Abstract
Fungi occupy diverse environments and are subjected to many extreme conditions. Among the stressful conditions faced by fungi are pH changes, osmotic changes, thermal changes, oxide radicals, nutrient deprivation, and exposure to chemicals. These adversities can be found either in the environment or in animal and human hosts. The cell wall integrity (CWI) pathway provides a means to fortify and repair damages to the cell wall in order to withstand stressful environments. The CWI pathway in comprised of cell wall stress sensors that lead to activation of a mitogen-activated protein kinase (MAPK) cascade. Signaling through the MAPK cascade leads to expression of transcription factors that facilitate biosynthesis of cell wall components and actin organization. Given the relatively limited number of components of the CWI pathway and the very diverse stimuli, there must be a means of expanding the pathway. To manage the diverse stress conditions, the CWI pathway cross talks with other pathways or proteins, and these cross talk events enhance the signaling capabilities of the CWI pathway. Lateral influences that facilitate maintaining the cell wall under stress conditions are TOR signaling, calcineurin signaling, the high-osmolarity glycerol pathway, the cyclic AMP-protein kinase A pathway, and additional proteins. In this article, we highlight several of the cross talk events that have been described for Saccharomyces cerevisiae and several other fungi.
Fungal environments range from soil to plants to animal and human hosts. Fungi can also inhabit extreme environments such as hydrothermal vents, bird excreta, the arctic, aquatic environments, salterns, mine drainages, or even bare rock surfaces in the case of lichens. Each environment presents challenges for fungi that must be overcome for them to survive and grow, including osmotic changes, oxidative stress, heat shock, pH changes, nutrient limitations, and chemical challenges. These stresses emanate either from natural environments or from the host immune system in response to pathogenesis. Exposure of fungal cells to any of these stress conditions results in altered gene expression to enable the cell to endure the adverse environment. Changes to gene expression require a coordinated effort from multiple pathways in order to allow a limited amount of proteins to achieve the complicated feat of surviving unfavorable conditions. The key defense to withstand environmental adversities is the fungal cell wall. Due to damage inflicted by the stressors, the cell wall is repaired and even fortified through cell wall biosynthesis and the integration of cell wall components into the cell wall when exposed to suboptimal or hostile environments. The cell wall is a cellular structure unique to fungi among eukaryotes (plant cells have a cell wall, but it is very different from the fungal cell wall). The cell wall varies between fungi, but the overall composition consists of α- and β-glucans (the principal polysaccharides of the bilayer-structured cell wall), N-acetylglucosamine, mannoproteins, and various other glycoproteins.
To elicit the necessary transcriptional changes due to extracellular stressors, fungal cells maintain transmembrane sensors that detect environmental changes and initiate signals that are conveyed from the cell surface to the nucleus. The model yeast Saccharomyces cerevisiae, for example, utilizes five separate mitogen-activated protein kinase (MAPK) cascades to respond appropriately to environmental changes (8). The cell wall integrity (CWI) pathway utilizes one of the MAPK cascades to facilitate the maintenance of the cell wall by mediating cell wall biosynthesis, actin organization, and other events necessary to maintain CWI (46). As S. cerevisiae is the model eukaryote, much of what is known about the CWI pathways as well as other signaling pathways has been defined in this organism. This review therefore highlights what is known about the pathways in S. cerevisiae an makes comparisons to other fungal species.
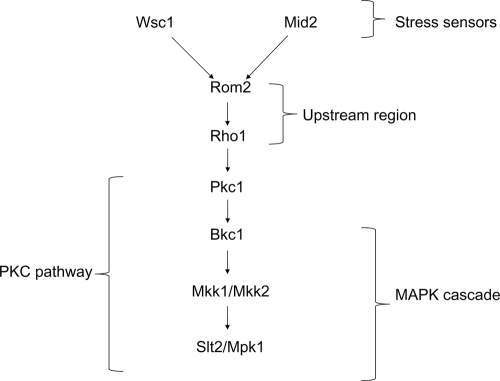
The CWI pathway utilizes GTPase-activating proteins and guanyl nucleotide exchange factors (GEFs) to regulate the activation of the kinase cascade that leads to the activation of transcription factors. In S. cerevisiae this cascade is initiated by cell wall-associated stress sensors Mid2 and Wsc1 (38, 78) (Fig. 1). These proteins bind to Rom2, which is a GEF for Rho1 (58, 62) (Table 1). Rho1 affects compositional changes in the cell wall through activation of the glucan synthase Fks1 (19, 53, 63), which facilitates the production of the major cell wall component 1,3-β-d-glucan (18). Rho1 also binds and activates Pkc1 (36, 56), which in turn regulates the MAPK cascade. Pkc1 phosphorylates Bck1, a MAPK kinase kinase (MAPKKK), which transmits the signal to MAPK kinases (MAPKKs) Mkk1 and Mkk2. These two kinases finally activate the MAPK Slt2/Mpk1 (5). The stimulation of Slt2/Mpk1 leads to phosphorylation of the transcription factors Rlm1 and SBF (consisting of the two transcription factors Swi4 and Swi6), both of which initiate the expression of cell wall synthesis genes (17, 34, 50, 81).
FIG. 1.
Diagram of the CWI pathway. Stress conditions stimulate the CWI integrity pathway through stress sensors. There are additional stress sensors capable of activating the CWI pathway that are not represented in the figure, but our review focuses primarily on the Wsc1 and Mid2 sensors. In this simplified diagram, either the Wsc1 or Mid2 stress sensor can bind to Rom2, which activates Rho1 as part of the upstream region of the pathway. Rho1 then initiates the PKC pathway by activating Pkc1. The PKC pathway includes the MAPK cascade of Bck1, Mkk1, Mkk2, and Slt2/Mpk1.
TABLE 1.
Genes of the CWI pathway in different fungi
| Gene function | Gene(s) in: |
||||||
|---|---|---|---|---|---|---|---|
| S. cerevisiae | S. pombe | A. nidulans | A. fumigatus | C. albicans | C. neoformans | N. crassa | |
| Rho1-GTP form activates Pkc1 | RHO1 | rho1+ | RHOA | RHO1 | RHO1 | rho-1 | |
| Serine/threonine kinase that regulates a MAPK activation pathway | PKC1 | pck1+, pck2+ | PKCA | PKC1 | PKC1 | pkc | |
| MAPKKK | BCK1/SLK1 | mkh1+ | BCK1 | mik-1 | |||
| MAPKK | MKK1, MKK2 | pek1+/skh1+ | MKK2 | mek-1 | |||
| MAPK | SLT2/MPK1 | pmk1+/spm1+ | MPKA | MPKA | MKC1 | MPK1 | mak-1 |
| Transcription factor activated by MAPK | RLM1 | ||||||
| Transcription factor activated by MAPK made up of Swi4/Swi6 | SBF genes | ||||||
Overall, the CWI pathway is conserved among fungi, including budding yeast, fission yeast, and filamentous fungi (61). However, components from some fungi are still being elucidated, and important differences in the pathway have been identified. An example is the redundant MAPKKs Mkk1 and Mkk2 of S. cerevisiae, for which there is only the MAPKK Mkk2 in Cryptococcus neoformans (27) (Table 1). Additionally, Slt2/Mpk1 is activated in S. cerevisiae in response to heat shock, cell wall stress compounds, or oxidative stress. However, in Schizosaccharomyces pombe the Slt2/Mpk1 homolog pmk1p is activated only in response to heat stress and sodium chloride (osmotic changes), indicating a more limited response by the CWI pathway of S. pombe (51).
Although there are slight differences, the CWI function of maintaining cell integrity is conserved between fungi, and CWI pathway activation is not restricted to an individual stimulus but can be elicited by a number of events. A genome-wide analysis of five cell wall mutants indicated transcriptional changes in 5% of the S. cerevisiae genome (or approximately 300 genes) (44), suggesting complex changes in gene expression not limited to a single signal cascade. Cross talk between the CWI pathway and other stress response pathways enable the CWI pathway to respond to numerous, diverse stress events with responses that appropriately alleviate cellular stress.
RESPONSE TO VARIABLE OSMOTIC CONDITIONS
Osmoregulation is needed to maintain fungal cell turgor, volume, and the appropriate intracellular environment for biochemical reactions (32). For example, some fungi, such as Aspergillus spp., are found in the soil, an environment that can undergo osmotic change due to drought, rain, or other climate conditions. Furthermore, this fungus is capable of surviving both in the soil environment and within a mammalian host as a human pathogen. Fungi that make this transition must be able to compensate for the changes in environmental osmolarity.
In S. cerevisiae, external osmotic changes elicit responses from both the CWI pathway and the high-osmolarity glycerol (HOG) response pathway. In general, the CWI pathway can respond to hypoosmotic conditions but the HOG pathway of S. cerevisiae responds to hyperosmotic conditions. However, the HOG pathway of pathogenic fungi in which it has been studied is broader in scope than governing just the osmotic response, because it also regulates stress responses to stimuli such as oxidative stress, UV light, heavy metals, and high temperature (1, 4, 7).
A cellular response to hypoosmotic conditions is manifested through the CWI pathway as indicated by the activation of the MAPK Slt2/Mpk1. The phosphorylation of Slt2/Mpk1 occurs without specificity toward osmotic solutes and is observed with hypotonic solutions of sorbitol, NaCl, or glucose (14). The CWI pathway response to hypoosmotic conditions relies on components of the MAPK module. Deletion of the MAPK module components prevents Slt2/Mpk1 phosphorylation under hypoosmotic conditions (14). Further, the resulting pkc1, bck1, and mkk1 mkk2 mutant strains lyse in the absence of osmotic stabilizers (33, 45, 47).
The role of the CWI pathway in hypoosmotic stabilization is retained in human-pathogenic fungi. In the case of Candida albicans, hypoosmotic conditions initiate the phosphorylation of Mkc1 (an Slt2/Mpk1 homolog) (Table 1) (54). Activation of Mkc1 is Pkc1 dependent, and a pkc1/pkc1 homozygous mutant lyses in the absence of osmotic support (60). A pkc1 deletion strain of C. neoformans also lyses in the absence of osmotic stabilization (26).
In contrast to the response elicited by hypoosmotic conditions, hyperosmotic conditions experienced by S. cerevisiae trigger a response from the HOG pathway (as noted above) in which the inactivation of Sln1 kinase leads to the dephosphorylation of Ssk1. The unphosphorylated Ssk1 activates the MAPKKKs Ssk22 and Ssk2 (32). Ssk2 then activates the MAPKK Pbs2. Pbs2 phosphorylates the MAPK Hog1, which then is transported to the nucleus to activate transcription factors. An alternative pathway to initiate Hog1, known as the Sho1-dependent pathway, also exists for S. cerevisiae. In this pathway, Sho1 activates Ste20 in response to osmotic stress via the Cdc42 GTPase, which then stimulates the MAPKKK Ste11. Ste11 with Ste50 is then able to activate Pbs2 for the phosphorylation of Hog1 (3). The Sho1-dependent alternative pathway for Hog1 activation does not appear to be conserved in all other fungi (3).
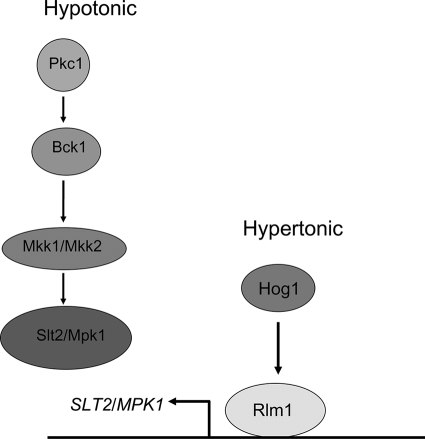
Thus, opposing osmotic conditions activate the CWI and HOG pathways; therefore, it is perceivable that there is some type of cross talk to coordinate the regulation of the two pathways. A cross talk event indeed occurs between the CWI and HOG pathways, involving Slt2/Mpk1. While hypotonic solutions induce the phosphorylation of Slt2/Mpk1 in a protein kinase C (PKC) pathway-dependent manner, hypertonic solutions induce transcription of SLT2/MPK1 that is Hog1 and Rlm1 dependent (28) (Fig. 2). The Hog1-dependent transcription of the CWI pathway gene SLT2/MPK1 under hyperosmotic conditions and the CWI-induced activation of Slt2/Mpk1 suggest regulation between the HOG and the CWI pathways for osmotic homeostasis.
FIG. 2.
Proposed mechanism of the osmotic stress response in S. cerevisiae. The CWI pathway is activated in response to hypoosmotic stress, leading to the dual phosphorylation of Slt2/Mpk1. The HOG pathway is activated in response to hyperosmotic stress conditions and leads to activation of the transcription factor Rlm1.
Though S. cerevisiae demonstrates a model in which hypoosmotic conditions trigger the CWI pathway and hyperosmotic conditions trigger the HOG pathway, this division to regulate osmolarity is not conserved in all fungi. S. pombe poses an exception where the CWI pathway component Slt2/Mpk1 homolog Pmk1 (72, 83) (Table 1) is activated regardless of whether the osmotic stressor is hyperosmotic or hypoosmotic (51). Madrid and colleagues demonstrated that the activation of Pmk1 relies on the MAPK cascade elements Mkh1 and Pek1 (Table 1) (51).
A general perspective is that the CWI pathway responds to osmotic stress regardless of the type of osmotic stress in some fungi but that in other types of fungi the HOG pathway is needed in conjunction with the CWI pathway to respond to osmotic stress. A cross talk event occurs to coordinate the effort of stabilizing the cell in hypertonic solutions.
RESPONSE TO pH STRESS
Much like the osmotic changes that can occur as fungal cells are transferred from one environment to another, pH changes can be encountered upon environmental transitions. The CWI pathway also functions to facilitate tolerance to pH changes. Alkaline stress in the environment is sensed by the Wsc1 transmembrane sensor of S. cerevisiae (68). Other genes from the CWI pathway important to alkaline tolerance are BCK1 and SLT2/MPK1, indicating that the Wsc1 signal is transmitted to the CWI MAPK cascade. This is exemplified by alkaline tolerance for a wsc1 mutant strain that contains a bck1-20 allele to induce constitutive activation of Slt2/Mpk1 (68).
It is interesting to note that the wsc1 mutation does not completely abolish phosphorylation of Slt2/Mpk1 in alkaline stress, but rather Slt2/Mpk1 phosphorylation is only decreased, indicating the potential for additional means of Slt2/Mpk1 activation either through activation of the CWI pathway utilizing a different sensor or through proteins outside of the linear CWI pathway in response to alkaline conditions. The remnant phosphorylation of Slt2/Mpk1 could possibly be contributed through activation of the CWI pathway via the Mid2 sensor under alkaline conditions. However, a mid2 mutant strain exhibits only a marginal decrease in Slt2/Mpk1 phosphorylation under alkaline conditions and is not alkali sensitive (68). Thus, if Mid2 does contribute to CWI activation under alkaline conditions it is likely a minor contribution. Whatever the additional alkaline-induced signaling that results in Slt2/Mpk1 phosphorylation may be, it is likely independent of the HOG pathway, which shows interactions with the CWI pathway under other stress conditions, because Hog1 is not phosphorylated under alkaline conditions (68).
The phosphorylated form of Slt2/Mpk1 has the capacity to activate either the Rlm1 or SBF transcription factor. Mutation of the transcription factor RLM1 results in resistance to alkaline conditions, but strains with SBF defects are sensitive to alkaline media (68), indicating that alkali tolerance is SBF dependent but Rlm1 independent.
Fungal cells adapt not only to alkaline conditions but also to low-pH conditions. This stress response is particularly important to withstand pH changes encountered within hosts. Phagocytic cells introduce low-pH conditions when foreign bodies are phagocytosed as a means of host defense. Further, acidic conditions are also produced in the environment occupied by growing fungal cells as a result of active proton extrusion into the medium by the plasma membrane ATPase (77). Low-pH conditions lead to Slt2/Mpk1 phosphorylation. Indeed, both SLT2/MPK1 and BCK1 from the CWI pathway were found to be required for growth under low-pH medium conditions (9), indicating the requirement for the MAPK cascade.
Under low-pH stress conditions, a mid2 mutant strain exhibits a decrease in the transcription of PST1, a gene regulated by the Slt2/Mpk1-activated transcription factor Rlm1 from the CWI pathway. Transcription of PST1 under acidic stress conditions is not particularly reliant on the stress sensor Wsc1 (9), but rather the stress sensor Mid2 mediates a response to acidic conditions that leads to activation of the Rlm1 transcription factor.
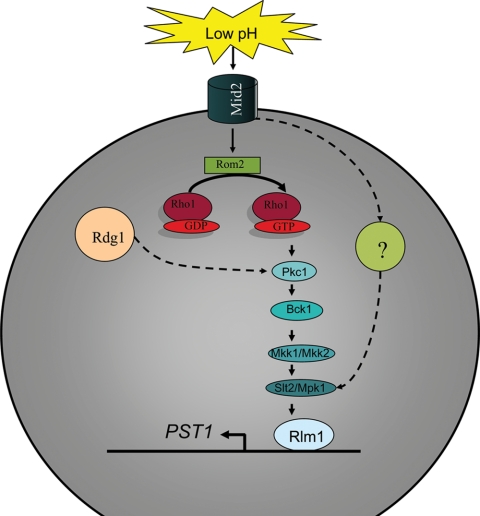
The CWI pathway receives lateral influence under acidic conditions from Rgd1 (Fig. 3). The RGD1 gene encodes a Rho GTPase-activating protein that acts on Rho3 and Rho4. It plays a role in tolerance to acidic conditions, which is exemplified by rgd1 mutant strain sensitivity to low-pH conditions. The defects observed for the rgd1 mutant strain are exacerbated in an rgd1 mid2 double mutant strain (9), suggesting that Rgd1 influences the CWI pathway. The rgd1 mutant strain viability phenotype is suppressed by an increase in PKC pathway activity, and defects in the RGD1 gene lead to defects in PKC pathway activity (15). It is likely that the PKC pathway functions downstream of Rgd1. Furthermore, the expression of RGD1 under acidic shock conditions was Hog1 dependent (25). Again, this is another stress condition indicating cross talk between the HOG and CWI pathways.
FIG. 3.
Proposed cellular response mechanism for S. cerevisiae to withstand extracellular low-pH conditions. Low-pH extracellular conditions activate the CWI pathway via the cell stress sensor Mid2. The activated signal transduction cascade leads to stimulation of the MAPK module. A potential lateral cross talk event with Rgd1 is suggested. There is also potentially an alternative mechanism by which the signal is mediated from the Mid2 sensor to Slt2/Mpk1, which is PKC pathway independent. Broken lines indicate potential signal transduction events.
Taken together, the findings so far indicate that Wsc1 is the CWI pathway stress sensor necessary for alkaline conditions and that Mid2 is the stress sensor required for acidic conditions. Alkaline-activated Wsc1 leads to transcription of SBF-dependent genes, and Mid2 low-pH activation leads to transcription of Rlm1-dependent genes. Hence, the difference stress sensors for the CWI pathway lead to activation of two different transcription factors. Further, the CWI pathway manages alkaline stress in a Hog1-independent manner but acidic stress conditions in a Hog1-dependent manner.
RESPONSE TO HEAT SHOCK
For fungi that maintain a niche within soil or plant environments, heat shock can occur with changes in climate temperature. Moreover, the difference in temperate conditions between a soil environment and a mammalian host can constitute a “heat shock” (for example, when fungi are relocated from the soil to the lungs of a host). In S. cerevisiae, heat shock causes the activation of Slt2/Mpk1 (28, 35, 78). However, phosphorylation of Slt2/Mpk1 is diminished under heat shock conditions in a wsc1 mutant strain (78). An S. cerevisiae wsc1 deletion mutant exhibits thermosensitive growth under high-temperature growth conditions at 37°C (78), indicating that Wsc1 plays a role as a sensor for heat shock.
There is evidence that Wsc1 mediates cross talk between the CWI pathway and the cyclic AMP (cAMP)-protein kinase A (PKA) pathway of S. cerevisiae for the heat stress response. The cAMP-PKA pathway is a nutrient-sensing mechanism that controls the cell cycle. In this signaling cascade, Ras is activated by a GEF, Cdc25, and is inactivated by GTPase-activating proteins Ira1 and Ira2. The activation of Ras yields an increase in adenylyl cyclase activity, which produces the second messenger cAMP for the activation of PKA, which targets several downstream proteins involved in metabolism. Among the downstream proteins regulated by PKA are the transcription factors Msn2/Msn4, which regulate the stress-inducible MAPK phosphatase Sdp1 (71).
Verna and colleagues (78) used an ira2 deletion strain, which is heat shock sensitive (70), to identify inhibitors of RAS activity. A wsc1 deletion mutation was found to suppress the heat shock phenotype of the ira2 mutant strain and is speculated to negatively regulate targets of RAS. Indeed, a ras2 deletion mutation rescues the heat shock sensitivity of a wsc mutant strain. Further, a wsc strain that overexpresses IRA2 is not sensitive to heat shock (78). Examination of which WSC gene is involved in this signaling cascade reveals that WSC1 but not WSC2 suppresses the heat shock sensitivity phenotype of a ras1Δ ras2Δ pCYR1 strain in which the RAS genes are deleted and adenylyl cyclase (CYR1) is overexpressed. This suggests that Wsc1 but not Wsc2 functions in conjunction with RAS signaling and that RAS and Wsc1 have opposing affects on a downstream target (78).
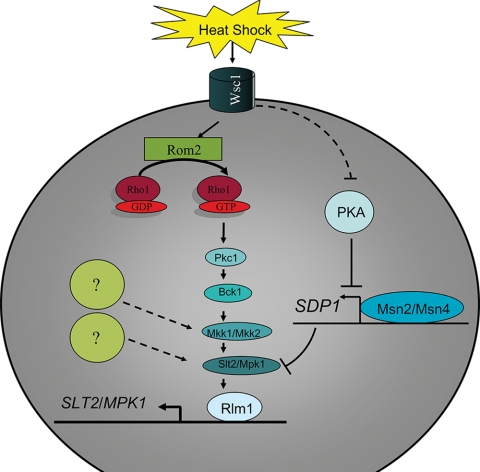
Thus, the Wsc1 transmembrane stress sensor of the CWI pathway facilitates cross talk with the cAMP-PKA pathway through the downstream activation of Slt2/Mpk1 in the CWI pathway and negative regulation of a downstream target of RAS in the cAMP-PKA pathway in response to thermal stress (Fig. 4). Additional cross talk with the cAMP-PKA pathway occurs at the Slt2/Mpk1 position of the CWI signaling cascade. Slt2/Mpk1 is negatively regulated by Msn2/Msn4-dependent Sdp1 from the cAMP-PKA pathway by direct dephosphorylation of Slt2/Mpk1 under heat shock conditions (28).
FIG. 4.
Heat stress is a means of activating the CWI pathway in S. cerevisiae. Wsc1 functions as the cell stress sensor to activate the CWI pathway in response to heat shock. There are several proteins within the CWI pathway that potentially participate in a lateral interaction to influence the CWI pathway response to heat shock. Further, there is indication of a cross talk event with the PKA pathway leading to Slt/Mpk1 phosphorylation during heat shock. Broken lines indicate potential signal transduction events.
The phosphorylation of Slt2/Mpk1 is dependent on the MAPK cascade of the CWI pathway during several stress responses, and thus it is presumable that the MAPK cascade is necessary for the heat shock response. Several genes in the PKC pathway are indeed required for thermal tolerance, including BCK1, MKK1/MKK2, and SLT2/MPK1 (33, 45, 74), indicating that the MAPK cascade is important in response to heat shock. Interestingly, the activation of Slt2/Mpk1 by heat shock is Pkc1 and Bck1 independent (29). In experiments by Harrison and colleagues (29), a pkc1 mutant strain harboring a bck1-20 allele that constitutively activates the MAPK pathway in the absence of PKC1 was used. Further, a bck1 mutant strain that contained an MKK1DD allele providing constitutive MAPK pathway activation in the absence of BCK1 was employed. In both of the strains a basal level of Slt2/Mpk1 activation was detected, but heat shock of these strains resulted in a notable increase of Slt2/Mpk1 phosphorylation (29). This suggests that Pkc1 and Bck1 are not required for Slt2/Mpk1 activation in response to heat stress. From these experiments we hypothesize that the individual components of the MAPK cascade are dispensable as long as the cascade signal conveyed from Mkk1/Mkk2 to Slt2/Mpk1 is intact. The dispensability of components of the CWI pathway provides an opportunity for potential lateral influences to activate Slt2/Mpk1 either through Mkk1/Mkk2 or at Slt/Mpk1 directly (Fig. 4).
The CWI pathway response to heat shock is not restricted to S. cerevisiae but also functions to mediate thermal tolerance in pathogenic fungi. Cryptococcus neoformans pkc1 and mpk1 deletion strains are temperature sensitive (26, 40). A rom2 deletion strain is also sensitive to thermal stress in combination with an additional cell wall stressor such as Congo red or sodium dodecyl sulfate (22). Heat sensitivity resulting from mutations in this set of genes suggests that the CWI pathway is required for a response to heat stress in C. neoformans. It is obvious that elucidation of cross talk events between the CWI pathway and other stress response mechanisms of pathogenic fungi during heat shock conditions needs further investigation.
RESPONSE TO LIMITED NITROGEN SOURCES
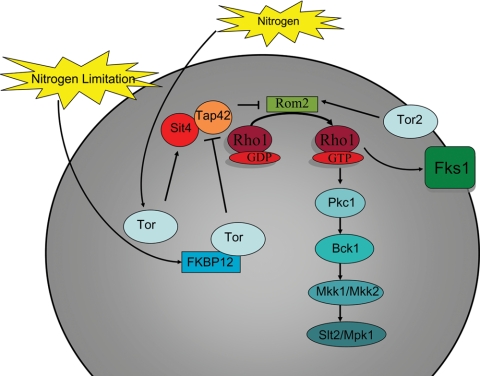
Nutrient stress occurs when fungal cells transition from a nutrient-rich to a nutrient-poor environment. The environment in this case could be the soil, a host, or even the in vitro conditions of yeast culture media. The availability of nutrients dictates cellular functions such as cell growth and cell cycle progression. TOR (target of rapamycin) is named for the ability of the agent rapamycin to inhibit this particular signaling cascade. The TOR signaling cascade senses the availability of nitrogen sources to regulate gene expression. To study the effects of nitrogen-limited conditions, rapamycin is used to inhibit the TOR signaling cascade. The agent rapamycin forms a complex with FKBP12, which is then able to complex with the serine/threonine kinase Tor, inhibit TOR signaling, and prevent association between Sit4 and Tap42. In contrast, Tor activated by nitrogen sources promotes the association of the type 2A phosphatase Sit4 with Tap42. Tor protein is conserved in many fungi (10), but two fungi from which much has been elucidated about TOR signaling are S. cerevisiae and S. pombe, each encoding two Tor proteins, Tor1 and Tor2 (30, 37).
There is evidence for cross talk between TOR-mediated signaling and the CWI pathway. S. cerevisiae cells with inhibited TOR signaling due to exposure to rapamycin exhibit activation of Slt2/Mpk1. Strains carrying a TOR1-1 or TOR2-1 allele are insensitive to rapamycin. The TOR1-1 strain exhibits no change in phosphorylation of Slt2/Mpk1 in the presence of rapamycin, and a TOR2-1 mutant experiences mild phosphorylation of Slt2/Mpk1 (73). Thus, inhibition of Tor1 leads to CWI activation. This is further illustrated by the lack of Slt2/Mpk1 phosphorylation in response to rapamycin in TAP42 and SIT4 mutant strains. Interestingly, the TOR inhibited-induced activation of Slt2/Mpk1 is dependent on components of the CWI pathway, including Pkc1 and Bck1, as indicated by a lack of Slt/Mpk1 activation in pkc1 and bck1 deletion strains. Examination for the requirement of the upper part of the CWI signal cascade reveals that deletion of ROM2 diminishes phosphorylation of Slt2/Mpk1 in the presence of rapamycin (73). The decrease in phosphorylation rather than the complete lack of phosphorylation may be in part due to the presence of Rom1, which has a role partially redundant with that of Rom2 (58). Thus, the activation of Slt2/Mpk1 by TOR inhibition is dependent on PKC pathway components as well as upstream elements of the CWI pathway.
TOR signaling and the CWI pathway share the common function of actin organization. Although there are two TOR genes in S. cerevisiae, only TOR2 plays a role in actin organization (67). ROM2 restores growth in a tor2ts mutant strain when overexpressed (31, 66). However, TOR2 overexpression does not repair the growth defect of a rom2 mutant strain (66). Further evidence that Tor influences Rom2 is demonstrated by the reduction in Rom2 GEF activity in a tor2 mutant strain. Thus, Rom2 likely functions downstream of Tor2 (66) (Fig. 5). Although it is unclear from the reports by Schmidt et al. (66) and Torres et al. (73) whether Tor directly or indirectly influences the CWI pathway, it is agreed by both groups that the most likely candidate to receive Tor-directed signals is Rom2.
FIG. 5.
Nitrogen limitation inhibits TOR signaling and the subsequent activation of the CWI pathway. S. cerevisiae carries the genes TOR1 and TOR2. TOR signaling is activated in response to nitrogen or inhibited due to cellular exposure to nitrogen limitations or synthetically to the agent rapamycin. Rapamycin binds to the prolyl-isomerase enzyme FKBP12. Binding of FKBP12 and thus inhibition of Tor leads to Slt2/Mpk1 phosphorylation. In this process, the Tor/FKBP12 complex prevents Tap42, a type 2A phosphatase-associated protein that binds to Sit4, a type 2A-related serine threonine phosphatase, from inhibiting Rom2. As a likely recipient of TOR signaling, Rom2 stimulates the CWI pathway in a PKC pathway-dependent manner that leads to Slt2/Mpk1 activation.
Cross talk events between the TOR signaling and CWI pathways in the pathogenic fungus C. albicans are also observed. In C. albicans there is a single copy of TOR (10). Cross talk occurs through a G protein of the Ras superfamily, Rhb1, involved in the activation of Tor. A rhb1/rhb1 deletion mutant strain is susceptible to rapamycin (75), indicating a relationship to Tor. Of note, the RHB1 ortholog in Aspergillus fumigatus is rhbA. A role for rhbA in TOR signaling is conserved in A. fumigatus, where an rhbA deletion mutant strain is sensitivity to rapamycin (59).
Further, wild-type C. albicans cells exposed to rapamycin exhibit enhanced phosphorylation of Mkc1 (encoded by a SLT2/MPK1 homolog) (Table 1), suggesting that TOR influences the CWI pathway (75). The rhb1/rhb1 strain is also sensitive to cell wall stress induced by Congo red and calcofluor white (75), which indicate defects in CWI. Congo red and calcofluor white exposure are known to stimulate MAPK induction of Mkc1 (16, 38). Exposure of cells to calcofluor white indeed induces Mkc1 phosphorylation, but in the rhb1/rhb1 strain activation of Mkc1 is diminished. This suggests that Mkc1 phosphorylation is Rhb1 dependent during cell wall stress (75).
CALCIUM SIGNALING-INDUCED STRESS RESPONSE
It has been established that calcium functions as a second messenger, and changes in calcium homeostasis have a number of physiological effects in cells. It is not well known what type of calcium exposure fungal cells encounter in the environment, but indeed they are able to react to calcium. Calcium has been shown to induce Mkc1 in C. albicans (54), and an mkc1/mkc1 mutant is sensitive to calcium (55). The calcium-induced phosphorylation of Mkc1 is a Hog1-dependent event (54). Calcium is also known to be an activator of calcineurin in cells. Calcineurin is a Ca2+/calmodulin-dependent serine/threonine protein phosphatase. It is the target of such drugs as cyclosporine and FK506. In the inactive state calcineurin is a heterodimer composed of A and B subunits. When activated, it is in a Ca2+/calmodulin-bound state, causing a change that frees the active site of an autoinhibitory domain (21). S. cerevisiae carries two redundant calcineurin A genes, CNA1 and CNA2 (12, 49), and a single copy of the calcineurin B gene, CNB1 (13, 42). Only a single copy of the calcineurin A subunit is encoded by the fungi S. pombe (82), C. albicans (65), C. neoformans (57), and Aspergillus nidulans (64). Calcineurin participates in a number of physiological processes in fungi, including cell cycle progression, morphological changes, and cell wall biosynthesis. Among pathogenic fungi, calcineurin is also important for virulence (57, 65). Inhibition of C. neoformans calcineurin function induces the phosphorylation of Mpk1 and the induction of FKS1. FKS1 is also induced by calcineurin in C. albicans (65). Both Mpk1 and Fks1 are under the control of the CWI pathway (40). Fks1 is activated by Rho1, which also activates Pkc1 to induce the MAPK cascade. Interestingly, the deletion of FKS1 in S. cerevisiae leads to increased sensitivity to the calcineurin inhibitor cyclosporine (20), suggesting an interplay between the CWI pathway and calcineurin.
The potential interplay between calcineurin and the CWI pathway is further strengthened by evidence from S. cerevisiae in which constitutively active calcineurin partially suppresses the lysis phenotypes of pkc1 and slt2/mpk1 mutant strains (24). Garrett-Engele and colleagues (24) further demonstrated that the pkc1 mutant strain grown with the osmotic stabilizer was sensitive to the calcineurin inhibitors cyclosporine and FK506. An slt2/mpk1 mutant strain also exhibited sensitivity to these calcineurin inhibitors (24). Thus, calcineurin compensates for some of the defects in the CWI pathway. The calcineurin overexpression may have produced more osmotically stable conditions for the pkc1 and slt2/mpk1 mutant strains, or it could have activated a subset of CWI pathway transcription factors.
RESPONSE TO OXIDATIVE STRESS
Oxidative stress occurs in the natural environment with exposure to aerobic conditions and UV light. For pathogenic fungi, oxidative stress is manifested through the host's production of reactive oxygen species, hydrogen peroxide, and hydroxyl radicals by phagocytic cells. Oxide radicals are also the consequence of normal cell metabolism. The first line of defense by fungi to prevent cell damage from exogenous oxide radicals is the cell wall. The cell senses oxide stress, and reactive measures are taken by signal transduction pathways to protect fungi from the adverse environment. A key defense against oxidative stress is the HOG pathway (1, 2, 69). The CWI pathway provides additional oxide defense through cross talk with the HOG pathway. Oxidative stress resistance is also dependent upon the transcription factors Skn7, Yap1, Msn2, and Msn4. These transcription factors control the expression of numerous genes in response to peroxide stress. Orthologous sequences for the transcription factors are shared between the budding yeast S. cerevisiae, the fission yeast S. pombe, and the pathogens C. albicans, Candida glabrata, and Ustilago maydis (11). A number of other enzymes are produced to combat oxide attack, including superoxide dismutases, catalases, peroxidases, and glutathione peroxidases.
In S. cerevisiae, diamide and hydrogen peroxide exposure aid in the effort to deduce the CWI pathway response to oxide stress. Diamide depletes glutathione and oxide thiol groups. Hydrogen peroxide promotes lipid peroxidation, protein oxidation, and DNA damage. Diamide or hydrogen peroxide is capable of activating the dual phosphorylation of Slt2/Mpk1, although with differing kinetic activity. Diamide-induced oxide stress is sensed in S. cerevisiae by the transmembrane proteins Mid2 and Wsc1 (79). In contrast, hydrogen peroxide does not require one of the known CWI stress sensors to provoke the phosphorylation of Slt2/Mpk1 and is suspected to elicit an intracellular means of activation (79). Exposure of a rom2 mutant strain to either diamide or hydrogen peroxide results in diminished phosphorylation of Slt2/Mpk1. Pkc1 is also required, but the MAPK module downstream of Pkc1 is not vital to the cellular response in defense of oxidative stress (79). Thus, the activation of the CWI pathway by oxidative stress by either inducer requires the upper part of the CWI pathway for cell survival (79).
A similar oxidative stress response occurs in C. neoformans (26). In this case, phosphorylation of Mpk1 indicates PKC pathway activity in response to oxidative stress elicited from diamide or hydrogen peroxide. PKC1 is required to defend against oxidative stress. However, as seen in S. cerevisiae, the C. neoformans MAPK module is dispensable for survival against oxidative stress (26). Those authors suggest that an alternative pathway extends from Pkc1 to provide for protection against oxidative stress (26). Thus, there is an alternative function of Pkc1 other than activation of the MAPK module that leads to an oxide-induced phosphorylation of Mpk1. The greater severity of the pkc1 mutation compared to the bck1 mutation evaluated for C. neoformans by Gerik and colleagues suggests further roles for Pkc1 that are not facilitated through the MAPK module (27), a hypothesis that has been postulated by others in regard to the role of PKC (39). Indeed, analysis of the synthetic genetic network around S. cerevisiae PKC1 reveals several genes that influence the CWI pathways but are not themselves CWI pathway genes (41). Additional evidence for an extended role of Pkc1 beyond the activation of the MAPK module is the lethality of pkc mutations in S. cerevisiae, indicating that PKC1 is essential (48), whereas mutations of the MAPK module are not lethal. Likewise, deletion of PKC1 of C. neoformans is lethal unless the cells are protected by an osmotic stabilizer such as sorbitol, whereas the MAPK module mutants show no growth defects under regular medium conditions (26).
The CWI pathway is also activated by oxidative stress in C. albicans (54). Mkc1 is phosphorylated in the presence of hydrogen peroxide, but interestingly, it is not required for cell viability in the presence of hydrogen peroxide (54). Phosphorylation of Mkc1 with oxide stress is Pkc1 dependent (as seen with other fungi) except when diamide is used as the inducing agent. In the case of diamide, Mkc1 phosphorylation is Pkc1 independent. The phosphorylation event is not simply a result of cell wall damage, because phosphorylation also occurs in the presence of the osmotic stabilizer sorbitol (54). The phosphorylation of Mkc1 is also Pbs2 and Hog1 dependent, indicating a cross talk event between the CWI and HOG pathways in response to oxide stress (2).
A slightly different oxide response was observed with A. fumigatus. The CWI pathway of A. fumigatus differentiates between the types of oxidative stressor. An mpkA (a homolog of SLT2/MPK1) deletion strain is sensitive to diamide and menadione, which generates superoxides, but resistant to hydrogen peroxide (76). Mechanistically there is similarity in that A. fumigatus, like C. albicans, does not require MAPK for cell survival when oxide stress is presented as hydrogen peroxide, indicating that various oxides elicit different cellular responses.
RESPONSE TO NITROSATIVE STRESS
The CWI pathway is not characterized to respond to nitrosative stress. However, C. neoformans presents a thus far unique situation by responding to nitrosative stress by activating the CWI pathway. In vitro exposure of C. neoformans to nitrosative stress in the form of sodium nitrite results in phosphorylation of Mpk1. PKC1 is vital to cell survival during nitrosative stress (26). Further examination of the cell response to nitrosative stress is needed to determine if the CWI pathway facilitates a response to nitrosative stress for other fungi. If the CWI pathway is not the primary means of responding to nitrosative stress in fungal cells, perhaps the CWI response to nitrosative stress observed in C. neoformans indicates a cross talk event with another pathway.
RESPONSE TO ANTIFUNGAL AGENTS AND CHEMICAL COMPOUNDS
There are several types of antifungal agents available for treating fungal diseases. Echinocandin antifungals such as caspofungin target (1,3)-β-d glucan synthase. When C. albicans cells are exposed to caspofungin, the chitin biosynthesis genes CHS2 and CHS8 are upregulated (80), among other genes. In an slt2/mpk1 mutant strain, CHS2 and CHS8 exhibit only marginal increases in expression upon caspofungin exposure compared to a wild-type strain exposed to caspofungin, which exhibits significant increases in CHS2 and CHS8 expression (80). Thus, the CWI pathway is required for chitin biosynthesis in response to stress induced by caspofungin.
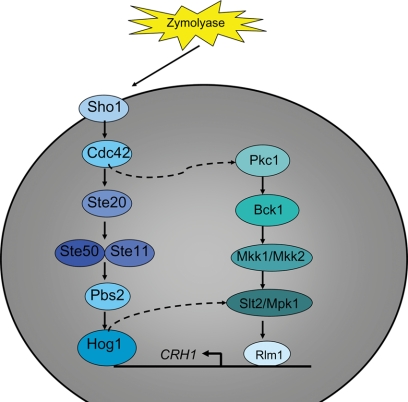
Additional compounds have been identified to elicit CWI stress; these compounds are not used to treat fungal infections in patients but can provide additional information about the pathway interactions that are induced to prevent or repair CWI damage. Zymolyase is an enzyme that affects the cell wall by hydrolyzing the β-(1,3)-glucan network. The cellular response to Zymolyase involves both the CWI and HOG pathways. Exposure of S. cerevisiae to Zymolyase results in the increased expression of CRH1, which encodes a glycosylphosphatidylinositol cell wall protein induced under cell wall damage growth conditions. Crh1 protein expression induced by Zymolyase is Hog1 and Slt2/Mpk1 dependent (6).
Zymolyase stimulation of the HOG pathway is through the Sho1-dependent branch. The genes SHO1, STE11, and HOG1, but not SSK1 or SSK2/SSK22, are required in the response to Zymolyase treatment (6, 23). Zymolyase induction of Slt2/Mpk1 phosphorylation from the CWI pathway requires PKC1, BCK1, MKK1/MKK2, and MPK1 but not the upstream element ROM2 or the stress sensors MID2 and WSC1 (6, 23). This type of activation indicates that the MAPK module is required for Slt2/Mpk1 phosphorylation by Zymolyase but not the upstream portion of the CWI pathway or the stress sensors. Thus, it is not a linear activation of Slt2/Mpk1 through the CWI pathway but rather the influence of a lateral element. The evidence provided suggests that the response to Zymolyase is mediated through the Sho1-dependent branch of the HOG pathway and that a cross talk event which initiates the MAPK module of the CWI pathway occurs. Bermejo et al. (6) demonstrated that Hog1 phosphorylation was Slt2/Mpk1 independent. Further, they demonstrated that Slt2/Mpk1 was overphosphorylated in response to Zymolyase and that the overphosphorylation event was Hog1 dependent. Thus, the HOG pathway functions to activate the MAPK module of the CWI pathway in the presence of Zymolyase-induced cell stress (Fig. 6).
FIG. 6.
Cellular exposure to Zymolyase leads to activation of both the HOG and CWI pathways. The cellular exposure to Zymolyase leads to phosphorylation of Slt2/Mpk1. Activation of the CWI pathway is independent of the known cell stress sensors that function in the CWI pathway but dependent on the HOG pathway. Activation of the HOG pathway in the presence of Zymolyase is Sho1 dependent and Sln1 independent. Expression of Crh1, a downstream target of the CWI pathway, is Hog1 and Slt2/Mpk1 dependent. Broken lines indicate potential signal transduction events.
A different manifestation of the cellular CWI response is observed with exposure to the chemical caffeine. Although it is not likely that caffeine is a stress-inducing agent in the natural environment, it does provide insight into fungal cell signaling interactions. It has not yet been fully elucidated how caffeine affects cells, but it does indeed act as a cell wall stress agent. Kuranda and colleagues (43) identified that caffeine induces a CWI response in S. cerevisiae through phosphorylation of Slt2/Mpk1 in a Tor-dependent manner. Activation of the CWI pathway is independent of the stress sensors WSC1 and MID2. It is, however, dependent on other members of the CWI pathway, including ROM2, RHO1, BCK1, and MKK1 (43, 52). Thus, there is potentially a sensor other than Wsc1 and Mid2 to initiate the signal cascade or a lateral influence.
The chemical activation of the CWI pathway is not a uniform response but rather depends on the chemical that elicits the response. We have seen that the CWI pathway can interact with the HOG pathway or TOR signaling, and it is possible that other signaling mechanisms could be recruited by different chemical stresses.
CONCLUSION
Maintaining CWI is a difficult task given the numerous external influences that challenge fungal cells. Plant and animal hosts in particular present adverse conditions to fungi in multiple ways simultaneously through changes in pH, temperature, and nutrient availability and by actively combating fungi with oxidative radicals and phagocytosis of fungal cells. It would be prudent for fungi to maintain constitutively high activation of the CWI pathway at all times, but this is not the case. Perhaps it is too costly in energy and cellular resources to maintain the CWI pathway at defensive levels at all times. It is therefore strategically activated when stress conditions or growth warrants the activity.
It would be difficult for a single pathway to correctly attend to each of the very different adverse conditions to which a fungal cell is exposed in a linear manner. Therefore, the CWI pathway is a multifunctional pathway that receives lateral influences at many points along the signaling cascade. Lateral interactions encompass Tor signaling, calcineurin signaling, the HOG pathway, cAMP-PKA signaling, and input from proteins not yet elucidated. This complicated network of signaling enables the CWI pathway to be activated by numerous types of stressors and provides the necessary responses to fortify the cell and preserve fungal cell viability.
Although much of the information presented in this article in based on pathway interactions learned through research involving the model yeast S. cerevisiae, with additional information provided through research on several pathogenic fungi, much more is likely to be revealed about the signal transduction interactions that lead to CWI maintenance from numerous other fungi that cope with a plethora of hostile environments. Further, there is still much that can be learned through fungal studies by creating double knockout and overexpression strains to elucidate how the pathways presented in this article interact to cope with stress conditions.
Acknowledgments
This work was supported by National Institutes of Health grants R01 AI075286 and R21 AI079569 to E.M.
We acknowledge three anonymous reviewers for helpful feedback and comments.
Footnotes
Published ahead of print on 28 August 2009.
REFERENCES
- 1.Alonso-Monge, R., F. Navarro-Garcia, E. Roman, A. I. Negredo, B. Eisman, C. Nombela, and J. Pla. 2003. The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans. Eukaryot. Cell 2:351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arana, D. M., C. Nombela, R. Alonso-Monge, and J. Pla. 2005. The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology 151:1033-1049. [DOI] [PubMed] [Google Scholar]
- 3.Bahn, Y. S. 2008. Master and commander in fungal pathogens: the two-component system and the HOG signaling pathway. Eukaryot. Cell 7:2017-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahn, Y. S., S. Geunes-Boyer, and J. Heitman. 2007. Ssk2 mitogen-activated protein kinase kinase kinase governs divergent patterns of the stress-activated Hog1 signaling pathway in Cryptococcus neoformans. Eukaryot. Cell 6:2278-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banuett, F. 1998. Signalling in the yeasts: an informational cascade with links to the filamentous fungi. Microbiol. Mol. Biol. Rev. 62:249-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermejo, C., E. Rodriguez, R. Garcia, J. M. Rodriguez-Pena, M. L. Rodriguez de la Concepcion, C. Rivas, P. Arias, C. Nombela, F. Posas, and J. Arroyo. 2008. The sequential activation of the yeast HOG and SLT2 pathways is required for cell survival to cell wall stress. Mol. Biol. Cell 19:1113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boisnard, S., G. Ruprich-Robert, M. Florent, B. Da Silva, F. Chapeland-Leclerc, and N. Papon. 2008. Insight into the role of HOG pathway components Ssk2p, Pbs2p, and Hog1p in the opportunistic yeast Candida lusitaniae. Eukaryot. Cell 7:2179-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, R. E., and J. Thorner. 2007. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1773:1311-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claret, S., X. Gatti, F. Doignon, D. Thoraval, and M. Crouzet. 2005. The Rgd1p Rho GTPase-activating protein and the Mid2p cell wall sensor are required at low pH for protein kinase C pathway activation and cell survival in Saccharomyces cerevisiae. Eukaryot. Cell 4:1375-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz, M. C., A. L. Goldstein, J. Blankenship, M. Del Poeta, J. R. Perfect, J. H. McCusker, Y. L. Bennani, M. E. Cardenas, and J. Heitman. 2001. Rapamycin and less immunosuppressive analogs are toxic to Candida albicans and Cryptococcus neoformans via FKBP12-dependent inhibition of TOR. Antimicrob. Agents Chemother. 45:3162-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuellar-Cruz, M., M. Briones-Martin-del-Campo, I. Canas-Villamar, J. Montalvo-Arredondo, L. Riego-Ruiz, I. Castano, and A. De Las Penas. 2008. High resistance to oxidative stress in the fungal pathogen Candida glabrata is mediated by a single catalase, Cta1p, and is controlled by the transcription factors Yap1p, Skn7p, Msn2p, and Msn4p. Eukaryot. Cell 7:814-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cyert, M. S., R. Kunisawa, D. Kaim, and J. Thorner. 1991. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc. Natl. Acad. Sci. USA 88:7376-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cyert, M. S., and J. Thorner. 1992. Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol. Cell. Biol. 12:3460-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davenport, K. R., M. Sohaskey, Y. Kamada, D. E. Levin, and M. C. Gustin. 1995. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J. Biol. Chem. 270:30157-30161. [DOI] [PubMed] [Google Scholar]
- 15.de Bettignies, G., D. Thoraval, C. Morel, M. F. Peypouquet, and M. Crouzet. 2001. Overactivation of the protein kinase C-signaling pathway suppresses the defects of cells lacking the Rho3/Rho4-GAP Rgd1p in Saccharomyces cerevisiae. Genetics 159:1435-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Nobel, H., C. Ruiz, H. Martin, W. Morris, S. Brul, M. Molina, and F. M. Klis. 2000. Cell wall perturbation in yeast results in dual phosphorylation of the Slt2/Mpk1 MAP kinase and in an Slt2-mediated increase in FKS2-lacZ expression, glucanase resistance and thermotolerance. Microbiology 146:2121-2132. [DOI] [PubMed] [Google Scholar]
- 17.Dodou, E., and R. Treisman. 1997. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17:1848-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas, C. M., F. Foor, J. A. Marrinan, N. Morin, J. B. Nielsen, A. M. Dahl, P. Mazur, W. Baginsky, W. Li, M. el-Sherbeini, et al. 1994. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-beta-d-glucan synthase. Proc. Natl. Acad. Sci. USA 91:12907-12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drgonova, J., T. Drgon, K. Tanaka, R. Kollar, G. C. Chen, R. A. Ford, C. S. Chan, Y. Takai, and E. Cabib. 1996. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science 272:277-279. [DOI] [PubMed] [Google Scholar]
- 20.Eng, W. K., L. Faucette, M. M. McLaughlin, R. Cafferkey, Y. Koltin, R. A. Morris, P. R. Young, R. K. Johnson, and G. P. Livi. 1994. The yeast FKS1 gene encodes a novel membrane protein, mutations in which confer FK506 and cyclosporin A hypersensitivity and calcineurin-dependent growth. Gene 151:61-71. [DOI] [PubMed] [Google Scholar]
- 21.Fox, D. S., and J. Heitman. 2002. Good fungi gone bad: the corruption of calcineurin. Bioessays 24:894-903. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs, B. B., G. P. Tegos, M. R. Hamblin, and E. Mylonakis. 2007. Susceptibility of Cryptococcus neoformans to photodynamic inactivation is associated with cell wall integrity. Antimicrob. Agents Chemother. 51:2929-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia, R., J. M. Rodriguez-Pena, C. Bermejo, C. Nombela, and J. Arroyo. 2009. The high osmotic response and cell wall integrity pathways cooperate to regulate transcriptional responses to zymolyase-induced cell wall stress in Saccharomyces cerevisiae. J. Biol. Chem. 284:10901-10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrett-Engele, P., B. Moilanen, and M. S. Cyert. 1995. Calcineurin, the Ca2+/calmodulin-dependent protein phosphatase, is essential in yeast mutants with cell integrity defects and in mutants that lack a functional vacuolar H+-ATPase. Mol. Cell. Biol. 15:4103-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gatti, X., G. de Bettignies, S. Claret, F. Doignon, M. Crouzet, and D. Thoraval. 2005. RGD1, encoding a RhoGAP involved in low-pH survival, is an Msn2p/Msn4p regulated gene in Saccharomyces cerevisiae. Gene 351:159-169. [DOI] [PubMed] [Google Scholar]
- 26.Gerik, K. J., S. R. Bhimireddy, J. S. Ryerse, C. A. Specht, and J. K. Lodge. 2008. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot. Cell 7:1685-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerik, K. J., M. J. Donlin, C. E. Soto, A. M. Banks, I. R. Banks, M. A. Maligie, C. P. Selitrennikoff, and J. K. Lodge. 2005. Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Mol. Microbiol. 58:393-408. [DOI] [PubMed] [Google Scholar]
- 28.Hahn, J. S., and D. J. Thiele. 2002. Regulation of the Saccharomyces cerevisiae Slt2 kinase pathway by the stress-inducible Sdp1 dual specificity phosphatase. J. Biol. Chem. 277:21278-21284. [DOI] [PubMed] [Google Scholar]
- 29.Harrison, J. C., T. R. Zyla, E. S. Bardes, and D. J. Lew. 2004. Stress-specific activation mechanisms for the “cell integrity” MAPK pathway. J. Biol. Chem. 279:2616-2622. [DOI] [PubMed] [Google Scholar]
- 30.Heitman, J., N. R. Movva, and M. N. Hall. 1991. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253:905-909. [DOI] [PubMed] [Google Scholar]
- 31.Helliwell, S. B., I. Howald, N. Barbet, and M. N. Hall. 1998. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics 148:99-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hohmann, S., M. Krantz, and B. Nordlander. 2007. Yeast osmoregulation. Methods Enzymol. 428:29-45. [DOI] [PubMed] [Google Scholar]
- 33.Irie, K., M. Takase, K. S. Lee, D. E. Levin, H. Araki, K. Matsumoto, and Y. Oshima. 1993. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol. 13:3076-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung, U. S., A. K. Sobering, M. J. Romeo, and D. E. Levin. 2002. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 46:781-789. [DOI] [PubMed] [Google Scholar]
- 35.Kamada, Y., U. S. Jung, J. Piotrowski, and D. E. Levin. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9:1559-1571. [DOI] [PubMed] [Google Scholar]
- 36.Kamada, Y., H. Qadota, C. P. Python, Y. Anraku, Y. Ohya, and D. E. Levin. 1996. Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 271:9193-9196. [DOI] [PubMed] [Google Scholar]
- 37.Kawai, M., A. Nakashima, M. Ueno, T. Ushimaru, K. Aiba, H. Doi, and M. Uritani. 2001. Fission yeast tor1 functions in response to various stresses including nitrogen starvation, high osmolarity, and high temperature. Curr. Genet. 39:166-174. [DOI] [PubMed] [Google Scholar]
- 38.Ketela, T., R. Green, and H. Bussey. 1999. Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 181:3330-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozubowski, L., S. C. Lee, and J. Heitman. 2009. Signaling pathways in the pathogenesis of Cryptococcus. Cell. Microbiol. 11:370-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraus, P. R., D. S. Fox, G. M. Cox, and J. Heitman. 2003. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 48:1377-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krause, S. A., H. Xu, and J. V. Gray. 2008. The synthetic genetic network around PKC1 identifies novel modulators and components of protein kinase C signaling in Saccharomyces cerevisiae. Eukaryot. Cell 7:1880-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuno, T., H. Tanaka, H. Mukai, C. D. Chang, K. Hiraga, T. Miyakawa, and C. Tanaka. 1991. cDNA cloning of a calcineurin B homolog in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 180:1159-1163. [DOI] [PubMed] [Google Scholar]
- 43.Kuranda, K., V. Leberre, S. Sokol, G. Palamarczyk, and J. Francois. 2006. Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Mol. Microbiol. 61:1147-1166. [DOI] [PubMed] [Google Scholar]
- 44.Lagorce, A., N. C. Hauser, D. Labourdette, C. Rodriguez, H. Martin-Yken, J. Arroyo, J. D. Hoheisel, and J. Francois. 2003. Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 278:20345-20357. [DOI] [PubMed] [Google Scholar]
- 45.Lee, K. S., and D. E. Levin. 1992. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12:172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levin, D. E. 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69:262-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levin, D. E., and E. Bartlett-Heubusch. 1992. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J. Cell Biol. 116:1221-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levin, D. E., F. O. Fields, R. Kunisawa, J. M. Bishop, and J. Thorner. 1990. A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell 62:213-224. [DOI] [PubMed] [Google Scholar]
- 49.Liu, Y., S. Ishii, M. Tokai, H. Tsutsumi, O. Ohki, R. Akada, K. Tanaka, E. Tsuchiya, S. Fukui, and T. Miyakawa. 1991. The Saccharomyces cerevisiae genes (CMP1 and CMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Mol. Gen. Genet. 227:52-59. [DOI] [PubMed] [Google Scholar]
- 50.Madden, K., Y. J. Sheu, K. Baetz, B. Andrews, and M. Snyder. 1997. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science 275:1781-1784. [DOI] [PubMed] [Google Scholar]
- 51.Madrid, M., T. Soto, H. K. Khong, A. Franco, J. Vicente, P. Perez, M. Gacto, and J. Cansado. 2006. Stress-induced response, localization, and regulation of the Pmk1 cell integrity pathway in Schizosaccharomyces pombe. J. Biol. Chem. 281:2033-2043. [DOI] [PubMed] [Google Scholar]
- 52.Martin, H., J. M. Rodriguez-Pachon, C. Ruiz, C. Nombela, and M. Molina. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275:1511-1519. [DOI] [PubMed] [Google Scholar]
- 53.Mazur, P., and W. Baginsky. 1996. In vitro activity of 1,3-beta-d-glucan synthase requires the GTP-binding protein Rho1. J. Biol. Chem. 271:14604-14609. [DOI] [PubMed] [Google Scholar]
- 54.Navarro-Garcia, F., B. Eisman, S. M. Fiuza, C. Nombela, and J. Pla. 2005. The MAP kinase Mkc1p is activated under different stress conditions in Candida albicans. Microbiology 151:2737-2749. [DOI] [PubMed] [Google Scholar]
- 55.Navarro-Garcia, F., M. Sanchez, J. Pla, and C. Nombela. 1995. Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol. Cell. Biol. 15:2197-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nonaka, H., K. Tanaka, H. Hirano, T. Fujiwara, H. Kohno, M. Umikawa, A. Mino, and Y. Takai. 1995. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 14:5931-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Odom, A., S. Muir, E. Lim, D. L. Toffaletti, J. Perfect, and J. Heitman. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16:2576-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ozaki, K., K. Tanaka, H. Imamura, T. Hihara, T. Kameyama, H. Nonaka, H. Hirano, Y. Matsuura, and Y. Takai. 1996. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15:2196-2207. [PMC free article] [PubMed] [Google Scholar]
- 59.Panepinto, J. C., B. G. Oliver, J. R. Fortwendel, D. L. Smith, D. S. Askew, and J. C. Rhodes. 2003. Deletion of the Aspergillus fumigatus gene encoding the Ras-related protein RhbA reduces virulence in a model of invasive pulmonary aspergillosis. Infect. Immun. 71:2819-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paravicini, G., A. Mendoza, B. Antonsson, M. Cooper, C. Losberger, and M. A. Payton. 1996. The Candida albicans PKC1 gene encodes a protein kinase C homolog necessary for cellular integrity but not dimorphism. Yeast 12:741-756. [DOI] [PubMed] [Google Scholar]
- 61.Pel, H. J., J. H. de Winde, D. B. Archer, P. S. Dyer, G. Hofmann, P. J. Schaap, G. Turner, R. P. de Vries, R. Albang, K. Albermann, M. R. Andersen, J. D. Bendtsen, J. A. Benen, M. van den Berg, S. Breestraat, M. X. Caddick, R. Contreras, M. Cornell, P. M. Coutinho, E. G. Danchin, A. J. Debets, P. Dekker, P. W. van Dijck, A. van Dijk, L. Dijkhuizen, A. J. Driessen, C. d'Enfert, S. Geysens, C. Goosen, G. S. Groot, P. W. de Groot, T. Guillemette, B. Henrissat, M. Herweijer, J. P. van den Hombergh, C. A. van den Hondel, R. T. van der Heijden, R. M. van der Kaaij, F. M. Klis, H. J. Kools, C. P. Kubicek, P. A. van Kuyk, J. Lauber, X. Lu, M. J. van der Maarel, R. Meulenberg, H. Menke, M. A. Mortimer, J. Nielsen, S. G. Oliver, M. Olsthoorn, K. Pal, N. N. van Peij, A. F. Ram, U. Rinas, J. A. Roubos, C. M. Sagt, M. Schmoll, J. Sun, D. Ussery, J. Varga, W. Vervecken, P. J. van de Vondervoort, H. Wedler, H. A. Wosten, A. P. Zeng, A. J. van Ooyen, J. Visser, and H. Stam. 2007. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat. Biotechnol. 25:221-231. [DOI] [PubMed] [Google Scholar]
- 62.Philip, B., and D. E. Levin. 2001. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell. Biol. 21:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qadota, H., C. P. Python, S. B. Inoue, M. Arisawa, Y. Anraku, Y. Zheng, T. Watanabe, D. E. Levin, and Y. Ohya. 1996. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science 272:279-281. [DOI] [PubMed] [Google Scholar]
- 64.Rasmussen, C., C. Garen, S. Brining, R. L. Kincaid, R. L. Means, and A. R. Means. 1994. The calmodulin-dependent protein phosphatase catalytic subunit (calcineurin A) is an essential gene in Aspergillus nidulans. EMBO J. 13:3917-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48:959-976. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt, A., M. Bickle, T. Beck, and M. N. Hall. 1997. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell 88:531-542. [DOI] [PubMed] [Google Scholar]
- 67.Schmidt, A., J. Kunz, and M. N. Hall. 1996. TOR2 is required for organization of the actin cytoskeleton in yeast. Proc. Natl. Acad. Sci. USA 93:13780-13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Serrano, R., H. Martin, A. Casamayor, and J. Arino. 2006. Signaling alkaline pH stress in the yeast Saccharomyces cerevisiae through the Wsc1 cell surface sensor and the Slt2 MAPK pathway. J. Biol. Chem. 281:39785-39795. [DOI] [PubMed] [Google Scholar]
- 69.Singh, K. K. 2000. The Saccharomyces cerevisiae Sln1p-Ssk1p two-component system mediates response to oxidative stress and in an oxidant-specific fashion. Free Radic. Biol. Med. 29:1043-1050. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka, K., M. Nakafuku, F. Tamanoi, Y. Kaziro, K. Matsumoto, and A. Toh-e. 1990. IRA2, a second gene of Saccharomyces cerevisiae that encodes a protein with a domain homologous to mammalian ras GTPase-activating protein. Mol. Cell. Biol. 10:4303-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thevelein, J. M., and J. H. de Winde. 1999. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33:904-918. [DOI] [PubMed] [Google Scholar]
- 72.Toda, T., S. Dhut, G. Superti-Furga, Y. Gotoh, E. Nishida, R. Sugiura, and T. Kuno. 1996. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol. Cell. Biol. 16:6752-6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torres, J., C. J. Di Como, E. Herrero, and M. A. De La Torre-Ruiz. 2002. Regulation of the cell integrity pathway by rapamycin-sensitive TOR function in budding yeast. J. Biol. Chem. 277:43495-43504. [DOI] [PubMed] [Google Scholar]
- 74.Torres, L., H. Martin, M. I. Garcia-Saez, J. Arroyo, M. Molina, M. Sanchez, and C. Nombela. 1991. A protein kinase gene complements the lytic phenotype of Saccharomyces cerevisiae lyt2 mutants. Mol. Microbiol. 5:2845-2854. [DOI] [PubMed] [Google Scholar]
- 75.Tsao, C.-C., Y.-T. Chen, and C.-Y. Lan. 2009. A small G protein Rhb1 and a GTPase-activating protein Tsc2 involved in nitrogen starvation-induced morphogenesis and cell wall integrity of Candida albicans. Fungal Genet. Biol. 46:126-136. [DOI] [PubMed] [Google Scholar]
- 76.Valiante, V., T. Heinekamp, R. Jain, A. Hartl, and A. A. Brakhage. 2008. The mitogen-activated protein kinase MpkA of Aspergillus fumigatus regulates cell wall signaling and oxidative stress response. Fungal Genet. Biol. 45:618-627. [DOI] [PubMed] [Google Scholar]
- 77.van der Rest, M. E., A. H. Kamminga, A. Nakano, Y. Anraku, B. Poolman, and W. N. Konings. 1995. The plasma membrane of Saccharomyces cerevisiae: structure, function, and biogenesis. Microbiol. Rev. 59:304-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verna, J., A. Lodder, K. Lee, A. Vagts, and R. Ballester. 1997. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94:13804-13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vilella, F., E. Herrero, J. Torres, and M. A. de la Torre-Ruiz. 2005. Pkc1 and the upstream elements of the cell integrity pathway in Saccharomyces cerevisiae, Rom2 and Mtl1, are required for cellular responses to oxidative stress. J. Biol. Chem. 280:9149-9159. [DOI] [PubMed] [Google Scholar]
- 80.Walker, L. A., C. A. Munro, I. de Bruijn, M. D. Lenardon, A. McKinnon, and N. A. Gow. 2008. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 4:e1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watanabe, Y., K. Irie, and K. Matsumoto. 1995. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 15:5740-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshida, T., T. Toda, and M. Yanagida. 1994. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J. Cell Sci. 107:1725-1735. [DOI] [PubMed] [Google Scholar]
- 83.Zaitsevskaya-Carter, T., and J. A. Cooper. 1997. Spm1, a stress-activated MAP kinase that regulates morphogenesis in S. pombe. EMBO J. 16:1318-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]