Abstract
Synovium is considered a candidate source of cells for cartilage tissue engineering. Compared to the mesenchymal stem cells (MSCs) from other sources, synovium-derived stem cells (SDSCs) have a higher capacity for chondrogenic differentiation. Our objective was to define cocktails of growth factors that support the growth and chondrogenic differentiation of SDSCs in chemically defined medium. We established a fast and highly selective technique of negative isolation of SDSC populations. The individual and combined effects of three growth factors – transforming growth factor-β1 (TGF-β1), insulin-like growth factor I (IGF-I), and basic fibroblast growth factor (FGF-2) – were evaluated in serum-free pellet cultures of SDSCs for the chondrogenesis of SDSCs using histology, biochemical analysis and real-time RT-PCR. In vitro studies identified TGF-β1 as the key factor for both the growth and chondrogenesis of SDSCs. The highest rates of SDSC growth were observed with the synergistic interaction of all three factors. With respect to chondrogenic differentiation of SDSCs, the interaction of TGF-β1 and IGF-I applied simultaneously was superior to the sequential application of these two factors or any other combination of growth factors studied. Based on these findings, we propose a two-step protocol for the derivation of chondrogenic SDSCs: a cocktail of TGF-β1, IGF-I and FGF-2 is applied first to induce cell growth followed by a cocktail of TGF-β1 and IGF-I applied to induce chondrogenesis.
Keywords: Synovium, Mesenchymal stem cell, Chondrogenesis, TGF-β1, FGF-2, IGF-I
Introduction
Cartilage formation is driven by mesenchymal stem cells (MSCs) that proliferate, condense and differentiate into chondrocytes. MSCs are an attractive cell source for cartilage tissue engineering because these cells can not only differentiate into skeletal cell lineages, but also have the ability to mediate the response of immune cells in vivo (Chamberlain et al., 2007). Compared to other sources of MSCs, synovium has a higher chondrogenic capacity, rendering this tissue an excellent candidate source of cells for engineering of cartilage (Sakaguchi et al., 2005; Shirasawa et al., 2006; Mochizuki et al., 2006; Yoshimura et al., 2007).
Sequential application of growth factors has been studied in an attempt to recapitulate the developmental events associated with chondrogenesis (Pei et al., 2002a). Developmentally relevant growth factors such as transforming growth factor β(TGF-β), insulin-like growth factor I (IGF-I), and basic fibroblast growth factor (FGF-2) have been investigated to identify their roles in regulating the in vitro chondrogenesis of MSCs (Lennon et al., 1995; Trippel, 1995; Yamaguchi, 1995; Johnstone et al., 1998). TGF-β1 has been shown to stimulate collagen type II and proteoglycan expression in MSCs (Yamaguchi, 1995). FGF, a factor homologous with the cartilage-derived growth factor, was shown to stimulate both DNA and proteoglycan synthesis in cartilage (Gospodarowicz et al., 1987; Crabb et al., 1990). IGF-I, a factor expressed in developing cartilage within the condensing region of the limb, and also in mature cartilage and synovial fluid, was shown to regulate chondrogenesis in MSCs and anabolism of cartilage matrix (Martel-Pelletier et al., 1998).
We previously used a method of fast negative selection to remove macrophages and isolate a population of cells (Pei et al., 2008a) that rapidly proliferated in pellet cultures when treated with FGF-2 (50 ng/mL) and underwent chondrogenesis when treated with a combination of IGF-I (500 ng/mL) and TGF-β1 (10 ng/mL) (Pei et al., 2005). However, there is no direct evidence that these negatively isolated cells are in fact the synovium-derived stem cells (SDSCs). Also, there is no data for the combination and sequence of growth factors that induce rapid proliferation of SDSCs with chondrogenic potential. It is still controversial if the chondrogenic effect of TGF-β1 and IGF-I is maximized using these two factors in a combination pattern (Fukumoto et al., 2003) or sequentially (Worster et al., 2001). We attempted to collect experimental data to help answer these important questions.
The present study was designed to test the hypotheses (1) that cells derived from synovial tissue by negative isolation are SDSCs, (2) that proliferation of SDSCs with chondrogenic potential can be markedly promoted by using a combination of FGF-2, IGF-I and TGF-β1, and (3) chondrogenic differentiation can be dramatically enhanced by using a combination of IGF-I and TGF-β1. Our goal was to investigate the individual and combined actions of FGF-2, IGF-I and TGF-β1 on SDSCs for the application of growth factors resulting in maximum SDSC-based chondrogenic differentiation.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), phosphate-buffered saline (PBS), fetal bovine serum (FBS), trypsin-EDTA solution, penicillin, streptomycin, NuPAGE® Novex® Bis-Tris Mini Gels, XCell SureLock™ Mini-Cell, nitrocellulose membrane, and XCell II™ Blot module were from Invitrogen (Carlsbad, CA). Ascorbic acid 2-phosphate was from Wako Chemicals USA Inc. (Richmond, VA). Dynabeads M-450 CD14 (clone RMO52) and Dynal Magnetic Particle Concentrator® were from Dynal Biotech (Oslo, Norway). Trypsin and collagenase P were from Roche (Indianapolis, IN). ITS+Premix, consisting of insulin (6.25 μg/mL), transferrin (6.25 μg/mL), selenous acid (6.25 μg/mL), linoleic acid (5.35 μg/mL) and bovine serum albumin (1.25 μg/mL), were from Collaborative Biomedical (BD, Bedford, MA). Hoechst 33258 dye was from Polysciences (Warrington, PA). Dimethylmethylene blue dye was from Aldrich (Milwaukee, WI). Recombinant human growth factors, including transforming growth factor β1 (TGF-β1), basic fibroblast growth factor (FGF-2), and insulin-like growth factor I (IGF-I), were from R&D Systems (Minneapolis, MN). The monoclonal antibodies used in this study include collagen type I (Sigma, St. Louis, MO), collagen type II (II-II6B3, DSHB, Iowa City, IA), collagen type X (Sigma), aggrecan (Abcam, Cambridge, MA), calprotectin (Abcam), vimentin (Abcam), CD105 (GeneTex, Inc., San Antonio, TX), CD90 (BD Biosciences, San Jose, CA), CD14 (AbD Serotec, Oxford, UK) and β-actin (Sigma). RIPA buffer, BCA™ Protein Assay Kit, the secondary antibody of HRP-Conjugated Goat anti-mouse, SuperSignal® West Femto Maximum Sensitivity Substrate, CL-XPosure Film and Restore™ Plus Western Blot Stripping Buffer were all from Pierce (Rockford, IL). All other reagents were from Sigma unless otherwise specified.
Harvest of synovial cells
Random biopsies of the intimal layer of synovial tissue (Fig. 1A) were obtained aseptically from knee joints of young pigs and for each experiment the cells from several knee joints were pooled together. After temporary storage in culture medium at 4°C, the synovial tissue was finely minced using scalpels and digested at 37°C on an x-y-z shaker (Clay Adams Nutator, BD, Bedford, MA) first for 30 min in PBS containing 0.1% trypsin and then for 2 h in 0.1% solution of collagenase P in DMEM/10%FBS. The cell suspension was filtered through a 70-μm nylon filter to eliminate aggregates and tissue remnants, and the cells were collected from the filtrate by centrifugation. Cells were kept in primary culture for 4 days (complete medium including DMEM/10%FBS, 100 U/mL penicillin, 100 μg/mL streptomycin). Nonadherent cells were removed by PBS wash on days 2 and 4, and the adherent cells were further treated as described below to isolate SDSCs.
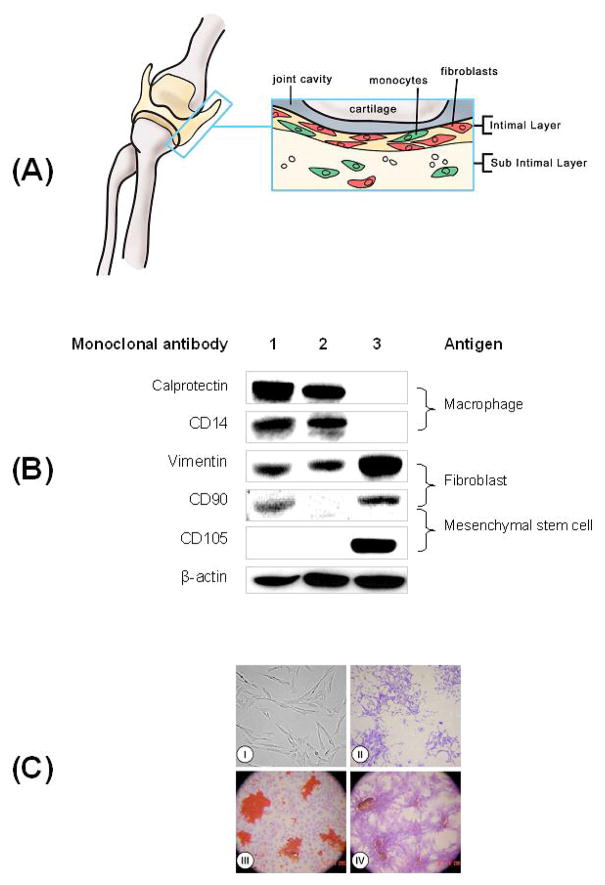
Figure 1. Isolation and multi-differentiation property of synovium-derived stem cells (SDSCs).
(A) Harvest sites and target tissue (intimal layer of synovium) in a porcine knee joint; anterior-posterior view is shown. (B) Selection of SDSCs from synovial tissue using negative isolation method. Western blot analysis of lysates from three different sources: synovial tissue (lane 1), conjugated cells (lane 2), and negatively isolated cells (lane 3) are shown. Immunoblots were probed with monoclonal antibodies against macrophages (Calprotectin and CD14), fibroblasts (Vimentin and CD90) and MSCs (CD105 and CD90). β-actin served as an internal control. (C) Morphology property (I/II) and multi-differentiation capacity (III/IV) of SDSCs in monolayer culture. (I) Phase contrast photomicrograph of SDSCs. (II) Four SDSC-colonies were stained with crystal violet. (III) Adipogenesis of SDSCs. After 14 days in complete medium to form single cell-derived colonies, SDSCs were incubated in adipogenesis medium for an additional 21 days, and stained with Oil Red O. Representative lipid vacules are shown. (IV) Osteogenesis of SDSCs. After 14 days in complete medium to form single cell-derived colonies, SDSCs were incubated in osteogenesis medium for an additional 21 days, and stained with Alizarin Red S for calcified nodules.
Negative isolation of SDSCs
For negative isolation of SDSCs from primary cultures of adherent synovial cells consisting of macrophages and fibroblasts (Zimmermann et al., 2001; Pei et al., 2008), cells were detached by trypsinization for less than 1 min (0.25% trypsin/0.2% EDTA). Cells were washed, suspended in PBS/2% FBS (107/mL) and incubated with 5 × 107/mL Dynabeads M-450 CD14 with the monoclonal antibody specific for macrophages for 1 h at 4°C on an x-y-z shaker. The conjugated cells and the unbound Dynabeads were collected using the Dynal Magnetic Particle Concentrator®. The depleted supernatant with fibroblast-like cells (also referred to as SDSCs) was transferred to a second tube for further study.
Adipogenesis and osteogenesis of SDSCs
SDSCs were plated in a six-well plate at low density (100 cells/cm2) and cultured in complete medium for 14 days. At day 7, single cell-formed colonies were stained with 0.07% crystal violet. For adipogenic differentiation, the medium was replaced with adipogenesis differentiation medium, which was complete medium supplemented with 10−6 M dexamethasone, 0.5 mM isobutyl-1-methyl xanthine, 100 μM indomethacin, and 10 μg/mL insulin. The cells were cultured for an additional 21 days followed by fixing in 4% paraformaldehyde for over 1 h and staining with fresh Oil Red-O solution for 2 h. For osteogenic differentiation, the medium was replaced with osteogenesis differentiation medium, which was complete medium supplemented with 10−7 M dexamethasone, 0.2 mM ascorbic acid, and 10 mM β-glycerolphosphate for an additional 21 days followed by fixing and staining with 2% Alizarin Red (pH 4.2).
Pellet cultures
Aliquots of 1 × 106 negatively isolated SDSCs were centrifuged at 500 g for 5 min in a 15 mL centrifuge tube to form an aggregated cell pellet. For cell characterization, the pellets were cultured in serum-free defined medium (high-glucose DMEM, 40 μg/mL proline, 100 nM dexamethasone, 0.1 mM ascorbic acid 2-phosphate, 100 U/mL penicillin, 100 mg/L streptomycin, and 1x ITS+Premix) with the supplementation of TGF-β1 at 1 and 10 ng/mL in orbitally mixed 24-well-plates for 7 days. To determine the purification effect on chondrogenesis, pellets of synovial cells (SCs) derived without negative isolation served as a control.
For optimization of growth factor cocktails to maximize chondrogenesis, negatively isolated SDSC-pellets were cultured in serum-free defined medium with or without the application of different sequences and combinations of growth factors, during a two-step culture period. Cell pellets were first cultured statically for 3 days in 15 mL tubes (one pellet per tube in 1 mL culture medium, proliferation phase), followed by a dynamic culture in orbitally mixed 24-well-plates (one pellet per well in 1 mL medium, differentiation phase). The medium was changed every two to three days with a chemically defined culture medium containing specific combinations of growth factors.
Regimens of application of growth factors
The concentrations of growth factors in culture medium were maintained at levels previously shown to support proliferation and differentiation of human mesenchymal stem cells from other sources: 10 ng/mL for TGF-β1 (T), 50 ng/mL for FGF-2 (F), and 500 ng/mL for IGF-I (I). To determine the proliferation growth factor cocktail, single growth factors (TGF-β1, FGF-2) and their combinations with IGF-I (TGF-β1/FGF-2/IGF-I, FGF-2/IGF-I, TGF-β1/FGF-2/IGF-I) were applied in the first 3 days of culture, followed by the application of 10 ng/mL TGF-β1 (a classic chondrogenic differentiation medium (Nöth et al., 2007)) during the subsequent 15 days of culture. To determine the differentiation growth factor cocktail, the application of FGF-2 (a potent mitogen for MSC (Tsutsumi et al., 2001)) for the first 3 days of culture was followed by the application of TGF-β1 and IGF-I, either sequentially or in combination, during the subsequent 15 days of culture. Nine different combinations of growth factors were studied. In each case, cells cultured in chemically defined medium without supplemental growth factors served as controls.
Histology and immunohistochemistry
Randomly selected pellets (n=2 per data point) were fixed overnight at 4°C in 4% paraformaldehyde in PBS (pH 7.4), paraffin embedded, and sectioned to 5 μm. Consecutive sections were stained with Safranin O/Fast Green for sulfated glycosaminoglycan (GAG) and were immunostained with monoclonal antibodies against collagen type II, I, and X, individually. Immunohistochemical sections were hydrated, treated with 1% hydrogen peroxide to inhibit endogenous peroxidase, and incubated for 30 min with 2 mg/mL testicular hyaluronidase in PBS (pH 5) at 37°C, This was followed by another 30 min with 1.5% normal goat serum and overnight at 4°C with the primary antibody. Finally, the sections were stained using a kit (Vectastain ABC, Burlingame, CA) and counterstained with hematoxylin.
Biochemical analyses
Randomly selected pellets (n=4 per data point) were frozen and digested for 6 h at 60°C with 125 μg/mL papain in PBE buffer (100 mM phosphate, 10 mM EDTA, pH 6.5) containing 10 mM cysteine, by using 100 μL of enzyme per sample. To quantify cell density, DNA content was measured with the use of Hoechst 33258 dye and a spectrofluorometer (QM-1; Photon Technology International, South Brunswick, NJ). GAG was measured by using dimethylmethylene blue dye and a spectrophotometer (Perkin-Elmer, Wellesley, MA) with bovine chondroitin sulfate as a standard (Pei et al., 2002b).
Western blots
Randomly selected samples (n=2 per data point) were snap-frozen in liquid nitrogen and stored at −80°C until analysis. RIPA buffer was used to extract sample proteins from synovial tissue, conjugated cells and negatively isolated cells. Total sample proteins were quantified using BCA™ Protein Assay Kit. 15 μg of sample proteins were loaded and separated by NuPAGE® Novex ® Bis-Tris Mini Gels using the XCell SureLock ™ Mini-Cell at 120 V for 3 h at 4°C. Bands were transferred onto a nitrocellulose membrane using XCell II™ Blot module at e15 V overnight at 4°C. Nonspecific binding was blocked with 5% non-fat milk in TBST (100 mM Tris-HCl, 0.9% NaCl, 1% Tween 20, pH 7.5) for 1 h, and the membrane was incubated with primary monoclonal antibodies Calprotectin and CD14 (macrophage/monocyte marker), Vimentin (fibroblast marker (Sappino et al., 1990)), CD90 (fibroblast and MSC marker), CD105 (MSC marker), and β-actin (internal control) individually in 1% non-fat milk in TBST for 3 h at room temperature. The membrane was incubated in the secondary antibody of HRP-Conjugated Goat anti-mouse (dilution factor was 1:5000) for 40 min at room temperature, followed by exposure in SuperSignal® West Femto Maximum Sensitivity Substrate using a CL-XPosure Film. The membrane could also be stripped using Restore™ Plus Western Blot Stripping Buffer at 37°C for 15 min and incubated with another primary antibody followed by the protocol described above.
Real-time quantitative reverse transcriptase (RT)-PCR
Pellets (n = 4 per group) were collected at the end of culture (day 18) for studies on gene expression. Total RNA was extracted from pellets by homogenizing using an RNase-free pestle in TRIzol reagent (Life Technologies Inc., Grand Island, NY) and RNeasy® Mini Kit (Qiagen, Valencia, CA) according to manufacturer’s directions. Quantification of mRNA was performed by real-time RT-PCR with DNA Engine Opticon™ system (MJ Research, Inc., Waltham, MA). This mRNA analysis focused on marker genes for chondrogenesis (collagen I for early phase and collagen II/aggrecan for late phase): aggrecan gene (forward primer GCCCTTTACATTTGCCCCTG; reverse primer CGGGCGGTAATGGAACACAA), collagen I (α1) gene (forward primer CTGGTGATGCTGGTCCTGTTG; reverse primer CCTTGGGGTTCTTGCTGATGT), collagen II (α1) gene (forward primer CCGCCTTTGCTGGCTTAGGCCCG; reverse primer CCACCATTGATGGTTTCTCCAAACC).
RNA (150 ng per sample) was used for oligo(dT)12–18-primed cDNA synthesis using SuperScript™II RT (Invitrogen, Carlsbad, CA). The DyNAmo™ SYBR Green qPCR kit (MJ Research, Inc., Waltham, MA) was used for quantitative real-time analysis of cDNA samples from different groups. The cycle parameters were 95°C for 10 min to activate the Tbr DNA polymerase, then 39 cycles at 94°C for 10 s denaturation, followed by 55°C for 20 s annealing, and 72°C for 20 s extension. The last extension was 72°C for 5 min. The 18S RNA (forward primer CGGCTACCACATCCAAGGAA and reverse primer GCTGGAATTACCGCGGCT) was amplified at the same time and used as an internal control. The cycle threshold (Ct) values for 18S RNA and that of samples were measured and calculated by computer software (Perkin-Elmer, Wellesley, MA). Relative transcript levels were calculated as χ = 2−ΔΔCt, in which ΔΔCt = ΔE−ΔC, and ΔE=Ctexp−Ct18s; ΔC=Ctct1−Ct18s (Livak and Schmittgen, 2001).
Statistical analysis
Statistics were assessed by one-way analysis of variance (ANOVA); P values less than 0.05 were considered statistically significant.
Results
Derivation of SDSCs by negative isolation
We established a protocol for fast and accurate isolation of SDSCs from synovial tissue using monodisperse superparamagnetic polystyrene beads (Dynabeads®) coated with a primary monoclonal antibody specific for CD14, a membrane antigen predominantly expressed on macrophages/monocytes. The first step involved enzyme digestion and primary culture of the synovial tissue intimal layer (Fig. 1A). The resulting detached cell suspension consisting of fibroblasts and macrophages/monocytes was incubated with Dynabeads® CD14 followed by the application of Dynal MPC®-1, a tool used to separate Dynabeads from diverse liquid sample matrices. The Dynabeads® CD14 with or without binding macrophages/monocytes were attracted to the tube wall adjacent to Dynal MPC®-1. The unattached, “negatively selected” cells in the supernatant were collected for further study.
In order to evaluate the efficiency of the negative isolation technique, monoclonal antibodies against macrophage/monocyte markers (Calprotectin and CD14) and stem cell markers (vimentin and CD90) were used to determine the protein levels of macrophages and fibroblasts in the synovial tissue, conjugated cells and negatively isolated cells (Fig. 1B). Macrophage markers were detected in the synovial tissue and conjugated cells, but not in the negatively isolated cells. On the contrary, stem cell markers were detected mainly in the negatively isolated cells. Notably, the isolated cells showed strong expression of mesenchymal stem cell markers CD105 and CD90, compared to negligible amounts of these two markers in synovial tissue and conjugated cells.
To evaluate proliferation ability, SDSCs of passage 2 were plated at a density of 100 cells/cm2 and cultured for 14 days with the complete culture medium to produce single cell-derived colonies (Fig. 1C-I). The SDSCs increased very rapidly and reached approximately 360-fold in 14 days (data not shown here). At day 7, SDSCs formed single cell-derived colonies and most cells kept their fibroblastic spindle shape (Fig. 1C-II). To evaluate multi-differentiation capacity, the adipogenesis and osteogenesis medium were used for an additional 21 days to differentiate SDSCs into adipocytes (Fig. 1C-III) and calcified nodules (Fig. 1C-IV). These data indicate that SDSCs have characteristics of MSCs.
Capacity of negatively isolated SDSCs for cartilage differentiation
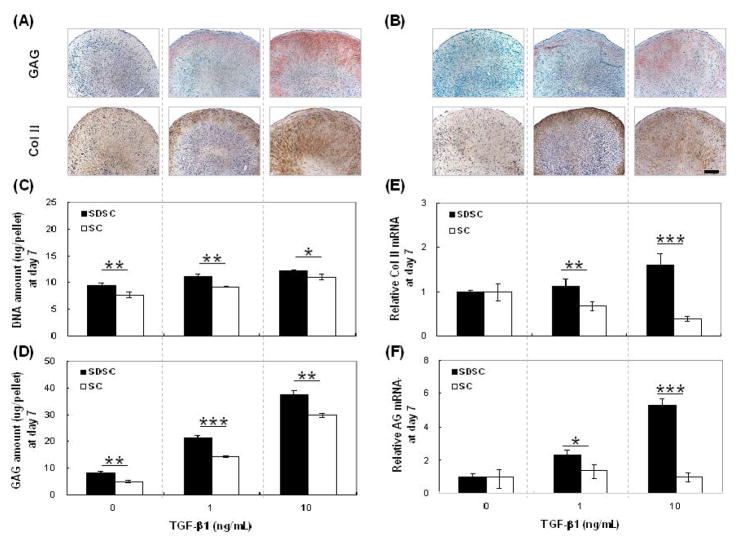
To compare the chondrogenic potential of synovial derived stem cells before isolation (primary SCs) and after negative isolation (purified SDSCs), cells were treated in the defined chondrogenic medium supplemented with varying doses of TGF-β1. At day 7 of culture, compared to SC-pellets (Fig. 2B), SDSC-pellets contained higher concentrations of sulfated GAG and collagen II – two key markers of chondrogenesis (Fig. 2A). Biochemical analysis also suggested that SDSC-pellets contained more cells (Fig. 2C) and GAG (Fig. 2D). Consistent with the measured protein expression levels, SDSC-pellets yielded higher mRNAs of collagen II (Fig. 2E) and aggrecan (Fig. 2F).
Figure 2. Higher chondrogenic differentiation capacity of negatively isolated SDSCs.
(A&B) histology data for glycosaminoglycan (GAG) and collagen II. (A): SDSC-pellets and (B): SC-pellets. All scale bars are 100 μm. (C&D): biochemical analysis. (C): DNA amount (μg/pellet) and (D): GAG amount (μg/pellet). (E&F): real time RT-PCR. (E): relative collagen II mRNA level. (F): relative aggrecan mRNA level. Data are shown as Ave ± SD (n = 4) after 7 days of culture. Statistically significant differences are shown with * for p<0.05, ** for p<0.01, and *** for p<0.001.
Effects of proliferative growth factors applied early in culture
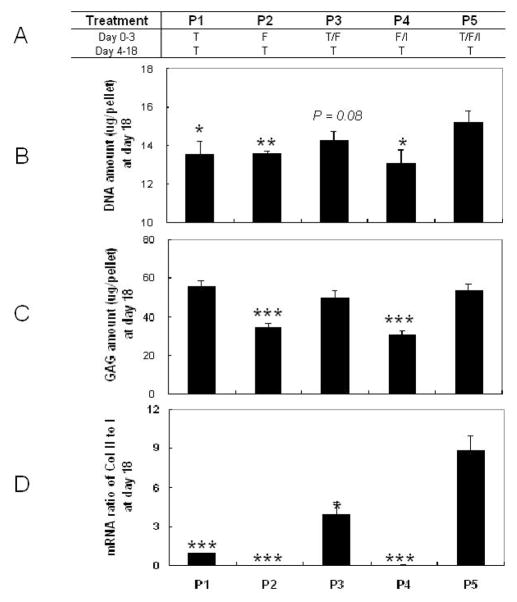
To define the proliferation capacity of the negatively isolated SDSCs in a pellet culture system, we studied the effects of five different cocktails of proliferative growth factors applied over the first 3 days of culture, followed by the application of TGF-β1 for an additional 15 days. The experimental groups are listed in Fig. 3A. After 3 days of culture, treatment with FGF-2 alone or FGF-2 combined with IGF-I yielded SDSC-pellets with DNA amounts that were comparable to the control group. However, supplementation with TGF-β1, TGF-β1/FGF-2 and TGF-β1/FGF-2/IGF-I yielded SDSC-pellets with significantly increased amounts of DNA (p<0.05 for TGF-β1 treatment) and GAG (p<0.001) compared to groups without TGF-β1 (data not shown).
Figure 3. Effects of proliferation growth factor cocktails: biochemical data.
(A) Experimental groups. (B): DNA contents (μg/pellet). (C): GAG contents (μg/pellet). (D): mRNA ratio of collagen II to collagen I. Data are shown as Ave ± SD (n = 4) after 18 days of culture. Statistically significant differences compared to P5 are shown with * for p<0.05, ** for p<0.01, and *** for p<0.001.
After the additional 15 days of culture, pre-treatment with IGF-I and FGF-2 resulted in decreased amounts of DNA when compared to the pretreatment with FGF-2 alone. In contrast, the pre-treatments with TGF-β1 and FGF-2 increased the DNA amount over levels observed in the FGF-2 group. In particular, the combined pretreatment with TGF-β1, FGF-2 and IGF-I yielded SDSC-pellets with the highest DNA amounts among all groups (p<0.05) (Fig. 3B). The pretreatments with TGF-β1, TGF-β1/FGF-2 and TGF-β1/FGF-2/IGF-I yielded SDSC-pellets with comparable amounts of GAG that were significantly and markedly higher (p<0.001) compared to the two groups cultured without TGF-β1 (Fig. 3C). Real time RT-PCR data also suggested that the SDSC-pellets pretreated with TGF-β1, TGF-β1/FGF-2 or TGF-β1/FGF-2/IGF-I had a higher mRNA ratio of type II to I collagen (an index of chondrogenic differentiation (Chua et al., 2005)), especially for pretreatment with TGF-β1/FGF-2/IGF-I (Fig. 3D).
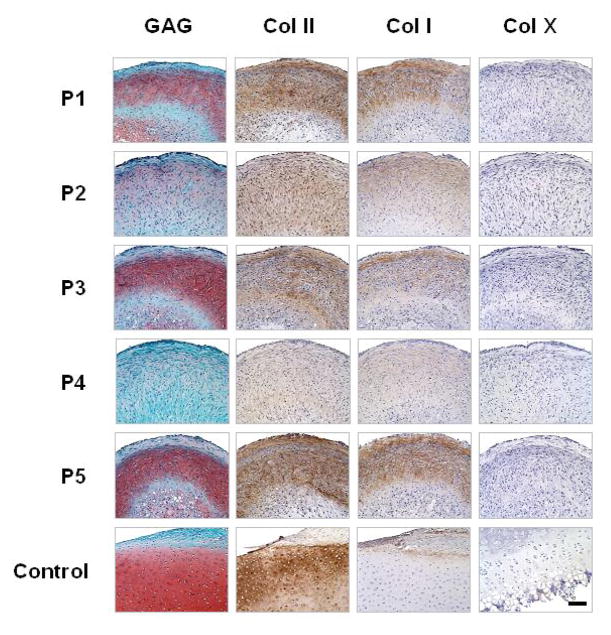
Histological data shown in Fig. 4 corroborate biochemical findings from Fig. 3. After 18 days of culture (3 days of proliferative growth factor cocktails followed by 15 days of TGF- β1), the pretreatments with TGF-β1, TGF- β1/FGF-2 or TGF-β1/FGF-2/IGF-I during the first 3 days of culture yielded SDSC-pellets with more densely sulfated GAG (as indicated by Safranin O staining) and type II collagen as well as type I collagen (as indicated by immunostains) when compared to the pretreatments without TGF-β1. There was no detectable type X collagen expression in any of the groups.
Figure 4. Effects of proliferation growth factor cocktails: histological data.
Distributions of sulfated GAG (Safranin-O), type II collagen (antibody), type I collagen (antibody) and type X collagen (antibody) for experimental groups shown in Fig. 2. Knee cartilage and attached synovial tissue from the same animal served as positive controls. All scale bars are 100 μm.
Effects of cocktails of differentiation growth factors applied late in culture
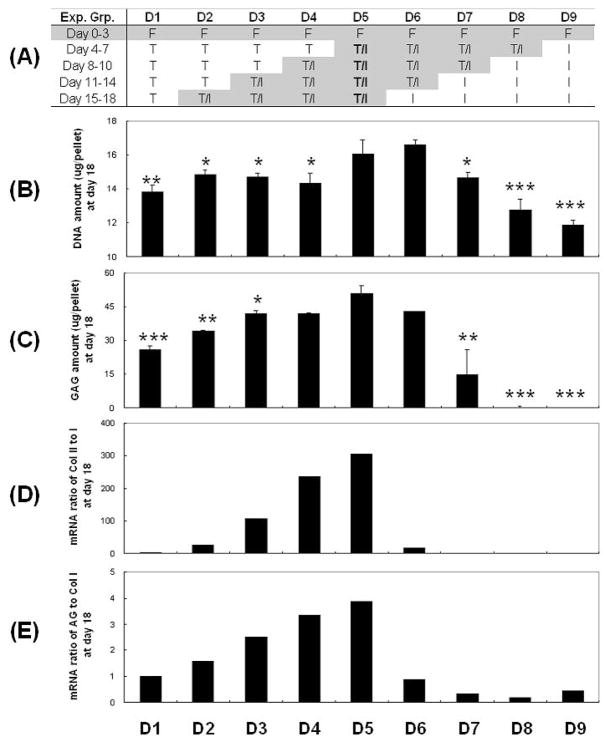
To define the differentiation capacity of the negatively isolated SDSCs, cell pellets were treated with FGF-2 during the first 3 days of culture, and then with nine different cocktails of growth factors during the additional 15 days of culture. The experimental groups were designed by systematically changing the application of growth factors in 3 day increments from TGF-β1 alone to TGF-β1/IGF-I (D1–D5), and TGF-β1/IGF-I to IGF-I (D5–D9) as shown in Fig. 5A.
Figure 5. Effects of differentiation growth factor cocktails: biochemical data.
(A) Experimental groups. (B): DNA contents (μg/pellet). (C): GAG contents (μg/pellet). (D): mRNA ratio of collagen II to collagen I. (E): mRNA ratio of aggrecan to collagen I. Data are shown as Ave ± SD (n = 4) after 18 days of culture. Statistically significant differences compared to D5 are shown with * for p<0.05, ** for p<0.01, and *** for p<0.001.
A combined TGF-β1 and IGF-I treatment yielded SDSC-pellets with higher DNA (Fig. 5B) and GAG amounts (Fig. 5C) than treatment with TGF-β1 or IGF-I alone. The combination of TGF-β1 and IGF-I applied throughout the culture after the first three days of treatment with FGF-2 yielded SDSC-pellets with the highest DNA and GAG amounts (group D5 in Fig. 5 B, C). Furthermore, the amounts of DNA and GAG increased with the duration of time during which the combination of TGF-β1 and IGF-I was applied (groups D2–D5 and D5–D8 in Fig, 5 B, C). For the same duration of application of TGF-β1 and IGF-I, the amounts of DNA and GAG were higher when the factors were applied early than late (groups D5–D8 vs. groups D2–D5, Fig. 5 B, C).
Notably, the chondrogenic differentiation indices - mRNA ratio of either collagen type II to I (Fig. 5D) or aggrecan to collagen type I (Fig. 5E) - also had the highest values when the combination of TGF- β1 and IGF-I was applied throughout the duration of the differentiation phase. Interestingly, these two indices had high values for applications of TGF-β1 and IGF-I late in culture and low values for application early in culture (Fig. 5 D,E), a trend opposite of that seen for DNA and GAG contents (Fig. 5 B,C).
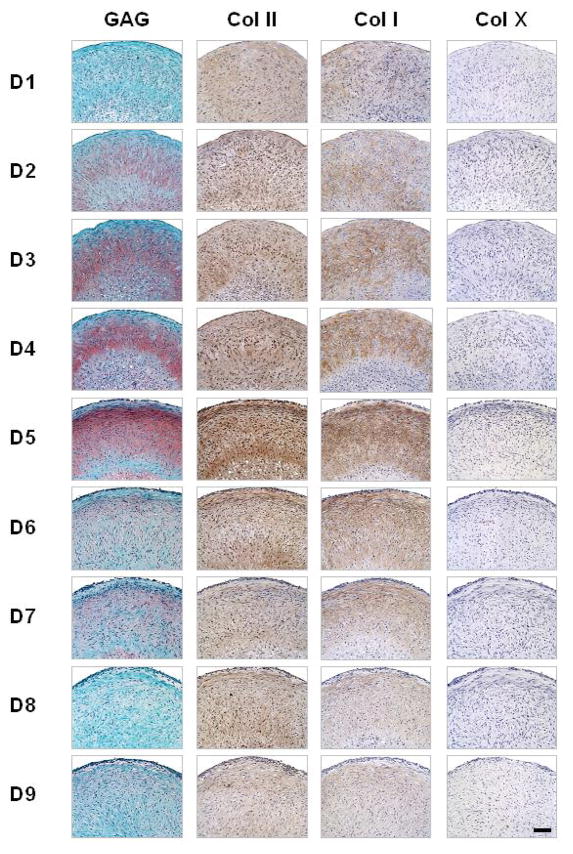
Histological data were completely consistent with these biochemical and RT-PCR data. The combinations of TGF-β1 and IGF-I yielded SDSC-pellets with more GAG and type II collagen as well as type I collagen expression compared to the treatments with TGF-β1 alone. Group D5 displayed the highest presence and best organization of GAG (as indicated by the Safranin O stain) and type II and type I collagens (immunostains). There was no detectable type X collagen expression in any of the groups (Fig. 6).
Figure 6. Effects of differentiation growth factor cocktails: histological data.
Distributions of sulfated GAG (Safranin O), type II collagen (antibody), type I collagen (antibody) and type X collagen (antibody) for the nine experimental groups (nine treatments) shown in Fig. 4. All scale bars are 100 μm.
Discussion
The present study suggests that negative isolation is a fast and reliable technique to derive MSCs, here termed SDSCs, from synovial tissue. Negatively isolated cells have high chondrogenic potential, which accounts for synovium-derived chondrogenesis. The combination of IGF-I, FGF and TGF-β1 applied early during culture, followed by the application of TGF-β1 alone (a classic chondrogenic differentiation medium (Nöth et al., 2007)) promoted proliferation of SDSCs with chondrogenic potential. The combination of TGF-β1 and IGF-I applied after the initial 3-day period of cell proliferation from FGF-2 (a potent mitogen for MSC (Tsutsumi et al., 2001)) was superior to all other combinations and sequences of growth factors. Our study supported the notion that sustained administration of TGF- β1 can effectively enhance chondrogenesis and suppress terminal cell differentiation, and thus may be effective for cartilage repair (Kawamura et al., 2005).
The synovial intimal cells, termed synoviocytes, contain macrophages (type A cells) and fibroblast-like cells (type B cells) (Edwards, 1994). With the rich rough endoplasmic reticulum and dendritic processes, synovial fibroblast-like cells are involved in the production of specialized matrix constituents including hyaluronan, collagens and fibronectin for the intimal interstitium and synovial fluid. The proliferative potential of these fibroblast-like cells in loco is also much higher than that of macrophages (Vandenabeele et al., 2003).
MSCs are pluripotent cells with the ability to differentiate along osteogenic, chondrogenic, and adipogenic lineages (Pittenger et al., 1999), but these cells lack a unique surface antigen for positive selection. Identification of MSCs is therefore based on differentiation properties and an extensive panel of monoclonal antibodies, including differentiation and lineage specific markers, growth factor receptors, and adhesion molecules. In particular, CD105 (SH2, endoglin) and CD90 (Thy-1) are present on MSC surfaces but are lost during the developmental progression into differentiated phenotypes (Bruder et al., 1998; Chen et al., 1999). Among these markers, CD105 was shown to bind TGF-β1 and TGF-β3 with high affinity, which may play a role in mediating TGF-βsignaling during chondrogenic differentiation of MSCs (Cheifetz et al., 1992), while CD90 (Thy-1) also served as a marker gene for fibroblasts (Saalbach et al., 1998).
Our previous study indicated that macrophages inhibit chondrogenic differentiation of SDSCs (Pei et al., 2008b). Since MSCs are negative for CD14 (Wiesmann et al., 2006), a macrophage/monocyte marker, negative isolation can be employed to derive SDSCs from synovial tissue. After negative isolation using Dynabeads® CD14, the monoclonal antibodies against CD14 and macrophage L1 protein indicated that synovial tissue and conjugated cells had high percentages of macrophages, which in turn were not detectable in negatively isolated cells. However, high expression of vimentin (Sappino et al., 1990) and CD90 (Saalbach et al., 1998) in negatively isolated cells was used to identify these cells as fibroblast-like cells with strong MSC properties (CD105 (Cheifetz et al., 1992)). Our study demonstrated that the negatively isolated cells had a pronounced proliferation capacity and could form single cell-derived colonies when they were plated at low density. After confluence, they were able to differentiate into adipose and bone tissue when incubated with adipogenesis and osteogenesis medium, respectively. Therefore, we designated these cells as SDSCs.
Our study also demonstrated that negative isolation could increase the percentage of mesenchymal cells and improve the capacity for chondrogenic differentiation. Compared to conventional passaging, this technique proved to be successful by providing satisfactory numbers of cells within short periods of time. Another advantage was that negative isolation with Dynabeads® M-450 CD14 avoids direct contact of isolated cells with monoclonal antibodies and/or magnetobeads, thereby reducing possible functional alterations of the cells (Zimmermann et al., 2001).
Because of the high chondrogenic capacity, SDSCs were chosen as an MSC model in a pellet culture system to characterize the sequence of events of chondrogenesis in vitro. In this study, several key growth factors and their combinations were added exogenously to elicit responses to the same growth factors that are found during embryonic cartilage development. TGF-βwas chosen due to its central role in the chondrogenic process (Serra et al., 1997; Dunker et al., 2002). TGF-βisoforms may regulate mesenchymal condensation - a critical event for cartilage differentiation - by recruitment of cells into pre-cartilage tissue. In the defined, serum-free medium, pretreatment with combinations of growth factors that included TGF- β1 initiated MSC proliferation and determined the exclusive commitment to chondroprogenitor cells (Mackay et al., 1998; Chimal-Monroy and Diaz de Leon, 1999; Pittenger et al. 2000; Sekiya et al., 2002; Vandenabeele et al., 2003); the cells eventually differentiated into chondrocytes. In this process, TGF- β1 was suggested to directly control the expression of SRY-box containing gene 9 (Sox 9), a master regulator of chondrogenesis (Wright et al., 1995; Healy et al., 1996).
Our finding that TGF-β1/FGF-2 pretreatment could increase DNA synthesis to levels higher than those observed with TGF-β1 alone was consistent with the previous report that TGF-β1 and FGF have synergistic effects on DNA synthesis (Crabb et al., 1990). FGFs are known to play vital roles regulating limb and facial primordium outgrowth, pattern formation, and skeletogenesis (Niswander, 2003; Richman and Lee, 2003). A recent study showed that treatment with TGF-β1 up-regulates tyrosine phosphorylation of the FGF receptor 3 (FGFR3) in chondrocytes in the proliferating zone (Peters et al., 1993), without alterations in total FGFR3 levels on embryonic bone formation (Mukherjee et al., 2005). Like TGF-β1, FGF also can stimulate the expression of the master chondrogenic factor Sox 9 through the mitogen-activated protein kinase/extracellular regulated kinase (MEK-ERK) cascade (Murakami et al., 2000), indicating there is crosstalk between these two growth factors in chondrogenesis through this specific pathway.
Furthermore, TGF-β1/FGF-2/IGF-I pretreatment could enhance DNA synthesis more than the TGF-β1/FGF-2 combination, which is supported by a previous study (O’Keefe et al., 1994). These effects of TGF-β1 and FGF-2 were further enhanced in a dose-dependent manner by IGF-I. The underlying mechanism is still unknown. Our data also suggest that there was no synergistic effect on chondrogenesis between FGF-2 and IGF-1, consistent with a previous study (Mukherjee et al., 2005). The biological action of IGFs is transduced by the type I tyrosine kinase receptor (IGFIR) that classically activates MEK1/2, ERK1/2 and the phosphatidylinositol-3-kinase (PI3K-Akt) pathways (Laviola et al., 2007). It has been reported that IGF-I had potent chondroinductive actions on MSCs. ERK1/2 mitogen-activated protein kinase (MAPK) pathway mediated the TGF-β1 mitogenic response and in part the IGF-I proliferative action in the chondrogenesis of MSCs (Longobardi et al., 2006), indicating the MAPK pathway might be involved in the proliferation of SDSCs pre-treated with TGF-β1/FGF-2/IGF-I.
To optimize the therapeutic use of SDSCs, we need to understand the mechanisms by which growth factors mediate self-replication and chondrogenic differentiation of these cells. TGF-β1, but not IGF-I, was shown to induce condensation and N-cadherin expression (Longobardi et al., 2006) and to inhibit terminal differentiation of chondrocytes (Ferguson et al., 2000). In turn, IGF-I has been demonstrated to play a key role in skeletal growth (DeChiara et al., 1990; Baker et al., 1993) by modulation of MSC chondrogenesis, proliferation, and apoptosis, and by inducing the expression of chondrocyte markers (Longobardi et al., 2006).
In serum-free medium, IGF-I alone has no proliferation effect on SDSCs, and IGF-I/TGF-β1 has strong differentiation effects (Pei et al., 2005). In our previous studies, there was no chondrogenesis in the SDSC-pellets in the absence of TGF-β1 (Pei et al., 2005), which is consistent with other published studies (Milne et al., 2001; Baddoo et al., 2003; Indrawattana et al., 2004; Kawamura et al., 2005). A sustained expression of TGF-β1 was more effective in inducing chondrogenic differentiation of SDSCs than was IGF-I. One possible reason was that insulin, a part of ITS+Premix in the defined chondrogenic medium, was present at levels that are 100 times above physiological values. When compared with IGF, insulin has an affinity for the IGF-I receptor (IGFIR) that is approximately two orders of magnitude lower. It is thus possible that the lack of biological activity of additional IGF-I was due to nonspecific stimulation of IGFIR by insulin present in the ITS+Premix (Osborn et al., 1989).
However, when TGF-β1 and IGF-I are applied in combination, TGF-β1 can act synergistically with IGF-I and increase its responsiveness (Sekiya et al., 2002; Fukumoto et al., 2003; Indrawattana et al., 2004; Sakimura et al., 2006). Our data demonstrated that the combination pattern of TGF-β1 and IGF-I yielded SDSC-pellets with more mature cartilage-like tissue. Our study was in accordance with a recent report that the treatment of chondrocyte cultures with IGF-I stabilizes chondrogenic potential, stimulates Sox 9 and promotes molecular interactions between ERK and Sox 9 (Shakibaei et al., 2006).
It has been reported that TGF-β1 has three discrete effects on IGF-I in cultured rat articular chondrocytes (Tsukazaki et al., 1994): (i) a decrease in IGF-I and 41-kDa IGF binding protein production, (ii) an increase in IGF-I receptor binding sites, (iii) down-regulation of IGF-I-induced receptor autophosphorylation without interference from IGF-I biological action. The combination of TGF-β1 and IGF-I was also found to augment the mitotic and matrix synthetic actions of chondrosarcoma chondrocytes (Matsumura et al., 2000). A recent study showed that in renal carcinoma cells Caki-2, IGF-I interacted directly with TGF-β1 activation of the TGF-βreceptor (TbR) complex by enhancing phosphorylation and nuclear translocation of SMAD2 (Rosendahl and Forsberg, 2006). However, whether this pathway was involved in the chondrogenesis of SDSCs is not entirely clear.
Although the slight effect of IGF-I on these metabolites implied that it does not exert a major influence on the maturational state of the cell, insulin and IGF-I have been shown to play key roles in the TGF-β-mediated re-expression of aggrecan and type II collagen in adult human articular chondrocytes (Yaeger et al., 1997).
Our findings imply that the SDSCs isolated by negative selection from synovial tissue can provide a large and durable stem cell reservoir for cartilage tissue engineering. TGF-β1 plays a key role in chondrogenesis of SDSCs by enhancing either cell proliferation when applied in combination with FGF-2 and IGF-I or chondrogenic differentiation when applied with IGF-I. The supplementation of TGF-β1/FGF-2/IGF-I to enhance SDSC proliferation and TGF-β1/IGF-I to enhance chondrogenic differentiation can form a basis for SDSC based cartilage tissue engineering. Crosstalk among these growth factors needs to be further investigated.
Acknowledgments
This study (project no. S-06-22P) was supported by the AO Research Fund of the AO Foundation. The authors also would like to thank Brett Kenney (Division of Animal and Veterinary Sciences, West Virginia University) for his help with harvesting pig tissue and Suzanne Smith for her help in editing the manuscript.
References
- Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89:1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Bruder SP, Ricalton NS, Boynton RE, Connolly TJ, Jaiswal N, Zaia J, Barry FP. Mesenchymal stem cell surface antigen SB-10 corresponds to activated leukocyte cell adhesion molecule and is involved in osteogenic differentiation. J Bone Miner Res. 1998;13:655–663. doi: 10.1359/jbmr.1998.13.4.655. [DOI] [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, Middleton J. Mesenchymal Stem Cells: their Phenotype, Differentiation Capacity, Immunological Features and Potential for Homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- Chen XD, Qian HY, Neff L, Satomura K, Horowitz MC. Thy-1 antigen expression by cells in the osteoblast lineage. J Bone Miner Res. 1999;14:362–375. doi: 10.1359/jbmr.1999.14.3.362. [DOI] [PubMed] [Google Scholar]
- Chimal-Monroy J, Diaz de Leon L. Expression of N-cadherin, N-CAM, fibronectin and tenascin is stimulated by TGF-beta1, beta2, beta3 and beta5 during the formation of precartilage condensations. Int J Dev Biol. 1999;43:59–67. [PubMed] [Google Scholar]
- Chua KH, Aminuddin BS, Fuzina NH, Ruszymah BH. Insulin-transferrin-selenium prevent human chondrocyte dedifferentiation and promote the formation of high quality tissue engineered human hyaline cartilage. Eur Cell Mater. 2005;9:58–67. doi: 10.22203/ecm.v009a08. [DOI] [PubMed] [Google Scholar]
- Crabb ID, O’Keefe RJ, Puzas JE, Rosier RN. Synergistic effect of transforming growth factor beta and fibroblast growth factor on DNA synthesis in chick growth plate chondrocytes. J Bone Miner Res. 1990;5:1105–1112. doi: 10.1002/jbmr.5650051103. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Efstratiadis A, Robertson EJ. A growth deficiency phenotype in heterozygous mice carrying an insulin like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- Dunker N, Schmitt K, Krieglstein K. TGF-beta is required for programmed cell death in interdigital webs of the developing mouse limb. Mech Dev. 2002;113:111–120. doi: 10.1016/s0925-4773(02)00015-1. [DOI] [PubMed] [Google Scholar]
- Edwards JC. The nature and origins of synovium: experimental approaches to the study of synoviocyte differentiation. J Anat. 1994;184:493–501. [PMC free article] [PubMed] [Google Scholar]
- Ferguson CM, Schwarz EM, Reynolds PR, Puzas JE, Rosier RN, O’Keefe RJ. Smad2 and 3 mediate transforming growth factor-beta1- induced inhibition of chondrocyte maturation. Endocrinology. 2000;141:4728–4735. doi: 10.1210/endo.141.12.7848. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, O’Driscoll SW. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthr Cartilage. 2003;11:55–64. doi: 10.1053/joca.2002.0869. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D, Ferrara N, Schweigerer L, Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987;8:95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Healy C, Uwanogho D, Sharpe PT. Expression of the chicken Sox9 gene marks the onset of cartilage differentiation. Ann NY Acad Sci. 1996;785:261–262. doi: 10.1111/j.1749-6632.1996.tb56278.x. [DOI] [PubMed] [Google Scholar]
- Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914–919. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Chu CR, Sobajima S, Robbins PD, Fu FH, Izzo NJ, Niyibizi C. Adenoviral-mediated transfer of TGF-beta1 but not IGF-1 induces chondrogenic differentiation of human mesenchymal stem cells in pellet cultures. Exp Hematol. 2005;33:865–872. doi: 10.1016/j.exphem.2005.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr Pharm Des. 2007;13:663–669. doi: 10.2174/138161207780249146. [DOI] [PubMed] [Google Scholar]
- Lennon DP, Haynesworth SE, Young RG, Dennis JE, Caplan AI. A chemically defined medium supports in vitro proliferation and maintains the osteochondral potential of rat marrow-derived mesenchymal stem cells. Exp Cell Res. 1995;219:211–222. doi: 10.1006/excr.1995.1221. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res. 2006;21:626–636. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J, Di Battista JA, Lajeunesse D, Pelletier JP. IGF/IGFBP axis in cartilage and bone in osteoarthritis pathogenesis. Inflamm Res. 1998;47:90–100. doi: 10.1007/s000110050288. [DOI] [PubMed] [Google Scholar]
- Matsumura T, Whelan WC, Li XQ, Trippel SB. Regulation by IGF-I and TGF-beta1 of Swarm-rat chondrosarcoma chondrocytes. J Orthop Res. 2000;18:351–355. doi: 10.1002/jor.1100180305. [DOI] [PubMed] [Google Scholar]
- Milne M, Quail JM, Rosen CJ, Baran DT. Insulin-like growth factor binding proteins in femoral and vertebral bone marrow stromal cells: Expression and regulation by thyroid hormone and dexamethasone. J Cell Biochem. 2001;81:229–240. doi: 10.1002/1097-4644(20010501)81:2<229::aid-jcb1038>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54:843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Dong SS, Clemens T, Alvarez J, Serra R. Coordination of TGF-beta and FGF signaling pathways in bone organ cultures. Mech Dev. 2005;122:557–571. doi: 10.1016/j.mod.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Murakami S, Kan M, McKeehan WL, de Crombrugghe B. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA. 2000;97:1113–1118. doi: 10.1073/pnas.97.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswander L. Pattern formation: Old models out on a limb. Nat Rev Genet. 2003;4:133–143. doi: 10.1038/nrg1001. [DOI] [PubMed] [Google Scholar]
- Nöth U, Rackwitz L, Heymer A, Weber M, Baumann B, Steinert A, Schütze N, Jakob F, Eulert J. Chondrogenic differentiation of human mesenchymal stem cells in collagen type I hydrogels. J Biomed Mater Res A. 2007;83:626–635. doi: 10.1002/jbm.a.31254. [DOI] [PubMed] [Google Scholar]
- O’Keefe RJ, Crabb ID, Puzas JE, Rosier RN. Effects of transforming growth factor-beta 1 and fibroblast growth factor on DNA synthesis in growth plate chondrocytes are enhanced by insulin-like growth factor-I. J Orthop Res. 1994;12:299–310. doi: 10.1002/jor.1100120302. [DOI] [PubMed] [Google Scholar]
- Osborn KD, Trippel SB, Mankin HJ. Growth factor stimulation of adult articular cartilage. J Orthop Res. 1989;7:35–42. doi: 10.1002/jor.1100070106. [DOI] [PubMed] [Google Scholar]
- Pei M, Aaron RK, Ciombor DM. TGF-β1-dependent chondrogenic effect of IGF-I and FGF-2 on synovial fibroblast. Trans Orthop Res Soc. 2005;30:1452. [Google Scholar]
- Pei M, He F, Kish V, Vunjak-Novakovic G. Engineering of Functional Cartilage Tissue Using Synovium-derived Stem Cells. Clin Orthop Relat Res. 2008b doi: 10.1007/s11999-008-0316-2. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei M, Luo JM, Chen Q. Enhancing and Maintaining Chondrogenesis of Synovial Fibroblasts by Cartilage Extracellular Matrix Protein Matrilins. Osteoarthr Cartilage. 2008a doi: 10.1016/j.joca.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei M, Seidel J, Vunjak-Novakovic G, Freed LE. Growth factors for sequential cellular de- and re-differentiation in tissue engineering. Biochem Biophys Res Commun. 2002a;294:149–154. doi: 10.1016/S0006-291X(02)00439-4. [DOI] [PubMed] [Google Scholar]
- Pei M, Solchaga LA, Seidel J, Zeng L, Vunjak-Novakovic G, Caplan AI, Freed LE. Bioreactors mediate the effectiveness of tissue engineering scaffolds. FASEB J. 2002b;16:1691–1694. doi: 10.1096/fj.02-0083fje. [DOI] [PubMed] [Google Scholar]
- Peters K, Ornitz D, Werner S, Williams L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev Biol. 1993;155:423–430. doi: 10.1006/dbio.1993.1040. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mosca JD, McIntosh KR. Human mesenchymal stem cells: progenitor cells for cartilage, bone, fat and stroma. Curr Top Microbiol Immunol. 2000;251:3–11. doi: 10.1007/978-3-642-57276-0_1. [DOI] [PubMed] [Google Scholar]
- Richman JM, Lee SH. About face: Signals and genes controlling jaw patterning and identity in vertebrates. Bioessays. 2003;25:554–568. doi: 10.1002/bies.10288. [DOI] [PubMed] [Google Scholar]
- Rosendahl AH, Forsberg G. IGF-I and IGFBP-3 augment transforming growth factor-b actions in human renal carcinoma cells. Kidney Int. 2006;70:1584–1590. doi: 10.1038/sj.ki.5001805. [DOI] [PubMed] [Google Scholar]
- Saalbach A, Kraft R, Herrmann K, Haustein UF, Anderegg U. The monoclonal antibody AS02 recognizes a protein on human fibroblasts being highly homologous to Thy-1. Arch Dermatol Res. 1998;290:360–366. doi: 10.1007/s004030050318. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Matsumoto T, Miyamoto C, Osaki M, Shindo H. Effects of insulin-like growth factor I on transforming growth factor beta1 induced chondrogenesis of synovium-derived mesenchymal stem cells cultured in a polyglycolic acid scaffold. Cells Tissues Organs. 2006;183:55–61. doi: 10.1159/000095509. [DOI] [PubMed] [Google Scholar]
- Sappino AP, Schurch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144–161. [PubMed] [Google Scholar]
- Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaei M, Seifarth C, John T, Rahmanzadeh M, Mobasheri A. Igf-I extends the chondrogenic potential of human articular chondrocytes in vitro: Molecular association between Sox9 and Erk1/2. Biochem Pharmacol. 2006;72:1382–1395. doi: 10.1016/j.bcp.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Shirasawa S, Sekiya I, Sakaguchi Y, Yagishita K, Ichinose S, Muneta T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006;97:84–97. doi: 10.1002/jcb.20546. [DOI] [PubMed] [Google Scholar]
- Trippel SB. Growth factor actions on articular cartilage. J Rheumatol Suppl. 1995;43:129–132. [PubMed] [Google Scholar]
- Tsukazaki T, Usa T, Matsumoto T, Enomoto H, Ohtsuru A, Namba H, Iwasaki K, Yamashita S. Effect of transforming growth factor-beta on the insulin-like growth factor-I autocrine/paracrine axis in cultured rat articular chondrocytes. Exp Cell Res. 1994;215:9–16. doi: 10.1006/excr.1994.1307. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, Takagishi K, Kato Y. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- Vandenabeele F, De Bari C, Moreels M, Lambrichts I, Dell’Accio F, Lippens PL, Luyten FP. Morphological and immunocytochemical characterization of cultured fibroblast-like cells derived from adult human synovial membrane. Arch Histol Cytol. 2003;66:145–153. doi: 10.1679/aohc.66.145. [DOI] [PubMed] [Google Scholar]
- Wiesmann A, Buhring HJ, Mentrup C, Wiesmann HP. Decreased CD90 expression in human mesenchymal stem cells by applying mechanical stimulation. Head Face Med. 2006;2:8. doi: 10.1186/1746-160X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worster AA, Brower-Toland BD, Fortier LA, Bent SJ, Williams J, Nixon AJ. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19:738–749. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- Wright E, Hargrave MR, Christiansen J, Cooper L, Kun J, Evans T, Gangadharan U, Greenfield A, Koopman P. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- Yaeger PC, Masi TL, de Ortiz JL, Binette F, Tubo R, McPherson JM. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997;237:318–325. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A. Regulation of differentiation pathway of skeletal mesenchymal cells in cell lines by transforming growth factor-beta superfamily. Semin Cell Biol. 1995;6:165–173. doi: 10.1006/scel.1995.0023. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- Zimmermann T, Kunisch E, Pfeiffer R, Hirth A, Stahl HD, Sack U, Laube A, Liesaus E, Roth A, Palombo-Kinne E, Emmrich F, Kinne RW. Isolation and characterization of rheumatoid arthritis synovial fibroblasts from primary culture-primary culture cells markedly differ from fourth-passage cells. Arthritis Res. 2001;3:72–76. doi: 10.1186/ar142. [DOI] [PMC free article] [PubMed] [Google Scholar]