.1046/j.1471-4159.1999.0722345.x. [DOI] [PubMed] [Google Scholar]
- Rodieck RW. Principles of Structure and Function. San Francisco, California: W. H. Freeman & Co; 1973. The Vertebrate Retina. [Google Scholar]
- Roepman R, Bernoud-Hubac N, Schick DE, Maugeri A, Berger W, Ropers HH, Cremers FP, Ferreira PA. The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Hum Mol Genet. 2000;9:2095–2105. doi: 10.1093/hmg/9.14.2095. [DOI] [PubMed] [Google Scholar]
- Rogers KK, Wilson PD, Snyder RW, Zhang X, Guo W, Burrow CR, Lipschutz JH. The exocyst localizes to the primary cilium in MDCK cells. Biochem Biophys Res Commun. 2004;319:138–143. doi: 10.1016/j.bbrc.2004.04.165. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Cowley GS, McGee TL, Sandberg MA, Berson EL, Dryja TP. A null mutation in the rhodopsin gene causes rod photoreceptor dysfunction and autosomal recessive retinitis pigmentosa. Nat Genet. 1992;1:209–213. doi: 10.1038/ng0692-209. [DOI] [PubMed] [Google Scholar]
- Saihan Z, Webster AR, Luxon L, Bitner-Glindzicz M. Update on Usher syndrome. Curr Opin Neurol. 2009;22:19–27. doi: 10.1097/wco.0b013e3283218807. [DOI] [PubMed] [Google Scholar]
- Sameshima M, Uehara F, Ohba N. Specialization of the interphotoreceptor matrices around cone and rod photoreceptor cells in the monkey retina, as revealed by lectin cytochemistry. Exp Eye Res. 1987;45:845–863. doi: 10.1016/s0014-4835(87)80101-x. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Jansen HG. Absence of receptor outer segments in the retina of rds mutant mice. Neurosci Lett. 1981;21:23–26. doi: 10.1016/0304-3940(81)90051-3. [DOI] [PubMed] [Google Scholar]
- Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, Sawai H, Kobayashi K, Tani A, Toda T, Usukura J, Tano Y, Fujikado T, Furukawa T. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 2008;11:923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- Satoh AK, O’Tousa JE, Ozaki K, Ready DF. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development. 2005;132:1487–1497. doi: 10.1242/dev.01704. [DOI] [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Early retinal development in the zebrafish, Danio rerio: light and electron microscopic analyses. J Comp Neurol. 1999;404:515–536. [PubMed] [Google Scholar]
- Schmitz F, Drenckhahn D. Dystrophin in the retina. Prog Neurobiol. 1997;53:547–560. doi: 10.1016/s0301-0082(97)00047-6. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Schwartz SB, Aleman TS, Cideciyan AV, Swaroop A, Jacobson SG, Stone EM. De novo mutation in the RP1 gene (Arg677ter) associated with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2003;44:3593–3597. doi: 10.1167/iovs.03-0155. [DOI] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Smith DS, Niethammer M, Ayala R, Zhou Y, Gambello MJ, Wynshaw-Boris A, Tsai LH. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat Cell Biol. 2000;2:767–775. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–1113. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Glas M, Lasarzik I, Vollrath L. Mouse photoreceptor synaptic ribbons lose and regain material in response to illumination changes. Eur J Neurosci. 2004;19:1559–1571. doi: 10.1111/j.1460-9568.2004.03198.x. [DOI] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Vollrath L, Seeliger MW, Jaissle G, Eshkind LG, Leube RE. Synaptic vesicle alterations in rod photoreceptors of synaptophysin-deficient mice. Neuroscience. 2001;107:127–142. doi: 10.1016/s0306-4522(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Starr DA, Fischer JA. KASH ‘n Karry: the KASH domain family of cargo-specific cytoskeletal adaptor proteins. Bioessays. 2005;27:1136–1146. doi: 10.1002/bies.20312. [DOI] [PubMed] [Google Scholar]
- Starr DA, Han M. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science. 2002;298:406–409. doi: 10.1126/science.1075119. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Fisher SK, Anderson DH. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol. 1980;190:501–508. doi: 10.1002/cne.901900307. [DOI] [PubMed] [Google Scholar]
- Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Stone J, van Driel D, Valter K, Rees S, Provis J. The locations of mitochondria in mammalian photoreceptors: relation to retinal vasculature. Brain Res. 2008;1189:58–69. doi: 10.1016/j.brainres.2007.10.083. [DOI] [PubMed] [Google Scholar]
- Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Ruther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:260–263. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- Sukumaran S, Perkins BD. Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 Intraflagellar Transport mutants. Vision Res. 2009 doi: 10.1016/j.visres.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan A, Nguyen T, Suter B. Drosophila Lissencephaly-1 functions with Bic-D and dynein in oocyte determination and nuclear positioning. Nat Cell Biol. 1999;1:444–449. doi: 10.1038/15680. [DOI] [PubMed] [Google Scholar]
- Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin’s carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Talts JF, Andac Z, Gohring W, Brancaccio A, Timpl R. Binding of the G domains of laminin alpha1 and alpha2 chains and perlecan to heparin, sulfatides, alpha-dystroglycan and several extracellular matrix proteins. Embo J. 1999;18:863–870. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Hurd LB, Papermaster DS. Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J Cell Biol. 2000;151:1369–1380. doi: 10.1083/jcb.151.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Papermaster DS. The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures. Mol Biol Cell. 2004;15:2027–2037. doi: 10.1091/mbc.E03-09-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K, Yamada E. The fine structure of the retina studied with the electron microscope. IV. Morphogenesis of outer segments of retinal rods. J Biophys Biochem Cytol. 1959;6:225–230. doi: 10.1083/jcb.6.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, Fejtova A, Bracko O, Gundelfinger ED, Brandstatter JH. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol. 2005;168:825–836. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tom Dieck S, Brandstatter JH. Ribbon synapses of the retina. Cell Tissue Res. 2006;326:339–346. doi: 10.1007/s00441-006-0234-0. [DOI] [PubMed] [Google Scholar]
- Townes-Anderson E, Dacheux RF, Raviola E. Rod photoreceptors dissociated from the adult rabbit retina. J Neurosci. 1988;8:320–331. doi: 10.1523/JNEUROSCI.08-01-00320.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes-Anderson E, MacLeish PR, Raviola E. Rod cells dissociated from mature salamander retina: ultrastructure and uptake of horseradish peroxidase. J Cell Biol. 1985;100:175–188. doi: 10.1083/jcb.100.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutt LL, Burnside B. Microtubule polarity and distribution in teleost photoreceptors. J Neurosci. 1988;8:2371–2380. doi: 10.1523/JNEUROSCI.08-07-02371.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang WY, Bossard C, Khanna H, Peranen J, Swaroop A, Malhotra V, Dynlacht BD. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell. 2008;15:187–197. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- Tsujikawa M, Omori Y, Biyanwila J, Malicki J. Mechanism of positioning the cell nucleus in vertebrate photoreceptors. Proc Natl Acad Sci U S A. 2007;104:14819–14824. doi: 10.1073/pnas.0700178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- Tyska MJ, Mooseker MS. Myosin-V motility: these levers were made for walking. Trends Cell Biol. 2003;13:447–451. doi: 10.1016/s0962-8924(03)00172-7. [DOI] [PubMed] [Google Scholar]
- van de Pavert SA, Kantardzhieva A, Malysheva A, Meuleman J, Versteeg I, Levelt C, Klooster J, Geiger S, Seeliger MW, Rashbass P, Le Bivic A, Wijnholds J. Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J Cell Sci. 2004;117:4169–4177. doi: 10.1242/jcs.01301. [DOI] [PubMed] [Google Scholar]
- Van Epps HA, Hayashi M, Lucast L, Stearns GW, Hurley JB, De Camilli P, Brockerhoff SE. The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. J Neurosci. 2004;24:8641–8650. doi: 10.1523/JNEUROSCI.2892-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan DK, Fisher SK. Cytochalasin D disrupts outer segment disc morphogenesis in situ in rabbit retina. Invest Ophthalmol Vis Sci. 1989;30:339–342. [PubMed] [Google Scholar]
- Vervoort R, Wright AF. Mutations of RPGR in X-linked retinitis pigmentosa (RP3) Hum Mutat. 2002;19:486–500. doi: 10.1002/humu.10057. [DOI] [PubMed] [Google Scholar]
- Vollrath L, Spiwoks-Becker I. Plasticity of retinal ribbon synapses. Microsc Res Tech. 1996;35:472–487. doi: 10.1002/(SICI)1097-0029(19961215)35:6<472::AID-JEMT6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Wan L, Almers W, Chen W. Two ribeye genes in teleosts: the role of Ribeye in ribbon formation and bipolar cell development. J Neurosci. 2005;25:941–949. doi: 10.1523/JNEUROSCI.4657-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans.?2UU. J Comp Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- Warren RH, Brunside B. Microtubules in cone myoid elongation in the teleost retina. J Cell Biol. 1978;78:247–259. doi: 10.1083/jcb.78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H. The Neural Basis of Early Vision. Tokyo: Springer Verlag; 2003. The cone pedicle, the first synapse in the retina; pp. 19–38. [Google Scholar]
- Wei X, Cheng Y, Luo Y, Shi X, Nelson S, Hyde DR. The zebrafish Pard3 ortholog is required for separation of the eye fields and retinal lamination. Dev Biol. 2004;269:286–301. doi: 10.1016/j.ydbio.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Wei X, Malicki J. nagie oko, encoding a MAGUK-family protein, is essential for cellular patterning of the retina. Nature Genetics. 2002;31:150–157. doi: 10.1038/ng883. [DOI] [PubMed] [Google Scholar]
- Wetts R, Serbedzija G, Fraser S. Cell Lineage Analysis Reveals Multipotent Precursors in the Ciliary Margin of the Frog Retina. Dev Bio. 1989;136:254–263. doi: 10.1016/0012-1606(89)90146-2. [DOI] [PubMed] [Google Scholar]
- Whited JL, Cassell A, Brouillette M, Garrity PA. Dynactin is required to maintain nuclear position within postmitotic Drosophila photoreceptor neurons. Development. 2004;131:4677–4686. doi: 10.1242/dev.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K, Ketema M, Truong H, Sonnenberg A. KASH-domain proteins in nuclear migration, anchorage and other processes. J Cell Sci. 2006;119:5021–5029. doi: 10.1242/jcs.03295. [DOI] [PubMed] [Google Scholar]
- Williams CL, Winkelbauer ME, Schafer JC, Michaud EJ, Yoder BK. Functional Redundancy of the B9 Proteins and Nephrocystins in Caenorhabditis elegans Ciliogenesis. Mol Biol Cell. 2008;19:2154–2168. doi: 10.1091/mbc.E07-10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS. Usher syndrome: animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Res. 2008;48:433–441. doi: 10.1016/j.visres.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Arikawa K, Paallysaho T. Cytoskeletal components of the adherens junctions between the photoreceptors and the supportive Muller cells. J Comp Neurol. 1990;295:155–164. doi: 10.1002/cne.902950113. [DOI] [PubMed] [Google Scholar]
- Williams DS, Linberg KA, Vaughan DK, Fariss RN, Fisher SK. Disruption of microfilament organization and deregulation of disk membrane morphogenesis by cytochalasin D in rod and cone photoreceptors. J Comp Neurol. 1988;272:161–176. doi: 10.1002/cne.902720202. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Gundersen GG. Here come the SUNs: a nucleocytoskeletal missing link. Trends Cell Biol. 2006;16:67–69. doi: 10.1016/j.tcb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol. 2005;12:879–885. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- Yacob A, Wise C, Kunz YW. The accessory outer segment of rods and cones in the retina of the guppy, Poecilia reticulata P. (Teleostei). An electron microscopical study. Cell Tissue Res. 1977;177:181–193. doi: 10.1007/BF00221080. [DOI] [PubMed] [Google Scholar]
- Yang Z, Chen Y, Lillo C, Chien J, Yu Z, Michaelides M, Klein M, Howes KA, Li Y, Kaminoh Y, Chen H, Zhao C, Al-Sheikh YT, Karan G, Corbeil D, Escher P, Kamaya S, Li C, Johnson S, Frederick JM, Zhao Y, Wang C, Cameron DJ, Huttner WB, Schorderet DF, Munier FL, Moore AT, Birch DG, Baehr W, Hunt DM, Williams DS, Zhang K. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest. 2008;118:2908–2916. doi: 10.1172/JCI35891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Shedding of discs from rod outer segments in the rhesus monkey. J Ultrastruct Res. 1971;34:190–203. doi: 10.1016/s0022-5320(71)90014-1. [DOI] [PubMed] [Google Scholar]
- Young RW, Droz B. The renewal of protein in retinal rods and cones. J Cell Biol. 1968;39:169–184. doi: 10.1083/jcb.39.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelhof AC, Hardy RW, Becker A, Zuker CS. Transforming the architecture of compound eyes. Nature. 2006;443:696–699. doi: 10.1038/nature05128. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hong DH, Pawlyk B, Yue G, Adamian M, Grynberg M, Godzik A, Li T. The retinitis pigmentosa GTPase regulator (RPGR)- interacting protein: subserving RPGR function and participating in disk morphogenesis. Proc Natl Acad Sci U S A. 2003;100:3965–3970. doi: 10.1073/pnas.0637349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Lathrop KL, Sun M, Wei X. Intact retinal pigment epithelium maintained by Nok is essential for retinal epithelial polarity and cellular patterning in zebrafish. J Neurosci. 2008;28:13684–13695. doi: 10.1523/JNEUROSCI.4333-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]