Abstract
Shewanella sp. strain HN-41 was previously shown to produce novel, photoactive, As-S nanotubes via the reduction of As(V) and S2O32− under anaerobic conditions. To determine if this ability was unique to this bacterium, 10 different Shewanella strains, including Shewanella sp. strain HN-41, Shewanella sp. strain PV-4, Shewanella alga BrY, Shewanella amazonensis SB2B, Shewanella denitrificans OS217, Shewanella oneidensis MR-1, Shewanella putrefaciens CN-32, S. putrefaciens IR-1, S. putrefaciens SP200, and S. putrefaciens W3-6-1, were examined for production of As-S nanotubes under standardized conditions. Of the 10 strains examined, three formed As-S nanotubes like those of strain HN-41. While Shewanella sp. strain HN-41 and S. putrefaciens CN-32 rapidly formed As-S precipitates in 7 days, strains S. alga BrY and S. oneidensis MR-1 reduced As(V) at a much lower rate and formed yellow As-S after 30 days. Electron microscopy, energy-dispersive X-ray spectroscopy, and extended X-ray absorption fine-structure spectroscopy analyses showed that the morphological and chemical properties of As-S formed by strains S. putrefaciens CN-32, S. alga BrY, and S. oneidensis MR-1 were similar to those previously determined for Shewanella sp. strain HN-41 As-S nanotubes. These studies indicated that the formation of As-S nanotubes is widespread among Shewanella strains and is closely related to bacterial growth and the reduction rate of As(V) and thiosulfate.
A number of bacterial strains have been shown to contribute to the formation of diverse arsenic minerals (4). If sulfide is present as a ligand for immobilization of arsenic, As-S precipitates often form. Desulfosporosinus auripigmentum, which can be isolated from lake sediments, reduces As(V) to As(III) and S(VI) to S(−II) during anaerobic respiration and forms a yellow arsenic sulfide precipitate (7). While Desulfovibrio strain Ben-RB also produces precipitated arsenic sulfide in culture media, As reduction was not correlated with energy conservation (6). Other taxonomically divergent microorganisms isolated from various arsenic-rich sites have also been shown to reduce As(V) to As(III) and form arsenic sulfide precipitates (1, 2).
We previously reported that Shewanella sp. strain HN-41 produces an extensive extracellular network of filamentous arsenic-sulfide (As-S) nanotubes via its dissimilatory metal-reducing activity (4). The As-S nanotubes, which formed via the reduction of As(V) and S2O32−, were initially amorphous As2S3 but evolved with increasing incubation time toward polycrystalline phases of the chalcogenide minerals realgar (AsS) and duranusite (As4S). Because the Shewanella As-S nanotubes behaved both as metals and as semiconductors, in terms of their electrical and photoconductive properties, respectively, it was postulated that they may provide useful materials for novel nano- and optoelectronic devices (4).
While several bacterial species have been shown to produce amorphous and particulate As-S precipitates (1, 2, 4, 7), the formation of the As-S nanotubes by other bacteria has not yet been described, suggesting that this may be a unique property of Shewanella strains. To test this hypothesis, 10 different Shewanella strains, including Shewanella sp. strains PV-4 and HN-41, Shewanella alga BrY, Shewanella amazonensis SB2B, Shewanella denitrificans OS217, Shewanella oneidensis MR-1, Shewanella putrefaciens CN-32, S. putrefaciens IR-1, S. putrefaciens SP200, and S. putrefaciens W3-6-1, were inoculated into HEPES-buffered basal medium (3, 5) containing 10 mM sodium dl-lactate as the electron donor and 5 mM arsenate (Na2HAsO4·7H2O) and 5 mM thiosulfate (Na2S2O3·5H2O) as the electron acceptors. All chemicals and methods for sample preparation and characterization used in this study were previously described (4).
Of the 10 different Shewanella strains examined, only four strains, Shewanella sp. strain HN-41, S. putrefaciens CN-32, S. alga BrY, and S. oneidensis MR-1, produced As-S yellow precipitates in culture medium following incubation in the presence of arsenate and thiosulfate. Shewanella sp. strain HN-41 and S. putrefaciens CN-32 produced yellow precipitates of As-S after 7 days of incubation, whereas S. alga BrY and S. oneidensis MR-1 produced only a small amount of visible precipitate after 30 days of incubation. The remainder of the tested Shewanella strains failed to produce yellow precipitates, regardless of incubation time.
The culture medium of the strains tested was periodically sampled during the bacterial incubation period to determine the concentrations of lactate, acetate, arsenic, and sulfide in the aqueous solution. Among the 10 strains examined, Shewanella strain HN-41, S. putrefaciens CN-32, S. alga BrY, and S. oneidensis MR-1 metabolized lactate in growth medium containing arsenate and thiosulfate (Table 1). Shewanella sp. strain HN-41 and S. putrefaciens CN-32 rapidly consumed lactate both as an electron donor and as a carbon source (see Fig. S1 in the supplemental material). Cultures of S. alga BrY and S. oneidensis MR-1 consumed ∼1.4 mM lactate after 7 days, while Shewanella sp. strain HN-41 and S. putrefaciens CN-32 consumed 1.7 mM and 2.3 mM lactate, respectively. Although S. putrefaciens CN-32 reduced As(V) in the culture medium supplemented with 5 mM As(V) as the sole electron acceptor, Shewanella sp. strain HN-41, S. alga BrY, and S. oneidensis MR-1 did not reduce As(V) and did not oxidize lactate to acetate (data not shown). Consequently, the latter three strains could not utilize As(V) as an electron acceptor for respiratory metabolism.
TABLE 1.
Influence of thiosulfate on the consumption of lactate, reduction of As(V), and formation of As-S nanotubes by Shewanella strains in medium containing lactate and 5 mM As(V)
| Shewanella strain | Consumption of lactate in medium supplemented with: |
Reduction of As(V) in medium supplemented with: |
Formation of As-S nanotubes in medium supplemented with As(V) and S2O32− after: |
|||
|---|---|---|---|---|---|---|
| S2O32− | No S2O32− | S2O32− | No S2O32− | 7 days | 30 days | |
| Shewanella sp. strain HN-41 | + | − | + | − | + | + |
| Shewanella sp. strain PV-4 | − | − | − | − | − | − |
| S. alga BrY | + | − | + | − | − | + |
| S. amazonensis SB2B | − | − | − | − | − | − |
| S. denitrificans OS217 | − | − | − | − | − | − |
| S. oneidensis MR-1 | + | − | + | − | − | + |
| S. putrefaciens CN-32 | + | + | + | + | + | + |
| S. putrefaciens IR-1 | − | − | − | − | − | − |
| S. putrefaciens SP200 | − | − | − | − | − | − |
| S. putrefaciens W3-6-1 | − | − | − | − | − | − |
In the presence of thiosulfate, however, Shewanella sp. strain HN-41 and S. putrefaciens CN-32 reduced As(V) to As(III) and thiosulfate to sulfide, and the lactate consumed was oxidized to acetate. Shewanella sp. strain HN-41 and S. putrefaciens CN-32 reduced 1.7 and 3 mM As(V) to As(III), respectively, based on determination of As(V) present at day 7. The reduction of As(V) by S. alga BrY (0.8 mM) and S. oneidensis MR-1 (0.5 mM) was relatively slower than that by Shewanella sp. strain HN-41 and S. putrefaciens CN-32 (see Fig. S1 in the supplemental material). The sulfide produced in aqueous phase by Shewanella sp. strain HN-41 and S. putrefaciens CN-32 initially increased to 150 μM and thereafter decreased to 20 μM, concomitantly with the formation of As-S precipitates (see Fig. S2 in the supplemental material).
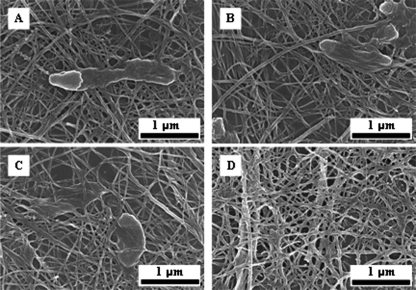
The As-S nanotubes produced by the Shewanella strains were examined for morphology by using scanning electron microscopy and for chemical analysis by using extended X-ray absorption fine-structure (EXAFS) spectroscopy at the Pohang Accelerator Laboratory in Pohang, Republic of Korea (4). Electron microscopic analyses revealed that S. alga BrY, S. oneidensis MR-1, and S. putrefaciens CN-32 produced filamentous As-S nanotubes (Fig. 1), similar to those formed by Shewanella sp. strain HN-41 (4). Energy-dispersive X-ray spectral analysis of single, filamentous, As-S nanotubes formed by S. alga BrY, S. oneidensis MR-1, and S. putrefaciens CN-32 showed As/S ratios of 1.23 ± 0.13, 1.34 ± 0.09, and 0.80 ± 0.03, respectively, which were greater than that (0.72 ± 0.03) found in the nanotubes produced by Shewanella sp. strain HN-41 (values are means ± standard deviations of six As-S nanotubes from each sample).
FIG. 1.
Scanning electron microscopic images of As-S nanotubes formed by Shewanella sp. strain HN-41 (A), S. putrefaciens CN-32 (B), S. alga BrY (C), and S. oneidensis MR-1 (D). Bars, 1 μm.
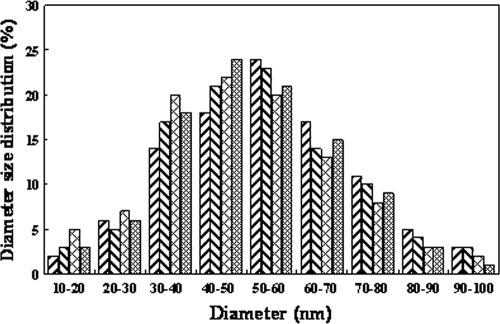
The main mineralogical components of the filamentous As-S nanotubes formed by S. alga BrY, S. oneidensis MR-1, and S. putrefaciens CN-32 were comprised of a mixture of several arsenic-rich As-S compounds, with increasing ratios of As to S (see above). The size distribution for the width of the As-S nanotubes formed by Shewanella sp. strain HN-41, S. putrefaciens CN-32, S. alga BrY, and S. oneidensis MR-1 was determined by measurement of 100 As-S nanotubes of each sample. Results of this analysis indicated that the As-S nanotubes had a major distribution range of 40 to 70 nm for Shewanella. sp. strain HN-41, whereas the other three strains examined produced nanotubes with widths of 30 to 60 nm (Fig. 2).
FIG. 2.
Diameter size distribution of As-S nanotubes produced by Shewanella sp. strain HN-41 ( ), S. putrefaciens CN-32 (
), S. putrefaciens CN-32 ( ), S. alga BrY (
), S. alga BrY ( ), and S. oneidensis MR-1 (
), and S. oneidensis MR-1 ( ). Diameter values were determined from the measurement of 100 As-S nanotubes.
). Diameter values were determined from the measurement of 100 As-S nanotubes.
Radial structure functions of the EXAFS spectra of the As-S nanotubes produced by S. alga BrY, S. oneidensis MR-1, and S. putrefaciens CN-32 showed single crest-peaks corresponding to As(III)-S(−II) bonding, similar to what was seen for the As-S nanotubes produced by Shewanella. sp. strain HN-41 (Fig. 3). Additional peaks found in the EXAFS data indicated that there were slight differences among the minerals formed by the strains.
FIG. 3.
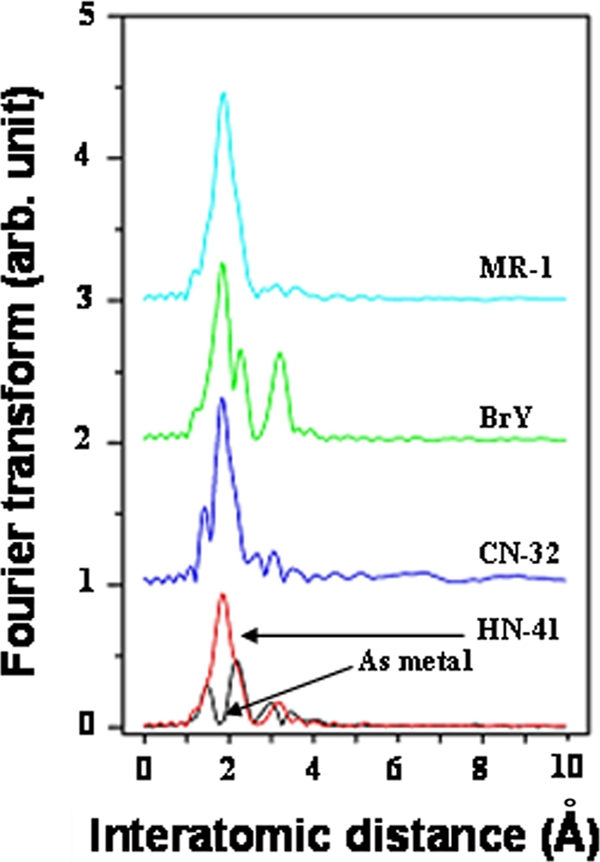
Fourier-transformed radial structure functions (in R-space Å) of EXAFS data from As metal and As-S nanotubes produced by Shewanella sp. strain HN-41, S. putrefaciens CN-32, S. alga BrY, and S. oneidensis MR-1.
The influence of temperature on the properties and formation of the As-S nanotubes by strains HN-41 and CN-32 was investigated. In addition to forming As-S nanotubes at 20°C, the two strains also formed As-S particle structures (see Fig. S3 in the supplemental material). Moreover, bacterial cultures incubated at 20°C produced about a twofold-greater concentration of sulfide in the liquid medium than that found at 30°C (see Fig. S4 in the supplemental material). Energy-dispersive X-ray spectroscopy analyses showed that the As-S particles produced at 20°C had an As/S ratio similar to that of the As-S nanotubes produced at 30°C (data not shown). Mineralogical alteration of the As-S nanotubes with time was also demonstrated by previous X-ray diffraction analyses, in which the ratio of As to S in the precipitates increased with time (4). This resulted in the formation of arsenic-rich phases consisting of As4S5, AsS, and As4S3. Taken together, these results indicate that physiological properties of the strains and abiological factors, including pH and concentration of S(−II) in the medium, also likely control the varied structures, properties, and stability of the As-S minerals and nanotubes formed by Shewanella strains (7).
In the past several years, various As-reducing microorganisms have been isolated (8, 9, 14, 15) and arsenic reduction has been explained by two mechanisms of respiratory and detoxification activities encoded by arr and ars genes, respectively (13). Shewanella sp. strain ANA-3 has been extensively studied to examine mechanisms of arsenate reduction (10-12).
In order to investigate the possible relationship between formation of the As-S nanotubes and arsenate reduction, four different Shewanella strains, which appeared to form the As-S nanotubes, were analyzed for the presence and structure of putative arrA and arsC genes found in the arsenic resistance operon found in Shewanella sp. strain ANA-3 (AY271310) (see Table S1 in the supplemental material). The ArrA and ArsC of Shewanella. sp. strain HN-41 and S. putrefaciens strain CN-32 showed 35.6 and 100%, and 93.7 and 100% protein sequence similarities, respectively, with the corresponding proteins encoded by the arr-ars operon from Shewanella sp. strain ANA-3 (AY271310). In contrast, S. oneidensis MR-1 did not have an identifiable arrA gene but contained a putative arsC gene with less than 60% protein sequence similarity with the ArsC from Shewanella sp. strain ANA-3. The genomic sequence of S. alga BrY is not available. While the mechanisms leading to the delayed formation of the As-S nanotubes by S. oneidensis MR-1 are not clearly understood, the rapid formation of the As-S nanotubes by Shewanella sp. strain HN-41 and S. putrefaciens CN-32 may be due to active arsenate reductase systems that are correlated with the presence of the arrA and/or arsC genes. Since control studies indicated that sulfide alone in a 20 mM concentration was not able to reduce arsenate (data not shown), arsenate reductase activity may be involved in formation of the As-S nanotubes by Shewanella. In addition, thiosulfate reduction may also influence the formation of As-S nanotubes.
In summary, the results of the current study indicate that several species and strains of Shewanella are able to synthesize As-S nanotubes via the combined reduction of arsenate and thiosulfate. Aside from important biogeological implications, the biogenic formation of one-dimensional As-S nanotubes may also greatly contribute to new, green, biosynthetic methods for the production of inorganic materials at nanoscales, which ultimately may find use in novel nano- and optoelectronic devices. However, to more fully utilize these new materials, more detailed physiological and biochemical studies are needed to better elucidate the mechanisms leading to the biogenic formation of the As-S nanotubes.
Supplementary Material
Acknowledgments
Support for this work from the 21C Frontier Microbial Genomics and Applications Center Program (M102KK010011-08K1101-01110), Ministry of Education, Science and Technology, Republic of Korea, to H.-G. Hur and the Korea Research Foundation (MOEHRD) (KRF-2007-357-D00141), Ministry of Education, Science and Technology, Republic of Korea, to J.-H. Lee is gratefully acknowledged.
Footnotes
Published ahead of print on 28 August 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Demergasso, C. S., C. D. Guillermo, E. G. Lorena, J. J. Pueyo Mur, and C. Pedrós-Alió. 2007. Microbial precipitation of arsenic sulfides in Andean salt flats. Geomicrobiol. J. 24:111-123. [Google Scholar]
- 2.Ledbetter, R. N., S. A. Connon, A. L. Neal, A. Dohnalkova, and T. S. Magnuson. 2007. Biogenic mineral production by a novel arsenic-metabolizing thermophilic bacterium from the Alvord Basin, Oregon. Appl. Environ. Microbiol. 73:5928-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee, J. H., J. H. Han, H. C. Choi, and H. G. Hur. 2007. Effects of temperature and dissolved oxygen on Se(IV) removal and Se(0) precipitation by Shewanella sp. HN-41. Chemosphere 68:1898-1905. [DOI] [PubMed] [Google Scholar]
- 4.Lee, J. H., M. G. Kim, B. Yoo, N. V. Myung, J. Maeng, T. Lee, A. C. Dohnalkova, J. K. Fredrickson, M. J. Sadowsky, and H. G. Hur. 2007. Biogenic formation of photoactive arsenic-sulfide nanotubes by Shewanella sp. strain HN-41. Proc. Natl. Acad. Sci. USA 104:20410-20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee, J. H., Y. Roh, K. W. Kim, and H. G. Hur. 2007. Organic acid-dependent iron mineral formation by a newly isolated iron-reducing bacterium, Shewanella sp. HN-41. Geomicrobiol. J. 24:31-41. [Google Scholar]
- 6.Macy, J. M., J. M. Santini, B. V. Pauling, A. H. O'Neill, and L. I. Sly. 2000. Two new arsenate/sulfate-reducing bacteria: mechanisms of arsenate reduction. Arch. Microbiol. 173:49-57. [DOI] [PubMed] [Google Scholar]
- 7.Newman, D. K., T. J. Beveridge, and F. M. M. Morel. 1997. Precipitation of arsenic trisulfide by Desulfotomaculum auripigmentum. Appl. Environ. Microbiol. 63:2022-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman, D. K., E. K. Kennedy, J. D. Coates, D. Ahmann, D. J. Ellis, D. R. Lovley, and F. M. M. Morel. 1997. Dissimilatory arsenate and sulfate reduction in Desulfotomaculum auripigmentum, sp. nov. Arch. Microbiol. 165:380-388. [DOI] [PubMed] [Google Scholar]
- 9.Oremland, R. S., and J. F. Stolz. 2005. Arsenic, microbes and contaminated aquifers. Trends Microbiol. 13:45-49. [DOI] [PubMed] [Google Scholar]
- 10.Saltikov, C. W., A. Cifuentes, K. Venkateswaran, and D. K. Newman. 2003. The ars detoxification system is advantageous but not required for As(V) respiration by the genetically tractable Shewanella species strain ANA-3. Appl. Environ. Microbiol. 69:2800-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saltikov, C. W., and D. K. Newman. 2003. Genetic identification of a respiratory arsenate reductase. Proc. Natl. Acad. Sci. USA 100:10983-10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saltikov, C. W., R. J. Wildman, and D. K. Newman. 2005. Expression dynamics of arsenic respiration and detoxification in Shewanella sp. strain ANA-3. J. Bacteriol. 187:7390-7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silver, S. 1998. Genes for all metals—a bacterial view of the periodic table. J. Ind. Microbiol. Biotechnol. 20:1-12. [DOI] [PubMed] [Google Scholar]
- 14.Stolz, J. F., P. Basu, J. M. Santini, and R. S. Oremland. 2006. Arsenic and selenium in microbial metabolism. Annu. Rev. Microbiol. 60:107-130. [DOI] [PubMed] [Google Scholar]
- 15.Stolz, J. F., and R. S. Oremland. 1999. Bacterial respiration of arsenic and selenium. FEMS Microbiol. Rev. 23:615-627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.