Abstract
T cells may interact with a number of bacterial surface antigens, an encounter which has the potential to downmodulate host immune responses. Neisseria meningitidis, a human colonizer and an agent of septicemia and meningitis, expresses Opa proteins which interact with the CEACAM1 receptor expressed on activated T cells. Since CEACAM1 can act as an inhibitory receptor and T cells in subepithelial tissues may encounter whole bacteria, which often express Opa proteins in vivo, this study assessed primarily if Opa proteins expressed on meningococci affect T-cell functions. In addition, Opa-containing outer membrane vesicles (OMV) have been used as vaccine antigens, and therefore Opa+ and Opa− OMV were also studied. While Opa+ bacteria adhered to CEACAM-expressing T cells, both the Opa+ and Opa− phenotypes induced no to a small transient depression, followed by a prolonged increase in proliferation as well as cytokine production. Such responses were also observed with heat-killed bacteria or OMV. In addition, while anti-CEACAM antibodies alone inhibited proliferation, on coincubation of T cells with bacteria and the antibodies, bacterial effects predominated and were Opa independent. Thus, while Opa proteins of N. meningitidis can bind to T-cell-expressed CEACAM1, this is not sufficient to overcome the T-cell recognition of bacterial factors, which results in a proliferative and cytokine response, an observation consistent with the ability of the host to establish lasting immunity to Opa-expressing meningococci that it frequently encounters. The data also imply that Opa-proficient vaccine preparations may not necessarily inhibit T-cell functions via CEACAM1 binding.
Neisseria meningitidis (meningococci) and Neisseria gonorrhoeae (gonococci), which are highly related at the genetic and antigenic levels, are human-specific mucosal bacteria capable of causing localized or systemic infection. N. meningitidis may colonize the human respiratory mucosal tissue of 3 to 30% of healthy individuals asymptomatically but, in some situations, may penetrate into deeper tissues to cause invasive diseases, such as septicemia and meningitis (5). N. gonorrhoeae may also be carried asymptomatically in a few individuals (13), but in most cases it causes localized infections of urogenital mucosa, and in a few untreated gonorrhea patients, disseminated infection may develop (20).
Immune responses to mucosal bacteria are initiated at mucosa-associated lymphoid tissue, where CD4+ T-cell priming occurs and results in the generation of effector and memory T cells (12). Bacterial colonization, and the subsequent disease process in susceptible individuals, begins with adhesion to specific receptors on human mucosal epithelial cells. N. meningitidis and N. gonorrhoeae express colony opacity-associated (Opa) proteins in vitro and in vivo that enable them to attach to human cells. It is now well established that the major receptors targeted by the Opa proteins belong to the CEACAM (carcinoembryonic antigen-related cell adhesion molecule) family of receptors (6, 44, 45). CEACAM1 is one of several related molecules expressed on human epithelial cells, endothelial cells, and leukocytes, but CEACAM1 is the only member of the family expressed on T cells (19, 28). CEACAM1 is a transmembrane molecule with either a long (L) or a short (S) cytoplasmic tail. CEACAM1-L, with a long cytoplasmic tail, contains two tyrosine residues which form part of modified immunoreceptor tyrosine-based activation/inhibition motifs (ITAM/ITIM motifs) (16). The relative abundance of the isoforms, which may be present simultaneously in CEACAM1-expressing tissues, may dictate the signaling outcomes of CEACAM1 ligation (35).
In addition, Opa structural variations may also affect bacterial specificity and affinity for distinct CEACAMs (10, 40, 44). N. meningitidis and N. gonorrhoeae possess multiple complete copies of opa genes (up to 4 and 11 genes, respectively), with the consequence that distinct isolates may express structurally variant Opa proteins. Variations within the Opa family of transmembrane proteins occur in three of the four surface-exposed loops. It has been shown for strains of distinct serogroups of N. meningitidis that these variations influence the specificity of Opa proteins for different members of the CEACAM family (10, 40). Different meningococcal isolates further possess a wide range of opa alleles, variable regions, and repertoires. Particular Opa repertoires appear to correlate with hyperinvasiveness and disease but not with the severity of meningococcal disease (4). The host cell interface where Opa proteins exert such an influence remains to be defined, and while several studies have assessed the potential of Opa proteins to influence meningococcal interactions with human epithelial cells (15, 44), a limited number of studies have examined their effect on T cells (23), and none have studied the potential of live Opa-expressing meningococci to influence T-cell functions.
Previous studies have shown that a number of neisserial outer membrane proteins can modulate T-cell function. Of these, TspA (T-cell-stimulating protein A), IgA1 protease, pili, and porins can induce a proliferative response in T cells (27, 31, 34, 38). In contrast, an interaction of Opa+ N. gonorrhoeae with CEACAM1 inhibited immune responses of CD4+ T cells (2) and B cells (26). In the case of T cells, the inhibitory signal delivered by the N. gonorrhoeae Opa-CEACAM1 interaction was reported to involve the phosphotyrosine phosphatases 1 and 2 (SHP-1 and SHP-2) that interact with ITIM (2, 24). Interestingly, engagement of Opa+ N. gonorrhoeae with CEACAM1 on B cells occurred independently of ITIM involvement (26). Overall, the above reports highlight the following two important points: (i) with respect to cellular activation, the end product of neisseria-target cell interactions may be determined by a number of distinct bacterial and host cell characteristics; and (ii) in the context of the consequences of bacterial engagement with CEACAM1, such an interaction may not always bring into play the expected consequences of its ITIM-like motifs. Other notable observations include the following. Outer membrane vesicles (OMV) of some Opa-expressing N. meningitidis strains have been reported to inhibit CD4+ T-cell function (23), which is in line with CD4+ T-cell-inhibiting effects of Opa+ N. gonorrhoeae (2). However, N. meningitidis carriage is regarded as an immunizing event and has been shown to induce lasting T-cell memory (7, 8).
Collectively, these previous reports highlight the need for comprehensive studies to define the consequences of meningococcal interactions with cells of the human immune system, particularly as mucosal bacteria are increasingly being shown to reside in subepithelial tissues, where they may encounter T cells. This may occur, and since no comprehensive studies are yet available on T-cell responses that may ensue upon encountering live N. meningitidis, and particularly on the influence of Opa proteins in general when presented in whole bacteria, the focus of this study was to assess the immune responses of CD4+ T cells to well-characterized phenotypes of live N. meningitidis and to compare these with responses to Escherichia coli cells either expressing or lacking meningococcal Opa proteins. In addition, we compared the effects of heat-killed N. meningitidis and OMV derived from a meningococcal serogroup B strain on T-cell proliferation. The latter preparations are likely to be used as vaccine preparations and therefore, in our view, warranted such studies. In order to assess the T-cell responses to other CEACAM-binding agents and to study the effects of bacterial presence when Opa-CEACAM interactions are inhibited, the studies included cross-linking antireceptor antibodies as well as a recombinant molecule, rD-7, which carries the CEACAM-binding motif of Moraxella catarrhalis UspA1 adhesin (17) and has the potential to block bacterial binding without inducing a signaling cascade in T cells because the recombinant peptide may not cross-link CEACAM1 on T cells.
In our studies, frequently, but not invariably, an early Opa-independent transient decrease in T-cell proliferation was observed. This phase was followed by a profound stimulatory effect on T-cell immune functions, as assessed by proliferation assays and cytokine responses. In contrast, using anti-CEACAM1 antibodies in analogous assays, a significant inhibition of T-cell proliferation was observed. Overall, these data show that a certain surface component(s) of pathogenic Neisseria, whose precise identity remains to be determined, can exert either mild inhibitory or strong stimulatory effects on CD4+ T cells and that, most importantly, the latter predominate. Thus, it appears that human CD4+ T cells respond positively to one or more bacterial antigens to overcome any inhibition that may be induced via the engagement of CEACAM1, perhaps representing an advanced counterstrategy of the host.
MATERIALS AND METHODS
Isolation of primary CD4+ T cells from peripheral blood.
Human blood was obtained from healthy volunteers, or alternatively, fresh buffy coats were obtained from the blood bank in accordance with local guidelines. No major quantitative differences were observed between T cells from distinct sources. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density gradient centrifugation. CD4+ T cells were isolated from PBMC by negative selection, using immunomagnetic separation (CD4+ T-cell isolation kit II; Miltenyi Biotec). The purity of isolated CD4+ T cells (including percentages of CD4+ CD3+ cells, B cells, and macrophages) was evaluated by flow cytometry. The cells were cultured in RPMI 1640 medium (Sigma) supplemented with 10% heat-inactivated fetal calf serum, 20 mM HEPES buffer (Sigma), and 1% l-glutamine and incubated at 37°C in a humidified atmosphere of 5% CO2 in air.
Flow cytometry antibodies.
The antibodies used for flow cytometry included anti-human CD4-allophycocyanin monoclonal antibody (MAb), anti-human CD69-phycoerythrin-cyanin 5 (PE-Cy5) (both purchased from Dako), anti-human CD3-allophycocyanin MAb (clone SK7; BD), and anti-human CD4-PE MAb (clone SK3; BD). For CEACAM1 staining, polyclonal rabbit anti-human CEA (A0115; Dako), which reacts with several CEACAM family members, was used as the primary antibody and anti-rabbit immunoglobulin G-PE (Sigma) was used as the secondary antibody. To estimate the numbers of contaminating B cells and macrophages, anti-human CD20-PE antibody (BD) was used for B cells and anti-human CD64-FITC antibody (BD) was used for myeloid cells.
Bacterial isolates and CEACAM-binding agents used in the study.
For studies on live bacteria, N. meningitidis strains used included Opa− Opc− (Opa−) and Opa+ Opc− (Opa+) strains, which were derived from a spontaneous acapsulate nonpiliated variant of serogroup A strain C751 (43) (Table 1). N. meningitidis cells were grown on brain heart infusion agar supplemented with 10% heated horse blood as described previously (42) or on GC agar (Difco) with IsoVitaleX enrichment (BD). N. gonorrhoeae strain P9 isolates used included P9-1 (Opa− Pil−) and P9-13 (Opa+ Pil−) and were cultured on GC agar (22, 41) (Table 1).
TABLE 1.
Bacterial strains and derivatives and their phenotypic characteristicsa
| Species | Serotype | Strain or variant | Presence of characteristic |
Reference | |||
|---|---|---|---|---|---|---|---|
| Capsule | Pili | Opc | Opa | ||||
| N. meningitidis | A | C751; Opa− | − | − | − | − | 43 |
| A | C751; Opa+ | − | − | − | OpaD | 43 | |
| B | H44/76 | − | + | − | − | 36; current study | |
| B | H44/76 | − | + | − | OpaJ | 36; current study | |
| E. coli | NA | PC2984 | NA | NA | NA | NA | 10 |
| NA | PC2984 | NA | NA | NA | OpaJ | 10 | |
| NA | PC2984 | NA | NA | NA | OpaB | 10 | |
| N. gonorrhoeae | P9-1 | NA | − | NA | − | 22, 41 | |
| P9-13 | NA | − | NA | Opaa | 22, 41 | ||
NA, not applicable. Note that other than the results presented in Fig. 6, all studies used derivatives of N. meningitidis strain C751 and/or N. gonorrhoeae strain P9.
For experiments using OMV or heat-killed N. meningitidis, acapsulate derivatives of strain H44/76 were used (39). These were an OpaJ-expressing strain (OpaJ+) and an Opa deletion mutant (Opa−) lacking all four Opa genes (our unpublished results). The Opa− strain was constructed by consecutive deletion of all four opa genes of H44/76, using the ermC-rpsL counterselection method described previously (18). Complete deletion of all four opa genes was confirmed by PCR. The absence or presence of Opa and Opc expression was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Escherichia coli strain PC2984 containing empty plasmid (wild type) or plasmid-containing derivatives expressing either OpaJ or OpaB derived from N. meningitidis strain H44/76 were grown at 37°C in Luria-Bertani medium supplemented with chloramphenicol (25 μg/ml) (9). E. coli strains with OpaB and OpaJ have been shown to bind to CEACAM1 (10).
N. meningitidis OMV were prepared as described previously, using a deoxycholate extraction method (14). OMV protein content was determined by the bicinchoninic acid method (Pierce). OMV suspensions were used at 50 μg/ml in the experiments. These OMV preparations were shown to contain Opa proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting (data not shown).
All bacteria were cultured at 37°C in a humidified atmosphere of 5% CO2 for 16 to 20 h and, when required, heat killed at 56°C for 30 min. Bacterial suspensions generally were used at a multiplicity of infection (MOI) of 200:1.
Adhesion of Neisseria to T cells and T-cell proliferation were examined in the absence or presence of the rabbit anti-CEACAM antibody A0115, previously used for similar studies (2), or the rat anti-CEACAM MAb YTH71.3, used at 25 to 100 μg/ml (2, 40). In some experiments, the CEACAM-binding recombinant polypeptide rD-7 (2 μg/ml) was used, with or without anti-rD-7 antiserum. The polypeptide rD-8Δ, which does not bind the receptor, was used as a control (17).
Bacterial adhesion and internalization assays.
Unstimulated or stimulated CD4+ T cells (see below) were infected with N. meningitidis or N. gonorrhoeae (MOI of 100 to 200:1) and incubated for 3 h at 37°C in a humidified atmosphere of 5% CO2. Cells and bacteria were transferred to a 5-μm Transwell filter (Costar, Corning, NY). To remove nonadherent bacteria, the filters were washed six times with Hanks' balanced salt solution (HBSS). The filters were then transferred to new wells, 200 μl of 1% saponin was added per filter for 30 min to release cell-associated bacteria, which were harvested by four 200-μl washes with HBSS, and suspensions of bacteria were plated after appropriate dilutions to estimate the CFU. To assess if bacteria were internalized, CD4+ T cells were activated with immobilized anti-CD3 (iCD3) antibody or interleukin-2 (IL-2) with soluble CD3 (sCD3) antibody before incubation with bacteria for 3 h, and then gentamicin (200 μg/ml) was added for 90 min to kill extracellular bacteria. At the end of this period, the cells were washed, 1% saponin was added to release any intracellular bacteria, and viable counts were performed.
IF detection of T-cell-associated bacteria.
For immunofluorescence (IF) detection experiments, CD4+ T cells were stimulated with IL-2 (200 U/ml) for 48 h before infection with Opa+ or Opa− N. meningitidis at an MOI of 100:1 and then were incubated for 3 h at 37°C. Cells and bacteria were transferred to 5-μm Transwell filters and washed as described above to remove nonadherent bacteria. CD4+ T cells with adherent bacteria were resuspended in HBSS, transferred to 96-well plates coated with poly-l-lysine (Sigma), and incubated for 1 h at room temperature. Once attached, the cells were fixed at −20°C for 10 min, using ice-cold methanol-acetone (1:1). After washing and blocking of cells with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 30 min, mouse anti-human CD3 MAb (OKT3; a gift from Neil Williams) and polyclonal antibodies raised against Neisseria were added, followed by anti-mouse-FITC and anti-rabbit-tetramethyl rhodamine isocyanate secondary antibodies (Sigma).
celELISA.
Aliquots of cells were removed for assessment of tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) levels by a cell-based enzyme-linked immunosorbent assay (celELISA) method as described previously (1). Under sterile conditions, 96-well plates (Maxisorp immunoplates; Nunc, Roskilde, Denmark) were coated overnight at 4°C with 4 μg/ml anti-TNF-α or anti-IFN-γ capture antibodies (Pharmingen) in carbonate-bicarbonate buffer (0.05 M, pH 9.6). Plates were washed in PBS-Tween (0.1%) and blocked with sterile PBS-BSA (1%) for 1 h at 37°C. The blocking solution was removed, and samples and standards were incubated at 37°C in a humidified atmosphere of 5% CO2 in air for 24 h. The plates were then washed and incubated with biotin-labeled anti-TNF-α or anti-IFN-γ antibodies (Pharmingen) at 1 μg/ml in PBS-BSA (1%). The plates were developed using ExtrAvidin peroxidase (Sigma) in PBS-BSA (1%) and tetramethylbenzidine (Sigma) substrate in phosphate citrate buffer, pH 5. The absorbance values were measured at 450 nm, and cytokine levels were calculated by regression analysis against a standard curve.
T-cell proliferation assay.
To examine the effect of Opa protein expression of live bacteria on proliferation of CD4+ T cells, two conditions that significantly upregulated CEACAM1 expression on CD4+ T cells were used. In the first condition (IL-2/sCD3), the cells were prestimulated with IL-2 (200 U/ml) for 48 h, and after washing of the cells, the sCD3 antibody OKT3 (1 μg/ml) was added simultaneously with bacteria and maintained throughout the experiment. In the second condition, the cells were coligated with iCD3 antibody by culturing the cells and bacteria simultaneously in 48-well plates coated with the anti-CD3 antibody (2 μg/ml). In a few experiments, anti-CD28 antibody was also used (see the legend to Fig. 2). In the majority of the experiments, CD4+ T cells (5 × 105 cells/well) were cultured in 48-well plates and infected with N. meningitidis or N. gonorrhoeae at an MOI of 100:1 to 200:1. Three hours after the start of infection, gentamicin (50 μg/ml) was added and maintained throughout the experimental time course to prevent bacterial overgrowth. At daily intervals after infection, T cells were pulsed with [3H]thymidine (0.5 μCi/well) for 6 h, and the incorporated radioactivity was measured using a liquid scintillation beta-counter (1450 Microbeta; LKB Wallac, Turku, Finland) and expressed as average counts per minute (cpm) of duplicate cultures.
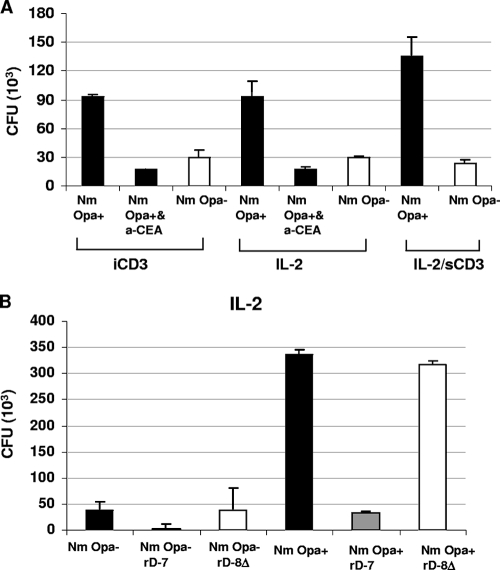
FIG. 2.
Effects of mode of CD4+ T-cell activation and blocking agents on bacterial adhesion. (A) For quantitative analysis of the binding of Opa+ or Opa− variants of N. meningitidis (Nm) to stimulated CD4+ T cells, various conditions were used. These included prestimulation of T cells with iCD3 antibody at 2 μg/ml and prestimulation with IL-2 at 200 U/ml. In both cases, after the stimulation period of 48 h, the cells were washed and infected with N. meningitidis in the absence or presence of anti-CEACAM antibody A0115 (a-CEA; 50 μg/ml). The condition labeled IL-2/sCD3 represents experiments in which IL-2-prestimulated T cells were inoculated with bacteria in the presence of anti-sCD3 antibody at 1 μg/ml. (B) CD4+ T cells were prestimulated with IL-2 for 48 h and then incubated for 1 h with recombinant polypeptide rD-7 (CEACAM-binding peptide) or rD-8Δ (control peptide) at 2 μg/ml before infection with N. meningitidis. Bacterial binding was assessed after a 3-h infection period by viable count assays. Binding of Opa− bacteria is also shown in each case for comparison.
To rule out any interference from bacterial presence on the [3H]thymidine cpm observed, the level of incorporation in bacteria alone (treated with gentamicin as described above) was determined. The counts recorded for blank wells and those containing bacteria alone were similar, and no increase in cpm was recorded over 2 days.
For evaluation of the effect of cell density on the cellular response, in some experiments T cells (2 × 105) were cultured in 24-well plates. The effect of bacterial numbers was also tested at MOIs of 20, 100, 200, and 500 bacteria/cell.
In addition, the effect of meningococcal Opa proteins on proliferation of CD4+ T cells was examined using OMV, heat-killed meningococci, or heat-killed transfected Escherichia coli. CD4+ T cells were prestimulated with IL-2 (1,000 U/ml) for 96 h. Following prestimulation, cells were washed and resuspended in PBS containing 5% heat-inactivated fetal calf serum and labeled by incubation with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) at 20°C for 5 min (29). Cells were then washed and resuspended in culture medium at 2 × 105/ml in a volume of 1 ml in flat-bottomed 24-well plates and incubated with anti-CEACAM1 antibody A0115 (2) or isotype control at 50 μg/ml, heat-killed bacteria at 4 × 107 CFU/ml (MOI, 200:1), or OMV at 50 μg/ml. Anti-sCD3 (1 μg/ml clone UCHT1; BD Pharmingen) and anti-sCD28 (1 μg/ml clone CD28.2; BD Pharmingen) were added simultaneously with heat-killed bacteria or OMV and maintained throughout the 72-h experiment. Proliferation was assessed by flow cytometric analysis of CFSE dilution (20,000 events/sample). Dead cells were excluded following staining with 7-aminoactinomycin D (Invitrogen).
Experimental replicates and data handling.
For bacterial adhesion experiments, assessments were done at least in triplicate, and mean values and standard errors of means are shown for one representative of at least two independent experiments. For proliferation assays, samples were set up in duplicate, and average values are shown for one typical experiment of several independent experiments. The range of values in most cases was within 20%, and was typically <12%, of the average value. The percent change in proliferation was expressed using the formula [(cpm test − cpm control)/cpm control] × 100. For CFSE proliferation assays, the numbers represent the percentages of divided cells, as measured by CFSE dilution (data shown are means of triplicate samples representative of three independent experiments).
RESULTS
Effects of neisserial Opa+ and Opa− phenotypes on CD69 expression and proliferation of unstimulated CD4+ T cells.
CD4+ T cells were isolated from PBMC by negative selection and contained mainly T cells (typically over 86% CD4+ CD3+ cells), with ≤1% contaminating B cells and ≤1% myeloid cells (monocytes, macrophages, and dendritic cells). Throughout the studies, T-cell proliferation was assessed either by [3H]thymidine incorporation or by CFSE dilution.
To observe Opa-CEACAM interactions without capsule interference, acapsulate N. meningitidis phenotypes were used (Table 1); notably, acapsulate N. meningitidis strains are found frequently in the nasopharynx. In initial studies, the relative effects of the bacteria on cell proliferation and CD69 expression of unstimulated CD4+ T cells were examined and are shown in Fig. S1 in the supplemental material. The data demonstrate an Opa-independent stimulation of naïve CD4+ T cells by neisserial isolates.
CEACAM1 expression on primary CD4+ T cells in response to distinct T-cell stimulatory factors.
In order to assess CEACAM1 expression on T cells stimulated via distinct agonists and to obtain optimum expression of CEACAM1 on CD4+ T cells, cellular responses to different stimuli were assessed, and CD69 was simultaneously monitored to confirm CD3-dependent activation (see Fig. S2 in the supplemental material). IL-2 prestimulation, IL-2 and anti-sCD3 preincubation, or iCD3 antibody preincubation increased CEACAM1 expression to high levels on more than 70% of CD4+ T cells.
Role of Opa proteins in direct interactions of Neisseria with activated T cells.
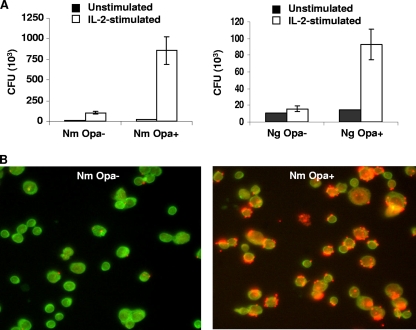
The binding of Opa− or Opa+ derivatives of N. meningitidis and N. gonorrhoeae to unstimulated and IL-2-prestimulated T cells was assessed by viable count assays. All Opa+ neisserial derivatives bound significantly more to stimulated than to unstimulated cells (Fig. 1A). Binding of N. meningitidis Opa+ and Opa− cells was confirmed by IF analysis (Fig. 1B), which showed that Opa+ N. meningitidis attached to about 90% of T cells.
FIG. 1.
Opa proteins increase direct binding of Neisseria to IL-2-stimulated CD4+ T cells. (A) Binding of Opa+ or Opa− variants of N. meningitidis (Nm) and N. gonorrhoeae (Ng) to unstimulated and IL-2-prestimulated CD4+ T cells. Bacterial binding was assessed after a 3-h infection period by viable count assays, and the means of quadruplicate estimations and standard errors (SE) are shown. (B) IF micrographs showing adherent N. meningitidis bacteria after 3 h of infection of T cells with Opa− or Opa+ variants. T cells were labeled with mouse anti-human CD3 antibody and anti-mouse FITC-conjugated secondary antibody, and bacteria were labeled with rabbit anti-neisseria antibody and anti-rabbit tetramethyl rhodamine isocyanate-conjugated secondary antibody. Data are from one representative of two experiments.
Bacterial internalization by activated CD4+ T cells was also assessed by gentamicin protection assays, as described in Materials and Methods. No intracellular bacteria could be demonstrated.
T-cell activation status versus bacterial adherence.
To examine if the activation status of T cells in the presence of different stimuli can affect the binding of N. meningitidis to T cells, CD4+ T cells were stimulated for 48 h with iCD3 antibody at 2 μg/ml or IL-2 at 200 U/ml, and then the cells were washed and infected with N. meningitidis. In some experiments, anti-CEA antibody A0115, known to disrupt Opa-CEACAM interaction, was included before bacterial infection. Bacterial adhesion was also assessed using IL-2-prestimulated cells and after the addition of anti-sCD3 at 1 μg/ml simultaneously with the addition of bacteria (IL-2/sCD3). Opa+ N. meningitidis cells bound to CD4+ T cells in larger numbers than did Opa− cells, and the activation status of T cells resulting from different stimuli did not affect this outcome (Fig. 2A). On blocking of CEACAM1 on T cells by treatment with the antibody A0115, the adhesion of Opa+ N. meningitidis was reduced significantly, demonstrating the role of CEACAM1 in adherence to activated T cells.
In our previous studies, the M. catarrhalis UspA1-based recombinant polypeptide rD-7 has been shown to inhibit the binding of several mucosal pathogens, including N. meningitidis, to a range of CEACAM-expressing cell lines (17). To assess its effect in the current studies, IL-2 stimulated CD4+ T cells were preincubated with rD-7 or a control peptide at 2 μg/ml for 1 h before the addition of N. meningitidis. The presence of rD-7, but not of control peptide, inhibited the binding of Opa+ N. meningitidis to T cells (Fig. 2B). These data demonstrate that the recombinant peptide rD-7 binds to T-cell CEACAM1 and additionally confirm the role of T-cell-expressed CEACAM1 in mediating Opa+ N. meningitidis adherence. These experiments also form the basis for further investigations described below.
Effect of neisserial Opa+ and Opa− phenotypes on proliferation of stimulated CD4+ T cells.
To choose the optimum assay design for the studies, the influences of bacterial MOI and cell density on CD4+ T-cell proliferation were first examined. The maximum effects were observed at an MOI of 200:1, and no significantly different responses were seen when T cells were seeded at different densities (see Fig. S3 in the supplemental material).
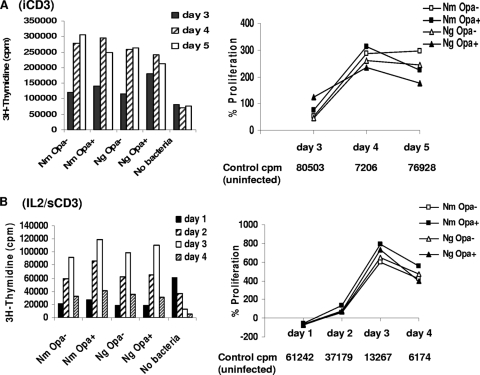
To assess further the effect of bacteria on CEACAM1 ligation, CD4+ T cells were stimulated under two sets of conditions that upregulated CEACAM1 expression significantly. As for adhesion, described above, these were (i) the cells and bacteria were incubated in 48-well plates coated with anti-CD3 antibody; and (ii) the cells were stimulated with IL-2 for 48 h, and after washing of the cells, anti-sCD3 was added together with bacteria. There were no significant differences in T-cell responses to Opa+ or Opa− cells under either of the conditions (Fig. 3), and both N. meningitidis and N. gonorrhoeae induced proliferation which was higher than that of controls on day 2 (Fig. 3B). The % proliferation compared to the controls reached values between 300 and 800% on days 3 and 4 (Fig. 3A and B). In IL-2-prestimulated cells (Fig. 3B), all neisserial strains caused transient depression at day 1, which was Opa independent. Summarizing early events observed in several experiments, it was apparent that in iCD3 antibody-stimulated cells, either no inhibition occurred at day 1 or there was initial inhibition at day 1, which recovered rapidly, a response similar to that for IL-2 shown in Fig. 3B.
FIG. 3.
Relative effects of neisserial Opa+ and Opa− phenotypes on proliferation of stimulated CD4+ T cells. To assess the effect of neisserial interactions with stimulated T cells on their proliferation, T cells were either stimulated with iCD3 antibody (A) or prestimulated with IL-2 followed by anti-sCD3 (IL-2/sCD3) (B). Raw data and percent changes in proliferation of infected T cells compared with uninfected controls (control cpm) are illustrated. Average values for [3H]thymidine incorporation from duplicate samples within an experiment are shown (SE, ±15%). The data are from one representative of four similar experiments. Nm, N. meningitidis; Ng, N. gonorrhoeae.
Effects of anti-CEACAM antibodies and recombinant polypeptide rD-7 on CD4+ T-cell proliferation.
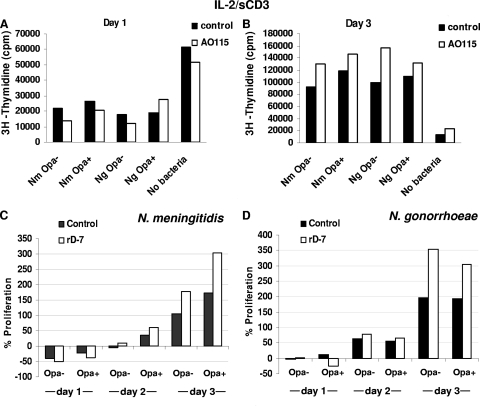
Since the ligation of bacteria with CEACAM1 did not affect T-cell proliferation, the effects of CEACAM-binding antibodies and recombinant peptide rD-7 were examined on T cells prestimulated under different conditions. The polyclonal anti-CEACAM antibody A0115 was expected to cross-link the receptor and accordingly inhibited T-cell proliferation (see Fig. S4 in the supplemental material). In contrast, the CEACAM-binding polypeptide rD-7 behaved as a monovalent ligand and did not affect T-cell proliferation, making it an ideal agent with which to investigate the whole neisserial effect on T cells when CEACAM1 binding is prevented without signaling (described below).
Effect of coincubation of T cells with bacteria and CEACAM-binding proteins on T-cell proliferation.
In order to examine how T cells respond to bacteria when their interactions with CEACAM1 are prevented by blocking antibodies, bacteria and blocking agents (A0115 and rD-7) were used simultaneously. IL-2-prestimulated T cells were incubated with rD-7 or the A0115 antibody for 1 h before bacteria were added in the presence of anti-sCD3 antibody. Under these conditions, Opa+ bacteria may be expected to behave like Opa− bacteria, since their adhesion to CEACAM will be inhibited by the agents, as shown in Fig. 2. In addition, CEACAM1 ligation with A0115 may also exert an additional effect, although rD-7 alone would be expected to affect only bacterial adhesion, as can be deduced from Fig. 2 and Fig. S4 in the supplemental material. In these experiments, A0115-mediated inhibition of T-cell proliferation was transient, and the effect was diminished by day 3 (see no-bacterium controls in Fig. 4A and B). With respect to N. meningitidis interactions, as in several other experiments, an Opa-independent transient inhibition was observed at early time points and recovered by day 2 onwards (Fig. 4).
FIG. 4.
Effect of costimulation of T cells with bacteria and CEACAM-binding proteins on proliferation of CD4+ T cells. CD4+ T cells were prestimulated with IL-2 for 48 h. After being washed, the cells were incubated with the antibody A0115 (50 μg/ml; A and B) or the recombinant polypeptide rD-7 (2 μg/ml; C and D) for 1 h before the addition of a live Opa+ or Opa− N. meningitidis (Nm) variant of strain C751 in the presence of anti-sCD3 antibody at 1 μg/ml. The results shown in panels C and D are % changes in proliferation relative to that in control cells and were calculated using average values for [3H]thymidine incorporation from duplicate samples. Ng, N. gonorrhoeae.
In general, the anti-CEACAM antibody A0115 had a slight inhibitory effect on bacterium-mediated T-cell proliferation on day 1 but not on day 3, and this effect was also independent of Opa expression (Fig. 4A and B). On the other hand, the addition of rD-7 with bacteria exaggerated bacterial effects on T-cell proliferation, but importantly, this was independent of Opa expression (Fig. 4C and D). Additionally, it can be surmised that since CEACAM-binding agents are unlikely to be affected in their interactions with T cells in the presence of largely nonadherent neisserial Opa− populations, these results suggest the overwhelming capacity of the bacteria to modulate T-cell function independently of CEACAM1 engagement.
Cytokine production by T cells in the presence of Neisseria.
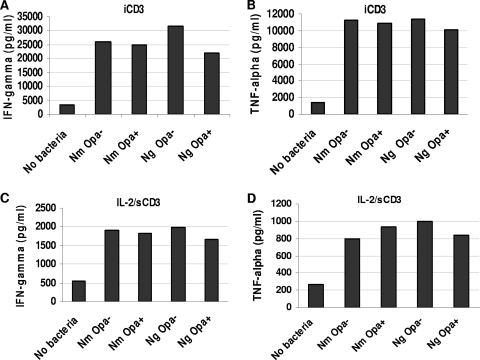
Proliferation of T cells is usually accompanied by cytokine production. To assess the profile and concentration of cytokines produced in response to the neisserial presence, primary CD4+ T cells were incubated with N. meningitidis or N. gonorrhoeae in the presence of iCD3 antibody or prestimulated with IL-2 for 48 h before the addition of bacteria in the presence of anti-sCD3 antibody. Both Opa+ and Opa− bacteria induced the production of IFN-γ and TNF-α significantly in the presence of anti-CD3 (Fig. 5). This increase in cytokine production was in accordance with the lasting and profound stimulatory effect of N. meningitidis and N. gonorrhoeae on T-cell proliferation, and notably, these data confirm the Opa/CEACAM-independent effects on T-cell functions.
FIG. 5.
Stimulation of cytokine production by CD4+ T cells upon neisserial infection. (A and B) CD4+ T cells were infected with N. meningitidis (Nm) or N. gonorrhoeae (Ng) in the presence of iCD3 antibody. (C and D) CD4+ T cells were prestimulated with IL-2 for 48 h before infection in the presence of anti-sCD3 antibody. Cytokine production was measured by celELISA on days 2 and 3 and expressed as pg/ml. The data are from one representative of two similar experiments.
T-cell response to Opa proteins presented in OMV, heat-inactivated N. meningitidis, or a heterologous background.
OMV of N. meningitidis have been used as vaccines, and therefore it was pertinent to examine if the presence of Opa proteins in OMV derived from a serogroup B strain of N. meningitidis could affect T-cell proliferation. In addition, since ligand accessibility may differ on whole bacteria versus vaccine preparations of OMV (deoxycholate extracted and lipopolysaccharide depleted) and may affect receptor binding, we further studied the effects of meningococcal Opa proteins presented in such OMV. In this context, cross-linking of multiple receptors by Opa proteins may be facilitated when presented on the OMV surface, since OMV with reduced levels of lipopolysaccharide are also smaller than whole bacteria. For comparison, we also studied the effects of whole heat-killed bacteria of the strains from which the OMV were derived. Opa− isolates of strain H44/76 expressed no Opa proteins, as all four opa genes were mutated, and none of the H44/76 isolates studied expressed Opc.
The CD4+ T cells used in the experiments were prestimulated with IL-2 (1,000 U/ml) for 96 h to upregulate CEACAM1 expression. To apply an independent method to assess T-cell proliferation, we used the CFSE dilution method. The validity of the experimental system was demonstrated by effective inhibition of T-cell proliferation by antibody ligation of CEACAM1 (Fig. 6A).
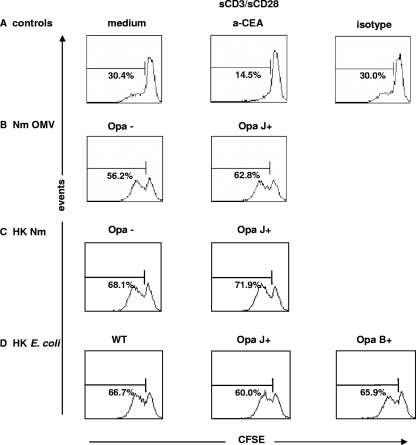
FIG. 6.
CD4+ T-cell responses to Opa proteins presented on OMV, heat-inactivated N. meningitidis, or a heterologous background. CD4+ T cells were prestimulated for 4 days with IL-2, labeled with CFSE, and then assessed for the ability to proliferate for 3 days in the presence of CD3/CD28. Additional agents included anti-CEACAM1 antibody A0115 or its isotype control (A), N. meningitidis strain H44/76 Opa− or OpaJ+ OMV (Nm OMV) (B), heat-killed N. meningitidis strain H44/76 Opa− or OpaJ+ (HK Nm) (C), and heat-killed wild-type OpaJ+ or OpaB+ E. coli (HK E. coli) (D). The number within each histogram represents the % divided cells in response to CD3/CD28, as measured by CFSE dilution (data are means of triplicate samples representative of three independent experiments).
In accordance with our observations with live meningococci, incubation with meningococcal OMV or heat-killed meningococci caused an increase in CD4+ T-cell proliferation compared to that with CD3/CD28 stimulation alone. Moreover, the presence of Opa proteins did not inhibit CD4+ T-cell proliferation (Fig. 6B and C). There was no significant difference in cell viability when using different stimuli.
In order to assess the role of Opa proteins in a delivery system independent of other meningococcal antigens, we used heat-killed E. coli transfected with meningococcal Opa proteins. Again, as with our previous findings, expression of Opa proteins did not inhibit CD3/CD28-induced CD4+ T-cell proliferation (Fig. 6D).
Finally, to assess if the live C751 isolates used in the earlier part of the study produced similar results in the CFSE dilution assays, IL-2-prestimulated T cells were infected with C751 Opa+ and Opa− bacteria. The flow cytometry profiles of controls and infected T cells were in agreement with the data presented in Fig. 6 (data not shown).
DISCUSSION
Natural immunity against N. meningitidis is thought to arise early in life through colonization by commensal Neisseria species, such as N. lactamica, and/or through carriage of N. meningitidis strains (37). N. meningitidis causes disease in only a small minority of carriers. However, N. gonorrhoeae appears to be controlled much less effectively by the human host. Such a lack of effective immunity may include factors such as frequent generation of new antigenic variants that allow immune evasion (33) or the ability of N. gonorrhoeae to suppress immune functions (2, 26). N. meningitidis and N. gonorrhoeae are highly related at the antigenic level. In addition, they express Opa proteins that bind to receptors on T cells and could downmodulate their functions. They also express other surface antigens that have been shown to induce T-cell proliferation to various extents (27, 31, 34, 38). However, such antigens may vary between species and strains.
Direct interactions of live N. meningitidis bacteria with T cells have not been investigated to date. This may also be important from the stance that the immune response to N. meningitidis, which in most adults is long-lived, is initiated in the mucosa-associated lymphoid tissue, where CD4+ T-cell priming could result in the generation of effector and memory T cells (7, 12).
In these studies, we aimed to assess how neisserial isolates expressing or lacking the expression of Opa proteins may interact directly with CD4+ T cells and to observe the resultant T-cell responses. In accordance with our previous observations and those of others for a variety of cell types expressing CEACAM1 (with constitutive expression as well as transfected or stimulated to express the receptor) (2, 3, 32, 40, 44), N. meningitidis variants expressing Opa proteins adhered in significantly greater numbers to CEACAM1-expressing T cells than did Opa− variants. Prior to this study, no formal demonstration of Opa-expressing N. meningitidis adhesion to T cells had been performed. Although this might have been expected, the subsequent T-cell responses to various neisserial phenotypes were surprising. N. meningitidis induced a lasting stimulatory effect on T-cell proliferation under all experimental conditions tested, a transient depression of T-cell proliferation was observed only under some experimental conditions, and these modulatory effects were Opa independent. In addition, there was no discernible difference between Opa+ and Opa− phenotypes in stimulating T-cell cytokine responses. Since our experiments used Opa− variants of strain C751 which retain the capacity to revert to Opa expression, we analyzed the potential enrichment of Opa+ bacteria in the T-cell-adherent population exposed to Opa− meningococci. Adhesion experiments and colony immunoblotting demonstrated that only a small proportion of T cells bound the CEACAM-binding (Opa+) revertants from Opa− inocula, and in very small numbers. Indeed, Opa− inocula represented an Opa+ MOI of <1:1 (data not shown). Thus, any modulation in proliferation induced by the revertants would be negligible (see Fig. S3 in the supplemental material, in which Opa+ N. meningitidis used at the minimum MOI of 20:1 had no significant effect on proliferation compared to controls without bacteria).
It was interesting that while Opa engagement with T cells had no lasting inhibitory effect on their proliferation, two anti-CEACAM antibodies mediated inhibition under similar experimental conditions, but the levels of inhibition varied with the T-cell stimulation conditions used. Accordingly, the responses of T cells upon CEACAM1 ligation reported previously have not been consistent. For example, under some experimental conditions, the engagement of CEACAM1 has been shown to enhance the proliferation of anti-CD3-stimulated T cells (11, 19). T-cell responses to Neisseria appear to be complex also. In vitro, a number of factors may contribute to the observed outcome, such as bacterial numbers, the nature of the stimulus, and the conditions of T-cell activation. In addition, CEACAM1 expression levels, and perhaps the relative ratios of receptor isoforms (CEACAM1-L versus CEACAM1-S), which could vary in different cells and according to the cellular activation state (35), could also determine the outcome. In the case of N. gonorrhoeae, it has been shown that both heparan sulfate proteoglycan-specific Opa50- and CEACAM-specific Opa52- expressing N. gonorrhoeae cells increased CD4+ T-cell proliferation in the presence of IL-2 but that only CEACAM-specific Opa52 inhibited proliferation in the presence of anti-CD3 (2). Increased proliferation of PBMC in response to purified Opa proteins has been reported previously (46). However, these studies used purified Opa proteins, which may not be functional and may not bind to T-cell CEACAMs. The T-cell response in this study is thus likely to be a response to the antigen presentation via the T-cell receptor rather than a direct response of Opa-CEACAM ligation on T cells.
Independently of CEACAM1, T-cell proliferative responses were also shown to be enhanced by meningococcal lysates or recombinant TspA (31). In studies on N. gonorrhoeae-T-cell interactions, it has been shown that Th1 and Th2 cytokines are upregulated by PBMC upon exposure to N. gonorrhoeae strain F62 (Opa− Pil+) (30). Additionally, the IgA1 protease of N. gonorrhoeae (MS11) induced T-cell proliferation, CD69 upregulation, and production of IFN-γ and TNF-α (38). Furthermore, gonococcal porin has been shown to induce the proliferation of T cells isolated from patients with urogenital gonococcal disease (34). In other studies, using piliated and nonpiliated gonococcal strains at MOIs of more than 20:1 resulted in CD4+ T-cell stimulation regardless of the piliation status of the bacterium. However, at an MOI of 20:1, the piliated strain induced greater T-cell proliferation than did the nonpiliated strain (27). Thus, clearly, both N. meningitidis and N. gonorrhoeae have been shown to stimulate T-cell responses.
To our knowledge, this is the first study to examine the direct interactions of live N. meningitidis isolates with T cells. However, previous studies have examined OMV of N. meningitidis containing CEACAM1-binding Opa proteins, which were found to suppress CD4+ T-lymphocyte function (23). In order to assess the role of Opa proteins of N. meningitidis in the context of other delivery systems that could be of interest in the development of novel vaccines against N. meningitidis, OMV of N. meningitidis, heat-killed bacteria, and Escherichia coli expressing CEACAM-binding Opa proteins were also examined. As for several other bacterial species, E. coli has also been shown to stimulate T-cell responses and to induce proliferation of human CD4+ T cells (21). Consistently, in our studies with transfected E. coli, CD4+ T-cell proliferation in response to CD3/CD28 was increased in the presence of heat-killed E. coli compared to that in medium-only controls. The data are consistent with the observations on live N. meningitidis.
Another notable point is that on inclusion of Opa+ or Opa− variants of N. meningitidis in addition to anti-CEACAM antibody in T-cell proliferation assays, the pattern of T-cell response was again not related to Opa expression but was dominated by other neisserial antigens not examined in the current study. Similar proliferative effects of unspecified gonococcal surface antigens have also been observed previously (27). Although somewhat unexpected, the data presented here suggest that human CD4+ T cells respond positively to one or more bacterial antigens to overcome any inhibition that may be induced via the engagement of CEACAM1, perhaps representing an advanced counterstrategy of the host. However, the pathogens have developed other strategies to overcome human immune responses, notably surface modulation, which enables evasion of innate and adaptive immunity.
Considerable variation has been reported for Opa alleles, variable regions, and repertoires between meningococcal isolates. Certain Opa repertoires appear to correlate with hyperinvasiveness and disease, although they do not appear to determine the severity of meningococcal disease (4). In view of the current studies, it would seem premature to assign any disease association of meningococcal Opa proteins in general to their effects on T-cell functions; their effects could be manifested with greater impact at other cellular interfaces (15, 25). In addition, strain differences may exist in Neisseria and may account for variations in the reported observations. Differences arising in neisserial strains that enable the depression of T-cell responses could prove to be beneficial to the survival of such clones. However, while from the studies reported here we can conclude that Opa proteins in several distinct genetic backgrounds do not necessarily inhibit T-cell functions, in view of the variations that exist in Opa alleles, the possibility remains that some of these may still have such an effect.
Supplementary Material
Acknowledgments
Research in M.V.'s laboratory is funded by The Wellcome Trust, BBSRC, and GlaxoSmithKline. M.V.D.F. was supported by an ESPID fellowship.
We thank Neil Williams for his helpful suggestions during the course of these studies and for his comments on the manuscript.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 31 August 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Beech, J. T., T. Bainbridge, and S. J. Thompson. 1997. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J. Immunol. Methods 205:163-168. [DOI] [PubMed] [Google Scholar]
- 2.Boulton, I. C., and S. D. Gray-Owen. 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 3:229-236. [DOI] [PubMed] [Google Scholar]
- 3.Bradley, C. J., N. J. Griffiths, H. A. Rowe, R. S. Heyderman, and M. Virji. 2005. Critical determinants of the interactions of capsule-expressing Neisseria meningitidis with host cells: the role of receptor density in increased cellular targeting via the outer membrane Opa proteins. Cell. Microbiol. 7:1490-1503. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan, M. J., C. Buckee, N. D. McCarthy, A. B. Ibarz Pavon, K. A. Jolley, S. Faust, S. J. Gray, E. B. Kaczmarski, M. Levin, J. S. Kroll, M. C. Maiden, and A. J. Pollard. 2008. Opa protein repertoires of disease-causing and carried meningococci. J. Clin. Microbiol. 46:3033-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartwright, K. A., and D. A. Ala'Aldeen. 1997. Neisseria meningitidis: clinical aspects. J. Infect. 34:15-19. [DOI] [PubMed] [Google Scholar]
- 6.Chen, T., and E. C. Gotschlich. 1996. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc. Natl. Acad. Sci. USA 93:14851-14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davenport, V., E. Groves, C. G. Hobbs, N. A. Williams, and R. S. Heyderman. 2007. Regulation of Th-1 T cell-dominated immunity to Neisseria meningitidis within the human mucosa. Cell. Microbiol. 9:1050-1061. [DOI] [PubMed] [Google Scholar]
- 8.Davenport, V., T. Guthrie, J. Findlow, R. Borrow, N. A. Williams, and R. S. Heyderman. 2003. Evidence for naturally acquired T cell-mediated mucosal immunity to Neisseria meningitidis. J. Immunol. 171:4263-4270. [DOI] [PubMed] [Google Scholar]
- 9.de Jonge, M. I., M. P. Bos, H. J. Hamstra, W. Jiskoot, P. van Ulsen, J. Tommassen, L. van Alphen, and P. van der Ley. 2002. Conformational analysis of opacity proteins from Neisseria meningitidis. Eur. J. Biochem. 269:5215-5223. [DOI] [PubMed] [Google Scholar]
- 10.de Jonge, M. I., H. J. Hamstra, L. van Alphen, J. Dankert, and P. van der Ley. 2003. Mapping the binding domains on meningococcal Opa proteins for CEACAM1 and CEA receptors. Mol. Microbiol. 50:1005-1015. [DOI] [PubMed] [Google Scholar]
- 11.Donda, A., L. Mori, A. Shamshiev, I. Carena, C. Mottet, M. H. Heim, C. Beglinger, F. Grunert, C. Rochlitz, L. Terracciano, P. Jantscheff, and G. De Libero. 2000. Locally inducible CD66a (CEACAM1) as an amplifier of the human intestinal T cell response. Eur. J. Immunol. 30:2593-2603. [DOI] [PubMed] [Google Scholar]
- 12.Ebert, L. M., P. Schaerli, and B. Moser. 2005. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol. Immunol. 42:799-809. [DOI] [PubMed] [Google Scholar]
- 13.Edwards, J. L., and M. A. Apicella. 2004. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 17:965-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frasch, C. E., L. van Alphen Holst, et al. 2001. Outer membrane protein vesicle vaccines for meningococcal disease. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 15.Griffiths, N. J., C. J. Bradley, R. S. Heyderman, and M. Virji. 2007. IFN-gamma amplifies NFkappaB-dependent Neisseria meningitidis invasion of epithelial cells via specific upregulation of CEA-related cell adhesion molecule 1. Cell. Microbiol. 9:2968-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammarstrom, S. 1999. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 9:67-81. [DOI] [PubMed] [Google Scholar]
- 17.Hill, D. J., A. M. Edwards, H. A. Rowe, and M. Virji. 2005. Carcinoembryonic antigen-related cell adhesion molecule (CEACAM)-binding recombinant polypeptide confers protection against infection by respiratory and urogenital pathogens. Mol. Microbiol. 55:1515-1527. [DOI] [PubMed] [Google Scholar]
- 18.Johnston, D. M., and J. G. Cannon. 1999. Construction of mutant strains of Neisseria gonorrhoeae lacking new antibiotic resistance markers using a two gene cassette with positive and negative selection. Gene 236:179-184. [DOI] [PubMed] [Google Scholar]
- 19.Kammerer, R., S. Hahn, B. B. Singer, J. S. Luo, and S. von Kleist. 1998. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur. J. Immunol. 28:3664-3674. [DOI] [PubMed] [Google Scholar]
- 20.Kerle, K. K., J. R. Mascola, and T. A. Miller. 1992. Disseminated gonococcal infection. Am. Fam. Physician 45:209-214. [PubMed] [Google Scholar]
- 21.Kersten, C. M., R. T. McCluskey, L. A. Boyle, and J. T. Kurnick. 1996. Escherichia coli and Pseudomonas aeruginosa induce expansion of V delta 2 cells in adult peripheral blood, but of V delta 1 cells in cord blood. J. Immunol. 157:1613-1619. [PubMed] [Google Scholar]
- 22.Lambden, P. R., J. E. Heckels, L. T. James, and P. J. Watt. 1979. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J. Gen. Microbiol. 114:305-312. [DOI] [PubMed] [Google Scholar]
- 23.Lee, H. S., I. C. Boulton, K. Reddin, H. Wong, D. Halliwell, O. Mandelboim, A. R. Gorringe, and S. D. Gray-Owen. 2007. Neisserial outer membrane vesicles bind the coinhibitory receptor carcinoembryonic antigen-related cellular adhesion molecule 1 and suppress CD4+ T-lymphocyte function. Infect. Immun. 75:4449-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, H. S., M. A. Ostrowski, and S. D. Gray-Owen. 2008. CEACAM1 dynamics during Neisseria gonorrhoeae suppression of CD4+ T lymphocyte activation. J. Immunol. 180:6827-6835. [DOI] [PubMed] [Google Scholar]
- 25.Muenzner, P., C. Dehio, T. Fujiwara, M. Achtman, T. F. Meyer, and S. D. Gray-Owen. 2000. Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infect. Immun. 68:3601-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantelic, M., Y. J. Kim, S. Bolland, I. Chen, J. Shively, and T. Chen. 2005. Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production. Infect. Immun. 73:4171-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plant, L. J., and A. B. Jonsson. 2006. Type IV pili of Neisseria gonorrhoeae influence the activation of human CD4+ T cells. Infect. Immun. 74:442-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prall, F., P. Nollau, M. Neumaier, H. D. Haubeck, Z. Drzeniek, U. Helmchen, T. Loning, and C. Wagener. 1996. CD66a (BGP), an adhesion molecule of the carcinoembryonic antigen family, is expressed in epithelium, endothelium, and myeloid cells in a wide range of normal human tissues. J. Histochem. Cytochem. 44:35-41. [DOI] [PubMed] [Google Scholar]
- 29.Quah, B. J., H. S. Warren, and C. R. Parish. 2007. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat. Protoc. 2:2049-2056. [DOI] [PubMed] [Google Scholar]
- 30.Rarick, M., C. McPheeters, S. Bright, A. Navis, J. Skefos, P. Sebastiani, and M. Montano. 2006. Evidence for cross-regulated cytokine response in human peripheral blood mononuclear cells exposed to whole gonococcal bacteria in vitro. Microb. Pathog. 40:261-270. [DOI] [PubMed] [Google Scholar]
- 31.Robinson, K., K. G. Wooldridge, D. B. Wells, A. Hasan, I. Todd, A. Robins, R. James, and D. A. Ala'Aldeen. 2005. T-cell-stimulating protein A elicits immune responses during meningococcal carriage and human disease. Infect. Immun. 73:4684-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowe, H. A., N. J. Griffiths, D. J. Hill, and M. Virji. 2007. Co-ordinate action of bacterial adhesins and human carcinoembryonic antigen receptors in enhanced cellular invasion by capsulate serum resistant Neisseria meningitidis. Cell. Microbiol. 9:154-168. [DOI] [PubMed] [Google Scholar]
- 33.Seifert, H. S., C. J. Wright, A. E. Jerse, M. S. Cohen, and J. G. Cannon. 1994. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Investig. 93:2744-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson, S. D., Y. Ho, P. A. Rice, and L. M. Wetzler. 1999. T lymphocyte response to Neisseria gonorrhoeae porin in individuals with mucosal gonococcal infections. J. Infect. Dis. 180:762-773. [DOI] [PubMed] [Google Scholar]
- 35.Singer, B. B., I. Scheffrahn, and B. Obrink. 2000. The tumor growth-inhibiting cell adhesion molecule CEACAM1 (C-CAM) is differently expressed in proliferating and quiescent epithelial cells and regulates cell proliferation. Cancer Res. 60:1236-1244. [PubMed] [Google Scholar]
- 36.Steeghs, L., H. de Cock, E. Evers, B. Zomer, J. Tommassen, and P. van der Ley. 2001. Outer membrane composition of a lipopolysaccharide-deficient Neisseria meningitidis mutant. EMBO J. 20:6937-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Troncoso, G., S. Sanchez, M. Moreda, M. T. Criado, and C. M. Ferreiros. 2000. Antigenic cross-reactivity between outer membrane proteins of Neisseria meningitidis and commensal Neisseria species. FEMS Immunol. Med. Microbiol. 27:103-109. [DOI] [PubMed] [Google Scholar]
- 38.Tsirpouchtsidis, A., R. Hurwitz, V. Brinkmann, T. F. Meyer, and G. Haas. 2002. Neisserial immunoglobulin A1 protease induces specific T-cell responses in humans. Infect. Immun. 70:335-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Ley, P., J. van der Biezen, and J. T. Poolman. 1995. Construction of Neisseria meningitidis strains carrying multiple chromosomal copies of the porA gene for use in the production of a multivalent outer membrane vesicle vaccine. Vaccine 13:401-407. [DOI] [PubMed] [Google Scholar]
- 40.Virji, M., D. Evans, A. Hadfield, F. Grunert, A. M. Teixeira, and S. M. Watt. 1999. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol. Microbiol. 34:538-551. [DOI] [PubMed] [Google Scholar]
- 41.Virji, M., and J. S. Everson. 1981. Comparative virulence of opacity variants of Neisseria gonorrhoeae strain P9. Infect. Immun. 31:965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virji, M., H. Kayhty, D. J. Ferguson, C. Alexandrescu, J. E. Heckels, and E. R. Moxon. 1991. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 5:1831-1841. [DOI] [PubMed] [Google Scholar]
- 43.Virji, M., K. Makepeace, D. J. Ferguson, M. Achtman, and E. R. Moxon. 1993. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol. Microbiol. 10:499-510. [DOI] [PubMed] [Google Scholar]
- 44.Virji, M., K. Makepeace, D. J. Ferguson, and S. M. Watt. 1996. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol. Microbiol. 22:941-950. [DOI] [PubMed] [Google Scholar]
- 45.Virji, M., S. M. Watt, S. Barker, K. Makepeace, and R. Doyonnas. 1996. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol. Microbiol. 22:929-939. [DOI] [PubMed] [Google Scholar]
- 46.Wiertz, E. J., A. Delvig, E. M. Donders, H. F. Brugghe, L. M. van Unen, H. A. Timmermans, M. Achtman, P. Hoogerhout, and J. T. Poolman. 1996. T-cell responses to outer membrane proteins of Neisseria meningitidis: comparative study of the Opa, Opc, and PorA proteins. Infect. Immun. 64:298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.